Ca increased the allocation of P to palisade mesophyll cells in Proteaceae, supporting our model of Ca-enhanced P toxicity and demonstrating its role in the distribution of Proteaceae.
Keywords: Calcifuge, calcium, calcium-enhanced phosphorus toxicity, cell type-specific distribution, elemental analysis, Jurien Bay chronosequence, phosphorus, Proteaceae, scanning electron microscopy, X-ray microanalysis
Abstract
Over 650 Proteaceae occur in south-western Australia, contributing to the region’s exceptionally high biodiversity. Most Proteaceae occur exclusively on severely nutrient-impoverished, acidic soils (calcifuge), whilst only few also occur on young, calcareous soils (soil-indifferent), higher in calcium (Ca) and phosphorus (P). The calcifuge habit of Proteaceae is explained by Ca-enhanced P toxicity, putatively linked to the leaf cell-specific allocation of Ca and P. Separation of these elements is essential to avoid the deleterious precipitation of Ca-phosphate. We used quantitative X-ray microanalysis to determine leaf cell-specific nutrient concentrations of two calcifuge and two soil-indifferent Proteaceae grown in hydroponics at a range of Ca and P concentrations. Calcium enhanced the preferential allocation of P to palisade mesophyll (PM) cells under high P conditions, without a significant change in whole leaf [P]. Calcifuges showed a greater PM [P] compared with soil-indifferent species, corresponding to their greater sensitivity. This study advances our mechanistic understanding of Ca-enhanced P toxicity, supporting the proposed model, and demonstrating its role in the calcifuge distribution of Proteaceae. This furthers our understanding of nutrient interactions at the cellular level and highlights its importance to plant functioning.
Introduction
Nutrients in leaves tend to be preferentially allocated to specific cell types (Karley et al., 2000b; Conn and Gilliham, 2010). This pattern of cell-specific nutrient allocation must be tightly regulated, as particular elements can interact with each other. For example, calcium (Ca) and phosphorus (P) must be allocated to separate cell types to avoid the deleterious precipitation of Ca-phosphates, which reduce the availability of both nutrients and severely impact cellular processes (McLaughlin and Wimmer, 1999; White and Broadley, 2003; Conn and Gilliham, 2010; Hayes et al., 2018).
The different cell types in which Ca and P are allocated vary among different groups of plants (Leigh and Tomos, 1993; Karley et al., 2000b; Conn and Gilliham, 2010; Guilherme Pereira et al., 2018; Hayes et al., 2018). Many eudicots tend to allocate P to epidermis and bundle sheath cells, and Ca to mesophyll cells (Outlaw et al., 1984; Treeby et al., 1987; Conn and Gilliham, 2010), whereas eudicots from severely P-impoverished habitats (Shane et al., 2004a; Hawkins et al., 2008; Guilherme Pereira et al., 2018; Hayes et al., 2018) and monocots (Boursier and Läuchli, 1989; Dietz et al., 1992; Leigh and Storey, 1993; Williams et al., 1993; Fricke et al., 1994; Karley et al., 2000a) tend to allocate P to mesophyll cells and Ca to specific non-mesophyll cells. However, the cell-specific allocation of Ca and P has only been studied under a narrow range of conditions, and we know little of how much it can vary or its impact on plant physiology.
The ability of eudicots from severely P-impoverished habitats to preferentially allocate P to mesophyll cells and Ca to other cell types represents an important adaptation increasing P-use efficiency (Guilherme Pereira et al., 2018; Hayes et al., 2018). By preferentially allocating P to where it is needed in the greatest amount—photosynthetic cells—these species can use P more efficiently (Stitt et al., 2010; Tsujii et al., 2017). However, this allocation pattern is also associated with P toxicity in P-sensitive Proteaceae from south-western Australia (Shane et al., 2004a; Hayes, 2018; Hayes et al., 2019). The low ability of these species to down-regulate P-uptake capacity, coupled with their preferential allocation of P to mesophyll cells, increases mesophyll [P], leading to P-toxicity symptoms, even under relatively low P supply (Hayes et al., 2019). Furthermore, Ca increases the severity of these P-toxicity symptoms (Grundon, 1972; Nichols and Beardsell, 1981), putatively linked to their cell-specific allocation of Ca and P (Hayes et al., 2019).
Proteaceae are mainly found across the southern hemisphere and are abundant in severely P-impoverished regions, such as south-western Australia, where >650 species occur (Weston, 2007). They represent an iconic component of the vegetation, contributing to the region’s exceptionally high biodiversity (Cowling and Lamont, 1998; George, 1998; Myers et al., 2000; Hopper and Gioia, 2004). Very few Proteaceae occur on young (<7000 years) calcareous soils in south-western Australia (Hayes et al., 2014; Zemunik et al., 2015). Instead, most occur on older, nutrient-impoverished acidic soils and are calcifuge (Hayes et al., 2014; Zemunik et al., 2015). The few Proteaceae that grow on calcareous soils also occur on acidic soils and are soil-indifferent. An example of this distribution is along the Jurien Bay dune chronosequence, south-western Australia (Hayes et al., 2014; Zemunik et al., 2015). The calcareous soils of this chronosequence exhibit relatively high total and plant-available [P], compared with the older acidic soils (Turner and Laliberté, 2015). However, this soil [P] (‘plant-available’ 1.2–2.1 mg P kg−1; Turner and Laliberté, 2015) is unlikely to be high enough to exclude P-sensitive Proteaceae (P toxicity is typically observed at ‘plant-available’ [P] >10 mg P kg−1; Lambers et al., 2002; Shane and Lambers, 2005). These young calcareous soils are also very high in [Ca] and have a low available micronutrient concentration (Turner and Laliberté, 2015).
Previous research has indicated that Ca-enhanced P toxicity, in addition to low soil micronutrient availability, can explain the exclusion of most Proteaceae from calcareous soils (Hayes, 2018; Hayes et al. 2019). Increasing Ca supply enhances sensitivity to P and the severity of P-toxicity symptoms in P-sensitive Proteaceae (Grundon, 1972; Nichols and Beardsell, 1981; Hayes et al., 2019). Calcium-enhanced P toxicity is thought to result from interactions between cell-specific Ca and P (Hayes et al., 2019). Increasing Ca supply is thought to increase PM [P], leading to P toxicity under high-P conditions (Hayes et al., 2019). However, this has never been quantitatively demonstrated, and its underpinning mechanism remains unknown. The main visual symptoms of P toxicity are leaf chlorosis and necrosis (Grundon, 1972; Groves and Keraitis, 1976; Nichols and Beardsell, 1981; Webb and Loneragan, 1990; Shane et al., 2004a, b; Parks et al., 2007; Hawkins et al., 2008; de Campos et al., 2013). Leaf chlorosis probably results from higher PM [P] reducing the physiological availability of Zn and other micronutrients (Chapman and Vanselow, 1937; Boawn and Legget, 1964; Cakmak and Marschner, 1987; Loneragan and Webb, 1993), whilst leaf necrosis may result from precipitation of Ca-phosphates, reducing the availability of both nutrients and interfering with numerous Ca-regulated cellular processes (McLaughlin and Wimmer, 1999; White and Broadley, 2003; Conn and Gilliham, 2010; Hayes et al., 2019).
We used quantitative X-ray elemental microanalysis to determine leaf cell-specific nutrient concentrations of four Proteaceae grown at a range of Ca and P concentrations. This included two calcifuge and two soil-indifferent Banksia and Hakea species. Our aims were to: (i) study the effects of Ca and P supply on the cell-specific allocation of these nutrients in Proteaceae; and (ii) compare responses in cell-specific nutrient allocation between calcifuge and soil-indifferent species and their link to Ca-enhanced P toxicity and species distribution.
First, we hypothesized that increasing Ca supply would alter the cell-specific allocation of P, that it would enhance the relative allocation of P to PM, and that this would be most evident under high P supply. Secondly, we hypothesized that calcifuge Proteaceae would show a greater response to increased Ca supply than those which were soil-indifferent, resulting in a greater PM [P] in calcifuges, particularly under high-P conditions. Finally, a comparison of calcifuge and soil-indifferent Proteaceae would allow us to understand the strategies of soil-indifferent species to tolerate high Ca and P supplies, thus providing a mechanistic understanding of Ca-enhanced P toxicity and its role in the calcifuge habit of most Proteaceae.
Materials and methods
Species selection
We selected four Proteaceae species, from two genera, Banksia and Hakea, which occur along the Jurien Bay dune chronosequence, south-western Australia, ~200 km north of Perth (Laliberté et al., 2012; Hayes et al., 2014; Zemunik et al., 2015). We selected two calcifuge (Hakea incrassata R.Br. and Banksia menziesii R.Br.) and two soil-indifferent species (H. prostrata R.Br. and B. prionotes Lindl.). Species distributions and their sensitivities to Ca-enhanced P toxicity were identified through published information (Hayes et al., 2014, 2019; Zemunik et al., 2015).
