Abstract
While antipsychotic medications provide important relief from debilitating psychotic symptoms, they also have significant adverse side effects, which might have relevant impact on human health. Several research studies, including ours, have shown that commonly used antipsychotics such as haloperidol and aripiprazole affect cholesterol biosynthesis at the conversion of 7-dehydrocholesterol (7-DHC) to cholesterol. This transformation is promoted by the enzyme DHCR7 and its inhibition causes increases in plasma and tissue levels of 7-DHC. The inhibition of this enzymatic step by mutations in the Dhcr7 gene leads to Smith-Lemli-Opitz syndrome, a devastating human condition that can be replicated in rats by small molecule inhibitors of DHCR7. The fact that two compounds, brexpiprazole and cariprazine, that were recently approved by the FDA have substructural elements in common with the DHCR7 inhibitor aripiprazole, prompted us to evaluate the effect of brexpiprazole and cariprazine on cholesterol biosynthesis. We report that cariprazine affects levels of 7-DHC and cholesterol in cell culture incubations at concentrations as low as 5 nM. Furthermore, a common metabolite of cariprazine and aripiprazole, 2,3-(dichlorophenyl) piperazine, inhibits DHCR7 activity at concentrations comparable to those of the potent teratogen AY9944. The cell culture experiments were corroborated in mice in studies showing that treatment with cariprazine elevated 7-DHC in brain and serum. The consequences of sterol inhibition by anti-psychotics in the developing nervous system and the safety of their use during pregnancy remains to be established.
Keywords: Antipyschotics, Teratogen, Cholesterol biosynthesis, Neurodevelopment
1. Introduction
Cholesterol is of critical importance in the brain during development and disruptions in its biosynthesis (Nes, 2011) have been associated with multiple disorders. Smith-Lemli-Opitz Syndrome (SLOS) is one such neurodevelopmental condition that arises from mutations in 7-dehydrocholesterol reductase (DHCR7),(Smith et al., 1964; Kelley and Hennekam, 2000; Dietschy and Turley, 2001; Dietschy and Turley, 2004; Witsch-Baumgartner et al., 2008; Porter and Herman, 2011; Kanungo et al., 2013) the enzyme that converts 7-dehydrocholesterol (7-DHC) to cholesterol, the last step in the complex biosynthesis. Scheme 1 shows the last steps in the pathway promoted by two reductase enzymes, DHCR7 that operates on the sterol B-ring double bond and DHCR24 that acts on the tail of the sterol at C24. SLOS is characterized biochemically by an increase of 7-DHC and a decrease of cholesterol in tissues and fluids.
Scheme 1.
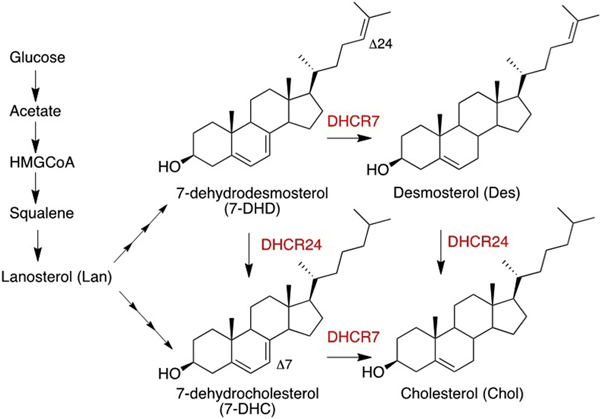
Sterol Structures and Reductase Enzymes in the Last Steps of Cholesterol Biosynthesis. The reductase enzymes, shown in red, act on the Δ7 and Δ24 double bonds.
