Leaves adapted to diurnal fluctuating light (FL) tend to have reduced photosynthetic parameters in comparison with those grown under constant light but intercepting the same daily photon integral (DPI). This reduction may result from a non-linear relationship between photosynthetic protein synthesis rate (PPSR) and incident photosynthetically active radiation (PAR). Models incorporating the PPSR–PAR relationship have quantitatively predicted the effects of FL reported in the literature. Further simulations suggest that the degree of this reduction varies with the FL pattern, DPI level and parameters describing the PPSR–PAR relationship.
Obtaining an understanding of the physiological responses of the plant to a FL regime has gained increasing attention in the past few years since FL reflects a more realistic situation for plants growing under natural conditions (Kaiser et al., 2018; Matsubara, 2018; Burgess et al., 2019). By hypothesizing that photosynthetic capacity (Amax) is determined by a mechanism leading towards maximal carbon assimilation, a higher Amax would be expected under FL (Retkute et al., 2015). However, this hypothesis leads to an overestimation of Amax by more than 50% under frequent light fluctuation, suggesting a more complex underlying mechanism. Recently, Vialet-Chabrand et al. (2017) have experimentally demonstrated that the daily carbon assimilation of plants grown under FL was lower in comparison with plants grown under a square wave light (SQ) regime but intercepting the same DPI (mol m−2 d−1). They highlighted the influence of diurnal light fluctuations on photosynthetic acclimation and photosynthetic capacity. One of their findings is that plants grown under FL had reduced photosynthetic parameters, particularly maximal electron transport rate (Jmax) and leaf absorptance of incident PAR. Biochemically, this reduction is due to a decrease in the photosynthetic protein abundance by 3–15%. However, the physiological mechanisms resulting in this difference in protein abundance between FL and SQ remain unknown. Here, we seek explanations for this reduction in photosynthetic proteins under FL by applying an hourly based dynamic model for photosynthetic acclimation.
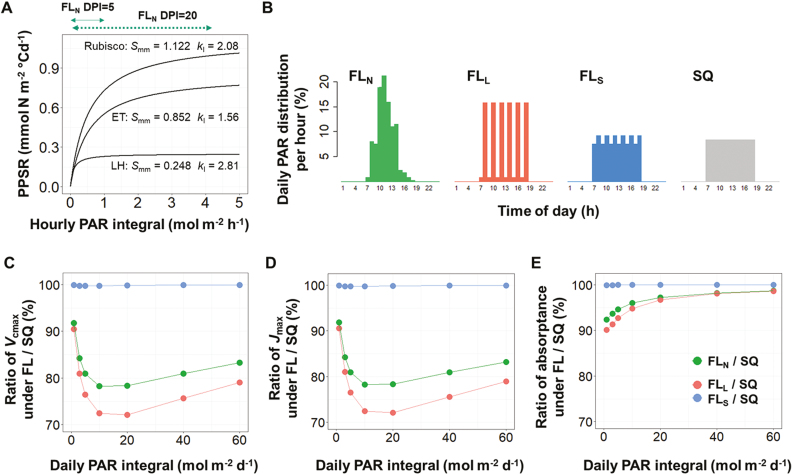
Protein abundance is regulated by the orchestration of multiple mechanisms and is the outcome of protein turnover: the continuous dynamics of protein synthesis and degradation (Kristensen et al., 2013; Nelson and Millar, 2015). Based on the concept of protein turnover, we have recently presented a mechanistic model describing photosynthetic acclimation (Pao et al., 2019) in which the experimental data suggested a non-linear relationship between the PPSR and PAR (see Supplementary Fig. S1 at JXB online). PPSR increases almost linearly with PAR under low light conditions (up to 200 µmol PAR m−2 s−1 for Rubisco and electron transport proteins), and then the slope of the PPSR–PAR curve decreases and the protein synthesis rate approaches a saturation level at high PAR (around 900 µmol PAR m−2 s−1). This form of a non-linear relationship is not surprising since it has been observed in the cause–effect relationships of many other biological phenomena. Of note is its implication that protein synthesis rate under FL conditions (ranging between 0 and 1500 µmol PAR m−2 s−1 in Vialet-Chabrand et al., 2017) is saturated occasionally during the course of the day and the protein