Abstract.
Lymphatic filariasis is a mosquito-borne parasitic disease responsible for morbidity and disability that affects 1.2 billion people worldwide, mainly the poor communities. Currently, filarial antigen testing is the method of choice for the detection of bancroftian filariasis, and to date, there are two commonly used tests. In the present study, a recently reported recombinant monoclonal antibody (5B) specific to BmSXP filarial antigen was used in developing an ELISA for the detection of circulating filarial antigen in sera of patients with bancroftian filariasis. The performance of the ELISA was evaluated using 124 serum samples. The ELISA was positive with all sera from microfilaremic bancroftian filariasis patients (n = 34). It also showed 100% diagnostic specificity when tested with sera from 50 healthy individuals and 40 patients with other parasitic diseases. The developed assay using the novel 5B recombinant monoclonal antibody could potentially be a promising alternative antigen detection test for bancroftian filariasis.
INTRODUCTION
Lymphatic filariasis (LF) is a debilitating mosquito-borne parasitic disease caused by three species of tissue/blood helminths, namely, Wuchereria bancrofti, Brugia malayi, and Brugia timori. Of the three species, W. bancrofti infection (bancroftian filariasis) is responsible for 90% of the infections. Lymphatic filariasis is a neglected tropical disease associated with poverty in 53 countries.1 The 2016 Global Burden of Disease reported that LF accounted for 189 million disability-adjusted life years.2 In the year 2000, the World Health Organization established the Global Programme to Eliminate Lymphatic Filariasis (GPELF) to eliminate LF as a public health problem by the year 2020. The GPELF requires accurate diagnostic tools for various phases of its program.
Good diagnostic tests for detecting and quantifying circulating filarial antigens in humans have been developed for bancroftian filariasis.3–9 These tests are sensitive and specific, they do not require the presence of circulating larvae called microfilariae (mf), the blood sample can be taken at any time, and they can detect both the microfilaremic and amicrofilaremic cases. To date, there are two established commercial antigen detection tests for patient diagnosis, research, or GPELF.10 The first is Og4C3 ELISA (Cellabs Pty Ltd., New South Wales, Australia), the first commercialized test to detect circulating W. bancrofti antigen in serum, plasma, or hydrocele fluid. The test showed high diagnostic sensitivity and was reported not to cross-react with other helminthic infections.11 The other antigen detection test is a point-of-care lateral flow cassette test, called Alere Filaria Test Strip (FTS) (Abbot, Scarborough, ME), which is a modified version of Alere BinaxNOW® Filariasis (Alere, Scarborough, ME) card test (immunochromatography test). Because of its field applicability and high diagnostic value, it is the tool used by the GPELF in bancroftian filariasis areas.12 The rapid test is easy to perform, is highly sensitive and specific, and requires no equipment.
We have previously produced a novel recombinant monoclonal antibody (5B) to a known filarial antigen, BmSXP.13 The protein is a highly immunogenic antigen for LF and is presently used as a test line in the commercially available PanLF Rapid and BLF Rapid tests (Reszon Diagnostics International, Selangor, Malaysia). In the present study, the 5B antibody was used as the detecting antibody together with a polyclonal antibody as the capture antibody in developing an ELISA for the detection of circulating W. bancrofti antigen in the human serum sample. The findings from this study may help to address some gaps in the serodiagnosis of bancroftian filariasis.
MATERIALS AND METHODS
Serum samples.
A total of 124 human serum samples were used in this study, comprising sera from individuals with W. bancrofti mf (n = 34), healthy people (n = 50), and individuals with other parasitic infections (n = 40). The latter comprised serum samples from the following infections: B. malayi (n = 7); Toxocara sp. (n = 4); Strongyloides stercoralis (n = 4); Trichuris trichiura (n = 4); Ascaris lumbricoides (n = 4); hookworm (n = 4); Toxoplasma gondii (n = 5); Echinococcus granulosus (n = 4); and Entamoeba histolytica (n = 4). The USM Human Research Ethics Committee approved the use of the stored and anonymized serum samples for diagnostic test evaluation.
Production and purification of polyclonal antibodies.