Glasshouse hydroponic experiment
Plants were grown from seed, as part of a larger experiment (Hayes et al., 2019), and transferred to hydroponics. Hakea species were grown between February and September 2014, and Banksia species between June 2014 and March 2015. Seeds were sterilized and germinated on moist filter paper (15 °C, 12 h:12 h, light:dark), before being transferred to trays containing 3 litres of continuously aerated nutrient solution. The strength of this nutrient solution (pH 5.8) was increased as plants became larger, from ‘25% growth’ to ‘100% growth’ (see Supplementary Table S1 at JXB online for concentrations). The solution was replenished 1–3 times per week. After 10 (Banksia) or 6 (Hakea) weeks, seedlings were transferred to 4.5 litre pots in a glasshouse.
Each 4.5 litre pot contained two plants and 4 litres of continuously aerated nutrient solution, maintained at 18 °C in a root-cooling tank. During acclimation in the glasshouse, the strength of the nutrient solution was increased from ‘25% growth’ to ‘50% growth’ (Banksia) or kept at ‘25% growth’ (Hakea). During growth and acclimation, P and Ca were supplied at 0.1 µM and 10 µM, respectively.
After 6 (Banksia) or 8 (Hakea) weeks of acclimation in the glasshouse, 64 (Banksia) or 80 (Hakea) plants of uniform size were selected for each species and exposed to four treatments. During the treatment period, plants were supplied with ‘50% basal’ (Banksia) or ‘25% to 33% basal’ (Hakea) nutrient solution (Supplementary Table S1) and supplemented with different P (0.1 µM, 10 µM; supplied as KH2PO4) and Ca (0 µM, 600 µM; supplied as CaCl2) concentrations. P treatments ranged from low/adequate (0.1 µM) up to toxic (10 µM) for P-sensitive Proteaceae (Shane et al., 2003, 2004a, b; de Campos et al., 2013), whilst Ca treatments covered a similarly wide range (0–600 µM; Jefferies and Willis, 1964; Grundon, 1972). All nutrient solutions were replenished three times per week. Despite no Ca being supplied under the 0 µM Ca treatment, there would be a very low background level of Ca in the deionized water. Plants were grown under treatment in a temperature-controlled glasshouse; mean temperature, 21 °C (Banksia) and 17 °C (Hakea). After 11 weeks of treatment, three representative plants were selected for each species/treatment combination and sampled for cell-specific element analysis.
Field collection
Samples for cell-specific element analysis were collected in November 2014, from three sites of contrasting soil type (two acidic, one calcareous) along the Jurien Bay dune chronosequence (for soil details, see Supplementary Table S2). Collections from acidic sites were part of a larger study (Hayes et al., 2018).
Two sites were selected on the oldest severely P-impoverished acidic dunes (Stage 6) and one on the much younger, relatively P-richer, calcareous dunes (Stage 3; Turner and Laliberté, 2015). All samples were collected within <100 m, at each site. Calcifuge and soil-indifferent species were collected from acidic sites, whilst soil-indifferent species were collected from both acidic and calcareous sites. At each site, three healthy mature individuals were selected and sampled for cell-specific element analysis.
Three soil samples were taken at each site (0–20 cm depth), in June 2015. Samples were sieved (<2 mm), air-dried, and homogenized prior to chemical analyses. Samples were analysed at the Smithsonian Tropical Research Institute (Panamá, Repúblic de Panamá). Soil pH was determined in a 1:2 soil to solution ratio in water using a glass electrode. Total soil [P] was measured by ignition and acid digestion, followed by molybdate colorimetry (Murphy and Riley, 1962). Readily exchangeable P was determined by extraction with anion-exchange membranes and molybdate colorimetry (Murphy and Riley, 1962; Turner and Romero, 2009). Total nitrogen (N) was measured by automated combustion and GC (gas chromatography) with thermal conductivity detection using a Thermo Flash 1112 analyser (CE Elantech, Lakewood, NJ, USA). Exchangeable Ca and other cations were determined by BaCl2 (0.1 M) extraction and inductively coupled plasma optical emission spectrometry on an Optima 7300 DV (Perkin-Elmer Ltd, Shelton, CT, USA) (Hendershot et al., 2008).
Leaf sampling for cell-specific element analysis
Mature, undamaged, fully expanded, and sun-exposed leaves were sampled from each individual plant, either grown in hydroponics or collected in the field. Three samples were collected from each individual plant, from 1–2 leaves. Upon removal of a leaf, sections (~2×3 mm) were cut from either side of the mid-rib, mid-way along the leaf, avoiding large secondary veins. These sections were then mounted onto aluminium pins using optimal cutting temperature compound and plunged into liquid N, thereby immediately immobilizing and preserving cellular ions. Samples were then stored in liquid N until preparation in a cryomicrotome and analysis using cryo-SEM. With this, all samples were collected fresh, immediately plunge-frozen in liquid N, and always kept under cryo-conditions, such that all elements were preserved in situ.
Cell-specific element analysis by X-ray microanalytical mapping
The method of cell-specific element analysis followed that of Hayes et al. (2018). Briefly, transverse regions of frozen hydrated leaf samples were prepared by cryoplaning a flat surface at –120 °C in a cryomicrotome (Leica EM FC6 cryochamber integrated with a Leica Ultracut EM UC6 microtome). The pin was then transferred to a Leica MED020 cryopreparation system and sputter-coated with 25–50 nm chromium, without sublimation. After coating, samples were transferred under vacuum to a Zeiss field emission scanning electron microscope fitted with a Leica VCT100 cryotransfer and stage, and an Oxford X-Max80 SDD X-ray detector interfaced to Oxford Instruments AZtecEnergy software. This preparation method and this fully integrated Oxford analytical system are highly suitable for elemental analysis and quantitation of biological samples (Huang et al., 1994; Marshall and Xu, 1998; Marshall and Clode, 2009; McCully et al., 2010; Marshall et al., 2012; Jin et al., 2017; Marshall, 2017; Hayes et al., 2018; Ondrasek et al., 2019).
Samples were analysed at –150°C, 15 kV, and a 2 nA beam current (measured using a Faraday cup), in high-current mode. Prior to each map, the instrument was calibrated, and the beam current measured using a pure copper standard. Elemental maps were acquired at a resolution of 512 pixels, for >3000 frames with a dwell time of 10 μs per pixel. Drift correction and pulse-pile up correction were activated. Quantitative numerical data were subsequently extracted from regions of interest drawn on the element maps, with individual spectra from each pixel within the region of interest summed and processed to yield element concentration data. Percentage hydrogen (H) and N were fixed at 11.11% and 3.3%, respectively, with oxygen (O) determined by difference. Regions of interest represented various areas and individual cells of interest. Only cells that were clearly identifiable and had a flat surface were analysed. Measurements of individual cells included the entire cell area, comprising both vacuole and cytoplasm. All cellular concentrations were the total element concentration per unit fresh weight, from fully hydrated cells. The relative distribution of nutrients within the leaf, described as preferential allocation, was based on relative differences in cell-specific concentrations.
The following six cell types were identified and analysed: epidermis (EP), hypodermis (HY), palisade mesophyll (PM), spongy mesophyll (SM), internal parenchyma (IP), and sclerenchyma (SC; excluding the hydrated sclerenchyma lumen). In total, >3000 cells were analysed in the glasshouse experiment, with 8–107 cells for each cell type within each species/treatment combination (Supplementary Table S3). For field-collected material, a total of >1200 cells were analysed, with 18–78 cells for each cell type within each species/soil type combination (Suppelementary Table S4). The analytical resolution was ~2 µm (sufficient for the analysis of individual plant cells; Marshall, 1982) and the detection limit was a few mmol kg−1 (Roomans and Dragomir, 2007).
Statistics
For glasshouse-grown plants, differences in [P] and [Ca] across all cell types and treatments, as well as across treatments within PM cells, were tested using general linear mixed-effect models, with individual plants as the random effect (Pinheiro and Bates, 2000). For field-collected plants, differences in [P] and [Ca] across cell types at the species level were tested using general linear mixed-effect models, with individual plants as the random effect (Pinheiro and Bates, 2000). The residuals of each model were visually inspected for heteroscedasticity. In the presence of heteroscedasticity, appropriate variance structures were specified if they significantly improved the model, based on Akaike and Bayesian Information Criterion (AIC and BIC, respectively) values (Pinheiro and Bates, 2000). Differences among cell types and treatments were defined using Tukey HSD post-hoc tests. All statistical analyses were performed using the R software platform (R Core Team, 2017), with the ‘nlme’ (Pinheiro et al., 2016), effects (Fox, 2003), and ‘multcomp’ (Hothorn et al., 2008) packages.
