Abstract.
Trypanosoma cruzi is the etiologic agent of Chagas disease (CD), which can result in severe cardiomyopathy. Trypanosoma cruzi is endemic to the Americas, and of particular importance in Latin America. In the United States and other non-endemic countries, rising case numbers have also been observed. The currently used drugs are benznidazole (BNZ) and nifurtimox, which have limited efficacy during chronic infection. We repurposed itraconazole (ICZ), originally an antifungal, in combination with amiodarone (AMD), an antiarrhythmic, with the goal of interfering with T. cruzi infection. Human pluripotent stem cells (hiPSCs) were differentiated into cardiomyocytes (hiPSC-CMs). Vero cells or hiPSC-CMs were infected with T. cruzi trypomastigotes of the II or I strain in the presence of ICZ and/or AMD. After 48 hours, cells were Giemsa stained, and infection and multiplication were evaluated microscopically. Trypanosoma cruzi infection and multiplication were evalutated also by electron microscopy. BNZ was used as a reference compound. Cell metabolism in the presence of test substances was assessed. Itraconazole and AMD showed strain- and dose-dependent interference with T. cruzi infection and multiplication in Vero cells or hiPSC-CMs. Combinations of ICZ and AMD were more effective against T. cruzi than the single substances, or BNZ, without affecting host cell metabolism, and better preserving host cell integrity during infection. Our in vitro data in hiPSC-CMs suggest that a combination of ICZ and AMD might serve as a treatment option for CD in patients, but that different responses due to T. cruzi strain differences have to be taken into account.
INTRODUCTION
Chagas disease (CD, American trypanosomiasis) is caused by infection with the protozoan Trypanosoma cruzi. Worldwide, an estimated 8–20 million people are infected. Although the disease is largely endemic to the Americas, particularly Latin America where most of the infected persons reside, case numbers are rising worldwide.1–5 It is estimated that more than 300,000 individuals in the United States have CD, with up to 45,000 persons having developed resulting cardiomyopathies.6,7
Trypanosoma cruzi is spread by more than 130 triatomine insects, also referred to as “kissing bugs,” which transfer T. cruzi during their blood meals.8 From the bloodstream, T. cruzi trypanomastigotes can infect and replicate in various organs and cell types, such as macrophages, smooth and skeletal muscles, cardiomyocytes, and endothelial cells. There are six known clinically important T. cruzi strains, TcI–VI,9 which seem to have different geographical locations and preferences for different organs.10,11 After infection, CD presents with an acute phase, with only signs at the locus of the insect bite, followed by an often asymptomatic lifelong chronic phase. Infected persons are at risk of developing cardiac and/or digestive pathology.4 The most common and severe manifestation of CD is the cardiac form (CARD), causing congestive heart failure, arrhythmias, and conduction abnormalities, and frequently leading to stroke and sudden death. Treatment is presently benznidazole (BNZ) or nitrofurans, which are only recommended for treatment of the acute phase and early in chronic infection,12,13 have limited efficacy,14 and cause severe side effects at prolonged high-dose treatment.
We have decades-long experience in testing antifungal drugs. Because T. cruzi and fungi are eukaryotes, it can be expected that they share common metabolic pathways that can be exploited as potential drug targets. In fact, a number of antifungals have been shown to be active against T. cruzi in vitro and in animal models, among those are posaconazole and itraconazole (ICZ).15,16,17 To combine antitrypanosomal therapy with a strategy to support heart function, we studied effects of the antifungal azole ICZ in combination with the anti-arrhythmic amiodarone (AMD) on T. cruzi infection in Vero cells, as well as in human-induced pluripotent cell–derived cardiomyocytes (hiPSC-CMs).
MATERIALS AND METHODS
Materials.
Itraconazole (R51211) was obtained from Janssen Pharmaceutica (Oud Turnhout, Belgium). Amiodarone, BNZ, amphotericin B (AmB), Giemsa solution, Bouin’s fixative solution, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT), and menadione were purchased from Sigma-Aldrich (St. Louis, MO). Fetal calf serum (FCS), RPMI 1640 medium, and B27 supplement (serum free, containing insulin) were derived from Gibco (New York, NY). Matrigel™ was obtained from BD Biosciences, San Jose, CA. Millicell EZ chamber slides were purchased from Millipore (Burlington, MA).
Trypanosoma cruzi strains.
Trypanosoma cruzi strains TcII (TcY, ATCC 50832) and TcI (Sylvio, ATCC 50800) were studied.
Isolation of trypanomastigotes.
Culture-derived trypanomastigotes (TCTs) of the TcI or TcII strains were obtained from monolayers of Vero cells (African green monkey kidney cells; ATCC; CCL-81) that had been infected at a ratio of 5:1 (TCTs: Vero cells). Vero cells were incubated at 37°C in RPMI 1640, enriched with 5% inactivated FCS, and supplemented with antibiotics (penicillin 500 μ/mL and streptomycin 0.5 mg/mL) for 7–9 days. Parasites were collected from the culture supernatants by centrifugation at 1000×g for 10 minutes and the sediment was suspended in RPMI + 5% fetal bovine serum. Parasites were counted using a Neubauer chamber and the number was adjusted according to assay needs.
Differentiation of hiPSC-CMs.
Reprogramming with Sendai virus was used to generate hiPSC lines from peripheral blood mononuclear cells of healthy individuals, according to previously published protocols.18,19 Human pluripotent stem cell lines were differentiated into hiPSC-CMs using a two-dimensional monolayer differentiation protocol and were maintained in a 5% CO2 + 95% air environment, and characterized as described previously, with respect to gene expression, protein expression, and electrophysiologic profiles.20–22
Infection with T. cruzi.
Monolayers of Vero cells or hiPSC-CMs were prepared on Matrigel-coated eight-well chamber slides at a density of 2 × 105 cells/well and cultivated for 48 hour at 37°C in a 5% CO2 atmosphere. When using hiPSC-CMs, chamber slides were pre-coated with Matrigel, and RPMI 1640 with 5% FCS was supplemented with B27. Infection was carried out at a target effector ratio of 1:5 (cell:parasite), with 24-hour interaction time in the presence of drugs, before replacing the medium with RPMI with 5% FCS ± B27. Twenty-four hours after infection, cells were washed with phosphate-buffered saline, fixed with Boudin’s solution, and Giemsa stained. The number of infected cells (defined as at least one amastigote/cell), as well as the number of amastigotes per infected cells (multiplication), was determined for each vision field by microscopy. About 25–40% of all cells in control wells (no drugs) were found to be infected by TcII, whereas TcI-infected cells to 80%, when used at the same infection ratio. In comparison to drug-treated wells, control infections (without the presence of drugs) were regarded as 100%.
Cell metabolism.
Viability of uninfected cells was determined by XTT metabolic assay.23 Into a 96-well plate, 105 cells were seeded and allowed to settle for 24 hours at 37°C at 5% CO2 and 80% humidity. After medium change, drugs were added, and cells were incubated for 24 hours at 37°C at 5% CO2 and 80% humidity. XTT with menadione (200 μg/mL, 40 μM in a volume of 100 μL) in RPMI with 5% FCS (containing B27 for cardiomyocyte tests) were added to each well and incubated at 37°C. Tests were evaluated using a plate reader (Opsys MR; DYNEX Technologies, Chantilly, VA).