Recombinant BmSXP protein was expressed and purified according to the method described in our previous report.14 The procedure for the production of polyclonal antibody against BmSXP was also based on a previously published report from our group.15 Briefly, the BmSXP antigen was emulsified with an equal volume of Freund’s complete adjuvant (Sigma Chemical Co., St. Louis, MO) and injected subcutaneously into eight sites on the rabbit. This was followed by three immunization boosts with 2-week intervals using the same volume of antigen in Freund’s incomplete adjuvant (Sigma). The rabbit was killed 10 days after the final boost to harvest the hyperimmune serum. The anti-BmSXP rabbit polyclonal antibody was purified using Melon IgG Purification Spin Column (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s instructions. The titer of the purified polyclonal antibody was determined by performing serial dilutions. The use of animals in this study was approved by the USM Animal Research Ethics Committee [Animal Ethics Approval/2011/(74)(343)].
Production of recombinant monoclonal antibody protein and conjugation with horseradish peroxidase (HRP).
The 5B recombinant monoclonal antibody protein was isolated using phage display technology and produced as previously described.13,16 Briefly, the 5B monoclonal plasmid was transformed into SHuffle® Express Escherichia coli cells (New England Biolabs) and expression was performed in 1 L 2-YT broth supplemented with 100 μg/mL ampicillin and 0.2% glucose. The culture was grown at 37°C at 200 rpm until the OD600 reached 0.6–0.7, then induced with 1 mM isopropyl-B-D-thiogalactopyranoside, and further cultured for 16 hours at 25°C with 180 rpm shaking. The purification was carried out using C-terminal His-tag with a nitrilotriacetic acid (Ni-NTA) purification column (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. The purified protein was then conjugated with HRP following the manufacturer’s instructions (Thermo Fisher Scientific). The success of HRP conjugation was validated by performing Western blot. The 5B-HRP was then used as a secondary antibody in the 5B-ELISA.
5B-ELISA.
Checkerboard titrations determined the optimal concentrations/dilutions of the anti-BmSXP IgG, serum sample, and 5B-HRP conjugate. Maxisorp microtiter plates (Nunc, Thermo Fisher Scientific, Rochester, NY) were coated with anti-BmSXP IgG polyclonal antibody in coating buffer (sodium carbonate/bicarbonate buffer, pH 9.6) for 1 hour at 37°C on a plate shaker (600 rpm). The coated plate was washed and incubated with 3% blocking solution (bovine serum albumin in phosphate-buffered saline [PBS], pH 7.2, containing 0.05% Tween) for 2 hours at 37°C at 600 rpm. Subsequently, the plate was washed, and the serum samples were added. It was first incubated for 1 hour at 37°C at 600 rpm, followed by overnight incubation at 4°C at 300 rpm. After another washing step, the plate was incubated with 5B-HRP conjugate for 1 hour at 37°C at 600 rpm. Finally, the substrate (2,2′-azino-bis [3-ethylbenzothiazoline-6-sulfonic acid] or ABTS) was added and incubated for 30 minutes at 37°C at 500 rpm. Absorbance readings were determined at 405 (reference 490) using a spectrophotometer (Multiskan Spectrum; Thermo Fisher Scientific, Walthman, MA). All serum samples were assayed in triplicate wells, and the average optical density (OD) readings of the wells were recorded. Repeat testing was performed on samples with a SD higher than one among the three wells.
ELISA using spiked dried blood spots.
One milliliter of venous blood from a healthy individual was collected into a tube containing ethylenediaminetetraacetic acid and aliquoted 30 μL each into five tubes. The tubes were centrifuged for 15 minutes at 3,000 × g. The plasma from each tube was removed and replaced with an equal volume of serum samples from five different individuals with W. bancrofti mf. The spiked blood mixture was spotted on the ears of a filter paper (TropBio, Sydney, AU) (10 μL/ear) and dried overnight at room temperature. The same volume of healthy blood was also dried on the filter paper as a negative control. Then, the dried blood spots were each eluted with 500 μL PBS with 0.05% Tween on a rotator for 4 hours at 4°C. The eluted supernatant was centrifuged and collected as samples for ELISA. For comparison, wells using the corresponding serum samples were included in the same ELISA plate.
Analytical sensitivity.
To determine the analytical sensitivity (limit of detection) of the ELISA, recombinant BmSXP antigen was added to a series of six tubes containing serum sample from a healthy individual to create spiked serum (200 uL/tube) containing 50, 10, 1, 0.1, and 0.01 ug/mL of BmSXP. Non-spiked serum was used as a negative control. The ELISA was performed as described previously.