synthesis per day per unit DPI is consequently less than that under SQ, the non-saturating condition (constantly at 460 µmol PAR m−2 s−1 for 12 h). By applying this PPSR–PAR relationship (Fig. 1A), it is possible to assess the differences in photosynthetic protein abundance between plants grown under FL and SQ. To simulate the effect of the diurnal light fluctuation, we first converted the parameters in the model of Pao et al. (2019) to an hourly basis by assuming a 12 h photoperiod and zero protein synthesis in the dark. Then, three FL patterns (Fig. 1B) and SQ with DPI between 1 and 60 mol m−2 d−1 were used as light input to simulate the abundance of Rubisco, electron transport, and light harvesting proteins (Pao et al., 2019), which were then converted to maximal Rubisco carboxylation rate (Vcmax), Jmax, and leaf PAR absorptance, respectively. Constants converting the amount of nitrogen in each functional protein pool into the corresponding capacities are used according to Buckley et al. (2013). Under natural diurnal light fluctuation (FLN in Fig. 1B) and light intensity (DPI = 10 and 20 mol PAR m−2 d−1) similar to the FL experiment in Vialet-Chabrand et al. (2017), the model predicted the effects of FL on photosynthetic parameters: Vcmax and Jmax were reduced by 21–22% (Fig. 1C, D) and leaf PAR absorptance by 2–4% (Fig. 1E). This prediction is within the range reported for leaf PAR absorptance (3–5%) but is different from that for the Vcmax (8–10%) and Jmax (11–15%) found in Arabidopsis (Vialet-Chabrand et al., 2017). These differences could be due to the lack of protein synthesis in the dark assumed in the simulations (see below) or due to the fact that their model was parameterized using greenhouse cucumber (Cucumis sativus), which might have different PPSR–PAR responses from Arabidopsis. However, both experimental and model studies suggest that Vcmax and Jmax were more affected by FL than leaf PAR absorptance. This can be explained by the fact that the synthesis rate of light harvesting proteins reaches saturation at a lower PAR level than Rubisco and electron transport proteins (Fig. 1A). Therefore, the effects of FL on light harvesting proteins under high DPI were almost negligible.
Fig. 1.
(A) The response curves of photosynthetic protein synthesis rate (PPSR) to photosynthetically active radiation (PAR) in Pao et al. (2019) are different between Rubisco, electron transport proteins, and light harvesting proteins, depending on the maximum synthesis rate (Smm) and the curvature (kI) of each functional protein group. The effect of fluctuating light (FL) on the photosynthetic parameters under different daily photon integral (DPI) and 12 h photoperiod was simulated with different diurnal FL patterns. (B) Daily PAR distribution (%) per hour for natural diurnal fluctuation (FLN; large fluctuation (FLL), small fluctuation (FLS), and square wave (SQ) light regimes. (C–E) The ratio of maximum carboxylation rate (Vcmax) (C), maximum electron transport rate (Jmax) (D), and leaf absorptance of PAR (E) between FL and SQ under different DPI levels. The solid and dotted green lines with arrows above (A) indicate the ranges of PAR under FLN pattern at DPI level of 5 and 20 mol m−2 d−1, respectively. (B) Adapted from Vialet-Chabrand et al., 2017. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiology 173, 2163–2179 (www.plantphysiol.org), ‘Copyright American Society of Plant Biologists.’