Electron microscopy (EM).
Human-induced pluripotent cell–derived cardiomyocytes were infected with T. cruzi type II as described previously. Infected cells were grown in multi-well chambers, fixed in a mixture of 4% paraformaldehyde with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3), and postfixed in 1% aqueous osmium tetroxide (1 hour) and 1% uranyl acetate (overnight). Samples were dehydrated in an increasing ethanol series (50–70–90–100%, 10 minutes each) and propylene oxide (100%, 2 × 15 minutes) before gradual infiltration with Embed 812 resin (25%, 50%, 75%, and 100% twice, 4 hours each), before final embedding in 100% EMbed 812 (SPI supplies, Johannesburg, South Africa) and polymerization for 48 hours at 60°C. Samples were trimmed for ultramicrotomy (Leica EM UC6, Leica Microsystems, Wetzlar, Germany) and ultrathin sections (100 nm) collected on silicon wafers and post-stained with 2% uranyl acetate (20 minutes) and Reynold’s lead citrate (2 minutes). Sections were visualized using a Zeiss MERLIN field emission scanning EM (Carl Zeiss Microscopy, Jena, Germany) operated at 6–8 kV acceleration voltage, 10 nA probe current, and using backscattered electron detection. Electron images were captured as tagged image file format files using a pixel averaging noise reduction algorithm.
Statistical analysis.
Results were analyzed using Student’s t-test if two equal groups were compared, or by Student’s t-test with Welsh modification if the two groups were of unequal sample sizes.
RESULTS
Effects of AMD and ICZ on infection of Vero cells with T. cruzi strain TcII.
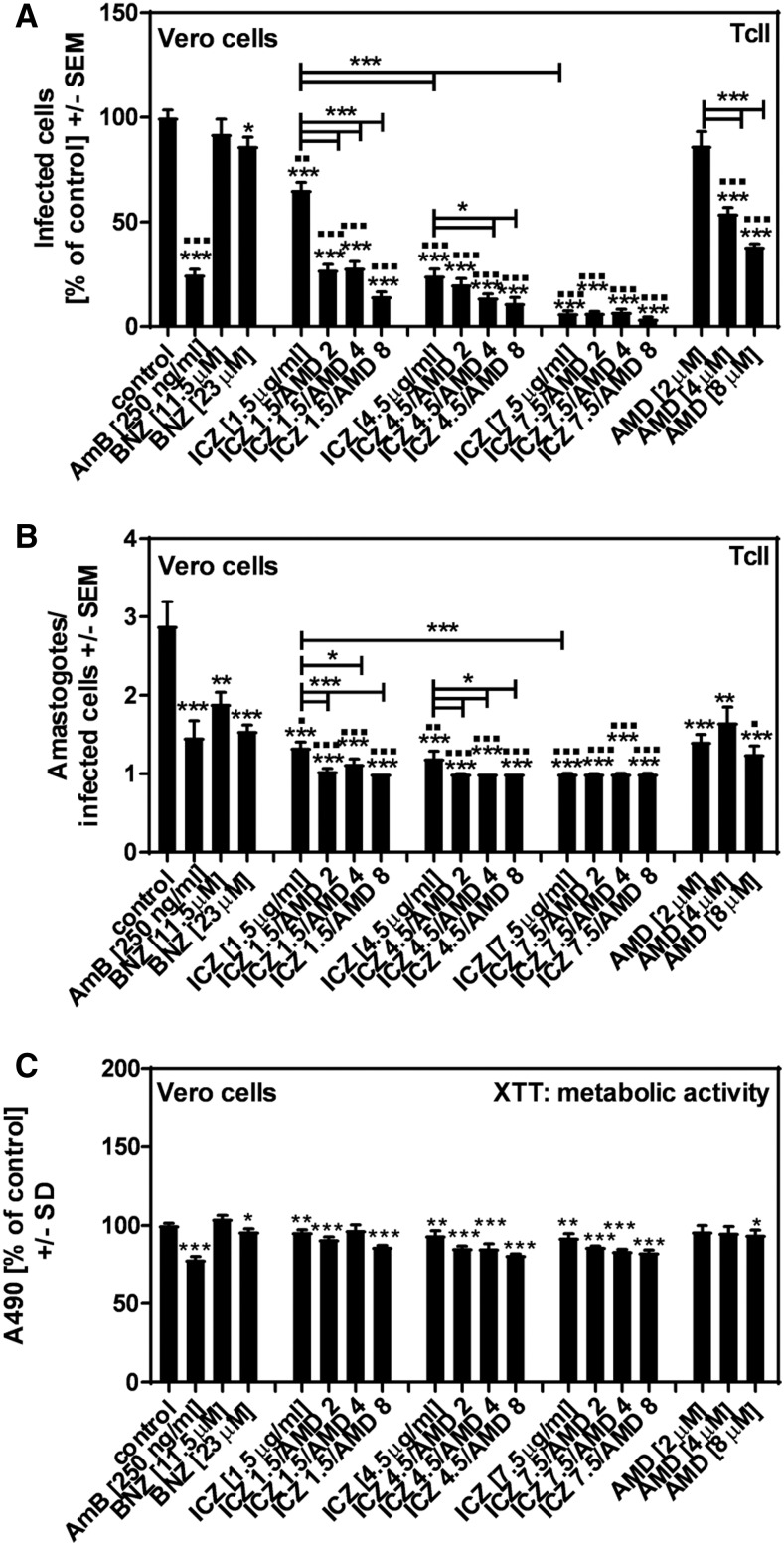
When Vero cells were infected with TcII in the presence of BNZ, we observed dose-dependent effects on both T. cruzi infection (Figure 1A) and multiplication (Figure 1B). Amphotericin B showed strong effects against TcII infection and multiplication as well (Figure 1A and B). Amiodarone or ICZ alone dose-dependently inhibited TcII infection (Figure 1A), and reduced multiplication (Figure 1B). In groups containing ICZ (1.5 μg/mL or 4.5 μg/mL), AMD dose-dependently increased effects against TcII infection (Figure 1A), and multiplication (Figure 1B). Anti-TcII effects of ICZ (7.5 μg/mL) were not further increased by AMD (Figure 1A and B). All ICZ combinations with AMD were more effective against TcII infection than AMD alone at a given concentration (all P ≤ 0.001). Regarding TcII multiplication, all AMD + ICZ combinations were more effective than AMD alone at a given concentration (Table 1).
Figure 1.
Effects of amiodarone (AMD) and itraconazole (ICZ) on infection of Vero cells with the Trypanosoma cruzi strain TcII. Vero cells (2 × 105/well) were infected with TcII at a ratio of five trypanosomes/cell for 24 hours at 37°C at 5% CO2 and 80% humidity. Infections took place in the presence of amphotericin B (250 ng/mL), benznidazole (BNZ) (11.5 or 23 μM), ICZ (1.5, 4.5, or 7.5 μg/mL), AMD (2, 4, or 8 μM), or ICZ/AMD combinations. Infection rates (A) and multiplication (B) were determined 48 hours after infection. Cell damage was determined by XTT assay (C). Statistics: Welch’s t-test. Comparisons without brackets: asterisks, RPMI vs. all other bars; squares, BNZ (23 μM) vs. all other bars. Other comparisons as indicated by the ends of the brackets. One, two, or three asterisks or squares = P ≤ 0.05, P ≤ 0.01, or P ≤ 0.001, respectively.