Statistical analysis.
The 5B-ELISA results were analyzed using MedCalc® software version 17 (MEDCALC statistical software, Ostend, Belgium). This software calculates the optimal reading of cutoff point, sensitivity, and specificity, and area under the curve (AUC). A P-value < 0.01 was considered statistically significant.
RESULTS
Polyclonal antibody, recombinant monoclonal antibody protein, and conjugation with HRP.
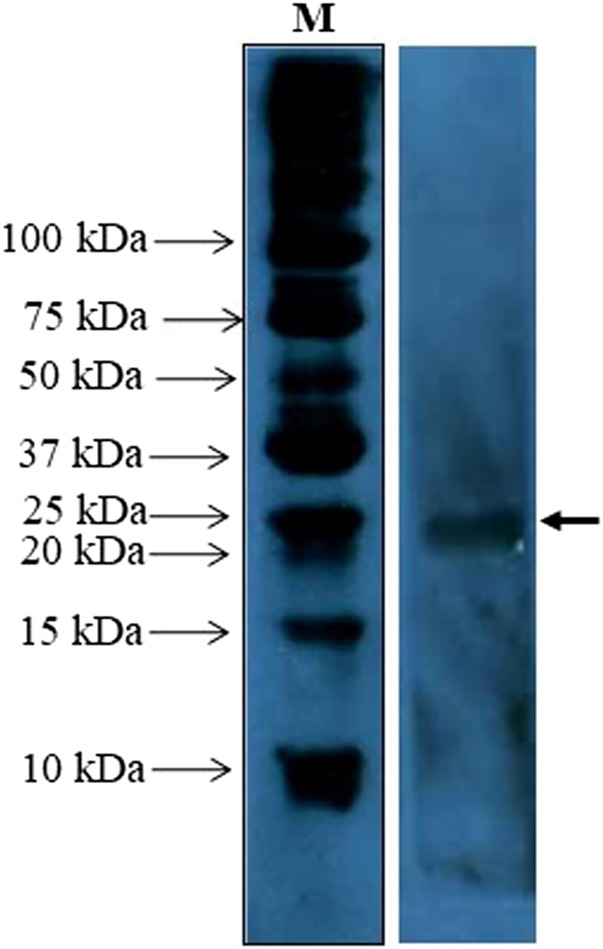
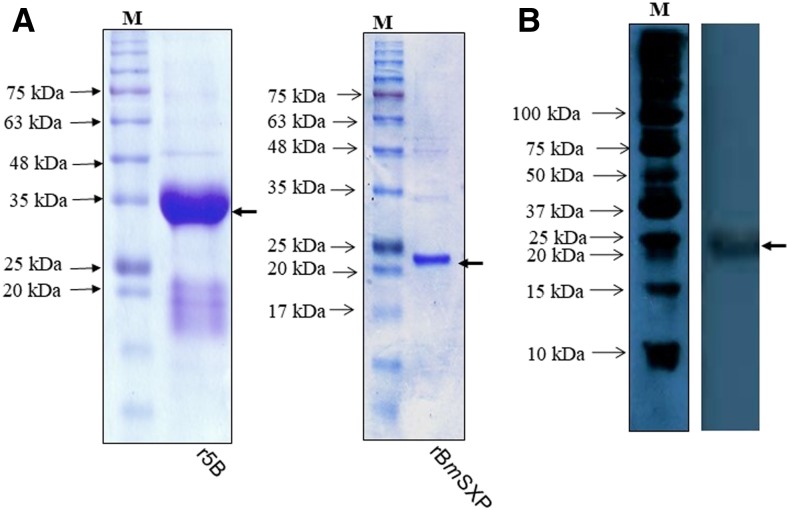
The titer of the anti-BmSXP polyclonal antibody was determined to be 1,024,000. The Western blot image in Figure 1 showed that the polyclonal antibody recognized the 22-kDa recombinant BmSXP protein. Figure 2A shows the sodium dodecyl sulfate (SDS) profiles of recombinant BmSXP and 5B monoclonal antibody proteins, and Figure 2B shows that the 5B-HRP conjugated protein successfully recognized the BmSXP protein band, thus confirming the success of the conjugation.
Figure 1.