Results
Hakea
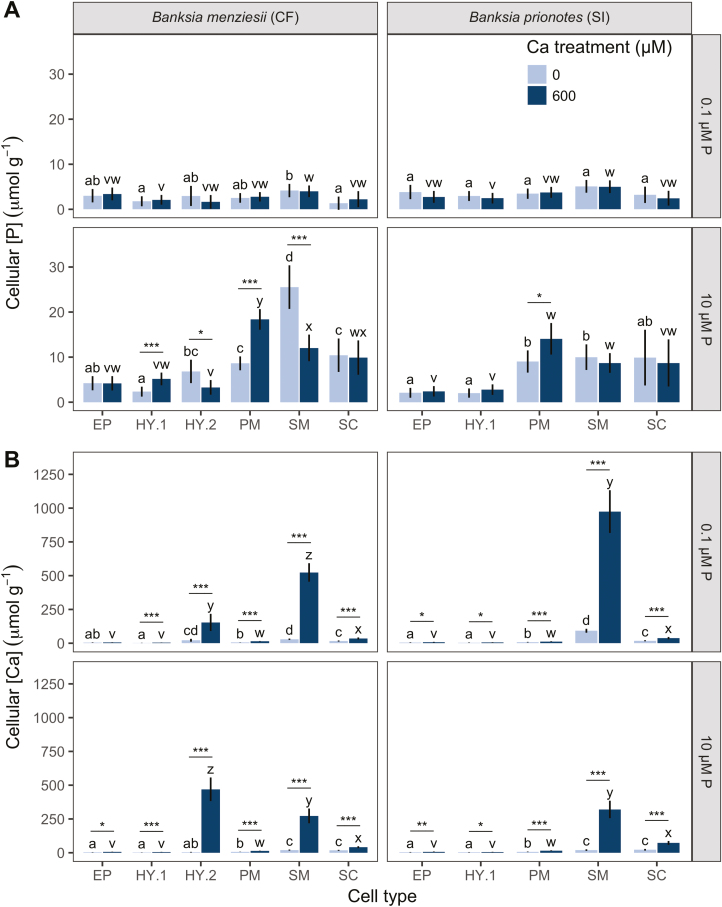
The cell-specific allocation of P depended on Ca and P supply in both Hakea species (P<0.01; Fig. 1A). The calcifuge H. incrassata showed preferential allocation of P to IP, under low Ca/high P supply, with at least an ~7-fold greater [P] than other cell types (EP, PM, and SC; Figs 1A, 2). Under the same high-P condition, increasing Ca supply caused an ~10-fold increase in PM [P], from 9 µmol g−1 to 100 µmol g−1 (Figs 1A, 2). Thus, PM [P] increased from ~13% of IP [P] under low Ca supply, to ~147% under high Ca supply, increasing the PM [P] to an exceptionally high level (100 µmol g−1) and increasing the relative distribution of [P] to PM.
Fig. 1.
Leaf cell-specific (A) phosphorus ([P]) and (B) calcium ([Ca]) concentrations of two Hakea species across different P and Ca treatments. Comparison of a typical calcifuge (CF) and soil-indifferent (SI) Hakea species. Only plants grown under high P (10 µM)/high Ca (600 µM) showed symptoms of P toxicity. Concentrations are per unit fresh weight, from fully hydrated cells. Bars are means, and error bars represent 95% confidence intervals, from linear mixed-effect models. Different letters indicate significant differences among cell types within each Ca treatment and panel (post-hoc Tukey test, P<0.05). P-values (*, <0.05; **, <0.01; ***, <0.001) represent significant differences between Ca treatments (post-hoc Tukey test). 0.1 µM P and 10 µM P, indicate the two P treatments. EP, epidermis; PM, palisade mesophyll; IP, internal parenchyma; SC, sclerenchyma.
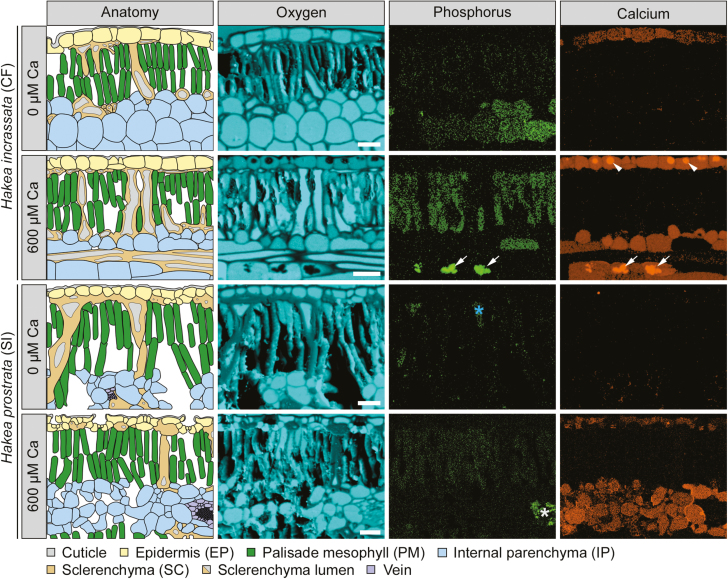
Fig. 2.
Qualitative element maps and corresponding anatomical schematics, showing phosphorus (P), calcium (Ca), and oxygen (O) distributions in transverse leaf sections of two Hakea species, grown under two Ca treatments (0 µM and 600 µM) and high P supply (10 µM). Comparison of a typical calcifuge (CF) and soil-indifferent (SI) Hakea species. All leaves were isobilateral, with images capturing at least half of the transverse section. P and Ca maps are corrected for peak overlaps and background subtraction, providing a visualization of the distribution, with quantified concentrations in Figs 1 and 3 (Supplementary Table S3). Only plants grown under high P (10 µM)/high Ca (600 µM) showed symptoms of P toxicity. Arrows indicate Ca-phosphate deposits; arrowheads indicate Ca-based crystals; the asterisk indicates accumulation of P in sclerenchyma lumen (blue) and xylem parenchyma (white) surrounding a vein. Scale bar=50 µm.
The soil-indifferent H. prostrata showed a preferential allocation of P to both IP and PM under low Ca/high P supply (Figs 1A, 2). Under the same high-P condition, increasing Ca supply decreased the [P] of all cell types, except PM (EP, from 16 µmol g−1 to 4 µmol g−1; IP, from 53 µmol g−1 to 7 µmol g−1; SC, from 12 µmol g−1 to 4 µmol g−1; Figs 1A, 2). This change resulted in a greater relative distribution of [P] to PM, reaching at least ~910% that of other cell types, without a significant increase in PM [P]. In summary, increasing Ca supply under high-P conditions significantly increased the relative distribution of [P] to PM in both Hakea species, increasing the PM [P] in H. incrassata, but not in H. prostrata.
Both Hakea species tended to show lower cell [P] under low P supply (H. incrassata, 2–6 µmol g−1; H. prostrata, 2–4 µmol g−1) compared with those under high P supply (H. incrassata, 2–100 µmol g−1; H. prostrata, 4–61 µmol g−1). Cellular [P] did not differ between Ca supplies under low P supply, except for a small change in SC of H. prostrata (Fig. 1A). Therefore, under low-P conditions, Hakea species showed lower cell [P], no major changes with Ca supply, and no clear preferential allocation of P.
The cell-specific allocation of Ca depended on Ca and P supply in both Hakea species (P<0.001; Fig. 1B). Hakea incrassata showed preferential allocation of Ca to EP under low Ca supply, irrespective of P supply (Fig. 1B). Under high Ca supply, IP and EP [Ca] increased substantially in H. incrassata (Figs 1B, 2). For example, under high-P conditions, IP [Ca] increased ~17-fold (from 7 µmol g−1 to 122 µmol g−1), whilst EP increased ~11-fold (from 96 µmol g−1 to 1040 µmol g−1). Other cell types also showed increases in [Ca] (P<0.01; Fig. 1B), but not to the same degree. Similar changes in Ca allocation were evident under low- and high-P conditions.
Hakea prostrata showed very low [Ca] in all cell types under low Ca supply, irrespective of P supply (Fig. 1B). However, under high Ca supply, it showed a preferential allocation of Ca to EP and IP, with an ~25-fold increase in both under high P supply (Figs 1B, 2). The [Ca] increased in almost all cell types, except in PM under high P supply (Figs 1B, 2). In summary, increasing Ca supply changed the cell-specific allocation of Ca in both Hakea species, with a strong preferential allocation of Ca to EP and IP under high Ca supply. However, unlike H. incrassata, H. prostrata showed no change in PM [Ca] under high-P conditions.
Under high Ca/high P supply, H. incrassata co-allocated Ca and P to IP cells. This was associated with the formation of Ca-phosphate deposits within IP (Fig. 2). Other Ca-based crystals, presumably oxalates, formed in the EP of H. incrassata (Fig. 2). We avoided all crystal regions when selecting cellular regions for analysis, so as not to misrepresent cellular [Ca] or [P]. Hakea prostrata did not co-allocate Ca and P within IP, and showed a decrease in IP [P], concomitant with an increase in [Ca]. In summary, the co-allocation of Ca and P in IP of H. incrassata at high Ca/high P supply resulted in precipitation of Ca-phosphates, absent under other treatments. In addition, the exceptionally high [Ca] in EP of H. incrassata under high Ca/high P supply was associated with formation of Ca-based crystals.