A number of small molecules, including some widely prescribed pharmaceuticals, modulate the activity of DHCR7, increasing cellular levels of 7-DHC and reducing cholesterol levels in a way that parallels the perturbations of sterol homeostasis found in SLOS patients (Giera et al., 2007, 2008, Sanchez-Wandelmer et al., 2010, Canfran-Duque et al., 2013, Hall et al., 2013, Boland and Tatonetti, 2016, Korade et al., 2016, Korade et al., 2017a,b). Given the parallel cellular biochemistry found in SLOS and in human exposures to DHCR7 inhibitors, there has been increased interest in describing the set of DHCR7-active compounds(Horling et al., 2012) that constitute potential exposure threats during neurodevelopment.
We have recently used Neuro2a cells to screen an NIH clinical collection of small molecules and found that some 5% of the compounds in the library increased 7-DHC levels by inhibiting DHCR7 (Kim et al., 2016). Among the most active compounds identified in that study were the psychotropic medications haloperidol, aripiprazole and trazodone. In another report, Korade and co-workers reported elevated levels of 7-DHC and 8-dehydrocholesterol (8-DHC) in the blood of patients using these same drugs (Korade et al., 2017a, 2017b). Hall and co-workers also reported elevated levels of 7-DHC in 22 individuals who had not been diagnosed with SLOS, but were on treatment with either aripiprazole or trazodone (Hall et al., 2013).
Compounds that modulate the DHCR7 enzyme have been a particular focus of a recent review that examined the effects of different drugs on the cholesterol biosynthesis pathway (Boland and Tatonetti, 2016). The authors of this paper suggested that a higher incidence of fetal malformations, spontaneous abortions and intrauterine death was associated with the use of several DHCR7 inhibitors taken during the first trimester of pregnancy, a critical period when a decrease in cholesterol has been linked to teratogenicity. As a conclusion, the authors proposed that the use of such pharmaceuticals during pregnancy could lead to what is essentially a chemically induced SLOS, a disorder with similar negative outcomes whose hallmark is the accumulation of 7-DHC (Boland and Tatonetti, 2016).
With the increased use of atypical antipsychotics in recent years, new drugs have been approved by the FDA that bear a striking structural resemblance to aripiprazole, a highly-prescribed drug and potent inhibitor of DHCR7. Two such compounds, brexpiprazole and cariprazine appeared in 2015 and both of these drugs have sub-structures that are identical to elements present in aripiprazole, see Fig. 1. Because of this close structural relationship and the suggestion that DHCR7 inhibitors such as aripiprazole may have a negative effect on fetal outcomes, we have assessed the potency of brexpiprazole and cariprazine on cholesterol biosynthesis. We report here the results of this study and we include here a comparison of the effects on sterol homeostasis of these two new antipsychotic medications with the effect of AY9944, a teratogen known to inhibit DHCR7 (Roux et al., 1980). Indeed, AY9944 is a notable molecule for comparison since it has been used to generate a pharmacological rat model of SLOS (Kolf-Clauw et al., 1996, Fliesler et al., 2004, Fliesler and Bretillon, 2010, Xu et al., 2011a,b). Finally, we tested the ability of cariprazine to alter cholesterol synthesis in mice. Sterol levels were measured in blood and brain in order to confirm the in vitro effect and to establish a link between drug treatment and 7-DHC levels.
Fig. 1.
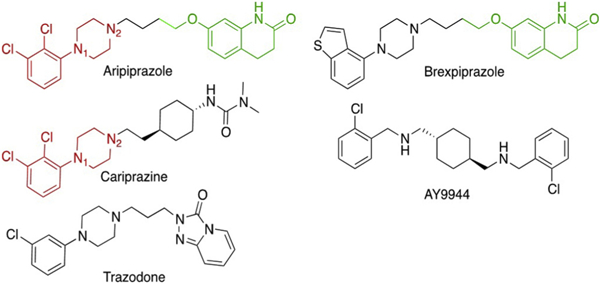
Structures of the Compounds Investigated. Common substructure features of aripiprazole and cariprazine are shown in red, those of aripiprazole and brexpiprazole are shown in green. AY9944 is a teratogenic compound used to generated a pharmacological rodent model for Smith-Lemli-Opitz syndrome, trazodone is a commonly prescribed psychoactive piperazine. The piperazine nitrogens are labeled N1 and N2 for purposes of discussion.