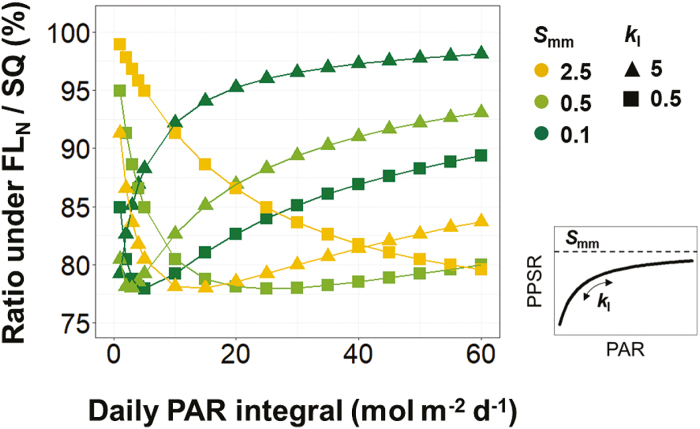
The different effects of FL on Vcmax, Jmax, and leaf absorptance (Fig. 1C–E) imply that the characteristics of the PPSR–PAR curve determine the impact of light fluctuation on the abundance of photosynthetic proteins. Hence, we further examined the extent to which the PPSR–PAR curve parameters, the maximum protein synthesis rate (Smm, equal to 0.1, 0.5, or 2.5) and the curvature (kI, equal to 0.5 or 5), affect the photosynthetic acclimation under natural diurnal light fluctuation (FLN in Fig. 1B) with DPI levels between 1 and 60 mol m−2 d−1 in combinations with nitrogen supply levels (2–10 mM) and leaf age (5–45 d). Age and nitrogen levels had no influence (<1%) on the impact of FLN (data not shown). The reduction of protein abundance due to FL was up to 22%, depending on the combinations of DPI, Smm, and kI (Fig. 2). Three types of response curves can be identified: (i) the combination of high kI and low Smm results in the strongest reduction under low light and this reduction decreases with DPI, resembling the light harvesting proteins (Fig. 1A); (ii) combining high kI and Smm or low kI and Smm shows the strongest reduction under low–intermediate DPI, resembling Rubisco and electron transport proteins (Fig. 1A); (iii) combining low kI and high Smm, the reduction in protein abundance increases with DPI. The third type of these response curves indicates that the PPSR will not be saturated even under high light conditions, probably an unfavorable strategy under natural selection. Altogether, these results suggest that variations in the parameters of the PPSR–PAR curve can form an explanation of the different acclimatory responses to FL between plant functional types, as reported by Watling et al. (1997).
Fig. 2.
The effects of FLN (Fig. 1B) on photosynthetic protein abundance depend on the values of maximum synthesis rate (Smm, equal to 0.1, 0.5, or 2.5) and the curvature (kI, equal to 0.5 or 5) of the PPSR–PAR response curves. For abbreviations see Fig. 1.
Mathematically, the hyperbolic characteristics of the PPSR–PAR response (Fig. 1A) suggest that the strongest impact of light fluctuation can be expected if the incident PAR fluctuates largely across the vertex of the PPSR–PAR curve and the impact of FL becomes smaller when it mostly fluctuates within the nearly linear range of the PPSR–PAR curve. This non-linear characteristic has two biological implications. Firstly, the influence of FL can be expected to be small under low or saturating PAR levels. In our simulation, the reductions of Vcmax and Jmax increase with DPI under low light level (DPI < 10 mol m−2 d−1, Fig. 1C, D). This result is similar to the observation in Arabidopsis that the impact of FL on the electron transport rate is stronger under a DPI of 5.1 than one of 3.6 mol m−2 d−1 (Alter et al., 2012). Also, in Alocasia macrorrhiza no reduction in Amax was observed under FL at a very low DPI level (1.4 mol m−2 d−1; Sims and Pearcy, 1993) while Amax tended to be 15% lower than SQ when DPI was 7 mol m−2 d−1 (Watling et al., 1997). Secondly, photosynthetic protein abundance will be more strongly affected by the large fluctuation (FLL in Fig. 1B) than by the small fluctuation (FLS in Fig. 1B, C–E). This agrees with the observations in Arabidopsis that, in comparison with SQ (85 µmol PAR m−2 s−1), FLL (ranging between 50 and 1250 µmol PAR m−2 s−1) reduces electron transport rate by 28%, while FLS (ranging between 50 and 650 µmol PAR m−2 s−1) reduces electron transport rate by only 8% (Alter et al., 2012). Grown under the FLS pattern (ranging between 30 and 525 µmol PAR m−2 s−1), Amax and nitrogen per unit leaf area (Narea, a proxy of photosynthetic protein abundance) of Shorea leprosula leaves were not different from those of leaves grown under SQ (170 µmol PAR m−2 s−1; Leakey et al., 2002), but in their following study Leakey et al. (2003) showed that Amax and Narea of the same species grown under FLL (ranging between 0 and 1700 µmol PAR m−2 s−1) were 20–30% lower than those of their counterparts grown under FLS (ranging between 0 and 750 µmol PAR m−2 s−1).