Banksia
The cell-specific allocation of P depended on Ca and P supply in B. menziesii (P<0.001), but not in B. prionotes (P=0.39; Fig. 3A). The calcifuge B. menziesii showed a preferential allocation of P to SM under low Ca/high P supply, with at least an ~2-fold greater [P] than in other cell types (EP, HY.1, HY.2, PM, and SC; Figs 3A, 4). Under the same high-P condition, increasing Ca supply increased the relative P allocation to PM, and away from SM, with an ~2-fold increase in PM [P] (from 9 µmol g−1 to 18 µmol g−1), coupled with an ~50% decrease in SM (from 26 µmol g−1 to 12 µmol g−1; Figs 3A, 4). The [P] also increased in HY.1, decreased in HY.2, and was unchanged in EP and SC.
Fig. 3.
Leaf cell-specific (A) phosphorus ([P]) and (B) calcium ([Ca]) concentrations of two Banksia species across different P and Ca treatments. Comparison of a typical calcifuge (CF) and soil-indifferent (SI) Banksia species. Only plants grown under high P (10 µM)/high Ca (600 µM) showed symptoms of P toxicity. Concentrations are per unit fresh weight, from fully hydrated cells. Bars are means, and error bars represent 95% confidence intervals, from linear mixed-effect models. Different letters indicate significant differences among cell types within each Ca treatment and panel (post-hoc Tukey test, P<0.05). P-values (*, <0.05; **, <0.01; ***, <0.001) represent significant differences between Ca treatments (post-hoc Tukey test). 0.1 µM P and 10 µM P indicate the two P treatments. EP, epidermis; HY.1, upper layer of hypodermis; HY.2, lower layer of hypodermis; PM, palisade mesophyll; SM, spongy mesophyll; SC, sclerenchyma.
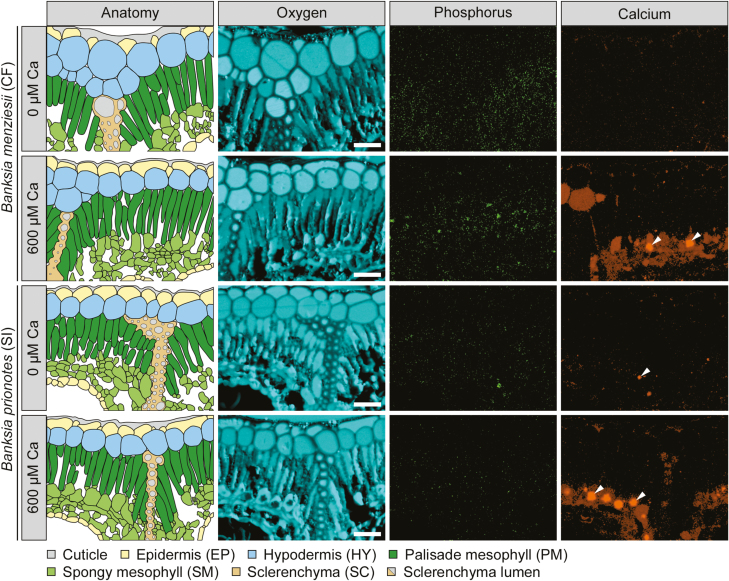
Fig. 4.
Qualitative element maps and corresponding anatomical schematics, showing phosphorus (P), calcium (Ca), and oxygen (O) distributions in transverse leaf sections of two Banksia species, grown under two Ca treatments (0 µM and 600 µM) and high P supply (10 µM). Comparison of a typical calcifuge (CF) and soil-indifferent (SI) Banksia species. All leaves were dorsiventral with stomatal crypts on the abaxial surface. Images capture the upper part of transverse sections, with the adaxial surface at the top. P and Ca maps are corrected for peak overlaps and background subtraction, and provide a visualization of the distribution, with quantified concentrations in Figs 1 and 3 (Supplementary Table S3). Only plants grown under high P (10 µM)/high Ca (600 µM) showed symptoms of P toxicity. Arrowheads indicate Ca-based crystals. Scale bar=50 µm.
The soil-indifferent B. prionotes showed a preferential allocation of P to PM, SM, and SC (9, 10, and 10 µmol g−1, respectively) under low Ca/high P supply (Figs 3A, 4). Under the same high-P condition, increasing Ca supply caused no change in [P] of most cell types, except PM, which showed a minor increase from 9 µmol g−1 to 14 µmol g−1 (P=0.02; Figs 3A, 4), which was non-significant (P=0.07) when PM [P] was analysed separately (Fig. 5). Although the increase in PM [P] was small, it did change the relative distribution of [P], increasing PM [P] from ~90% to ~162% of that in SM and SC. In summary, increasing Ca supply under high-P conditions increased the relative distribution of [P] to PM, resulting in an increase in the PM [P] of B. menziesii, and less of an increase in B. prionotes.
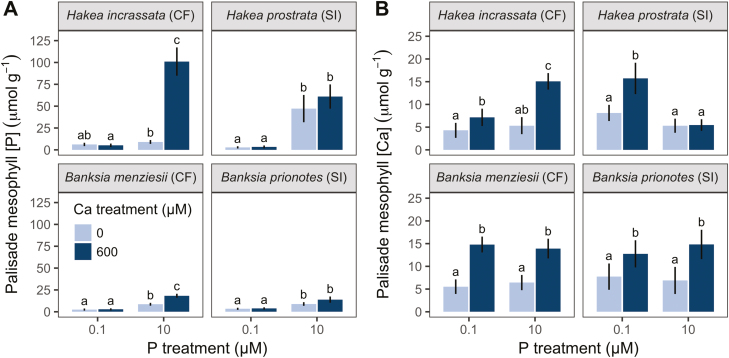
Fig. 5.
(A) Phosphorus ([P]) and (B) calcium ([Ca]) concentrations of palisade mesophyll cells across different P and Ca treatments. Comparison of typical calcifuge and soil-indifferent Hakea and Banksia species. Only plants grown under high P (10 µM)/high Ca (600 µM) showed symptoms of P toxicity. Concentrations are per unit fresh weight, from fully hydrated cells. Bars are means, and error bars represent 95% confidence intervals, from linear mixed-effect models. Different letters indicate significant differences among treatments within each panel (post-hoc Tukey test, P<0.05).
Both Banksia species tended to show lower [P] under low P supply (B. menziesii, 1–4 µmol g−1; B. prionotes, 2–5 µmol g−1), compared with those under high P supply (B. menziesii, 2–26 µmol g−1; B. prionotes, 2–14 µmol g−1). Cellular [P] did not differ between Ca supplies under low P supply (Fig. 3A). There were some differences in [P] among cell types, but there was no clear preferential allocation. Therefore, under low-P conditions, both Banksia species showed a low cell [P], no change in P allocation with increasing Ca supply, and no preferential allocation of P.
The cell-specific allocation of Ca depended on Ca and P supply in both Banksia species (P<0.001; Fig. 3B). Banksia menziesii showed low [Ca] under low Ca supply (2–29 µmol g−1), regardless of P supply (Fig. 3B). However, increasing Ca supply caused a major change in the allocation of Ca, depending on P conditions. For example, under high Ca/low P supply, Ca was preferentially allocated to SM (524 µmol g−1), followed by HY.2 (154 µmol g−1). Conversely, under the same high-Ca condition, but with high P supply, Ca was instead preferentially allocated to HY.2 (470 µmol g−1), and secondarily to SM (273 µmol g−1).
Banksia prionotes preferentially allocated Ca to SM (92 µmol g−1) under low Ca/low P supply (Fig. 3B). In contrast, under low-Ca conditions, but with high P supply, B. prionotes showed low [Ca] across all cell types (3–22 µmol g−1). Increasing Ca supply caused an acute increase in SM [Ca], at least ~26-fold and ~4-fold greater than that in other cell types, under low- and high-P conditions, respectively (Figs 3B, 4). In summary, increasing Ca supply significantly changed the cell-specific allocation of Ca in both Banksia species, with Ca generally allocated to SM under high-Ca conditions.
Both Banksia species contained Ca-based crystals, presumably oxalates, throughout SM cells, primarily under high Ca supply (Fig. 4).
Palisade mesophyll
A direct comparison of PM [P] and [Ca] across treatments revealed differences between calcifuge and soil-indifferent species (Fig. 5A). Calcifuges showed an increase in PM [P] with increasing Ca supply under high-P conditions (P<0.05; Fig. 5A), whilst soil-indifferent species did not (P >0.05; Fig. 5). Furthermore, within each genus, calcifuges showed a higher PM [P] than soil-indifferent species under high P supply. No species showed a difference in PM [P] under low P supply.
In both Banksia species, the PM [Ca] depended only on Ca treatment (P<0.001; Fig. 5B). In contrast, cellular [Ca] in both Hakea species changed between Ca treatments, dependent on P treatment (Ca treatment×P treatment: P<0.001). For example, H. prostrata showed no increase in PM [Ca] under high-P conditions, only under low-P conditions (Fig. 5B). Also, the PM [Ca] in H. incrassata increased more strongly with increasing Ca supply under high-P conditions than under low-P conditions.