Table 1.
Effects of AMD + ICZ combinations vs. AMD alone on TcII multiplication in Vero cells (P-value)
| ICZ (1.5 μg/mL) | ICZ (4.5 μg/mL) | ICZ (7.5 μg/mL) | |
|---|---|---|---|
| AMD (2 μM) | 0.0003 | < 0.0001 | < 0.0001 |
| AMD (4 μM) | 0.0139 | 0.0018 | 0.0018 |
| AMD (8 μM) | 0.0202 | 0.0204 | 0.0216 |
AMD = amiodarone; ICZ = itraconazole. The P-value is derived from the combinations of ICZ dose (columns), and AMD dose (rows) vs. the same AMD dose alone.
Itraconazole, AMD, and all ICZ + AMD combinations (with the exception of AMD [2 μM]), as well as AmB, showed stronger effects on TcII infection than BNZ (23 μM), a concentration equivalent to the highest dose therapeutic serum concentration (squares in Figure 1A). Effects of ICZ, and ICZ + AMD combinations on TcII multiplication also were stronger than effects of BNZ (23 μM) (squares in Figure 1B).
Amphotericin B, as well as ICZ, and all AMD + ICZ combinations significantly affected Vero cell metabolism (Figure 1C).
Effects of AMD and ICZ on infection of hiPSC-CMs with TcII.
Infection of hiPSC-CMs with TcII, as well as TcII multiplication, was affected by BNZ (dose-dependently), and by AmB (Figure 2A and B). Itraconazole or AMD alone dose-dependently inhibited TcII infection (Figure 2A), and reduced multiplication (Figure 2B). In all groups, AMD dose-dependently increased effects of ICZ against TcII infection (Figure 2A). In the ICZ (1.5 μg/mL) + AMD groups, AMD also dose-dependently increased effects against TcII multiplication (Figure 2B). Several ICZ + AMD combinations were more effective against TcII infection (Table 2) or multiplication (Table 3) than AMD alone at a given concentration.
Figure 2.
Effects of amiodarone (AMD) and itraconazole (ICZ) on infection of human-induced pluripotent cell–derived cardiomyocytes with TcII. Human-induced pluripotent cell–derived cardiomyocytes (2 × 105/well) were infected with TcII at a ratio of five trypanosomes/cell for 24 hours at 37°C at 5% CO2 and 80% humidity. Infections took place in the presence of amphotericin B (250 ng/mL), benznidazole (BNZ) (11.5 or 23 μM), ICZ (1.5, 4.5, or 7.5 μg/mL), AMD (2, 4, or 8 μM), or ICZ + AMD combinations. Infection rates (A) and multiplication (B) were determined 48 hours after infection. Statistics: Welch’s t-test. Comparisons without brackets: asterisks, RPMI vs. all other bars; squares, BNZ (23 μM) vs. all other bars. Other comparisons as indicated by the ends of the brackets. One, two, or three asterisks or squares = P ≤ 0.05, P ≤ 0.01, or P ≤ 0.001, respectively.
Table 2.
Effects of AMD + ICZ combinations vs. AMD alone on TcII infection in human-induced pluripotent cell–derived cardiomyocytes (P-value)
| ICZ (1.5 μg/mL) | ICZ (4.5 μg/mL) | ICZ (7.5 μg/mL) | |
|---|---|---|---|
| AMD (2 μM) | – | < 0.0001 | < 0.0001 |
| AMD (4 μM) | < 0.0001 | < 0.0001 | < 0.0001 |
| AMD (8 μM) | – | < 0.0001 | < 0.0001 |
AMD = amiodarone; ICZ = itraconazole. The P-value is derived from the combinations of ICZ dose (columns), and AMD dose (rows) vs. the same AMD dose alone.
Table 3.
Effects of AMD + ICZ combinations vs. AMD alone on TcII multiplication in human-induced pluripotent cell–derived cardiomyocytes (P-value)
| ICZ (1.5 μg/mL) | ICZ (4.5 μg/mL) | ICZ (7.5 μg/mL) | |
|---|---|---|---|
| AMD (2 μM) | – | < 0.0114 | < 0.0001 |
| AMD (4 μM) | – | < 0.0001 | < 0.0001 |
| AMD (8 μM) | – | – | < 0.0001 |
AMD = amiodarone; ICZ = itraconazole. The P-value is derived from the combinations of ICZ dose (columns), and AMD dose (rows) vs. the same AMD dose alone.
When combining the results shown in Figure 2A and Table 2, the following ICZ + AMD combinations were found to be more effective against TcII infection than both single substances: ICZ (4.5 μg/mL) + AMD (2, 4, or 8 μM), and ICZ (7.5 μg/mL) + AMD (2, 4, or 8 μM). Benznidazole had stronger effects on cardiomyocyte infection than were observed for Vero cell infection (compare Figure 2A with Figure 1A). Itraconazole (7.5 μg/mL) alone, as well as all combinations of ICZ (4.5 or 7.5 μg/mL) with AMD, showed significantly stronger effects on TcII infection than BNZ (23 μM) (Figure 2A). Only the combination ICZ (7.5 μg/mL) + (AMD 8 μM) showed stronger effects against TcII multiplication than BNZ (23 μM) (Figure 2B).
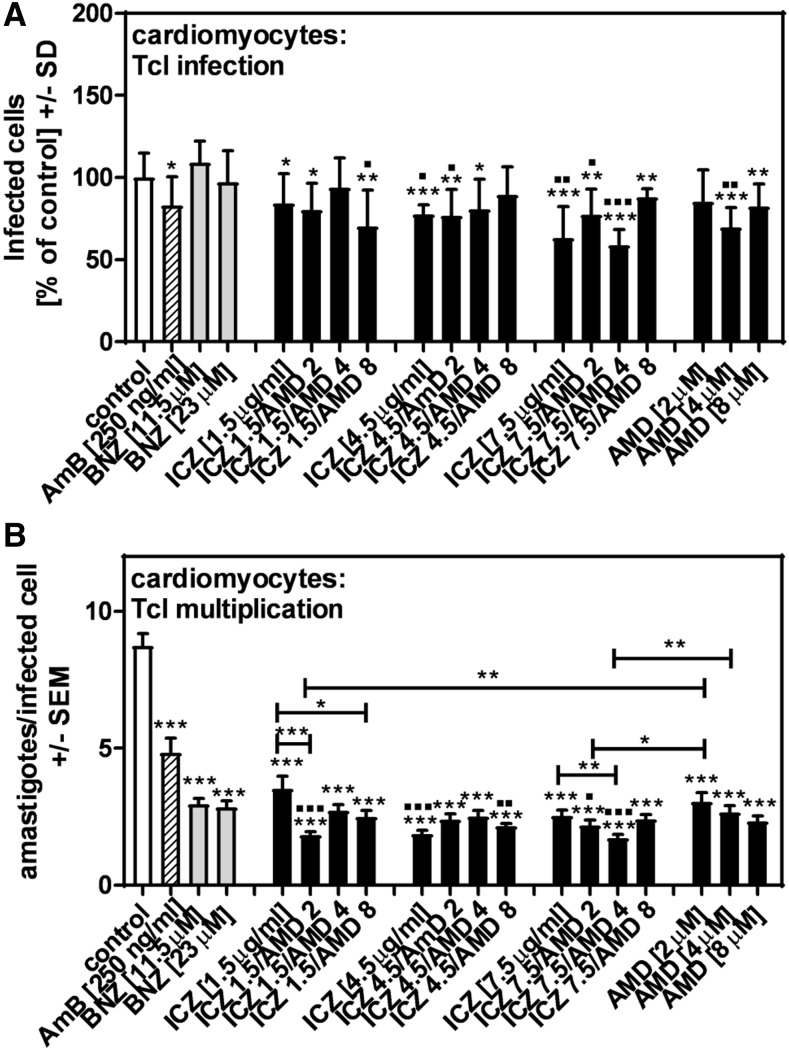
Effects of AMD and ICZ on infection of hiPSC-CMs with TcI.