As with any other model, this model is a simplification of the real system. For example, it assumes zero protein synthesis rate under darkness, which is unlikely for Rubisco (Ishihara et al., 2015). If a low rate of Rubisco synthesis during the dark period under FL and SQ is assumed (as suggested by Ishihara et al., 2015), the relative impact of FL will be lower than our prediction and thus closer to the reduction measured by Vialet-Chabrand et al. (2017). The current model also assumed the same degradation rate constants for different PAR levels although this does not hold true in planta especially under high light (Li et al., 2018). The available information is so far restricted for parameterizing this effect (Nelson et al., 2014; Li et al., 2017). Theoretically, if the degradation is enhanced under excess light while the synthesis rate remains stable, it can be expected that the reduction in protein abundance under FL would be even more severe. However, if the synthesis rate is coordinated with the degradation rate as reported for photosystem II subunit D1 protein (Aro et al., 1993), similar results to our simulation could be expected due to restored balance in the net rate of change. In addition, there are still unknown mechanisms involved in the acclimation to FL that are not considered in the model. The effects of the frequency of light fluctuations and the length of individual light events on photo-acclimation, as shown in previous studies (Yin and Johnson, 2000; Alter et al., 2012) and implying that protein synthesis does not react to a light signal instantaneously (e.g. Retkute et al., 2015), cannot be reproduced by our model (data not shown). Besides photosynthetic proteins, many physiological processes are also involved in the acclimation mechanism to FL, especially when tackling excess light energy. Photo-oxidative damage caused by the excess light events under FL may increase the need for photoprotection, photorespiration, and cyclic electron flow, which together alter the metabolism and partitioning of nitrogen and carbon (Matsubara, 2018; Annunziata et al., 2018; Schneider et al., 2019). Also, our model does not account for any photoperiodic regulation, which is also known to affect long-term acclimation (Seaton et al., 2018).
In summary, the hyperbolic PPSR–PAR response provides a mechanistic explanation of the reported reduction in photosynthetic protein abundances caused by diurnal light fluctuation. Our results suggest that the differences in protein abundances between FL and SQ conditions are determined by three components: the pattern of FL, the DPI level, and the species-specific PPSR–PAR curve parameters. Although a model cannot account for all details of the acclimation response under all environmental scenarios, our model delivers a systematic view of this phenomenon and thus can be a useful tool for designing FL scenarios for future experiments (see Supplementary Dataset S1 for the R script of the model). Our analyses point out the avenues for further investigations in the interspecific and genotypic variations of the PPSR–PAR relationship and in the response time to the light signal, as well as photoperiodic regulation and the combined effects of different environmental factors on photosynthetic protein turnover.
Supplementary data
Supplementary data are available at JXB online.
Dataset S1. R script of the protein turnover model and input file of light fluctuation patterns.
Fig. S1. Non-linear relationship between photosynthetic protein synthesis rate and light intensity.
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (DFG, Project number 403510751). We are grateful for the valuable comments of the two reviewers.