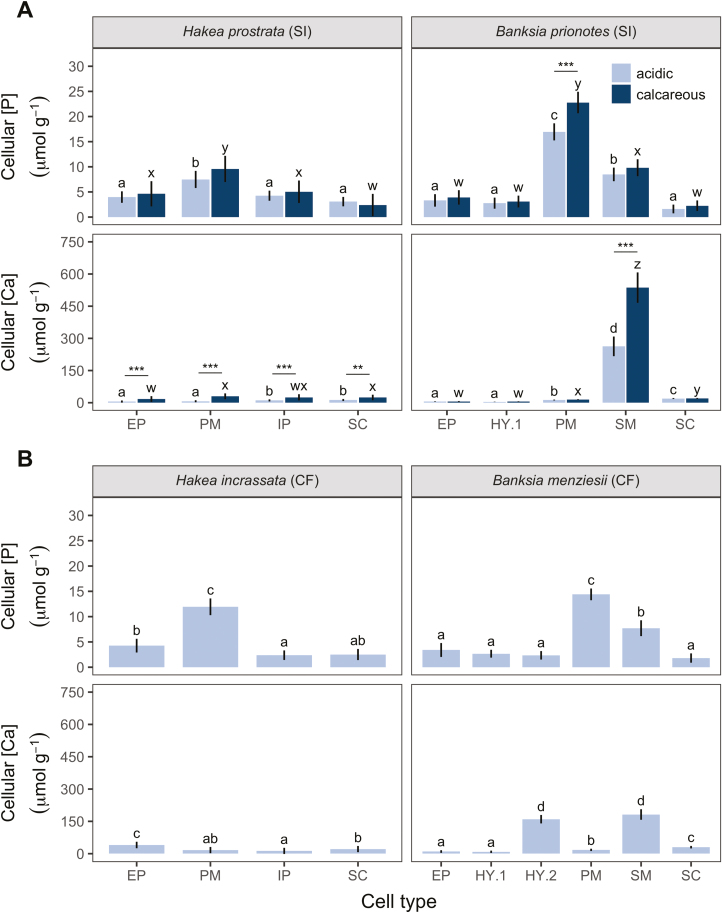
Field-collected material
All species collected from acidic soils showed a clear preferential allocation of P to PM cells (Fig. 6). Cellular [P] did not differ between H. prostrata plants collected from different soil types (P=0.84; Fig. 6A). However, in B. prionotes, the effect of soil type on [P] depended on cell type (soil type×cell type interaction: P<0.01). For example, PM [P] in B. prionotes was 17 µmol g−1 on acidic soils, versus 23 µmol g−1 on calcareous soils, with no difference in other cell types.
Fig. 6.
Leaf cell-specific phosphorus ([P]) and calcium ([Ca]) concentrations in natural populations of Proteaceae collected from field locations of contrasting soil type along the Jurien Bay chronosequence, south-western Australia. Comparison of typical (A) soil-indifferent (SI), and (B) calcifuge (CF) Hakea and Banksia species, indicating soil type(s) on which they were collected. (A) SI species are found on both acidic and calcareous soils; (B) CF species are only found on acidic soils. Calcareous soils are higher in total and available soil [P], and exchangeable [Ca], compared with acidic soils (Supplementary Table S2). None of the field-collected plants showed signs of P toxicity. Concentrations are per unit fresh weight, from fully hydrated cells. Bars are means, and error bars represent 95% confidence intervals, from linear mixed-effect models. Different letters indicate significant differences among cell types within each soil type and panel (post-hoc Tukey test, P<0.05). (A) P-values (*, <0.05; **, <0.01; ***, <0.001) represent significant differences between soil types (post-hoc Tukey test) in SI species. EP, epidermis; HY.1, upper layer of hypodermis; HY.2, lower layer of hypodermis; PM, palisade mesophyll; SM, spongy mesophyll; IP, internal parenchyma; SC, sclerenchyma. Data for plants collected on acidic soils are from Hayes et al. (2018).
Both Hakea species showed relatively low cellular [Ca] on acidic soils, with H. incrassata showing a preferential allocation of Ca to EP (Fig. 6B). Both Banksia species preferentially allocated Ca to SM, with B. menziesii also allocating Ca to HY.2. The effect of soil type on [Ca] depended on cell type in both soil-indifferent species (P<0.001; Fig. 6B). For example, [Ca] increased across all cell types in H. prostrata. In contrast, B. prionotes showed a much lower SM [Ca] on acidic soils, 263 µmol g−1 versus 537 µmol g−1 on calcareous soils. In summary, B. prionotes allocated extra P to PM and Ca to SM on calcareous soils, whilst H. prostrata maintained a stable PM [P], but increased [Ca] across cell types.
Discussion
Ca supply modulated the cell-specific allocation of leaf P in all species, enhancing the relative allocation of P to PM with increasing Ca supply, under high-P conditions. Calcifuges showed a greater response than soil-indifferent species, with greater PM [P] in calcifuges. Furthermore, through our comparison of calcifuge and soil-indifferent species, we were able to demonstrate quantitatively the mechanism by which Ca enhances P toxicity, and propose several strategies with which soil-indifferent species were able to tolerate high Ca and P supplies.
Calcium supply altered the cell-specific allocation of P
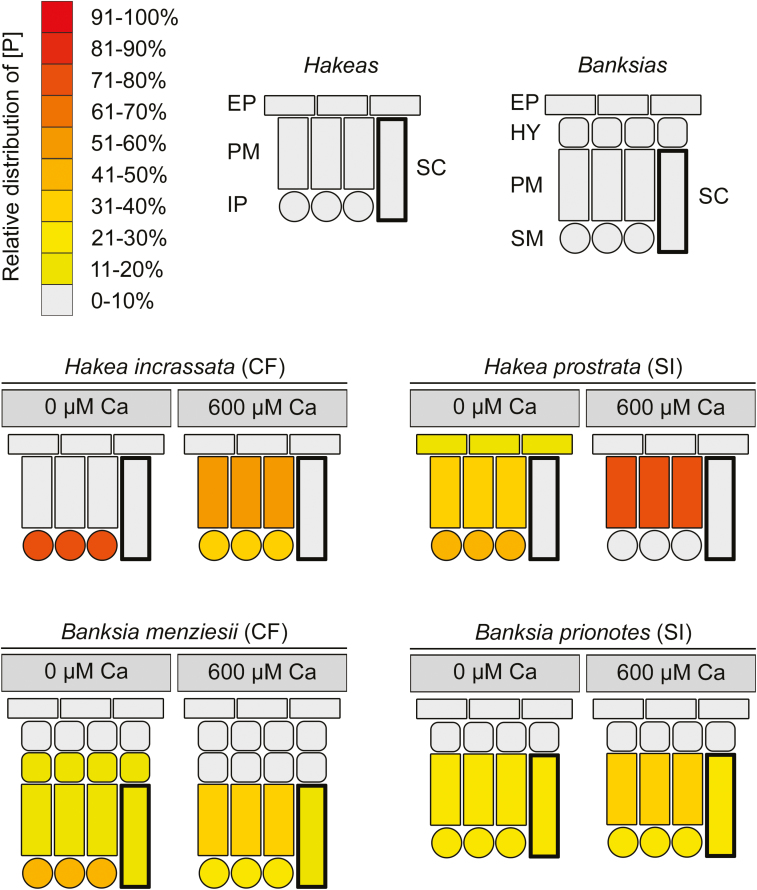
Increased Ca supply altered the cell-specific allocation of P under high-P conditions in all species, enhancing the relative allocation of [P] to PM (Fig. 7), resulting in PM showing the highest mean [P]. The effect of Ca supply on P allocation was only evident at high P supply. At low P supply, the cellular [P] was very low, showed no clear preferential allocation, and was independent of Ca supply.
Fig. 7.
Relative distribution patterns of phosphorus (P) within leaves of calcifuge (CF) and soil-indifferent (SI) Proteaceae grown under two calcium (Ca) treatments (0 µM and 600 µM) and high P supply (10 µM). Stylized leaf cross-sections show the relative proportion of [P] across the indicated cell types. Cell types vary among species due to differences in anatomy. EP, epidermis; HY hypodermis; PM, palisade mesophyll; SM, spongy mesophyll; IP, internal parenchyma; SC, sclerenchyma.
We surmise that Ca supply altered the cell-specific allocation of P through the following mechanism. Under low Ca supply, all cells had relatively low [Ca] (typically <30 µmol g−1). This low [Ca] allowed for excess P under high P supply to accumulate in cells other than PM (Fig. 7). For example, P typically accumulated in IP in Hakea species and in PM, SM, and SC in Banksia species, under low Ca supply. However, under high Ca supply, Ca also accumulated in non-PM cells, i.e. EP and IP in Hakea species, and SM and HY.2 in Banksia species. Associated with this increase in non-PM [Ca] was a strong change in the relative distribution of [P], away from non-PM (high [Ca]) and towards PM (low [Ca]). This change in relative distribution of [P] to PM was due to either an increase in PM [P] or a decrease in non-PM [P], or both, and was evident across all species.