Infection of hiPSC-CMs with TcI was not significantly affected by BNZ, and only slightly affected by AmB (Figure 3A). Itraconazole or AMD alone inhibited TcI infection (Figure 3A). There was no clear evidence for a benefit or detriment of ICZ + AMD combinations over the single substances (Figure 3A). With regard to TcI multiplication, BNZ as well as AmB showed significant effects, along with ICZ or AMD, as well as all ICZ + AMD combinations (Figure 3B). The following ICZ + AMD combinations were found to be more effective against TcI multiplication than either single substances: ICZ (4.5 μg/mL) + AMD (2 μM), and ICZ (4.5 μg/mL) + AMD (4 μM). In comparison to BNZ (23 μM), ICZ (4.5 or 7.5 μM) alone, AMD (4 μM) alone, and the following ICZ + AMD combinations showed stronger effects on TcI infection: ICZ (1.5 μg/mL) + AMD (8 μM), ICZ (4.5 μg/mL) + AMD (4 μM), and ICZ (7.5 μg/mL) + AMD (2 or 4 μM) (Figure 3A). Only ICZ (4.5 μg/mL) and the combinations ICZ (4.5 μg/mL) + AMD (8 μM), and ICZ (7.5 μg/mL) + AMD (2 or 4 μM) showed stronger effects against TcI multiplication than BNZ (23 uM) (Figure 3B).
Figure 3.
Effects of amiodarone (AMD) and itraconazole (ICZ) on infection of human-induced pluripotent cell–derived cardiomyocytes with TcI. Human-induced pluripotent cell–derived cardiomyocytes (2 × 105/well) were infected with TcI at a ratio of five trypanosoma/cell for 24 hours at 37°C at 5% CO2 and 80% humidity. Infections took place in the presence of amphotericin B (250 ng/mL), benznidazole (BNZ) (11.5 or 23 μM), ICZ (1.5, 4.5, or 7.5 μg/mL), AMD (2, 4, or 8 μM), or ICZ + AMD combinations. Infection rates (A) and multiplication (B) were determined 48 hours after infection. Statistics: Welch’s t-test. Comparisons without brackets: asterisks, RPMI vs. all other bars; squares, BNZ (23 μM) vs. all other bars. Other comparisons as indicated by the ends of the brackets. One, two, or three asterisks or squares = P ≤ 0.05, P ≤ 0.01, or P ≤ 0.001, respectively.
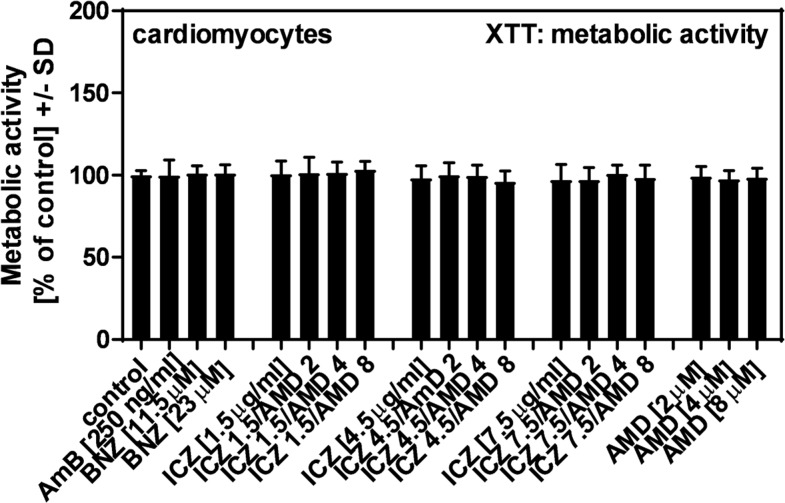
Effects of ICZ, AMD, or their combinations on hiPSC-CM metabolism.
As shown in Figure 4, none of the single substances or combinations indicated cell damage of hiPSC-CMs. This result corresponded to observations during microscopy, where no signs of cell stress were detected while analyzing Giemsa-stained cells (data not shown).
Figure 4.
Effects of itraconazole (ICZ), amiodarone (AMD), or their combinations on human-induced pluripotent cell–derived cardiomyocyte metabolism. Human-induced pluripotent cell–derived cardiomyocytes (2 × 105/well) were incubated with amphotericin B (250 ng/mL), benznidazole (11.5 or 23 μM), ICZ (1.5, 4.5, or 7.5 μg/mL), AMD (2, 4, or 8 μM), or ICZ + AMD combinations for 24 hours at 37°C at 5% CO2 and 80% humidity. Cell damage was determined by XTT assay. Statistics: t-test, RPMI vs. all other bars. No significant differences were detected.
