Abstract
Lateral root (LR) proliferation is a major determinant of soil nutrient uptake. How resource allocation controls the extent of LR growth remains unresolved. We used genetic, physiological, transcriptomic, and grafting approaches to define a role for C-TERMINALLY ENCODED PEPTIDE RECEPTOR 1 (CEPR1) in controlling sucrose-dependent LR growth. CEPR1 inhibited LR growth in response to applied sucrose, other metabolizable sugars, and elevated light intensity. Pathways through CEPR1 restricted LR growth by reducing LR meristem size and the length of mature LR cells. RNA-sequencing of wild-type (WT) and cepr1-1 roots with or without sucrose treatment revealed an intersection of CEP–CEPR1 signalling with the sucrose transcriptional response. Sucrose up-regulated several CEP genes, supporting a specific role for CEP–CEPR1 in the response to sucrose. Moreover, genes with basally perturbed expression in cepr1-1 overlap with WT sucrose-responsive genes significantly. We found that exogenous CEP inhibited LR growth via CEPR1 by reducing LR meristem size and mature cell length. This result is consistent with CEP–CEPR1 acting to curtail the extent of sucrose-dependent LR growth. Reciprocal grafting indicates that LR growth inhibition requires CEPR1 in both the roots and shoots. Our results reveal a new role for CEP–CEPR1 signalling in controlling LR growth in response to sucrose.
Keywords: Arabidopsis, carbon availability, CEP, CEPR1, growth, lateral root, peptide hormone, sucrose
Pathways that coordinate lateral root growth with carbon availability are poorly understood. We reveal a key role for CEP–CEPR1 signalling in curtailing lateral root growth expenditure in response to sucrose.
Introduction
Lateral root (LR) proliferation is instrumental in determining the overall size of the root network and the effectiveness of anchorage to the soil, and maximizing the opportunities for acquiring resources. LRs initiate post-embryonically from the primary root under the influence of an ordered developmental process involving the repeated division of specific ‘founder’ pericycle cells (Malamy and Benfey, 1997; Dubrovsky et al., 2008; Moreno-Risueno et al., 2010). The number, deployment, and growth of the LRs determine the overall patterning of the root system, which is developmentally malleable and influenced by the environment. Complex local and systemic processes, which integrate the supply of shoot resources with a myriad of environmental influences, combine to determine root system patterning (Malamy and Ryan, 2001; Krouk et al., 2010; Ruffel et al., 2011; Kircher and Schopfer, 2012; Huault et al., 2014; Tabata et al., 2014; Mohd-Radzman et al., 2016).
In plate-grown Arabidopsis plants, light is generally limiting. Therefore, when present, a major driver of LR growth is the uptake of externally supplied sucrose mediated by shoot contact with the medium (MacGregor et al., 2008) in combination with leaf-derived photosynthate (Kircher and Schopfer, 2012). How sucrose supply is precisely titrated and utilized to support LR growth is unknown, but in plate-grown plants this can be explored by varying the amount of externally supplied sucrose (MacGregor et al., 2008).
The interaction of C-TERMINALLY ENCODED PEPTIDES (CEPs) with CEP receptors (CEPRs) regulate several aspects of lateral organ proliferation in Arabidopsis and Medicago roots (Ohyama et al., 2008; Delay et al., 2013; Imin et al., 2013; Tabata et al., 2014; Mohd-Radzman et al., 2016; Roberts et al., 2016; Taleski et al., 2016, 2018; Ohkubo et al., 2017; Patel et al., 2018). CEPs negatively affect LR proliferation in several genera (Ohyama et al., 2008; Delay et al., 2013; Imin et al., 2013; Mohd-Radzman et al., 2016; Roberts et al., 2016). Low nitrogen (N) up-regulates CEP transcription in roots, which promotes the production of secreted CEP hormones that move from the root to the shoot via the xylem (Tabata et al., 2014; Okamoto et al., 2015; Patel et al., 2018). Two Arabidopsis receptors, CEPR1 (aka XIP1; Bryan et al., 2012) and CEPR2, specifically bind CEP hormones (Tabata et al., 2014); however, the extent to which these receptors shape LR growth remains elusive. Analysis of a CEPR1 knockout allele, cepr1-1, suggests a role in the systemic control of key nitrate transporters in N-demand signalling, and this mutant has longer LRs when grown on agar support medium (Tabata et al., 2014). In Medicago truncatula, however, >10 independent mutants affected in the CEPR1 orthologue, COMPACT ROOT ARCHITECTURE 2, have grossly altered LR development when grown in soil (Huault et al., 2014). Since cepr1 and cra2 knockout mutants are unresponsive to the negative root growth effects of CEPs (Huault et al., 2014; Tabata et al., 2014; Mohd-Radzman et al., 2016), this suggests that a core function of CEPR1/CRA2-dependent pathways is to control root growth.
The current understanding of the function of CEP–CEPR interactions is focused on the transcriptional activation of CEP genes in roots in response to very low N (Delay et al., 2013; Imin et al., 2013; Tabata et al., 2014), and the role of CEPs in N-demand signalling via the systemic control of nitrate transporter expression (Tabata et al., 2014; Ohkubo et al., 2017; Taleski et al., 2018). A CEP–CEPR1 interaction in the shoot primarily mediates this systemic N-demand signalling (Tabata et al., 2014; Ohkubo et al., 2017). In M. truncatula, however, preliminary evidence suggests that high CO2 also up-regulates CEP expression independently of low N (Imin et al., 2013), but the potential role for carbon (C) status in CEP–CEPR interactions is uncharacterized. In addition, there is evidence for local and systemic CEP–CEPR1/CRA2 functions affecting root growth in Arabidopsis and Medicago (Huault et al., 2014; Mohd-Radzman et al., 2015; Roberts et al., 2016; Taleski et al., 2016).
Several core and highly conserved pathways control plant growth responses to available C. For example, the coordination of growth under C limitation is carried out by sucrose non-fermenting 1-related protein kinase 1 (SnRK1) signalling (Baena-González et al., 2007). The sugar signalling molecule, trehalose-6-phosphate (T6P), is also critical for utilizing C for growth, and T6P levels correlate with sucrose availability (Schluepmann et al., 2003, 2004; Lunn et al., 2006). T6P and SnRK1 signalling appears to interact to control growth responses to C availability, with T6P inhibiting SnRK1 activity (Zhang et al., 2009; Delatte et al., 2011). Although the role of SnRK1 signalling in controlling shoot growth is well established (Baena-González et al., 2007), its potential role in the control of root growth is much less studied. In addition, TOR (TARGET OF RAPAMYCIN) kinase signalling is critical for root meristem activation in response to photosynthetically derived sugars (Xiong et al., 2013). It is unknown whether CEP–CEPR1 signalling intersects with any of these pathways to control root growth.
Here we show that independent Arabidopsis cepr1 knockout mutants in Columbia (Col-0) and Nössen (No-0) ecotypes display an increased LR growth phenotype that depends on supplied sucrose, other metabolizable sugars, and light intensity. Non-metabolizable sugars did not influence this phenotype. Using microscopy, we determined whether LR cell elongation or the size of the meristem zone (MZ) accounted for the increased LR growth phenotype of these cepr1 mutants. To investigate the potential underlying mechanisms, we conducted RNA-sequencing (RNA-Seq) and quantitative reverse transcription–PCR (RT–PCR) analyses of wild-type (WT) and cepr1 roots grown in the presence and absence of sucrose. This revealed that (i) sucrose highly up-regulates several CEP genes and (ii) genes with a basally perturbed expression in cepr1-1 significantly overlap with the WT sucrose transcriptional response, which included many SnRK1 target genes. We then examined the effect of synthetic CEP on LR growth. CEP addition repressed LR growth by inhibiting both the final length of mature LR cells and MZ size, and this effect depended on CEPR1. The results of reciprocal WT–cepr1 hypocotyl grafting suggest that CEPR1 acts in both roots and shoots to influence LR growth. Our data show that CEPR1 attenuates the extent of sucrose-dependent growth, and we present a new model for the role of CEP–CEPR1 interactions in regulating LR growth.