In summary, all species showed a change in P allocation with increased Ca supply, under high-P conditions. We surmise that this change in P allocation was strongly influenced by cellular [Ca] and the requirement of plants to separate cellular Ca and P (McLaughlin and Wimmer, 1999; White and Broadley, 2003; Conn and Gilliham, 2010). This is the first example of Ca influencing cell-specific allocation of P, significantly advancing our understanding of P nutrition and highlighting the importance of nutrient interactions at the cellular level.
A mechanism for Ca-enhanced P toxicity
The main symptoms of P toxicity are leaf chlorosis and necrosis (Grundon, 1972; Groves and Keraitis, 1976; Nichols and Beardsell, 1981; Webb and Loneragan, 1990; Lambers et al., 2002; Shane et al., 2004a, b; Parks et al., 2007; Hawkins et al., 2008; Hayes et al., 2019). We propose that P-toxicity symptoms and their enhancement under high Ca supply involve two processes. First, an inability to strongly down-regulate P-uptake capacity, and, secondly, cell-specific allocation of P and Ca.
P-sensitive Proteaceae show a weak ability to down-regulate P uptake, resulting in excessive P uptake and P toxicity, even at relatively low P availability (Handreck, 1997; Shane et al., 2004b; Shane and Lambers, 2006; de Campos et al., 2013). This is considered the primary physiological mechanism causing P toxicity and has been demonstrated for several P-sensitive species (Handreck, 1997; Shane et al., 2004b; Shane and Lambers, 2006; de Campos et al., 2013). The second mechanism is the preferential allocation of P to photosynthetic mesophyll cells in species from severely P-impoverished habitats (Shane et al., 2004a; Hawkins et al., 2008; Lambers et al., 2015; Hayes et al., 2018). Preferential allocation of P to photosynthetic cells increases photosynthetic P-use efficiency, but also leads to very high [P] in these cells at high P supply (Shane et al., 2004a; Hawkins et al., 2008; Hayes et al., 2018). Therefore, preferential allocation of P to mesophyll cells disproportionally increases mesophyll [P], enhancing their sensitivity to P, even at relatively low P availability. Furthermore, under high-P conditions, increased Ca supply significantly increases the preferential allocation of P to PM cells, resulting in higher PM [P], enhancing P-toxicity symptoms. This mechanism explains why Ca enhances P-toxicity symptoms, without changes in whole-leaf [P] (see Hayes et al., 2019 for whole-leaf nutrient concentrations).
An increase in PM [P] can reduce the physiological availability of micronutrients, such as Zn, manganese (Mn), and iron (Fe), resulting in leaf chlorosis, a symptom of P toxicity (Cakmak and Marschner, 1987; Broadley et al., 2012; Hayes et al., 2019). Furthermore, in species showing co-allocation of high [Ca] and [P], they may form Ca-phosphates, as in IP of H. incrassata. Although little is known regarding the impact of Ca-phosphate precipitation within cells, it probably reduces the availability of both nutrients, interfering with cellular processes, and may also result in physical cellular damage, all probably resulting in cell death and the associated leaf necrosis. In summary, this study supports the model of Ca-enhanced P toxicity, demonstrating, for the first time, the mechanism by which Ca enhances P toxicity.
Calcifuge species showed a greater response to increased Ca supply than soil-indifferent species
Compared with soil-indifferent species, calcifuges showed a greater increase in PM [P] with increasing Ca supply. For example, the calcifuge H. incrassata showed the greatest increase in PM [P] in response to increased Ca supply, from 9 µmol g−1 to 100 µmol g−1. This was an extreme increase in [P], considering that the PM [P] in field-collected plants was 12 µmol g−1 (Hayes et al., 2018). It is expected that such high PM [P] would severely reduce physiological [Zn] (Hayes et al., 2019). In contrast, the soil-indifferent H. prostrata showed no increase in PM [P]. Its PM [P] was less than that of H. incrassata under the same conditions and less than the 136–233 µmol g−1 in H. prostrata under highly P-toxic conditions (Shane et al., 2004a).
The calcifuge B. menziesii showed an ~2-fold increase in PM [P] with increasing Ca supply. However, this PM [P] was still only ~4 µmol g−1 greater than that in field-collected plants, yet it severely impacted glasshouse-grown plants (severe chlorosis; Hayes et al., 2019). The severe impact of this change may be partly explained by differences in cellular inorganic P concentrations ([Pi]) between glasshouse-grown and field-collected plants. A high [Pi] would severely reduce physiological [Zn] and result in chlorosis (Cakmak and Marschner, 1987; Zhao et al., 1998; Broadley et al., 2012). In this study, we measured total cellular [P] (including Pi and organic P); however, leaves with high [P] show a greater proportion of Pi and hence should show a greater cellular [Pi] (Veneklaas et al., 2012). Therefore, since glasshouse-grown plants showed leaf [P] ~6-fold greater than field-collected plants (Hayes et al., 2019), we would expect glasshouse-grown plants to show a greater proportion of Pi. We surmise that although PM [P] was only marginally greater in glasshouse-grown plants than in field-collected plants, the glasshouse-grown plants presumably contained a greater [Pi], which would more severely reduce the physiological [Zn], thus explaining the severe impact of this relatively small increase on the glasshouse-grown B. menziesii. In contrast, the soil-indifferent B. prionotes showed only a small increase in PM [P] with increasing Ca supply. This PM [P] was below that of B. menziesii and of field-collected B. prionotes. The development of mild chlorosis in glasshouse-grown B. prionotes under the most severe treatments suggests that glasshouse-grown plants had a greater cellular [Pi] than field-collected plants. This notion is supported by the ~11-fold greater whole-leaf [P] of glasshouse-grown plants, expected to result in a greater [Pi] (Veneklaas et al., 2012).
In summary, calcifuges showed a greater PM [P] compared with soil-indifferent species of the same genus and consequently calcifuges showed more severe chlorotic symptoms, indicating a reduced micronutrient availability and probably contributing to their increased sensitivity to Ca-enhanced P toxicity.
Some calcifuge species showed a low ability to separate Ca and P. For example, H. incrassata co-allocated Ca and P in IP under high P/high Ca supply. The inability of H. incrassata to separate Ca and P resulted in the precipitation of Ca-phosphates, explaining the severe symptoms of necrosis. This is the first evidence of a direct interaction between cellular Ca and P, resulting in the formation of Ca-phosphate deposits (Treeby et al., 1987; McLaughlin and Wimmer, 1999; Karley et al., 2000b; White and Broadley, 2003; Storey and Leigh, 2004; Conn and Gilliham, 2010). Hakea incrassata also showed an increase in [P] with increasing Ca supply across most cell types. In contrast, H. prostrata showed a reduction in [P] in non-PM cells. Consequently, H. prostrata was potentially better able to avoid co-allocation of Ca and P in non-PM cells, thus contributing towards its lower sensitivity to Ca-enhanced P toxicity.
The reduction in [P] of non-PM cells with increasing Ca supply in H. prostrata was probably achieved by a displacement of P to non-reported regions (i.e. xylem parenchyma). Similarly, in H. incrassata, an increase in [P] of all cells, with increasing Ca supply, may have resulted from a displacement of P within the leaf, from non-reported Ca-rich regions (i.e. apoplastic regions, xylem parenchyma, and sclerenchyma lumen) to reported cell regions (i.e. EP, PM, IP, and SC). These P displacements within the leaf may also account for the lack of a significant change in whole-leaf [P], despite significant changes in cell [P] (Hayes et al., 2019).
Banksia menziesii reduced co-allocation of Ca and P by shifting P allocation away from Ca-accumulating cell types (SM and HY.2) towards non-Ca-accumulating types (PM and HY.1). Therefore, B. menziesii altered its allocation of P under high Ca and high P supply. The soil-indifferent B. prionotes, however, showed no change in the [P] of any non-PM cell type. In addition to minimizing changes in P allocation with increasing Ca supply, B. prionotes also showed a greater whole-leaf [Zn], compared with B. menziesii (Hayes et al., 2019). A greater leaf [Zn] further explains why B. prionotes is less sensitive to Ca-enhanced P toxicity, because an increased [Zn] allows it to tolerate P-enhanced Zn requirements. Therefore, B. prionotes is more tolerant of Ca-enhanced P toxicity than B. menziesii, due to its ability to minimize changes in cellular [P], particularly PM [P], and because it operates at a higher leaf [Zn], allowing it to tolerate higher [P], without critically reducing physiological [Zn].