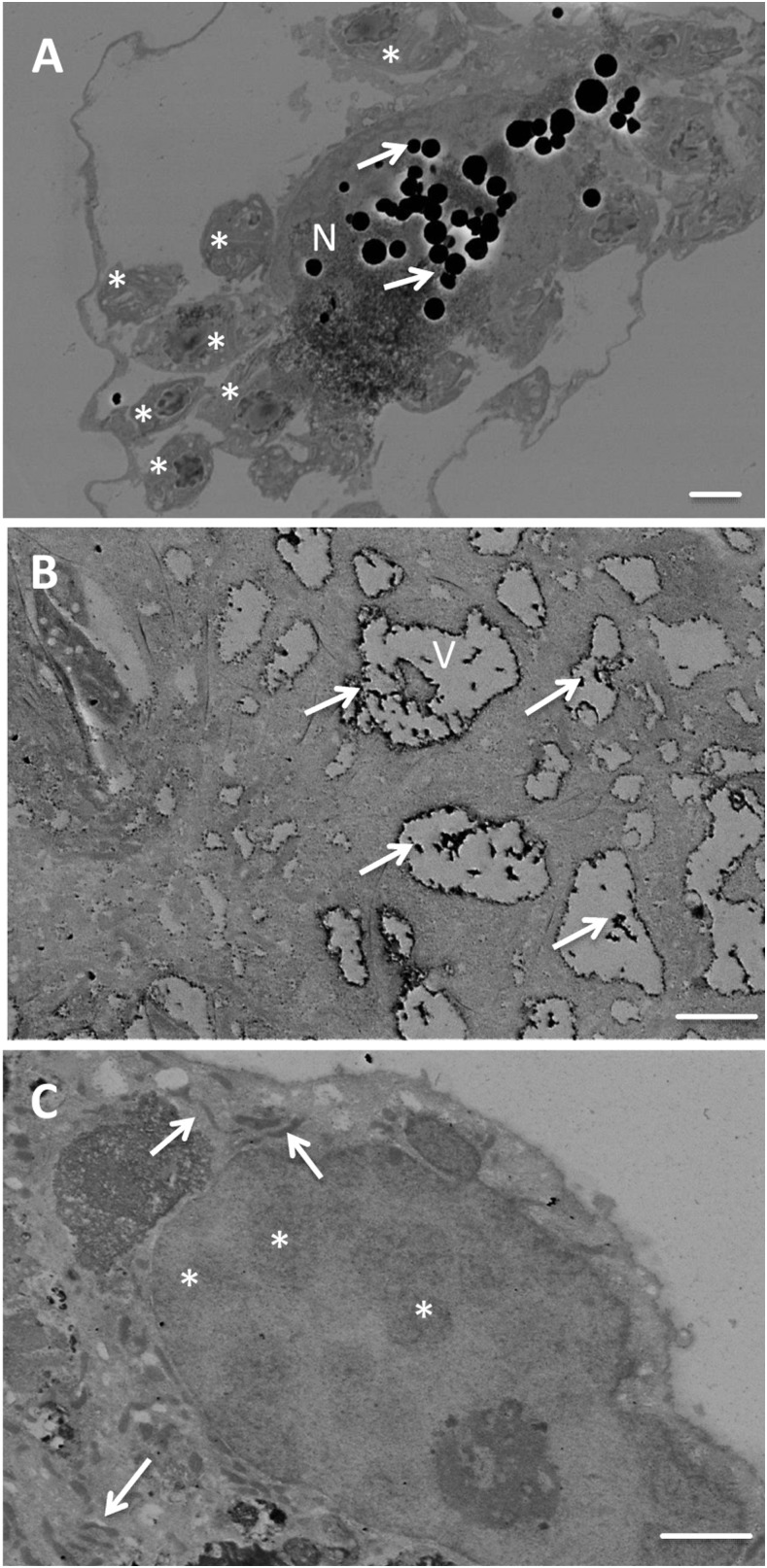
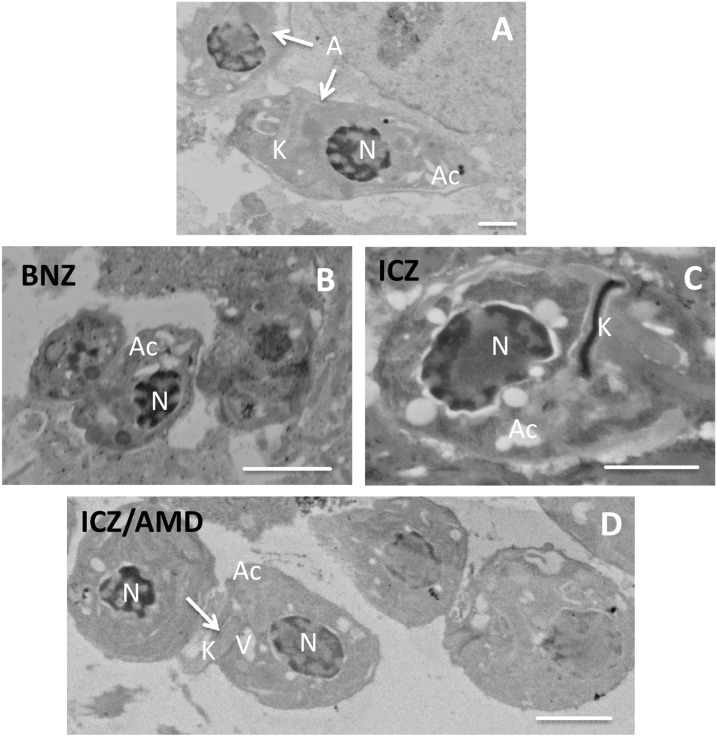
Effects of ICZ, AMD, or their combinations on T. cruzi–infected hiPSC-CM cellular and amastigote structure.
Human-induced pluripotent cell–derived cardiomyocytes, infected with T. cruzi type II, were evaluated for effects of different treatments on cellular (Figure 5) or amastigote (Figure 6) structure. In infected but untreated cells, amastigotes (asterisks in Figure 5A) with acidocalcisomes were visible as electron-transparent areas. Cell structures seemed devoid of cytoplasm, presumably due to infection. Electron-dense inclusions (lipid bodies, arrows in Figure 5A) occurred in the nucleus (N, Figure 1A) and vacuoles of a different cell (V, Figure 5B) of infected, untreated cells. Human-induced pluripotent cell–derived cardiomyocytes, infected with T. cruzi and treated with ICZ/AMD, appeared “healthier,” with a denser cytoplasm, and improved preservation of ultrastructure (Figure 5C). The nucleus (N) included various electron-dense areas (asterisks) distributed throughout the nucleoplasm. Mitochondria (arrows) appeared as flattened electron-dense structures. In amastigotes (arrows in Figure 6A) from untreated hiPSC-CMs, infected with T. cruzi type II, their nucleus (N), kinetoplast (K), and acidocalcisomes (Ac) were visible. Host cell structures were partially damaged by infection. Amastigotes from T. cruzi–infected and BNZ (Figure 6B), ICZ (Figure 6C), or ICZ/AMD (Figure 6D)-treated hiPSC-CMs showed increased numbers of vacuoles, which may be both acidocalcisomes (Ac) and contractile vacuoles (V). Nuclei showed increased electron density, which likely indicates physiological stress. In ICZ-treated cells, the kinetosomes appeared highly electron dense, whereas apparent extrusion of the kinetosome may occur in ICZ/AMD-treated cells (K with arrow). Taken together, ICZ/AMD treatment improved hiPSC-CM structure and damaged the T. cruzi amastigote structure.
Figure 5.
Effects of itraconazole (ICZ) and amiodarone (AMD) on the Trypanosoma cruzi–infected human-induced pluripotent cell–derived cardiomyocyte (hiPSC-CM) structure. Untreated (A and B) or ICZ/AMD-treated (C) hiPSC-CMs infected with T. cruzi type II. (A) and (B): Asterisks, amastigotes; electron transparent areas, acidocalcisomes. Arrows: Electron-dense inclusions, lipid bodies. N: nucleus (N). V: vacuoles (V). (C): N, nucleus including electron-dense areas (asterisks) distributed throughout the nucleoplasm. Arrows: mitochondria. Scale bars for (A–C): 2 μm.
Figure 6.
Effects of benznidazole (BNZ), itraconazole (ICZ), or ICZ/amiodarone (AMD) on amastigote structure. (A): Amastigotes (A, arrows) from untreated human-induced pluripotent cell–derived cardiomyocytes (hiPSC-CMs) infected with T. cruzi type II. Nucleus (N), kinetoplast (K), and acidocalcisomes (Ac) are visible. Scale bar: 2 μm. (B–D): Amastigotes from BNZ (B), ICZ (C), and ICZ/AMD (D) treated hiPSC-CMs infected with T. cruzi type II. Amastigotes contain acidocalcisomes (Ac) and contractile vacuoles (V). Nuclei show increased electron density. In ICZ-treated cells (C), the kinetosome appears highly electron dense, whereas apparent extrusion of the kinetosome may occur in ICZ/AMD-treated cells (D; K with arrow). Scale bar: 2 μm.