Western blot of the BmSXP recombinant antigen probed with the anti-BmSXP IgG polyclonal antibody. Arrow indicates the size of the BmSXP recombinant antigen at ∼22 kDa. This figure appears in color at www.ajtmh.org.
Figure 2.
Verification of the 5B monoclonal antibody conjugated with horseradish peroxidase (HRP). (A) SDS PAGE profile of the 5B monoclonal antibody protein and BmSXP antigen. (B) Western blot analysis of the BmSXP antigen probed with 5B-HRP. Arrow indicates the size of BmSXP recombinant antigen at ∼22 kDa. This figure appears in color at www.ajtmh.org.
Antigen detection ELISA.
Preliminary experiments were conducted to determine the best choice of protein to be used as the capture antibody and the secondary antibody for the detection of circulating W. bancrofti antigen in serum. The best result was obtained with anti-BmSXP IgG antibody as the capture antibody and 5B protein as the detecting antibody. The 5B-ELISA was first performed using BmSXP-spiked pooled and individual serum samples. The optimum parameters were found to be as follows: 20 μg anti-BmSXP IgG antibody, 1:50 serum sample, and 1:500 5B-HRP.
Detection of circulating W. bancrofti antigen in serum samples.
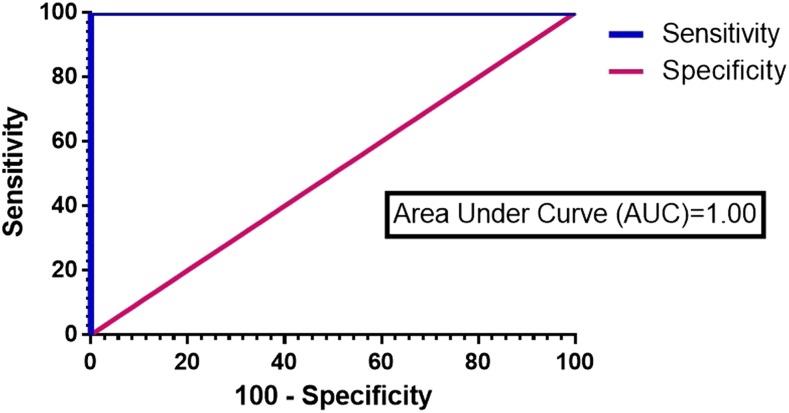
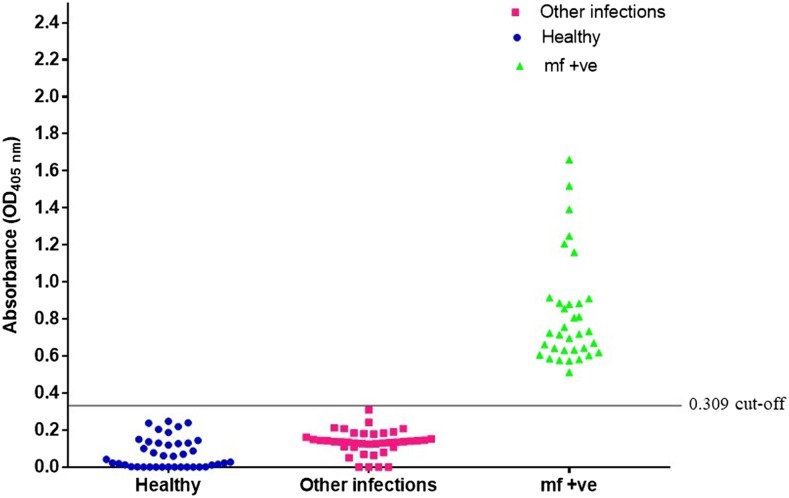
The 5B-ELISA was performed using 124 samples comprising sera from 34 W. bancrofti mf + individuals, 50 healthy individuals, and 40 with other infections. The results were used to plot a receiver operating characteristic curve (Figure 3) and distribution graph (Figure 4). It showed an excellent AUC value of 1.0 (95% CI = 0.952–1.0. P < 0.0001) and a cutoff value (COV) of 0.309. The 5B-ELISA showed a diagnostic sensitivity of 100% (95% CI: 88.4–100.0%) and diagnostic specificity of 100% (95% CI: 92.1–100.0%).
Figure 3.