Conclusions
Calcium enhanced the relative allocation of P to PM under high-P conditions, generally resulting in a greater PM [P], thus supporting our model of Ca-enhanced P toxicity (Hayes, 2018; Hayes et al., 2019). Calcifuge Proteaceae showed a greater response to increased Ca supply than soil-indifferent species, corresponding with their high sensitivity to Ca-enhanced P toxicity. Specifically, calcifuges showed a greater PM [P] compared with soil-indifferent species of the same genus. Therefore, we surmise that Ca-enhanced P toxicity is due to a weak ability to down-regulate P-uptake capacity, in conjunction with leaf cell-specific interactions between Ca and P. This results in higher leaf PM [P] under high Ca supply, thus interfering with the physiological [Zn] and/or precipitating with Ca.
We surmise that it is the higher soil [P] of young calcareous soils, in combination with higher soil [Ca] and low available [Zn], that excludes most Proteaceae from calcareous habitats and that soil-indifferent Proteaceae can overcome this restriction: by limiting changes in PM [P], separating Ca and P, and through several traits that increase leaf [Zn] (Hayes, 2018; Hayes et al., 2019). These strategies reduce the impact of Ca on PM [P], reduce deleterious interactions between Ca and P, and compensate for P-enhanced Zn requirement.
The mechanisms and pathways that regulate cell-specific nutrient allocation in leaves are not yet fully understood (White and Broadley, 2003; Conn et al., 2011; Conn and Gilliham, 2010; Gilliham et al. 2011). Differences in the cell-specific expression of transporters and channels probably play an important role in regulating cell-specific preferential allocation of nutrients and should be the focus of future research. Furthermore, knowledge of the interactions among nutrients at the cell-specific level highlights that there can be physiologically significant changes in cellular concentrations, with no apparent change at the leaf level. Therefore, this research highlights the importance of considering nutrient interactions at the cell level.
This study is critically important for the management of Proteaceae in restoration projects and in the horticultural industry (Bunn and Dixon, 1992; Enright and Lamont, 1992; Fuss et al., 1992; Stephenson, 2005; Cross and Lambers, 2017). This research also has important implications for our understanding of plant P nutrition, particularly regarding species that preferentially allocate P to mesophyll cells (i.e. monocot crop species and eudicots from severely P-impoverished habitats; Conn and Gilliham, 2010; Guilherme Pereira et al., 2018; Hayes et al., 2018). Species that preferentially allocate P to the mesophyll may be particularly susceptible to Ca-enhanced P toxicity and possibly other Ca-driven changes in cell-specific nutrient allocation. We speculate that similar relationships may also be observed between P and metals such as Fe or Mn, warranting further investigation (Robson and Pitman, 1983; Lambers et al., 2002; Broadley et al., 2012).
Supplementary data
Supplementary data are available at JXB online.
Table S1. Concentrations of nutrient solutions used in hydroponic experiments.
Table S2. Soil chemical properties from selected sites along the Jurien Bay dune chronosequence, south-western Australia.
Table S3. Cellular phosphorus (P) and calcium (Ca) concentrations of plants grown under different P and Ca supplies.
Table S4. Cellular phosphorus (P) and calcium (Ca) concentrations of Proteaceae species collected from contrasting soil types along the Jurien Bay dune chronosequence, south-western Australia.
Author contributions
PEH, CGP, and HL planned and designed the glasshouse experiment; PEH, PLC, and HL planned and designed the field collections; PEH and CGP performed the glasshouse experiment; PEH and PLC collected and analysed glasshouse and field-collected samples; PEH, PLC, and HL interpreted data and wrote the paper. All authors contributed critically to the development of the manuscript.
Acknowledgements
PEH and CGP were supported by an Australian Government Research Training Program Scholarship supplemented by a top-up scholarship from the Australian Research Council (ARC; DP130100005). We would like to thank Lyn Kirilak for her technical support. We would like to thank Daniel Beeck, Calum Irvine, Nickolas Mendez, Shahab Nikabadi, and Katie White for their assistance in the glasshouse experiment. This research was supported by an ARC-funded Discovery Project grant (DP130100005) awarded to HL and PLC. We acknowledge the facilities of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments. We acknowledge the Department of Parks and Wildlife (Western Australia) and the Shires of Dandaragan and Coorow for permission to conduct research on land under their administration.
References
- Boawn LC, Leggett GE. 1964. Phosphorus and zinc concentrations in Russet Burbank potato tissues in relation to development of zinc deficiency symptoms. Soil Science Society of America Journal 28, 229–232. [Google Scholar]
- Boursier P, Läuchli A. 1989. Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiologia Plantarum 77, 537–544. [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. 2012. Function of nutrients: micronutrients. In: Marschner P, ed. Marschner’s mineral nutrition of higher plants. Waltham, MA: Elsevier/Academic Press, 191–248. [Google Scholar]
- Bunn E, Dixon KW. 1992. Micropropagation of Stirlingia latifolia (Proteaceae), an important cut flower from Western Australia. HortScience 27, 368–368. [Google Scholar]
- Cakmak I, Marschner H. 1987. Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants. Physiologia Plantarum 70, 13–20. [Google Scholar]
- Chapman HD, Vanselow AP. 1937. The production of citrus mottle-leaf in controlled nutrient cultures. Journal of Agricultural Research 55, 365–379. [Google Scholar]
- Conn S, Gilliham M. 2010. Comparative physiology of elemental distributions in plants. Annals of Botany 105, 1081–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, et al. 2011. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell 23, 240–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RM, Lamont BB. 1998. On the nature of Gondwanan species flocks: diversity of Proteaceae in Mediterranean south-western Australia and South Africa. Australian Journal of Botany 46, 335–355. [Google Scholar]
- Cross AT, Lambers H. 2017. Young calcareous soil chronosequences as a model for ecological restoration on alkaline mine tailings. The Science of the Total Environment 607–608, 168–175. [DOI] [PubMed] [Google Scholar]
- de Campos MC, Pearse SJ, Oliveira RS, Lambers H. 2013. Downregulation of net phosphorus-uptake capacity is inversely related to leaf phosphorus-resorption proficiency in four species from a phosphorus-impoverished environment. Annals of Botany 111, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Schramm M, Lang B, Lanzl-Schramm A, Dürr C, Martinoia E. 1992. Characterization of the epidermis from barley primary leaves: II. The role of the epidermis in ion compartmentation. Planta 187, 431–437. [DOI] [PubMed] [Google Scholar]
- Enright N, Lamont B. 1992. Recruitment variability in the resprouting shrub Banksia attenuata and non-sprouting congeners in the Northern Sandplain Heaths of Southwestern Australia. Acta Oecologica-International Journal of Ecology 13, 727–741. [Google Scholar]
- Fox J. 2003. Effect displays in R for generalised linear models. Journal of Statistical Software 8, 1–27. [Google Scholar]
- Fricke W, Leigh RA, Tomos AD. 1994. Concentrations of inorganic and organic solutes in extracts from individual epidermal, mesophyll and bundle-sheath cells of barley leaves. Planta 192, 310–316. [Google Scholar]
- Fuss AM, Pattison SJ, Aspinall D, Sedgley M. 1992. Shoot growth in relation to cut flower production of Banksia coccinea and Banksia menziesii (Proteaceae). Scientia Horticulturae 49, 323–334. [Google Scholar]
- George AS. 1998. Proteus in Australia. An overview of the current state of taxonomy of the Australian Proteaceae. Australian Systematic Botany 11, 257–266. [Google Scholar]
- Gilliham M, Athman A, Tyerman SD, Conn SJ. 2011. Cell-specific compartmentation of mineral nutrients is an essential mechanism for optimal plant productivity—another role for TPC1? Plant Signaling & Behavior 6, 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves RH, Keraitis K. 1976. Survival and growth of seedlings of three sclerophyll species at high levels of phosphorus and nitrogen. Australian Journal of Botany 24, 681–690. [Google Scholar]
- Grundon NJ. 1972. Mineral nutrition of some Queensland heath plants. Journal of Ecology 60, 171–181. [Google Scholar]
- Guilherme Pereira C, Clode PL, Oliveira RS, Lambers H. 2018. Eudicots from severely phosphorus-impoverished environments preferentially allocate phosphorus to their mesophyll. New Phytologist 218, 959–973. [DOI] [PubMed] [Google Scholar]
- Handreck KA. 1997. Phosphorus requirements of Australian native plants. Soil Research 35, 241–290. [Google Scholar]
- Hawkins H-J, Hettasch H, Mesjasz-Przybylowicz J, Przybylowicz W, Cramer MD. 2008. Phosphorus toxicity in the Proteaceae: a problem in post-agricultural lands. Scientia Horticulturae 117, 357–365. [Google Scholar]
- Hayes PE. 2018. Does calcium-enhanced phosphorus toxicity explain the absence of most Proteaceae species from calcareous habitats? PhD Thesis. The University of Western Australia, Perth, WA, Australia. [Google Scholar]
- Hayes PE, Clode PL, Oliveira RS, Lambers H. 2018. Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant, Cell & Environment 41, 605–619. [DOI] [PubMed] [Google Scholar]