DISCUSSION
For decades, antimicrobial therapy against CD has been limited to the nitroimidazole derivative BNZ, most used, and the nitroheterocyclic compound nifurtimox. Both drugs are predominantly used in treatment of acute and early chronic phase CD.14,24 Benznidazole has poor tissue penetration25 and no accumulation within macrophages.26 Its relatively short half-life may make it difficult to maintain desirable tissue concentrations.27 Benznidazole is approved for use in pediatric patients for the treatment of CD in the United States; however, lackluster performance has been noted in large population studies of adults in the chronic stages of infection. Results of the BNZ Evaluation for Interrupting Trypanosomiasis trial revealed that BNZ treatment did not result in a statistically significant improvement in cardiac clinical outcomes, or radical cure of trypanosomiasis in humans.28,29 Long-term treatment with BNZ and nifurtimox in the chronic phase of infection is limited, owing to the development of severe side effects, as toxicity is cumulative in prolonged dosing.30 To determine better treatment options, inhibitors of ergosterol biosynthesis, trypanothione metabolism, cysteine protease, pyrophosphate metabolism, protein and purine synthesis, lysophospholipid analogs, and natural drugs have been investigated.31 In past years, much effort has been put into investigating trypanosoma-specific drug targets, such as cruzipain32 or trypanothione, hoping to avoid severe side effects in long-term treatment. Inhibitors against both molecules are under investigation.33
Polyenes and azoles are antifungals that act on ergosterol or interfere with ergosterol biosynthesis, and have shown effects against T. cruzi membrane integrity. Some of those drugs have been studied in human clinical trials, but unfortunately none have demonstrated improved efficacy over conventional therapy, either as monotherapy or as adjuncts. Our recent publication34 explored the possibility that amphotericin B could be curative with only intermittent administration because of prolonged blood levels, measured in weeks, and good tissue penetration. However, although our regimens significantly prolonged life in mice, and rapidly cleared the blood of all parasites, tissue infection was not cleared.34
Several azoles have been tested for their anti-trypanosoma activity, including ketoconazole, fluconazole, ICZ, voriconazole, and posaconazole.35 Although in vitro and animal model activity generally was promising, only ICZ and posaconazole thus far showed effects in clinical studies.36 Itraconazole (a synthetic triazole) has profound activity against T. cruzi infection in vitro and in vivo, presumably by interfering with terminal enzymes in the cell membrane ergosterol synthesis pathway.17,37 Because of the intracellular nature of T. cruzi and its capacity to reside in sanctuary sites such as adipose tissue,38 mononuclear phagocytic cells,39 and cardiomyocytes,40 treatment delivery poses a significant challenge. From a pharmacokinetic point of view, ICZ concentrates in macrophages, cardiomyocytes, spleen, and adipocytes in higher-than-plasma levels, thus increasing the parasite exposure to higher levels in infected tissues.40,41 McCabe et al.42 described in vitro activity of ICZ against T. cruzi in macrophages, and parasitologic cure in mice. Itraconazole has also been shown to enhance the anti-trypanosomal activity of BNZ in mice.16 A clinical study revealed parasitological cure in 53% of ICZ-treated patients with mostly tolerable side effects, as well as normalization of echocardiograms (EKG) in almost half of the patients.43 In a follow-up study, patients were reevaluated by polymerase chain reaction-based assays and a hybridization assay, showing that only about 8% of the patients treated with ICZ had been cured.44 In another study, only a 33% cure was achieved in patients followed up for 20 years.45 Our data obtained in the hiPSC-CMs, on the early events after infection, support these earlier reports on anti-trypanosomal effects of ICZ in a new and relevant system that allows drug testing in human cardiomyocytes. We also found that combining ICZ with AMD intensified ICZ effects, and in many cases was even more effective than either of the single compounds alone, without showing toxic effects on the cardiomyocytes.
Amiodarone is not only an anti-arrhythmic agent frequently prescribed to prevent complex arrhythmias in cases of CD cardiomyopathy17 but also has direct anti–T. cruzi activity.46,47 Amiodarone disrupts Ca2+ homeostasis in the parasite by inducing its release from intracellular stores (such as the parasites’ giant mitochondrion and acidocalcisomes, a unique organelle involved in the parasite bioenergetics). Amiodarone also blocks oxidosqualene cyclase activity in T. cruzi,46,48 thereby causing ultrastructural damage to the parasite and blocking biosynthesis of ergosterol.49 Amiodarone also accumulates in macrophages, where it concentrates rapidly and intensely at therapeutic levels.50 Amiodarone could be very useful for treatment of chronic CD. Arrhythmogenic events in CD appear related to the parasites’ capacity to disrupt gap junctional communication through reduction in the essential gap protein connexin 43 and its interaction with actin filaments. Amiodarone promotes cardiac cell recovery and homeostasis by recovering this F-actin fibrillar organization,49 as well as preventing the potential development of exercise-induced and potentially lethal ventricular arrhythmias.46 Although there are reports of anti-trypanosomal effects of AMD in vivo,46,49 CD patients who were treated with AMD on a regular basis did not show reduced parasitemia, compared with CD patients who had not been treated with AMD.51 A recent study investigated effects of AMD and/or BNZ, and came to the conclusion that although both compounds have considerable anti-trypanosomal effects of their own, or in combination, effects of their combination were not synergistic.52 Effects we observed when combining ICZ with AMD were, in many instances, in both effects on inhibition and on multiplication, more pronounced than effects of the single compounds, giving rise to the hope that increased anti-trypanosomal effects of the combination will be accompanied by positive effects on patient EKGs. Amiodarone has also been combined with posaconazole in vitro and in vivo, revealing synergistic effects on T. cruzi infection as well.46
Our results suggest T. cruzi strain specificity with respect to infection rates. In our hands, some combinations of ICZ and AMD acted cooperatively, with respect to reducing TcII infection, and multiplication, compared with either drug alone, in Vero cells, and cardiomyocytes. In both cell types, some concentrations of the single drugs, and their combinations were superior to BNZ in reducing infection, parasite multiplication, host cell structure, and damage to amastigotes. TcI in hiPSC-CMs was not affected by BNZ with respect to infection, although BNZ did favorably affect multiplication. With TcI, by contrast, ICZ or AMD reduced infection. However, their combinations were not better than either alone. Cooperative effects on TcI multiplication were seen with some combinations of ICZ and AMD. ICZ or AMD, and combinations, in contrast to BNZ, were active against TcI infection. Itraconazole and some combinations were superior to BNZ against TcI multiplication.