Materials and methods
Plant materials and growth conditions
The No-0 cepr1-1 and cepr2-1 mutants (RATM11-2459 and RATM15-3532) and the cepr1-1 cepr2-1 double mutant were obtained from RIKEN (Tabata et al., 2014). The homozygous Col-0 cepr1-3 mutant was isolated from the T-DNA line 467C01 generated by the GABI-Kat program and provided by Bernd Weisshaar (Kleinboelting et al., 2012). Sterilized seeds were grown on solidified medium (1% Type M agar) containing half-strength Murashige and Skoog (1/2 MS) basal salts (Sigma) at pH 5.7, and sugars added as described. Plates were grown in chambers at 22 °C with a 16 h photoperiod with 100–120 µmol m−2 s−1 light. For the light treatment experiment, seedlings were grown on 1/2 MS without sucrose under "low" (40 µmol m−2 s−1) or "high" (150 µmol m−2 s−1) light for 10 d. Roots were scanned and measured using ImageJ with the SMARTROOT plugin (Lobet et al., 2011).
Synthetic peptide
Synthetic CEP3 (i.e. TFRhyPTEPGHShyPGIGH) and CEP5 (DFRhyPTTPGHShyPGIGH) were used at 1 µM (Delay et al., 2013; Imin et al., 2013; Mohd-Radzman et al., 2015). Both peptides were synthesized by GL Biochem, Shanghai, and their structures were validated independently by MS.
Cell measurements
LRs were stained with 100 µM propidium iodide for 2 min, washed, and mounted on slides. Cortical cells in the meristematic and differentiation zones were measured using a Leica DM5500 microscope with a 560 nm excitation filter.
LR staging assay
The roots of seedlings were cleared after 7 d growth on 1/2 MS medium with or without 1% sucrose and observed by differential interference contast (DIC) microscopy using the Leica DM5500 microscope (Malamy and Benfey, 1997).
Hypocotyl grafting
Seedlings were grown for 6 d on 1/2 MS with 0.5% sucrose prior to hypocotyl grafting (Branco and Masle, 2019). Five days after grafting, plants were transferred to 1/2 MS medium with 1% sucrose.
RT–PCR analysis of cepr1-3
For RT–PCR analysis of cepr1-3, leaves were harvested from 27-day-old plants. RT–PCR was carried out over 35 cycles with primers targeting the CEPR1 coding sequence (F- CTTGTGGACAAGAACATCGTAGG, R- GATCAGAAGCTGAACAACTTCGTT) or UBQ10 (F- GATCTTTGCCGGAAAACAATTGGAGGATGGT, R- CGACTTGTCATTAGAAAGAAAGAGATAACAGG) (Ahn, 2009).
RNA-Seq
No-0 WT and cepr1-1 plants were grown vertically on 1/2 MS medium (no sucrose) for 6 d before transfer to 1/2 MS (control) or 1/2 MS+1% sucrose for 4 h. Three biological samples containing ~50 whole roots for each treatment were cut, snap-frozen in liquid nitrogen, and total RNA was isolated by a modified Trizol extraction method using columns from the RNeasy plant mini kit (QIAGEN) (Delay et al., 2013). mRNA library preparation and sequencing using the Next Seq 500 (Illumina) system was carried out at the ACRF Biomolecular Resource Facility (Australian National University).
RNA-Seq analysis
RNA-Seq reads were filtered and trimmed to improve read quality using fastp version 0.12.5 (Chen et al., 2018). Automatic 3' trimming was enabled, but otherwise default settings were used. Reads were mapped to the TAIR10 Arabidopsis genome assembly (Lamesch et al., 2012), with a custom annotation file, using STAR aligner version 2.5.4b (Dobin et al., 2013) with default settings. The recommended setting of 75 (read length – 1) was used for the ‘sjdbOverhang’ parameter during genome index generation. The annotation was based on Araport11 (Cheng et al., 2017), but with manual curation of CEP genes (Supplementary Table S1 at JXB online) based on the reads in our data set, and on the predicted CEP coding sequences (Ogilvie et al., 2014).
Raw read numbers were computed using HTSeq (Anders et al., 2015) before using edgeR (Robinson et al., 2010) to construct a model including each combination of genotype (WT and cepr1-1) and treatment (with and without sucrose addition). The baseline was the untreated WT (no sucrose), and the three alternative conditions were sucrose-treated WT, untreated cepr1-1, and sucrose-treated cepr1-1. We used edgeR to calculate the log2 fold change (FC) in gene expression. The edgeR statistical test ‘glmTreat’ was run to test for differential expression at least 25% above, or equivalently 20% below, the reference samples (magnitudes identical on a log scale). Genes with false discovery rate- (FDR) corrected P-values <0.05 were considered to have biologically and statistically significantly different expression from the reference samples.
Evaluation of overlaps between differentially expressed gene sets
We evaluated how many genes were differentially up- or down-regulated in sucrose-treated WT and untreated cepr1-1, compared with untreated WT. If a gene was differentially expressed in both comparisons, it was added to the count for the respective intersection. To evaluate whether the intersection was statistically significant, we calculated the expected number of overlapping genes if the two gene sets were independent using 10 000 Monte Carlo simulations (Hope, 1968). For each simulation, genes were randomly sampled without replacement to be up- or down-regulated in sucrose-treated WT, and then randomly sampled again to be up- or down-regulated in untreated cepr1-1. Excluded from sampling as unrealistic choices were genes with no mapped reads across any of our RNA-Seq libraries. For each comparison and direction of differential regulation, the number of genes sampled was set to match the observed count from our RNA-Seq analysis. If the observed number of overlapping genes was outside the 95% interval (2.5% and 97.5% quantiles) of the expected overlap, the intersection was considered to be statistically significant (P<0.05).
Intersections with KIN10 targets were evaluated in a similar manner. We compared genes coordinately up- or down-regulated in both sucrose-treated WT and untreated cepr1-1 with the global list of genes up- or down-regulated by KIN10 as determined by microarrays (Baena-González et al., 2007). For determining expected counts, we included only genes present on the microarray for which there was non-zero expression in any one of our RNA-Seq libraries.
qRT–PCR analyses
RNA was extracted from harvested tissue as described for RNA-Seq. Total RNA was used for cDNA synthesis using oligo(dT)12–18 primers and Superscript III reverse transcriptase (Invitrogen). For qRT–PCR, Fast SYBR Green fluorescent dye (Applied Biosystems) was used and samples were run on a ViiA 7 Real-Time PCR System (Applied Biosystems) following manufacturer’s specifications. Data were analysed using the ΔΔCT method (Livak and Schmittgen, 2001). EF1α (At1g07920) expression was used for normalization (Czechowski et al., 2005). Primers used are listed in Supplementary Table S2.