Receiver operating characteristic curve from analysis of results from 75 serum samples tested with the newly developed antigen detection 5B-ELISA. This figure appears in color at www.ajtmh.org.
Figure 4.
Distribution of the optical density readings of human serum samples tested using the antigen detection 5B-ELISA. The cutoff value of 0.309 is indicated as a line on the graph. This figure appears in color at www.ajtmh.org.
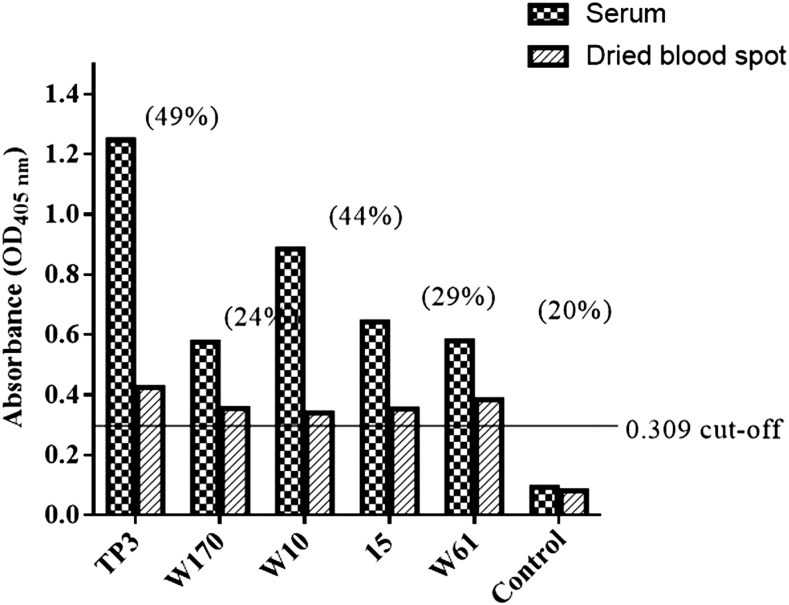
ELISA using spiked dried blood spots.
The results are shown in Figure 5. All five samples eluted from the spiked dried blood spots showed positive results (Figure 5). In comparison with the corresponding serum samples, the dried blood showed reductions in the OD values of 20–49%. Nevertheless, all the results were above assay COV.
Figure 5.
ELISA results using spiked dried blood samples. The cutoff optical density (OD) value of 0.309 is indicated as a line on the graph. For each sample, the percentage reductions in OD values of serum compared with eluted blood spots were TP3 (49%), W170 (24%), W10 (44%), 15 (29%), and W61 (20%).
Analytical sensitivity.
The least concentration of BmSXP that was detectable in the spiked serum sample was found to be 0.1 μg/mL.
DISCUSSION
Phage display technology is a robust method to produce recombinant monoclonal antibodies with high affinity to their targets.17,18 This method enables a relatively rapid generation of monoclonal antibodies against various targets through the clonal selection of antibody fragments and antibody protein expression in a prokaryotic system. Monoclonal antibodies have been applied with success in various fields such as molecular biology, diagnostics, therapeutics, and medical research.19,20
The application of recombinant monoclonal antibodies in place of conventional hybridoma monoclonal antibodies is becoming commonplace.21 Recently, a phage-derived engineered recombinant monoclonal antibody was produced against cystic echinococcosis and was applied in clinical immunohistochemistry for pathological diagnosis.22 In another study, a single-chain fragment variable (scFv) recombinant monoclonal antibody was produced against Bacillus anthracis spores. The antibody was applied in three different detection platforms (ELISA, immunofluorescence assays, and flow cytometry) for the detection of the bacterial spores.23 In medical imaging, radioisotope-labeled recombinant monoclonal antibodies are widely used to locate the tumor and differentiate between cancerous and noncancerous growths.24,25 Also, another form of a monoclonal antibody called nanobody was produced to detect Trypanosoma congolense infections in livestock. This recombinant nanobody was applied in the development of rapid diagnostic tests such as ELISA and lateral flow assay.26
A recent study reported the construction of an scFv antibody clone against Wb-SXP-1 protein. It was found to recognize recombinant Wb-SXP-1 and native Wb-SXP-1 protein in sera of microfilaremic patients.27 The scFV was constructed using the monoclonal antibody clone derived from the mouse hybridoma cell line against Wb-SXP-1 protein. The protein produced was insoluble and, thus, was purified under denatured condition, and then refolded. In comparison, the clone in our present study was phage-derived, and the scFv was produced as a soluble protein. Also, in the previous study, the scFv monoclonal antibody was used to coat the ELISA plate, whereas in our study, rabbit polyclonal against Wb-SXP-1 was used as the capture antibody.