The relative lack of efficacy of BNZ for TcI may be particularly important for the cases in the United States, as strains of the TcI type predominate in the United States.53 Such strains tend to be more resistant than others.54 In dogs, BNZ fails regularly even in acute infection.55 Although a concentration of 23 μM BNZ in human serum has been recommended,56 that is unlikely to be achieved in tissue in chronic infection,57 and tissue concentrations may be closer to the less effective 11.5 μM concentration we studied.57 Prior in vitro tests have sometimes found inhibition requires more than 23 μM BNZ.25 The package insert for BNZ27 indicates the Cmax resulting from the largest tablet to be dosed is < 11.5 μM, and tissue levels would be even lower. Strains isolated from clinical samples have higher IC50s in both the amastigote and epimastigote stages of the parasite25 than isolates from other sources. Benznidazole is delivered in less-than-plasma concentrations of the drug to target tissues, including 32% of plasma concentrations to the heart, 27% to the spleen, and 30% to the colon.25,27,57
Itraconazole was studied concomitantly to obtain its synergistic effect when coupled with AMD.17 Amiodarone concentrations are 10 times higher in the myocardium than plasma, and ICZ five times, thus accumulating at a primary target organ for T. cruzi.50,58,59 Fat is also a reservoir from which infection may be reactivated; therefore, lipid penetration is critical to eliminating the chronic phase.60 Both drugs are highly lipophilic, with levels of ICZ exceeding 25 times that of plasma in adipose tissue.61 The effective ICZ concentrations against TcI in our study correspond to ICZ concentrations in heart tissue, and the similar results in combination with various AMD concentrations may suggest concurrent AMD may be useful, and that a lower AMD dose can be used clinically. This is important because there is a concern about QTc prolongation in combinations of azoles with AMD, leading to tachyarrhythmia.62 However, although QTc prolongation occurs with this combination, a recent study has suggested that the prolongation was not associated with cardiac events.62 Because of variable absorption of oral ICZ.,63 therapeutic drug monitoring when ICZ is used in CD therapy will be important, to assure the desirable ICZ concentrations discussed are achieved.
Acknowledgments:
We thank Alberto Paniz-Mondolfi for his initial contributions to ICZ-AMD combinations, in patients.
REFERENCES
- 1.World Health Organization , 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90: 33–44. [PubMed] [Google Scholar]
- 2.Jurberg C, 2009. Chagas: one hundred years later. Bull World Health Organ 87: 491–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhoff LV, 2011. Epidemiology of American trypanosomiasis (Chagas disease). Adv Parasitol 75: 1–18. [DOI] [PubMed] [Google Scholar]
- 4.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M, 1992. Chagas’ disease. Clin Microbiol Rev 5: 400–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonney KM, 2014. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 21: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bern C, Kjos S, Yabsley MJ, Montgomery SP, 2011. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev 24: 655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bern C, Montgomery SP, 2009. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49: e52–e54. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro FA, Weirauch C, Felix M, Lazoski C, Abad-Franch F, 2018. Evolution, systematics, and biogeography of the triatominae, vectors of Chagas disease. Adv Parasitol 99: 265–344. [DOI] [PubMed] [Google Scholar]
- 9.Zingales B, et al. 2012. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol 12: 240–253. [DOI] [PubMed] [Google Scholar]
- 10.Vago AR, Andrade LO, Leite AA, d’Avila Reis D, Macedo AM, Adad SJ, Tostes S, Jr., Moreira MC, Filho GB, Pena SD, 2000. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol 156: 1805–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zingales B, 2018. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 184: 38–52 [Review]. [DOI] [PubMed] [Google Scholar]
- 12.de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM, 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 13.Fragata Filho AA, da Silva MA, Boainain E, 1995. Ethiologic treatment of acute and chronic Chagas’ Disease [corrected]. Sao Paulo Med J 113: 867–872. [DOI] [PubMed] [Google Scholar]
- 14.Urbina JA, 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115: 55–68. [DOI] [PubMed] [Google Scholar]
- 15.Morillo CA, et al. 2017. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: the STOP-CHAGAS trial. J Am Coll Cardiol 69: 939–947. [DOI] [PubMed] [Google Scholar]
- 16.Assíria Fontes Martins T, de Figueiredo Diniz L, Mazzeti AL, da Silva do Nascimento ÁF, Caldas S, Caldas IS, de Andrade IM, Ribeiro I, Bahia MT, 2015. Benznidazole/itraconazole combination treatment enhances anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS One 10: e0128707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paniz-Mondolfi AE, Pérez-Alvarez AM, Lanza G, Márquez E, Concepción JL, 2009. Amiodarone and itraconazole: a rational therapeutic approach for the treatment of chronic Chagas’ disease. Chemotherapy 55: 228–233. [DOI] [PubMed] [Google Scholar]
- 18.Churko JM, Burridge PW, Wu JC, 2013. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods Mol Biol 1036: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP, 2012. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 109: E1848–E1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade D, et al. 2012. Trypanosoma cruzi invades host cells through the activation of endothelin and bradykinin receptors: a converging pathway leading to chagasic vasculopathy. Br J Pharmacol 165: 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burridge PW, et al. 2014. Chemically defined generation of human cardiomyocytes. Nat Methods 11: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozzi A, Sayed N, Matsa E, Sass G, Neofytou E, Clemons KV, Correa-Oliveira R, Stevens DA, Wu JC, 2019. Using human induced pluripotent stem cell-derived cardiomyocytes as a model to study Trypanosoma cruzi infection. Stem Cell Reports pii: S2213–S6711(19)30139-0. doi: 10.1016/j.stemcr.2019.04.017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR, 1988. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48: 4827–4833. [PubMed] [Google Scholar]
- 24.Coura JR, de Castro SL, 2002. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97: 3–24. [DOI] [PubMed] [Google Scholar]
- 25.Molina I, Salvador F, Sánchez-Montalvá A, Artaza MA, Moreno R, Perin L, Esquisabel A, Pinto L, Pedraz JL, 2017. Pharmacokinetics of benznidazole in healthy volunteers and implications in future clinical trials. Antimicrob Agents Chemother 61: e01912–e01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perdomo VG, Rigalli JP, Luquita MG, Pellegrino JM, Ruiz ML, Catania VA, 2016. Up-regulation of ATP-binding cassette transporters in the THP-1 human macrophage cell line by the antichagasic benznidazole. Mem Inst Oswaldo Cruz 111: 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exeltis USA, Inc. , 2017. Benznidazole Package Insert. Florham Park, NJ: Exeltis USA, Inc. [Google Scholar]
- 28.Morillo CA, et al. BENEFIT Investigators , 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373: 1295–1306. [DOI] [PubMed] [Google Scholar]