Results
Characterization of cepr1-3
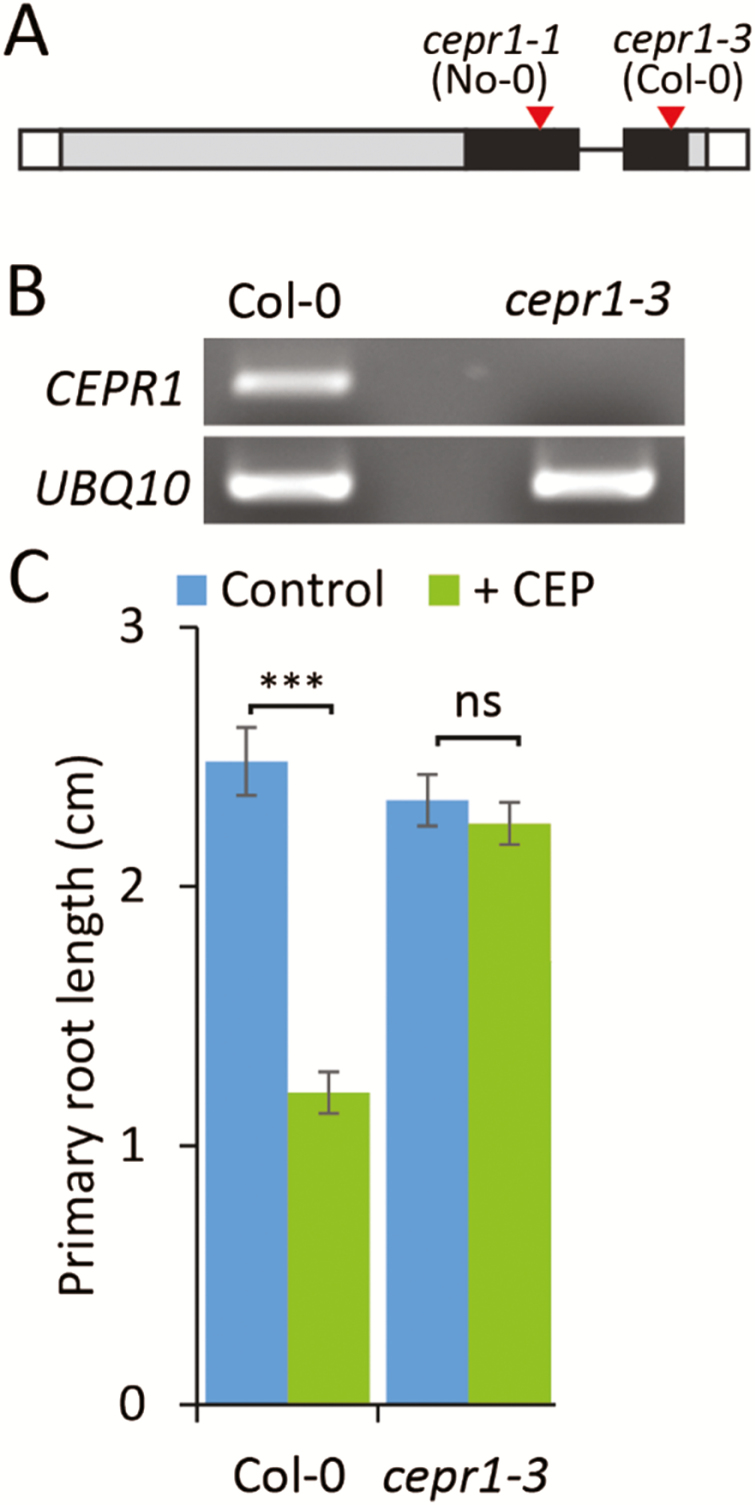
We used two CEPR1 knockout alleles, cepr1-1 and cepr1-3, in the No-0 and Col-0 backgrounds, respectively, to assess whether root growth responses to sucrose depend on the activity of this receptor. Both alleles have T-DNA insertions in the coding sequence corresponding to the kinase domain (Fig. 1A). We confirmed in the newly characterized cepr1-3 allele that full-length transcripts could not be detected (Fig. 1B). Like cepr1-1, the growth of the main root of cepr1-3 was insensitive to CEP addition (Fig. 1C; see Supplementary Fig. S1). Collectively, these data indicate that both lines are null mutants.
Fig. 1.
Characterization of the cepr1-3 mutant. (A) Diagram of the CEPR1 gene showing T-DNA insertion sites (red triangles) in the kinase domain (black) for the cepr1-1 and cepr1-3 alleles. (B) The full-length CEPR1 transcript could not be detected in the cepr1-3 mutant in an RT–PCR analysis with a UBQ10 control. (C) The cepr1-3 mutant is insensitive to CEP inhibition of primary root growth. Primary root length of Col-0 and cepr1-3 plants after 7 d of growth on 1/2 MS medium with or without 1 µM CEP3 (n=6). Statistically significant differences were determined using a Student’s t-test; ns, not significant, P>0.05; ***P<0.001.
CEPR1 restricts LR growth in response to metabolizable sugars and higher light availability
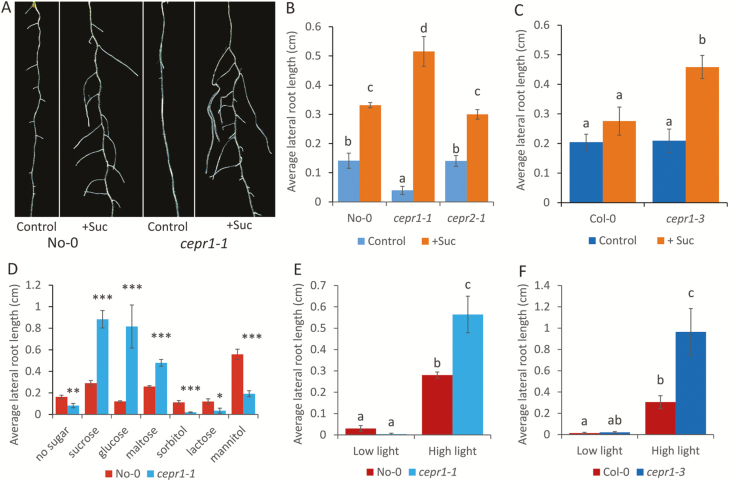
Sucrose addition resulted in significantly increased LR growth in both CEPR1 mutants when compared with the corresponding WT lines (Fig. 2A–C). The response of LRs to sucrose in the CEPR2 knockout mutant cepr2-1, however, was the same as in the WT (Fig. 2B). In addition, cepr2-1 retained WT sensitivity to CEP peptide addition (Supplementary Fig. S1). This indicated that the CEPR2 receptor plays no obvious role in the sucrose-dependent enhancement of LR growth or the CEP-mediated inhibition of primary root growth. The enhancement of LR growth in cepr1-1 depended on the presence of metabolizable sugars (sucrose, glucose, or maltose), but not non-metabolizable sugars (mannitol, lactose, or sorbitol; Fig. 2D), indicating that this phenotype is independent of the osmotic effects of sugar addition.
Fig. 2.
CEPR1 restricts LR growth in response to metabolizable sugars and higher light availability. (A) Representative images of 10-day-old No-0 and cepr1-1 grown on medium with no added sucrose (control) or with 1% sucrose (w/v) (+Suc). Scale bar=5 mm. (B, C) The average LR length of 12-day-old No-0, cepr1-1 and cepr2-1 seedlings (B) or Col-0 and cepr1-3 seedlings (C) in the presence or absence of 1% sucrose (n≥7). (D) Average LR length of No-0 and cepr1-1 plants after 12 d growth on medium supplemented with different sugars (1% w/v) (n≥9). Statistically significant differences between No-0 and cepr1-1 were determined using a Student’s t-test: *P≤0.05; **P≤0.01; ***P≤0.001. (E, F) Average LR length of WT and cepr1 10-day-old seedlings in the No-0 (E) and Col-0 (F) backgrounds under low (40 µmol m−2 s−1) or high (150 µmol m−2 s−1) light in the absence of sucrose (n≥6). Different letters indicate a statistically significant difference (P≤0.05, two-way ANOVA followed by Bonferroni multiple comparisons test). Bars indicate the SE.