Previously, we have reported on the production of a novel monoclonal antibody (5B) specific to an important filarial antigen, BmSXP, using RNA from microfilaremic persons infected with a lymphatic filarial parasite, B. malayi.13 It was the first reported monoclonal antibody for lymphatic filaria isolated from the human lymphatic filaria IgG immune library using phage display technology. The 5B monoclonal antibody was derived from the IgHV3-LV3 gene family.
In the present study, the 5B monoclonal antibody was used to develop an ELISA to detect the circulating filarial antigen in sera of patients with bancroftian filariasis. This initial evaluation of this assay showed excellent sensitivity and specificity. It was able to detect all W. bancrofti microfilaremic individuals and did not cross-react with sera of people who were healthy and with other infection. The ELISA was also able to detect W. bancrofti antigens in eluted dried blood samples, albeit with reduced OD values. Furthermore, the analytical sensitivity (limit of detection) of the ELISA was found to be 0.01 μg/mL.
The 5B monoclonal antibody is an scFv, which consist of the antigen-binding domains from the heavy and light chain linked by a flexible linker that retains its complete antigen-binding site.28 The advantages of smaller fragments of antibodies include excellent tissue penetration, can be produced in a prokaryotic system which saves time and cost, highly specific, and can be applied to various detection applications. The high specificity of the antibody is evident from the fact that it did not bind antigens in sera of patients with B. malayi, which is a very closely related lymphatic filaria species. It is also interesting because, as described previously, the phage library used in panning for the binder came from a B. malayi patient. It is also of note that assays to detect filarial-specific antibodies using BmSXP recombinant antigen or a very similar molecule called Bm14 showed cross-reactivity with sera from patients with B. malayi infection.29,30
Currently, there are two antigen detection diagnostic test kits for bancroftian filariasis in the market, that is, FTS and Og4C3 ELISA. The first is being used as the diagnostic tool for GPELF, whereas the latter is an established assay used by many scientists because of its high-throughput and quantitative features. These commercial antigen detection kits use hybridoma-derived monoclonal antibodies from heterologous sources. The FTS lateral rapid test uses AD12 monoclonal antibody derived from Dirofilaria immitis, a canine filaria which can infect humans, and recognizes a 200-kDa filarial antigen.30 Meanwhile, the Og4C3 ELISA uses Og4C3 monoclonal antibody of the IgM isotype derived from Onchocerca gibsoni, a bovine filaria.31 As mentioned previously, the present antigen detection 5B-ELISA uses a human recombinant monoclonal antibody against B. malayi, a species which is very close to W. bancrofti. Because 5B-ELISA and Og4C3 ELISA use different types of monoclonal antibodies, it would, thus, be interesting to compare the performance characteristics of the two ELISAs using the same panel of serum samples.
Both the commercial antigen detection tests have been reported to be highly sensitive and specific for bancroftian filariasis. However, recently, there have been reports of cross-reactivity of both antigen detection tests with sera from patients with non-lymphatic human filaria, in particular, Loa loa.32–34 Thus, in the context of the GPELF, the tests would not be useful in detecting W. bancrofti infection in areas co-endemic with loiasis.34
If the 5B-ELISA can exhibit specificity against Loa loa infection, it may have some practical applications for the GPELF activities in some parts of Africa. Unfortunately, currently, we do not have serum samples of Loa loa patients and will be a subject of a future study. The 5B-ELISA should also be evaluated using a much bigger panel of serum and eluted dried blood samples, including samples from W. bancrofti–infected individuals from different regions of the world, those who are antigen positive but amicrofilaremic (endemic normals) and posttreatment samples. Also, attempts should also be made to convert the 5B-ELISA into a point-of-care lateral flow test to make it field applicable for potential use in the GPELF.