- Hayes PE, Guilherme Pereira C, Clode PL, Lambers H. 2019. Calcium-enhanced phosphorus toxicity in calcifuge and soil-indifferent Proteaceae along the Jurien Bay chronosequence. New Phytologist 221, 764–777. [DOI] [PubMed] [Google Scholar]
- Hayes P, Turner BL, Lambers H, Laliberté E. 2014. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. Journal of Ecology 102, 396–410. [Google Scholar]
- Hendershot W, Lalande H, Duquette M. 2008. Ion exchange and exchangeable cations. In: Carter MR, Gregorich E, eds. Soil sampling and methods of analysis. Boca Raton, FL: Canadian Society of Soil Science, CRC Press, 173–178. [Google Scholar]
- Hopper SD, Gioia P. 2004. The South West Australian Floristic Region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623–650. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363. [DOI] [PubMed] [Google Scholar]
- Huang CX, Canny MJ, Oates K, McCully ME. 1994. Planing frozen hydrated plant specimens for SEM observation and EDX microanalysis. Microscopy Research and Technique 28, 67–74. [DOI] [PubMed] [Google Scholar]
- Jefferies RL, Willis AJ. 1964. Studies on the calcicole–calcifuge habit: II. The influence of calcium on the growth and establishment of four species in soil and sand cultures. Journal of Ecology 52, 691–707. [Google Scholar]
- Jin Q, Paunesku T, Lai B, Gleber SC, Chen SI, Finney L, Vine D, Vogt S, Woloschak G, Jacobsen C. 2017. Preserving elemental content in adherent mammalian cells for analysis by synchrotron-based x-ray fluorescence microscopy. Journal of Microscopy 265, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. 2000a Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiology 122, 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. 2000b Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends in Plant Science 5, 465–470. [DOI] [PubMed] [Google Scholar]
- Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll K-H, Zemunik G, Lambers H. 2012. Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. Journal of Ecology 100, 631–642. [Google Scholar]
- Lambers H, Clode PL, Hawkins H-J, Laliberté E, Oliveira RS, Reddell P, Shane MW, Stitt M, Weston P. 2015. Metabolic adaptations of the non-mycotrophic Proteaceae to soils with low phosphorus availability. In: Plaxton WC, Lambers H, eds. Annual plant reviews. Vol. 48 Hoboken, NJ: John Wiley & Sons, Inc., 289–335. [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martínez-Ferri E. 2002. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil 238, 111–122. [Google Scholar]
- Leigh RA, Storey R. 1993. Intercellular compartmentation of ions in barley leaves in relation to potassium nutrition and salinity. Journal of Experimental Botany 44, 755–762. [Google Scholar]
- Leigh RA, Tomos AD. 1993. Ion distribution in cereal leaves: pathways and mechanisms. Philosophical Transactions of the Royal Society B: Biological Sciences 341, 75–86. [Google Scholar]
- Loneragan JF, Webb MJ. 1993. Interactions between zinc and other nutrients affecting the growth of plants . In: Robson AD, ed. Zinc in soils and plants. Developments in plant and soil sciences, Vol. 55 Dordrecht: Springer, 119–134. [Google Scholar]
- Marshall AT. 1982. X-ray depth distribution (φ (ϱz)) curves for X-ray microanalysis of frozen-hydrated bulk biological samples. Micron 13, 317–318. [Google Scholar]
- Marshall AT. 2017. Quantitative X-ray microanalysis of model biological samples in the SEM using remote standards and the XPP analytical model. Journal of Microscopy 266, 231–236. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Clode PL. 2009. X-ray microanalysis of Rb+ entry into cricket Malpighian tubule cells via putative K+ channels. Journal of Experimental Biology 212, 2977–2982. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Goodyear MJ, Crewther SG. 2012. Sequential quantitative X-ray elemental imaging of frozen-hydrated and freeze-dried biological bulk samples in the SEM. Journal of Microscopy 245, 17–25. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Xu W. 1998. Quantitative elemental X-ray imaging of frozen-hydrated biological samples. Journal of Microscopy 190, 305–316. [DOI] [PubMed] [Google Scholar]
- McCully ME, Canny MJ, Huang CX, Miller C, Brink F. 2010. Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology: energy dispersive X-ray microanalysis (CEDX) applications. Functional Plant Biology 37, 1011–1040. [Google Scholar]
- McLaughlin SB, Wimmer R. 1999. Calcium physiology and terrestrial ecosystem processes. New Phytologist 142, 373–417. [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Nichols DG, Beardsell DV. 1981. Interactions of calcium, nitrogen and potassium with phosphorus on the symptoms of toxicity in Grevillea cv. ‘Poorinda Firebird’. Plant and Soil 61, 437–445. [Google Scholar]
- Ondrasek G, Clode P, Kilburn M, Guagliardo P, Romić D, Rengel Z. 2019. Zinc and cadmium mapping in the apical shoot and hypocotyl tissues of radish by high-resolution secondary ion mass spectrometry (NanoSIMS) after short-term exposure to metal contamination. International Journal of Environmental Research and Public Health 16, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Tarczynski MC, Miller WI. 1984. Histological compartmentation of phosphate in Vicia faba L. leaflet: possible significance to stomatal functioning. Plant Physiology 74, 430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks SE, Haigh AM, Harris AM. 2007. Responses of six species of Proteaceae, in containers, to controlled-release fertilizer. Communications in Soil Science and Plant Analysis 38, 2227–2237. [Google Scholar]
- Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-PLUS. New York: Springer. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team. 2016. nlme: linear and nonlinear mixed effects models. R package version 3.1-128. [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Robson AD, Pitman MG. 1983. Interactions between nutrients in higher plants . In: Läuchli A, Bieleski RL, eds. Inorganic plant nutrition. Encyclopedia of plant physiology, vol. 15 Berlin: Springer, 147–180. [Google Scholar]
- Roomans GM, Dragomir A. 2007. X-ray microanalysis in the scanning electron microscope. Electron Microscopy 507–528. [DOI] [PubMed] [Google Scholar]
- Shane MW, de Vos M, de Roock S, Cawthray GR, Lambers H. 2003. Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea prostrata R.Br. Plant and Soil 248, 209–219. [Google Scholar]
- Shane MW, Lambers H. 2005. Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiologia Plantarum 124, 441–450. [Google Scholar]
- Shane MW, Lambers H. 2006. Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. Journal of Experimental Botany 57, 413–423. [DOI] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Lambers H. 2004a Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). Journal of Experimental Botany 55, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Shane MW, Szota C, Lambers H. 2004b A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant, Cell & Environment 27, 991–1004. [Google Scholar]
- Stephenson R. 2005. Macadamia: domestication and commercialization. Chronica Horticulture 45, 11–15. [Google Scholar]
- Stitt M, Lunn J, Usadel B. 2010. Arabidopsis and primary photosynthetic metabolism—more than the icing on the cake. The Plant Journal 61, 1067–1091. [DOI] [PubMed] [Google Scholar]
- Storey R, Leigh RA. 2004. Processes modulating calcium distribution in citrus leaves. An investigation using X-ray microanalysis with strontium as a tracer. Plant Physiology 136, 3838–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeby MT, van Steveninck RF, de Vries HM. 1987. Quantitative estimates of phosphorus concentrations within Lupinus luteus leaflets by means of electron probe X-ray microanalysis. Plant Physiology 85, 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii Y, Oikawa M, Kitayama K. 2017. Significance of the localization of phosphorus among tissues on a cross-section of leaf lamina of Bornean tree species for phosphorus-use efficiency. Journal of Tropical Ecology 33, 237–240. [Google Scholar]
- Turner BL, Laliberté E. 2015. Soil development and nutrient availability along a 2 million-year coastal dune chronosequence under species-rich mediterranean shrubland in southwestern Australia. Ecosystems 18, 287–309. [Google Scholar]
- Turner BL, Romero TE. 2009. Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Science Society of America Journal 73, 1972–1979. [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. 2012. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist 195, 306–320. [DOI] [PubMed] [Google Scholar]
- Webb MJ, Loneragan JF. 1990. Zinc translocation to wheat roots and its implications for a phosphorus/zinc interaction in wheat plants. Journal of Plant Nutrition 13, 1499–1512. [Google Scholar]
- Weston PH. 2007. Proteaceae. In: Kubitzki K, eds. Flowering plants. Eudicots. The families and genera of vascular plants, vol. 9 Berlin Heidelberg: Springer-Verlag, 364–404. [Google Scholar]
- White PJ, Broadley MR. 2003. Calcium in plants. Annals of Botany 92, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Thomas BJ, Farrar JF, Pollock CJ. 1993. Visualizing the distribution of elements within barley leaves by energy dispersive X-ray image maps (EDX maps). New Phytologist 125, 367–372. [DOI] [PubMed] [Google Scholar]
- Zemunik G, Turner BL, Lambers H, Laliberté E. 2015. Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nature Plants 1, 15050. [Google Scholar]
- Zhao FJ, Shen ZG, McGrath SP. 1998. Solubility of zinc and interactions between zinc and phosphorus in the hyperaccumulator Thlaspi caerulescens. Plant, Cell & Environment 21, 108–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.