Next we tested whether endogenous C supply differentially affects LR growth in the cepr1 mutants. To do this, we grew the WT and cepr1 under low and high light regimes in the absence of added sugars. Under low light, the LR growth of both cepr1 mutants was not different from that of the WT, whereas under high light both cepr1 mutants had significantly greater LR growth (Fig. 2E, F). This suggests that CEPR1 represses LR growth in response to C derived from photosynthesis.
We assessed if the LR growth phenotype of cepr1 was an indirect effect of changes in the distribution of growth across the root system. To do this, we measured primary root length, LR number, and LR primordia staging in the presence and absence of sucrose (Supplementary Fig. S2A–H). Relative to their respective WTs, the cepr1 lines displayed ecotype- and sucrose treatment-specific differences in primary root length, LR number, and LR staging (Supplementary Fig. S2A–H). This points to an influence of the genetic background on CEPR1 activity with respect to these traits. The increased LR growth in response to sucrose observed in both alleles, however, could not be explained by differences in primary root growth, LR number, or LR primordia staging.
CEPR1 restricts MZ size and the extent of cell elongation in LRs
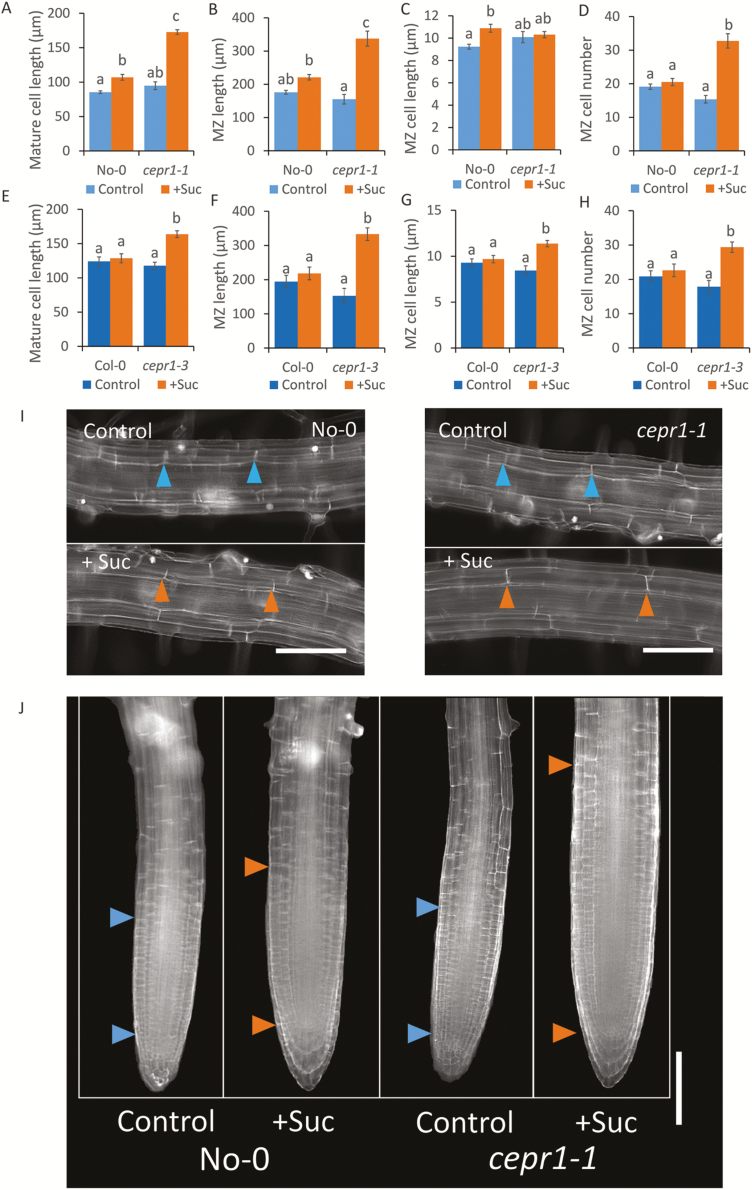
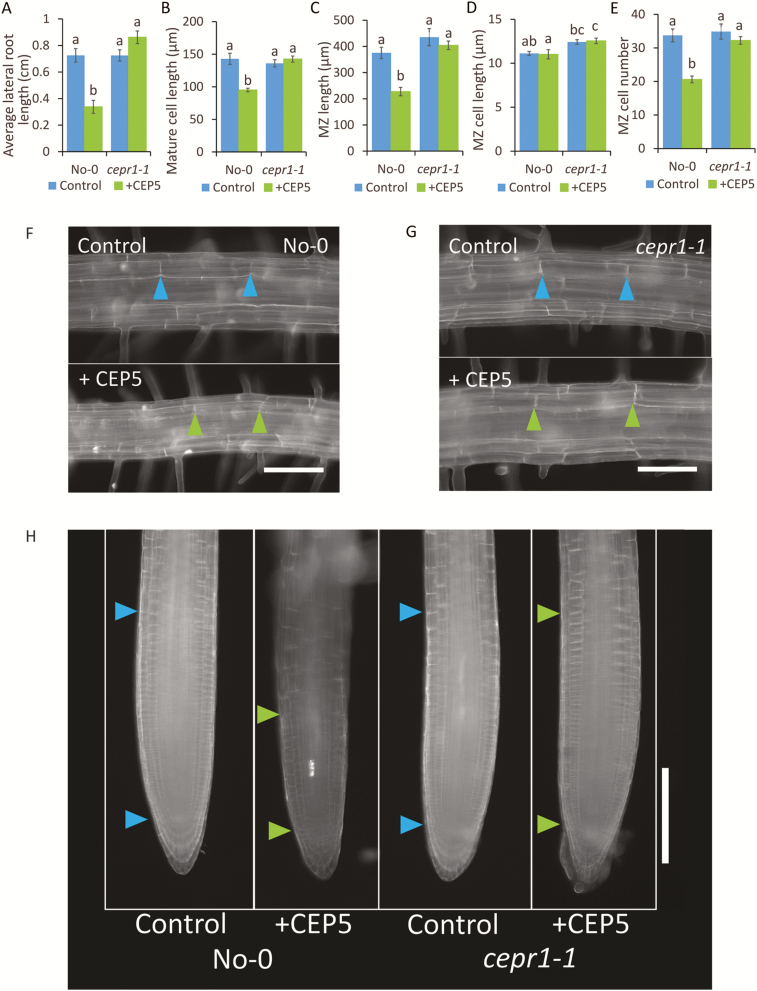
From the above results, we hypothesized a specific role for CEPR1 in controlling LR growth in response to sucrose. To test this, we examined if the physiological basis for the sucrose-dependent increase in LR growth in the cepr1 mutants was due to differences in cell elongation and/or MZ size. Sucrose addition resulted in longer mature cells in both cepr1-1 and cepr1-3 than in the corresponding WT lines (Fig. 3A, E, I); however, this alone did not account for the total increase in LR growth. Sucrose also promoted a significant increase in MZ size in cepr1-1 and cepr1-3 LRs (Fig. 3B, F, J). There were some differences in the underlying basis for the sucrose-dependent increase in LR growth between the ecotypes. The enhancement in cepr1 MZ size was primarily due to a sucrose-dependent increase in MZ cell number in cepr1-1 (Fig. 3C, D) and an increase in both MZ cell number and MZ cell size in cepr1-3 (Fig. 3G, H). These results demonstrate that CEPR1 inhibits the extent of the increase in LR mature cell length and MZ size in response to sucrose application.