- 29.Pecoul B, et al. 2016. The BENEFIT Trial: where do we go from here? PLoS Negl Trop Dis 10: e0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancado JR, 2002. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo 44: 29–37. [PubMed] [Google Scholar]
- 31.Apt W, 2010. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des Devel Ther 4: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murta AC, Persechini PM, Padron Tde S, de Souza W, Guimarães JA, Scharfstein J, 1990. Structural and functional identification of GP57/51 antigen of Trypanosoma cruzi as a cysteine proteinase. Mol Biochem Parasitol 43: 27–38. [DOI] [PubMed] [Google Scholar]
- 33.Sueth-Santiago V, Decote-Ricardo D, Morrot A, Freire-de-Lima CG, Lima ME, 2017. Challenges in the chemotherapy of Chagas disease: looking for possibilities related to the differences and similarities between the parasite and host. World J Biol Chem 8: 57–80 [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clemons KV, Sobel RA, Martinez M, Correa Oliveira R, Stevens DA, 2017. Lack of efficacy of liposomal amphotericin B against acute and chronic Trypanosoma cruzi infection in mice. Am J Trop Med Hyg 97: 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner FS, 2008. Sterol 14-demethylase inhibitors for Trypanosoma cruzi infections. Adv Exp Med Biol 625: 61–80. [DOI] [PubMed] [Google Scholar]
- 36.Sales Junior PA, Molina I, Fonseca Murta SM, Sánchez-Montalvá A, Salvador F, Corrêa-Oliveira R, Carneiro CM, 2017. Experimental and clinical treatment of Chagas disease: a review. Am J Trop Med Hyg 97: 1289–1303 [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbina JA, 2001. Specific treatment of Chagas disease: current status and new developments. Curr Opin Infect Dis 14: 733–741 [Review]. [DOI] [PubMed] [Google Scholar]
- 38.Tanowitz HB, Scherer PE, Mota MM, Figueiredo LM, 2017. Adipose tissue: a safe haven for parasites? Trends Parasitol 33: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanmarco LM, Eberhardt N, Ponce NE, Cano RC, Bonacci G, Aoki MP, 2018. New insights into the immunobiology of mononuclear phagocytic cells and their relevance to the pathogenesis of cardiovascular diseases. Front Immunol 8: 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvet CM, Melo TG, Garzoni LR, Oliveira FO, Jr., Neto DTS, Maria NSL, Meirelles L, Pereira MC, 2012. Current understanding of the Trypanosoma cruzi-cardiomyocyte interaction. Front Immunol 3: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perfect JR, Savani DV, Durack DT, 1993. Uptake of itraconazole by alveolar macrophages. Antimicrob Agents Chemother 37: 903–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCabe RE, Remington JS, Araujo FG, 1986. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg 35: 280–284. [DOI] [PubMed] [Google Scholar]
- 43.Apt W, Aguilera X, Arribada A, Pérez C, Miranda C, Sánchez G, Zulantay I, Cortés P, Rodriguez J, Juri D, 1998. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. Am J Trop Med Hyg 59: 133–138. [DOI] [PubMed] [Google Scholar]
- 44.Apt W, Arribada A, Zulantay I, Solari A, Sánchez G, Mundaca K, Coronado X, Rodríguez J, Gil LC, Osuna A, 2005. Itraconazole or allopurinol in the treatment of chronic American trypanosomiasis: the results of clinical and parasitological examinations 11 years post-treatment. Ann Trop Med Parasitol 99: 733–741. [DOI] [PubMed] [Google Scholar]
- 45.Apt W, Arribada A, Zulantay I, Rodríguez J, Saavedra M, Muñoz A, 2013. Treatment of Chagas’ disease with itraconazole: electrocardiographic and parasitological conditions after 20 years of follow-up. J Antimicrob Chemother 68: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 46.Benaim G, et al. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem 49: 892–899. [DOI] [PubMed] [Google Scholar]
- 47.Benaim G, Paniz Mondolfi AE, 2012. The emerging role of amiodarone and dronedarone in Chagas disease. Nat Rev Cardiol 9: 605–609 [Review]. [DOI] [PubMed] [Google Scholar]
- 48.Benaim B, Garcia CR, 2011. Targeting calcium homeostasis as the therapy of Chagas’ disease and leishmaniasis–a review. Trop Biomed 28: 471–481 [Review]. [PubMed] [Google Scholar]
- 49.Adesse D, Azzam EM, Meirelles MNL, Urbina JA, Garzoni LR, 2011. Amiodarone inhibits Trypanosoma cruzi infection and promotes cardiac cell recovery with gap junction and cytoskeleton reassembly in vitro. Antimicrob Agents Chemother 55: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirk RG, Lee P, Reasor MJ, 1990. Quantitative X-ray microanalysis of alveolar macrophages after long-term treatment with amiodarone. Exp Mol Pathol 52: 122–131. [DOI] [PubMed] [Google Scholar]
- 51.Carmo AA, Rocha MO, Silva JL, Ianni BM, Fernandes F, Sabino EC, Ribeiro AL, 2015. Amiodarone and Trypanosoma cruzi parasitemia in patients with Chagas disease. Int J Cardiol 189: 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lourenço AM, Faccini CC, Costa CAJ, Mendes GB, Fragata Filho AA, 2018. Evaluation of in vitro anti-Trypanosoma cruzi activity of medications benznidazole, amiodarone hydrochloride, and their combination. Rev Soc Bras Med Trop 51: 52–56. [DOI] [PubMed] [Google Scholar]
- 53.Curtis-Robles R, Zecca IB, Roman-Cruz V, Carbajal ES, Auckland LD, Flores I, Millard AV, Hamer SA, 2017. Trypanosoma cruzi (agent of Chagas disease) in sympatric human and dog populations in “colonias” of the lower Rio Grande Valley of Texas. Am J Trop Med Hyg 96: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teston AP, Monteiro WM, Reis D, Bossolani GD, Gomes ML, de Araújo SM, Bahia MT, Barbosa MG, Toledo MJ, 2013. In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Trop Med Int Health 18: 85–95. [DOI] [PubMed] [Google Scholar]
- 55.Guedes PM, Veloso VM, Tafuri WL, Galvão LM, Carneiro CM, Lana Md, Chiari E, Ataide Soares K, Bahia MT, 2002. The dog as model for chemotherapy of the Chagas’ disease. Acta Trop 84: 9–17. [DOI] [PubMed] [Google Scholar]
- 56.Richle RW, Raaflaub J, 1980. Difference of effective antitrypanosomal dosages of benznidazole in mice and man. Chemotherapeutic and pharmacokinetic results. Acta Trop 37: 257–261. [PubMed] [Google Scholar]
- 57.Perin L, Moreira da Silva R, Fonseca KD, Cardoso JM, Mathias FA, Reis LE, Molina I, Correa-Oliveira R, Vieira PM, Carneiro CM, 2017. Pharmacokinetics and tissue distribution of benznidazole after oral administration in mice. Antimicrob Agents Chemother 61: e02410–e02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debbas NM, du Cailar C, Bexton RS, Demaille JG, Camm AJ, Puech P, 1984. The QT interval: a predictor of the plasma and myocardial concentrations of amiodarone. Br Heart J 51: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janssen Pharmaceutica , 2012. Sporanox (R) Package Insert N.V. Olen, Belgium: Janssen Pharmaceutica. [Google Scholar]
- 60.Combs TP, et al. 2005. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem 280: 24085–24094. [DOI] [PubMed] [Google Scholar]
- 61.Prentice AG, Glasmacher A, 2005. Making sense of itraconazole pharmacokinetics. J Antimicrob Chemother 56 (Suppl 1): i17–i22 [Review]. [DOI] [PubMed] [Google Scholar]
- 62.Cook K, Straubol T, Biva Campbell K, Mourad A, Stiber J, Perfect JR, Johnson M, 2017. QTc prolongation in patients receiving triazoles and amiodarone. Open Forum Infect Dis 4 (Suppl 1): S84. [Google Scholar]
- 63.Hostetler JS, Heykants J, Clemons KV, Woestenborghs R, Hanson LH, Stevens DA, 1993. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother 37: 2224–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]