References
- Alter P, Dreissen A, Luo FL, Matsubara S. 2012. Acclimatory responses of Arabidopsis to fluctuating light environment: comparison of different sunfleck regimes and accessions. Photosynthesis Research 113, 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata MG, Apelt F, Carillo P, Krause U, Feil R, Koehl K, Lunn JE, Stitt M. 2018. Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. Journal of Experimental Botany 69, 4881–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, McCaffery S, Anderson JM. 1993. Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiology 103, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Cescatti A, Farquhar GD. 2013. What does optimization theory actually predict about crown profiles of photosynthetic capacity when models incorporate greater realism? Plant, Cell & Environment 36, 1547–1563. [DOI] [PubMed] [Google Scholar]
- Burgess AJ, Gibbs JA, Murchie EH. 2019. A canopy conundrum: can wind-induced movement help to increase crop productivity by relieving photosynthetic limitations? Journal of Experimental Botany 70, 2371–2380. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Obata T, Sulpice R, Fernie AR, Stitt M. 2015. Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein. Plant Physiology 168, 74–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Morales A, Harbinson J. 2018. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology 176, 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AR, Gsponer J, Foster LJ. 2013. Protein synthesis rate is the predominant regulator of protein expression during differentiation. Molecular Systems Biology 9, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey AD, Press MC, Scholes JD. 2003. Patterns of dynamic irradiance affect the photosynthetic capacity and growth of dipterocarp tree seedlings. Oecologia 135, 184–193. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Press MC, Scholes JD, Watling JR. 2002. Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant, Cell & Environment 25, 1701–1714. [Google Scholar]
- Li L, Aro EM, Millar AH. 2018. Mechanisms of photodamage and protein turnover in photoinhibition. Trends in Plant Science 23, 667–676. [DOI] [PubMed] [Google Scholar]
- Li L, Nelson CJ, Trösch J, Castleden I, Huang S, Millar AH. 2017. Protein degradation rate in Arabidopsis thaliana leaf growth and development. The Plant Cell 29, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S. 2018. Growing plants in fluctuating environments: why bother? Journal of Experimental Botany 69, 4651–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Alexova R, Jacoby RP, Millar AH. 2014. Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiology 166, 91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Millar AH. 2015. Protein turnover in plant biology. Nature Plants 1, 15017. [DOI] [PubMed] [Google Scholar]
- Pao Y-C, Chen T-W, Moualeu-Ngangue DP, Stützel H. 2019. Environmental triggers for photosynthetic protein turnover determine the optimal nitrogen distribution and partitioning in the canopy. Journal of Experimental Botany 70, 2419–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retkute R, Smith-Unna SE, Smith RW, Burgess AJ, Jensen OE, Johnson GN, Preston SP, Murchie EH. 2015. Exploiting heterogeneous environments: does photosynthetic acclimation optimize carbon gain in fluctuating light? Journal of Experimental Botany 66, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Bolger A, Zeier J, et al. 2019. Fluctuating light interacts with time of day and leaf development stage to reprogram gene expression. Plant Physiology 179, 1632–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton DD, Graf A, Baerenfaller K, Stitt M, Millar AJ, Gruissem W. 2018. Photoperiodic control of the Arabidopsis proteome reveals a translational coincidence mechanism. Molecular Systems Biology 14, e7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DA, Pearcy RW. 1993. Sunfleck frequency and duration affects growth rate of the understorey plant, Alocasia macrorrhiza. Functional Ecology 7, 683–689. [Google Scholar]
- Vialet-Chabrand S, Matthews JS, Simkin AJ, Raines CA, Lawson T. 2017. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiology 173, 2163–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling JR, Ball MC, Woodrow IE. 1997. The utilization of lightflecks for growth in four Australian rain‐forest species. Functional Ecology 11, 231–239. [Google Scholar]
- Yin ZH, Johnson GN. 2000. Photosynthetic acclimation of higher plants to growth in fluctuating light environments. Photosynthesis Research 63, 97–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.