Fig. 3.
CEPR1 represses LR mature cell length and MZ size in response to applied sucrose. The effect of sucrose on LR mature cell length and MZ size for WT and cepr1 in the No-0 (A–D) and Col-0 (E–H) backgrounds was measured after 10 d growth on medium with or without sucrose. (A–E) Length of mature cortical cells in emerged LRs (n≥54 cells). (B–F) MZ total length; (C, G) MZ cell length; and (D, H) MZ cell number in emerged LRs (n≥7 roots). Different letters indicate statistically significant differences (P≤0.05, two-way ANOVA followed by Bonferroni multiple comparisons test). Bars indicate the SE. Representative images of mature cortical cells (I) and MZ (J) of LRs in No-0 and cepr1-1. Arrows indicate mature cell length and MZ size, respectively. Bars indicate the SE. Scale bars=100 µm.
RNA-Seq reveals that multiple CEP genes are up-regulated in response to sucrose independently of CEPR1
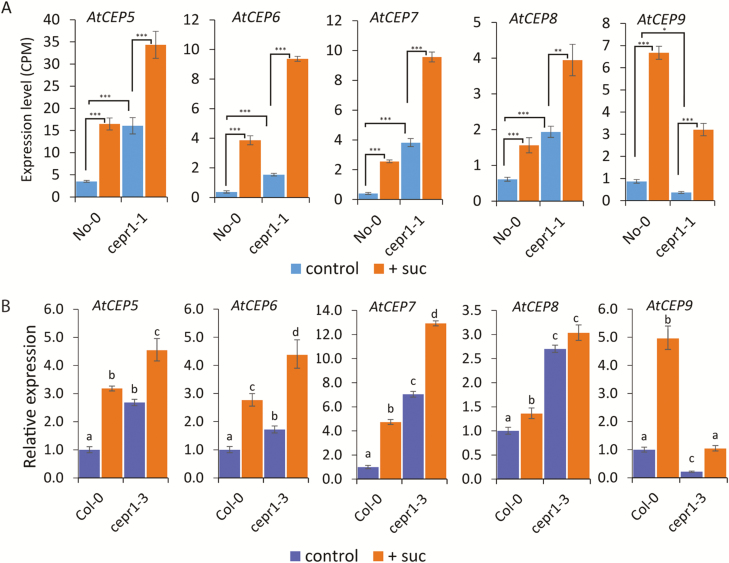
We used RNA-Seq to elucidate how CEPR1 represses LR growth in response to sucrose. We investigated the transcriptome of WT and cepr1-1 roots 4 h after transferring the seedlings to media with or without sucrose (Supplementary Dataset S1). Notably, the CEP ligand-encoding genes, AtCEP5–AtCEP9, were significantly up-regulated by sucrose in the No-0 and cepr1-1 backgrounds (Fig. 4A). qRT–PCR also showed that sucrose up-regulated AtCEP5–AtCEP9 in Col-0, and AtCEP5–AtCEP7 and AtCEP9 in cepr1-3 (Fig. 4B). Therefore, AtCEP5–AtCEP7, and AtCEP9 were robustly up-regulated by sucrose addition in the WT and cepr1 across both ecotypes. This supports a specific role for these CEP genes in the plant’s response to sucrose. In addition, the basal level of transcription of AtCEP5–AtCEP8 was elevated in cepr1-1, whereas the transcriptional level of AtCEP9 was reduced (Fig. 4A). This pattern of expression also occurred in cepr1-3 (Fig. 4B), demonstrating feedback regulation of AtCEP5–AtCEP8 and feedforward regulation of AtCEP9 through CEPR1.
Fig. 4.
Multiple AtCEP genes are up-regulated in response to sucrose. (A) Expression of AtCEP5–AtCEP9 in response to sucrose in No-0 and cepr1-1 (CPM, counts per million; *P≤0.05; **P≤0.01; ***P≤0.001; FDR corrected). (B) Relative expression of AtCEP5–AtCEP9 in roots in Col-0 and cepr1-3. Whole roots were harvested for gene expression analysis using qRT–PCR. Letters indicate significant differences (two-way ANOVA followed by Fisher’s least significant difference test, α=0.05). Bars indicate the SE, n=3.
CEPR1 signalling intersects with the sucrose transcriptional response in roots
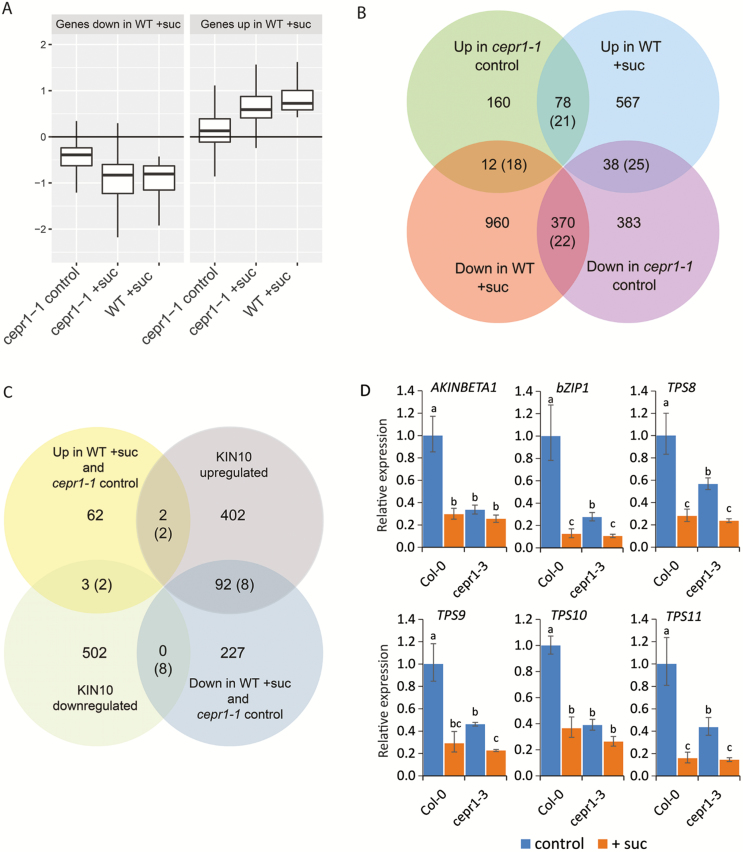
We assessed the number of genes differentially regulated as a result of sucrose addition, genotype, or the combination of both (Supplementary Table S3). Sucrose addition resulted in the differential expression of 2267 different genes in total across genotypes. Of these genes, 753 displayed a congruent response in both genotypes. Strikingly, there were 1268 genes differentially regulated by sucrose in the WT that did not significantly respond to sucrose in cepr1-1. To assess this further, we compared the mean expression of 2025 differentially expressed genes that constituted the WT sucrose response (irrespective of their expression in cepr1-1) across all treatment groups (Fig. 5A). Compared with the untreated WT, their mean expression in untreated cepr1-1 was shifted towards levels observed for sucrose-treated WT. We reasoned that this trend might reflect a basal perturbation in the expression of WT sucrose response genes in cepr1-1. To determine this, we tested whether the genes significantly up- or down-regulated in sucrose-treated WT or in untreated cepr1-1 significantly overlapped (Fig. 5B; Supplementary Table S4). There were strong and significant overlaps between genes differentially regulated in sucrose-treated WT, and in untreated cepr1-1 in the same direction; 17.1- and 3.8-fold higher than expected for down- or up-regulated genes, respectively (listed in Supplementary Tables S5 and S6). We observed a significant but comparatively weaker overlap between genes up-regulated by sucrose in WT and down-regulated in untreated cepr1-1 (1.5-fold higher than expected; listed in Supplementary Table S7). These results demonstrate that genes basally perturbed in cepr1-1 significantly overlap with the WT sucrose transcriptional response.