REFERENCES
- 1.World Health Oraganization , 2017. Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec 92: 594–607. [PubMed] [Google Scholar]
- 2.DALYs GBD, Collaborators H, 2017. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390: 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheirmaraj K, Reddy MV, Harinath BC, 1992. Detection of filarial antigen using antibodies raised against Wuchereria bancrofti microfilarial SDS soluble antigen. Southeast Asian J Trop Med Public Health 23: 444–449. [PubMed] [Google Scholar]
- 4.Forsyth KP, Spark R, Kazura J, Brown GV, Peters P, Heywood P, Dissanayake S, Mitchell GF, 1985. A monoclonal antibody-based immunoradiometric assay for detection of circulating antigen in bancroftian filariasis. J Immunol 134: 1172–1177. [PubMed] [Google Scholar]
- 5.Hamilton RG, Hussain R, Ottesen EA, 1984. Immunoradiometric assay for detection of filarial antigens in human serum. J Immunol 133: 2237–2242. [PubMed] [Google Scholar]
- 6.Lalitha P, Eswaran D, Gnanasekar M, Rao KV, Narayanan RB, Scott A, Nutman T, Kaliraj P, 2002. Development of antigen detection ELISA for the diagnosis of brugian and bancroftian filariasis using antibodies to recombinant filarial antigens Bm-SXP-1 and Wb-SXP-1. Microbiol Immunol 46: 327–332. [DOI] [PubMed] [Google Scholar]
- 7.Ramzy RM, Gad AM, Faris R, Weil GJ, 1991. Evaluation of a monoclonal-antibody based antigen assay for diagnosis of Wuchereria bancrofti infection in Egypt. Am J Trop Med Hyg 44: 691–695. [DOI] [PubMed] [Google Scholar]
- 8.Theodore JG, Kaliraj P, 1996. Wuchereria bancrofti recombinant antigen-derived poly- and monoclonal antibodies for the detection of circulating antigen(s) in the sera of lymphatic filarial patients. J Helminthol 70: 69–74. [DOI] [PubMed] [Google Scholar]
- 9.Zheng HJ, Tao ZH, Reddy MV, Harinath BC, Piessens WF, 1987. Parasite antigens in sera and urine of patients with bancroftian and brugian filariasis detected by sandwich ELISA with monoclonal antibodies. Am J Trop Med Hyg 36: 554–560. [DOI] [PubMed] [Google Scholar]
- 10.Rocha A, Braga C, Belem M, Carrera A, Aguiar-Santos A, Oliveira P, Texeira MJ, Furtado A, 2009. Comparison of tests for the detection of circulating filarial antigen (Og4C3-ELISA and AD12-ICT) and ultrasound in diagnosis of lymphatic filariasis in individuals with microfilariae. Mem Inst Oswaldo Cruz 104: 621–625. [DOI] [PubMed] [Google Scholar]
- 11.More SJ, Copeman DB, 1990. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol 41: 403–406. [PubMed] [Google Scholar]
- 12.World Health Organization , 2011. Lymphatic Filariasis: Monitoring and Epidemiological Assessment of Mass Drug Administration: A Manual for National Elimination Programmes. Geneva, Switzerland: World Health Organization; WHO/HTM/NTD/PCT/2011.4. [Google Scholar]
- 13.Rahumatullah A, Ahmad A, Noordin R, Lim TS, 2015. Delineation of BmSXP antibody V-gene usage from a lymphatic filariasis based immune scFv antibody library. Mol Immunol 67: 512–523. [DOI] [PubMed] [Google Scholar]
- 14.Abdul Rahman R, Hwen-Yee C, Noordin R, 2007. Pan LF-ELISA using BmR1 and BmSXP recombinant antigens for detection of lymphatic filariasis. Filaria J 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saidin S, Yunus MH, Othman N, Lim YA, Mohamed Z, Zakaria NZ, Noordin R, 2017. Development and initial evaluation of a lateral flow dipstick test for antigen detection of Entamoeba histolytica in stool sample. Pathog Glob Health 111: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahumatullah A, Abdul Karim IZ, Noordin R, Lim TS, 2017. Antibody-based protective immunity against helminth infections: antibody phage display derived antibodies against BmR1 antigen. Int J Mol Sci 18: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazemi-Lomedasht F, Behdani M, Habibi-Anbouhi M, Shahbazzadeh D, 2016. Production and characterization of novel camel single domain antibody targeting mouse vascular endothelial growth factor. Monoclon Antib Immunodiagn Immunother 35: 167–171. [DOI] [PubMed] [Google Scholar]