Fig. 5.
Transcriptional responses to sucrose intersect with CEP–CEPR1 signalling. The transcriptional response of WT and cepr1-1 roots was assessed by RNA-Seq 4 h after transfer to medium without (control) or with 1% sucrose (+suc). (A) Mean expression in each treatment group for genes significantly up- and down-regulated by sucrose in the WT, relative to levels in WT control. Outlier points are not shown. (B) Intersection of genes significantly up- and down-regulated in WT+suc and cepr1-1 control compared with WT control. (C) Genes significantly down- or up-regulated in the same direction in WT+suc and in cepr1-1 control (Supplementary Tables S5 and S6, respectively) compared with the global list of KIN10 up- or down-regulated genes from Baena-González et al. (2007) as determined by microarray analysis. The evaluation of overlapping genes was restricted to genes present on the microarray, as described in the Materials and methods. Values in parentheses indicate the expected number of overlapping genes if the two gene sets were independent. For a summary of 95% confidence intervals for the overlaps, see Tables S4 and S8, respectively. (D) Expression of a shortlist of overlapping KIN10 target genes in the Col-0 and cepr1-3 backgrounds in response to sucrose. Seedlings were treated as described for the RNA-Seq experiment and whole roots were harvested for gene expression analysis using qRT–PCR. Letters indicate significant differences (two-way ANOVA followed by Fisher’s least significant difference test, α=0.05). Bars indicate the SE, n=3.
To investigate further the strong overlap of genes differentially expressed in the same direction in sucrose-treated WT and untreated cepr1-1, we determined whether these genes intersected with those regulated by KIN10, a catalytic subunit of SnRK1, which coordinates transcription in response to C status and energy levels (Baena-González et al., 2007; Ramon et al., 2013). There was a significant overlap (11.4-fold higher than expected) between genes down-regulated in sucrose-treated WT and in untreated cepr1-1 and the known up-regulated targets of KIN10 from Baena-González et al. (2007), as determined by microarray analysis (Fig. 5C; Supplementary Table S8). These overlapping genes included AKINBETA1, which encodes a subunit of the SnRK1 complex, the transcription factor gene bZIP1, which is involved in sugar signalling and responses to low energy (Kang et al., 2010; Dietrich et al., 2011), and the putative trehalose-6-phosphate synthase genes TPS8– TPS11 (Supplementary Table S9). Moreover, qRT–PCR demonstrated a congruent pattern of expression for these SnRK1 target genes in cepr1-3, with expression in untreated cepr1-3 partially or fully shifted towards levels in sucrose-treated Col-0 (Fig. 5D). Together, these results demonstrate that the expression of these sucrose-responsive genes is basally uncoupled in the cepr1 mutants. This suggests that CEPR1 is required to maintain the transcriptional homeostasis of this gene subset, which is closely associated with C signalling.
CEP5 peptide represses LR growth through CEPR1 activity
In Arabidopsis, the addition of exogenous CEP hormones inhibits root proliferation by inhibiting primary root growth (Ohyama et al., 2008; Delay et al., 2013) and LR initiation (Roberts et al., 2016), and these effects depended on CEPR1 (Tabata et al., 2014; Roberts et al., 2016). The effect of CEPs on LR growth, however, are unknown. Based on the enhanced LR growth phenotype of both cepr1 mutants under sucrose treatment, we hypothesized that the addition of a synthetic CEP would inhibit LR growth. To test this, we applied CEP5 to No-0 and cepr1-1. To avoid indirect effects of CEP5 on LR growth resulting from primary root inhibition (Delay et al., 2013), we excised primary root tips prior to the treatment. CEP5 treatment inhibited LR growth in No-0, but not in cepr1-1 (Fig. 6A). LR growth inhibition was due to a CEPR1-dependent reduction in mature cell length (Fig. 6B, F, G), and inhibition of MZ size (Fig. 6C, H) via a reduction in MZ cell number (Fig. 6D, E). These results are consistent with a role for CEP–CEPR1 signalling in restricting the extent of sucrose-dependent LR growth.
Fig. 6.
CEP5 peptide inhibits LR growth by repressing mature cell length and MZ size via CEPR1. Seedlings were grown for 6 d on 1/2 MS medium before excising primary root tips. Plants were then transferred to 1/2 MS medium with or without 1 µM CEP5 and grown for an additional 4 d. (A) Average LR length (n≥6), (B) mature cortical cell length (n=54 cells), (C) MZ length, (D) MZ cell length, and (E) MZ cell number (n=8). Different letters indicate statistically significant differences (P≤0.05, two-way ANOVA followed by Bonferroni multiple comparisons test). Bars indicate the SE. (F, G) Representative images of LR mature cortical cells in No-0 (F) and cepr1-1 (G) (scale bars=100 µm). (H) Representative images of LR MZ in No-0 and cepr1-1 (scale bar=200 µm). Arrows indicate mature cell length and MZ size, respectively.
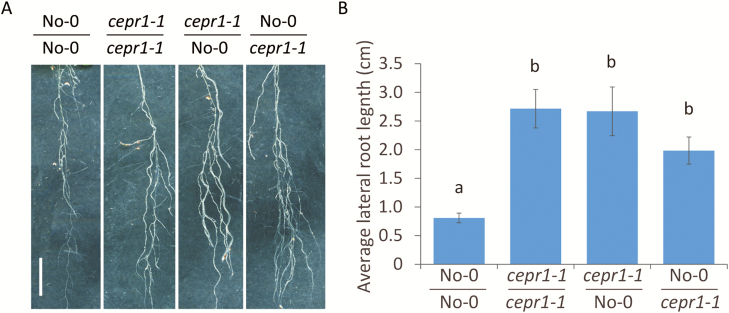
Grafting demonstrates that CEPR1 activity in both roots and shoots is required to repress LR growth
To determine if CEPR1 signalling influenced LR growth locally in the root and/or systemically via the shoot, we performed reciprocal hypocotyl grafting of the WT and cepr1-1. We observed that grafting cepr1-1 to the root or shoot increased LR growth (Fig. 7). This indicates that a functional CEPR1 is required in both the roots and the shoots to control LR growth.
Fig. 7.
Repression of LR growth requires CEPR1 activity in both the root and shoot. (A) Representative images of LRs of grafted plants. Scale bar=1 cm. (B) LR growth of grafted plants after 13 d on 1% sucrose (n≥5). Different letters indicate statistically significant differences (P≤0.05, two-way ANOVA followed by Bonferroni multiple comparisons test).
Discussion
During indeterminate growth, the meristematic centres of shoots and roots compete for resources to expand and grow. A major question in plant developmental biology is how resource allocation, and utilization, influences the competing growth demands of the root and shoot system. Several phytohormones, including auxin and cytokinin, play a role in influencing the competing demands of root and shoot growth (Wolters and Jürgens, 2009; Su et al., 2011); however, little is known about the pathways controlling LR growth in response to C levels. Our data provide insights into the molecular components controlling LR growth on a per root basis in response to C availability. We show that CEPR1 is critical in determining the extent of LR growth in response to metabolizable sugars, and C derived from photosynthesis. Therefore, this study reveals a new and important role for CEP–CEPR1 interactions beyond that previously identified in N-demand signalling (Tabata et al., 2014; Ohkubo et al., 2017).
CEPR1 controls the extent of LR growth in response to metabolizable sugars and light availability
We determined whether LR growth responses to sucrose depended on CEP receptor function. CEPR1 and CEPR2 have been implicated previously in the control of LR growth in response to sucrose (Dimitrov and Tax, 2018). In our work, two independent cepr1 knockout mutants displayed enhanced LR growth in response to sucrose, whilst the LR growth of the CEPR2 knockout mutant cepr2-1 was the same as in the WT. These data indicate that CEPR1 is the major receptor contributing to this response. Moreover, only metabolizable sugars, but not non-metabolizable sugars, enhanced LR growth in cepr1-1. This indicates that the osmotic effects of sugars do not play a detectable role in this phenotype. Therefore, we conclude that CEPR1 represses LR growth in response to added metabolizable sugars. Both cepr1 mutants show an increased LR growth response under elevated light in the absence of externally supplied sucrose. This result suggests that the control of C-dependent LR growth by CEPR1 is physiologically relevant.
We determined the underlying physiological basis for CEPR1 restriction of LR growth and found that CEPR1 restricted LR mature cell length and MZ size in response to sucrose. Sucrose enhanced MZ cell number in both cepr1 mutants. Known pathways controlling primary root MZ size in response to C levels include bZIP11–IAA3/SHY2, which inhibits MZ cell number in response to low C (Weiste et al., 2017). bZIP11 and other bZIPs (including bZIP1) mediate a subset of responses to C availability downstream of SnRK1 (Baena-González et al., 2007; Pedrotti et al., 2018). In addition, the TOR pathway promotes MZ cell number in response to increased C supply (Xiong et al., 2013). It would be of interest to determine whether these pathways are mechanistically involved in CEP–CEPR1 inhibition of LR growth.
Several CEP genes are up-regulated by sucrose
RNA-Seq and qRT–PCR approaches showed that AtCEP5–AtCEP7 and AtCEP9 responded to sucrose addition in WT and cepr1 mutant backgrounds. This shows that these CEP genes are up-regulated by sucrose independently of CEPR1. This suggests a direct role for CEP–CEPR1 signalling in LR growth responses to sucrose. In addition, the basal level of CEP gene expression was uncoupled in cepr1. This result demonstrated that there is feedback or feedforward regulation of specific CEP genes through CEPR1.
CEP–CEPR1 signalling intersects with the sucrose transcriptional response
As expected, sucrose regulated the expression of many WT genes (>2000). Compared with untreated WT, many of these genes were differentially expressed in untreated cepr1-1 in the same direction as in the sucrose-treated WT. In addition, we found that many of the genes co-down-regulated in sucrose-treated WT and untreated cepr1-1 were known up-regulated SnRK1 targets (Baena-González et al., 2007). We confirmed that there was a congruent response in cepr1-3 in a number of SnRK1 targets by qRT–PCR. This shows that CEPR1 affects the basal expression of these genes and suggests a perturbation of C signalling in cepr1 roots.
CEP5 peptide acts through CEPR1 to inhibit LR growth
We showed that CEP5 inhibited LR growth by restricting mature cell length and MZ size in a CEPR1-dependent manner. CEP5 decreased MZ size by reducing the number of MZ cells. The effect of CEP5 on LR growth in the present study was opposite to the effect of cepr1 knockout in the presence of sucrose. This is consistent with a model where CEP5 along with the other sucrose-induced CEP genes produce ligands, that act as agonists of CEPR1 activity and thereby inhibit the extent of sucrose-dependent LR growth. A potential role, however, for CEP5 as an antagonist of CEPR1 function in the regulation of LR initiation has also been proposed based on a CEP5 knockdown line and a CEPR1 point mutant xip1-1 displaying opposite phenotypes (Lee and De Smet, 2016; Roberts et al., 2016).
CEPR1 control of LR growth is mediated by local and systemic effects
Grafting data show that CEPR1 knockout in either roots or shoots results in elevated LR growth in the presence of sucrose. CEPR1 is expressed in the shoot vascular tissues where it has a role in systemic N-demand signalling (Tabata et al., 2014), however, CEPR1 is also expressed in the root vasculature (Bryan et al., 2012). Root-specific roles for CEP–CEPR1/CRA2 function exist in Arabidopsis and Medicago, respectively (Huault et al., 2014; Mohd-Radzman et al., 2016; Roberts et al., 2016). In addition, precedents exist in grafting experiments that show that specific phenotypic outcomes require gene function in both roots and shoots (Taochy et al., 2017). Possible explanations include the need for both root and shoot CEPR1 function to achieve a threshold level of a required signal(s) or that CEPR1 activity in the root and shoot results in distinct signal(s) that are both required. A requirement for CEPR1 activity in the shoot implies that a long-distance (mobile) signalling component, in part, controls LR growth. In principal, this would enable a coordination of root and shoot growth to occur through a systemic CEP–CEPR1 interaction.
A model for CEP–CEPR control of LR growth in response to sucrose
From these results, we present a model for CEP–CEPR1-dependent restriction of LR growth in response to increased C supply either from the addition of metabolizable sugars or through an increase in photosynthetically derived C (Fig. 8). An increase in sucrose availability induces several CEP ligand-encoding genes, leading to an increased production/secretion of mature peptides, which interact with CEPR1 in roots and shoots. The resulting CEP–CEPR1 signalling curtails the extent of LR growth in response to the increased supply of sucrose. This study shows that the CEP–CEPR1 peptide hormone system reduces sucrose-dependent growth expenditure in the root system. Therefore, CEP–CEPR1 signalling may represent a newly discovered route to coordinate overall C expenditure to balance root and shoot growth.
Fig. 8.
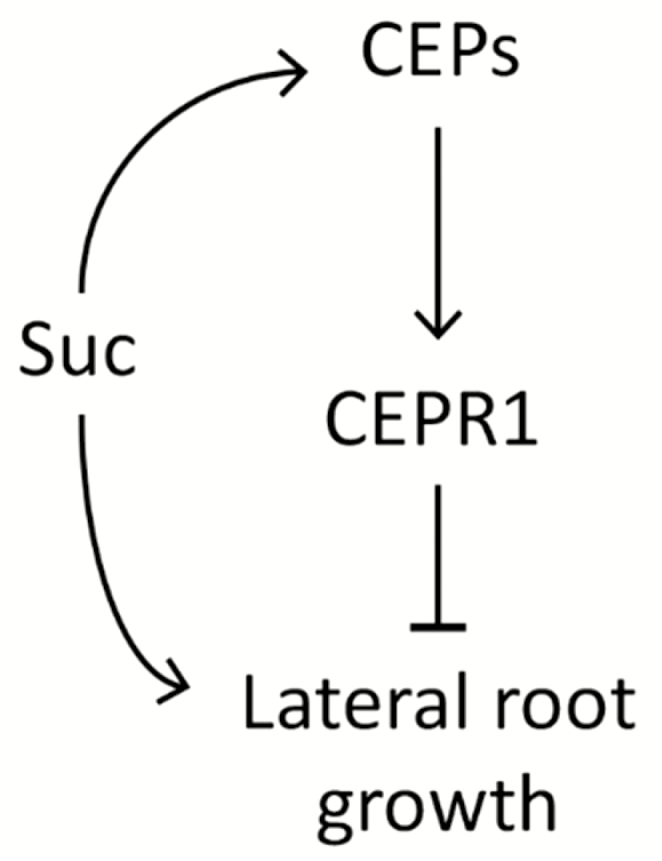
A model for CEP–CEPR1 inhibition of LR growth in response to sucrose. Increased sucrose supply leads to up-regulation of CEP genes and increased production of CEP ligands, which act through CEPR1 to restrict the promotion of LR growth by sucrose.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Loss of CEPR1 is sufficient to confer insensitivity to CEP addition.