- 18.Kazemi-Lomedasht F, Behdani M, Rahimpour A, Habibi-Anbouhi M, Poshang- Bagheri K, Shahbazzadeh D, 2015. Selection and characterization of specific nanobody against human immunoglobulin G. Monoclon Antib Immunodiagn Immunother 34: 201–205. [DOI] [PubMed] [Google Scholar]
- 19.Baert F, Noman M, Vermeire S, Van Assche G, D’Haens G, Carbonez A, Rutgeerts P, 2003. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 348: 601–608. [DOI] [PubMed] [Google Scholar]
- 20.Cavalli-Bjorkman N, Osby E, Lundin J, Kalin M, Osterborg A, Gruber A, 2002. Fatal adenovirus infection during alemtuzumab (anti-CD52 monoclonal antibody) treatment of a patient with fludarabine-refractory B-cell chronic lymphocytic leukemia. Med Oncol 19: 277–280. [DOI] [PubMed] [Google Scholar]
- 21.Hairul Bahara NH, Tye GJ, Choong YS, Ong EB, Ismail A, Lim TS, 2013. Phage display antibodies for diagnostic applications. Biologicals 41: 209–216. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Zhang R, Chen X, 2017. Application of a single-chain fragment variable (scFv) antibody for the confirmatory diagnosis of hydatid disease in non-endemic areas. Electron J Biotechnol 29: 57–62. [Google Scholar]
- 23.Mechaly A, Zahavy E, Fisher M, 2008. Development and implementation of a single-chain Fv antibody for specific detection of Bacillus anthracis spores. Appl Environ Microbiol 74: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg DM, 2007. Radiolabelled monoclonal antibodies in the treatment of metastatic cancer. Curr Oncol 14: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawashima H, 2014. Radioimmunotherapy: a specific treatment protocol for cancer by cytotoxic radioisotopes conjugated to antibodies. ScientificWorldJournal 2014: 492061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto Torres JE, et al. 2018. Development of a Nanobody-based lateral flow assay to detect active Trypanosoma congolense infections. Sci Rep 8: 9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamatchi R, Charumathi J, Ravishankaran R, Kaliraj P, Meenakshisundaram S, 2016. Construction and bacterial expression of a recombinant single-chain antibody fragment against Wuchereria bancrofti SXP-1 antigen for the diagnosis of lymphatic filariasis. J Helminthol 90: 74–80. [DOI] [PubMed] [Google Scholar]
- 28.Weil GJ, Ramzy RM, 2007. Diagnostic tools for filariasis elimination programs, Trends Parasitol 23: 78–82. [DOI] [PubMed] [Google Scholar]
- 29.Noordin R, Itoh M, Kimura E, Abdul Rahman R, Ravindran B, Mahmud R, Supali T, Weerasooriya M, 2007. Multicentre evaluations of two new rapid IgG4 tests (WB rapid and panLF rapid) for detection of lymphatic filariasis. Filaria J 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weil GJ, Curtis KC, Fischer PU, Won KY, Lammie PJ, Joseph H, Melrose WD, Brattig NW, 2011. A multicenter evaluation of a new antibody test kit for lymphatic filariasis employing recombinant Brugia malayi antigen Bm-14. Acta Trop 120 Suppl 1: S19–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weil GJ, Blair LS, Ewanciw DV, Malatesta PF, 1986. Use of parasite antigen detection to monitor the success of drug therapy in Dirofilaria immitis-infected dogs. J Parasitol 72: 737–740. [PubMed] [Google Scholar]
- 32.Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, Weil GJ, Fischer PU, 2014. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg 91: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanji S, et al. 2015. Cross-reactivity of filariais ICT cards in areas of contrasting endemicity of Loa loa and Mansonella perstansin Cameroon: implications for shrinking of the lymphatic filariasis map in the Central African Region. Plos Negl Trop Dis 9: e0004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanji S, Amvongo-Adjia N, Njouendou AJ, Kengne-Ouafo JA, Ndongmo WP, Fombad FF, Koudou B, Enyong PA, Bockarie M, 2016. Further evidence of the cross-reactivity of the Binax NOW(R) Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa loa and Onchocerca ochengi. Parasit Vectors 9: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]