Fig. S2. Effect of CEPR1 on primary root length, LR number, and LR primordia.
Table S1. Details of Arabidopsis CEP genes in Araport11 and manual curations for RNA-Seq analysis.
Table S2. List of primers used for qRT–PCR.
Table S3. Number of genes differentially regulated in the RNA-Seq as a result of sucrose treatment, genotype, or a combination of both.
Table S4. Observed versus expected overlaps between differentially expressed genes in WT+suc and cepr1-1 control.
Table S5. List of genes down-regulated in both sucrose-treated WT and untreated cepr1-1.
Table S6. List of genes up-regulated in both sucrose-treated WT and untreated cepr1-1.
Table S7. List of genes up-regulated in sucrose-treated WT and down-regulated in untreated cepr1-1.
Table S8. Observed versus expected overlaps between genes co-regulated in the same direction in WT+suc and cepr1-1 control, and genes differentially regulated by KIN10 in Baena-González et al. (2007).
Table S9. List of genes down-regulated in both sucrose-treated WT and untreated cepr1-1 that overlap with KIN10 up-regulated genes from Baena-González et al. (2007).
Dataset S1. RNA-Seq output for each gene showing log2 FC and P-values for each comparison, and counts per million for each sample.
Acknowledgements
An Australian Research Council grant to MAD (DP150104250) supported this work. KC was supported by an ANU PhD scholarship. MT was supported by an Australian Post Graduate award. We thank Bernd Weisshaar, MPI for Plant Breeding Research, Cologne for the T-DNA mutant line 467C01 and the ACRF Biomolecular Resource Facility for undertaking the RNA-sequencing. We thank Bernard Carroll (University of Queensland) and Rémi Branco (Australian National University) for hypocotyl grafting technical advice.
Author contributions
MAD and NI initiated the research; KC and MT conducted laboratory work and contributed equally to this manuscript; HO conducted the RNA-Seq analyses with contributions from MT; all authors contributed to data analysis; MT, KC, MAD, and HO wrote the manuscript with critical assessment from NI.
References
- Ahn JH. 2009. Semiquantitative analysis of Arabidopsis RNA by reverse transcription followed by noncompetitive PCR. Cold Spring Harbor Protocols 2009, pdb.prot5296. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942. [DOI] [PubMed] [Google Scholar]
- Branco R, Masle J. 2019. Systemic signalling through TCTP1 controls lateral root formation in Arabidopsis. Journal of Experimental Botany. doi: 10.1093/jxb/erz204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AC, Obaidi A, Wierzba M, Tax FE. 2012. XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta 235, 111–122. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD. 2017. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. The Plant Journal 89, 789–804. [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H. 2011. Growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiology 157, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay C, Imin N, Djordjevic MA. 2013. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. Journal of Experimental Botany 64, 5383–5394. [DOI] [PubMed] [Google Scholar]
- Dietrich K, Weltmeier F, Ehlert A, Weiste C, Stahl M, Harter K, Dröge-Laser W. 2011. Heterodimers of the Arabidopsis transcription factors bZIP1 and bZIP53 reprogram amino acid metabolism during low energy stress. The Plant Cell 23, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov I, Tax FE. 2018. Lateral root growth in Arabidopsis is controlled by short and long distance signaling through the LRR RLKs XIP1/CEPR1 and CEPR2. Plant Signaling & Behavior 13, e1489667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. 2008. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences, USA 105, 8790–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope ACA. 1968. A simplified Monte Carlo significance test procedure. Journal of the Royal Statistical Society:s B 30, 582–598. [Google Scholar]
- Huault E, Laffont C, Wen J, Mysore KS, Ratet P, Duc G, Frugier F. 2014. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genetics 10, e1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA. 2013. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. Journal of Experimental Botany 64, 5395–5409. [DOI] [PubMed] [Google Scholar]
- Kang SG, Price J, Lin PC, Hong JC, Jang JC. 2010. The arabidopsis bZIP1 transcription factor is involved in sugar signaling, protein networking, and DNA binding. Molecular Plant 3, 361–373. [DOI] [PubMed] [Google Scholar]
- Kircher S, Schopfer P. 2012. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109, 11217–11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B. 2012. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Research 40, D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. . 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937. [DOI] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, et al. . 2012. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research 40, D1202–D1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, De Smet I. 2016. Fine-tuning development through antagonistic peptides: an emerging theme. Trends in Plant Science 21, 991–993. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X. 2011. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology 157, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochemical Journal 397, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor DR, Deak KI, Ingram PA, Malamy JE. 2008. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. The Plant Cell 20, 2643–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. 1997. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. 2001. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiology 127, 899–909. [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Binos S, Truong TT, Imin N, Mariani M, Djordjevic MA. 2015. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. Journal of Experimental Botany 66, 5289–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Frugier F, Imin N, Djordjevic MA. 2016. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiology 171, 2536–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. 2010. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329, 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie HA, Imin N, Djordjevic MA. 2014. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genomics 15, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y. 2017. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nature Plants 3, 17029. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Ogawa M, Matsubayashi Y. 2008. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. The Plant Journal 55, 152–160. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Suzuki T, Kawaguchi M, Higashiyama T, Matsubayashi Y. 2015. A comprehensive strategy for identifying long-distance mobile peptides in xylem sap. The Plant Journal 84, 611–620. [DOI] [PubMed] [Google Scholar]
- Patel N, Mohd-Radzman NA, Corcilius L, et al. . 2018. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Molecular and Cellular Proteomics 17, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrotti L, Weiste C, Nägele T, et al. . 2018. Snf1-RELATED KINASE1–Controlled C/S1–bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. The Plant Cell 30, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, Ruelens P, Li Y, Sheen J, Geuten K, Rolland F. 2013. The hybrid four-CBS-domain KINβγ subunit functions as the canonical γ subunit of the plant energy sensor SnRK1. The Plant Journal 75, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I, Smith S, Stes E, et al. . 2016. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. Journal of Experimental Botany 67, 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD Coruzzi GM. 2011. Nitrogen economics of root foraging: transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. demand. Proceedings of the National Academy of Sciences, USA 108, 18524–18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. 2003. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 100, 6849–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S. 2004. Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiology 135, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, Liu YB, Zhang XS. 2011. Auxin–cytokinin interaction regulates meristem development. Molecular Plant 4, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. 2014. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346. [DOI] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2016. New role for a CEP peptide and its receptor: complex control of lateral roots. Journal of Experimental Botany 67, 4797–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2018. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. Journal of Experimental Botany 69, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Taochy C, Gursanscky NR, Cao J, et al. . 2017. A genetic screen for impaired systemic RNAi highlights the crucial role of DICER-LIKE 2. Plant Physiology 175, 1424–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiste C, Pedrotti L, Selvanayagam J, Muralidhara P, Fröschel C, Novák O, Ljung K, Hanson J, Dröge-Laser W. 2017. The Arabidopsis bZIP11 transcription factor links low-energy signalling to auxin-mediated control of primary root growth. PLOS Genetics 13, e1006607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Jürgens G. 2009. Survival of the flexible: hormonal growth control and adaptation in plant development. Nature Reviews. Genetics 10, 305–317. [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. 2013. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. 2009. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiology 149, 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.