2. Experimental section
2.1. Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO). HPLC grade solvents were purchased from Thermo Fisher Scientific Inc. (Waltham, MA). All cell culture reagents were from Mediatech (Manassas, VA), Life Technologies (Grand Island, NY), and Greiner Bio-One GmBH (Monroe, NC). Cariprazine (marketed as VRAYLAR in the US and REAGILA in Europe), Brexpiprazole (marketed as REXULTI), Aripiprazole (marketed as ABILIFY) and AY9944 were dissolved in DMSO for the experiments. Ergosterol was purchased from TCI America. All sterol standards, natural and isotopically labeled, used in this study are available from Kerafast, Inc. (Boston MA). D-13C6-glucose was purchased from Cambridge Isotope Laboratories, Inc. Delipidated fetal bovine serum was prepared as described previously and did not have detectable cholesterol levels (Gibson et al., 1990).
2.2. Cell cultures
The neuroblastoma cell line Neuro2a was purchased from American Type Culture Collection (Rockville, MD). The A549 human lung carcinoma cell line was obtained from the European Collection of Authenticated Cell Cultures (ECACC). Control and SLOS fibroblasts were described previously (Korade et al., 2016; Korade et al., 2017a,b). All cultured control human fibroblasts used were passages of 5–18. All cells were subcultured once a week, and the culture medium was changed every two days. All cell lines were maintained in DMEM with high glucose (25 mM), 1mM pyruvate, 1 mM L-glutamine, 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT), and penicillin/streptomycin at 37 °C and 5% CO2(medium 1). For the drug exposure experiments, human fibroblasts were cultured in DMEM with 10 mM 13C6-glucose, 1 mM L-glutamine, 10% delipidated fetal bovine serum (FBS) and penicillin/streptomycin (medium 2). Neuro2a and A549 cells were cultured in DMEM with 10 mM 13C6-glucose, 1 mM L-glutamine, N2-supplement and penicillin/streptomycin.
2.3. Cell culture incubations
Control human fibroblasts and Neuro2a cells were deposited in a 96-well plate at 10,000 cells per well in medium 1. A549 cells were deposited in a 96-well plate at 5000 cells per well in medium 1. After 24 h, medium was replaced with medium 2 in the presence and absence of drugs (AY9944, aripiprazole, brexpiprazole and cariprazine). Medium was replaced every 2 days. Experiments using Neuro2a and A549 cells were carried out for 48 h and experiments using human fibroblasts were carried out for 7 days. At the end point of the incubation, 10 μL of Hoechst dye (40 ng/μL) (Molecular Probes) was added to each well. The cells were incubated at 37 °C for 30 min in the dark and imaged using an ImageXpress Micro XL (Molecular Devices, Sunnyvale, CA) with a 10× objective. After removing the medium, 200 μL of MeOH containing internal standards was added. For experiments done with 13C6-glucose we used 0.5 nmol of ergosterol as the internal standard and for experiments done with regular glucose we used a cocktail containing isotopic labeled sterols as reported previously (d7-cholesterol, d7–7-DHC, 13C3-desmosterol and 13C3-lanosterol) (Korade et al., 2017a,b). The plate was placed on an Orbital shaker for 30 min at room temperature and centrifuged for 10 min using a Sorvall swing rotor. An aliquot (80 μL) of the supernatant was transferred to a PTAD-pre-deposited plate, sealed with Easy Pierce Heat Sealing Foil followed by 30 min agitation at room temperature, and analyzed by LC-MS/MS (as described in a following section). The remaining sample in each well (~ 120 μL) was transferred to vials and dried on a SpeedVac concentrator for GC/MS analysis. To each vial was added N,O-bis(trimethylsilyl)tri-fluoroacetamide (BSTFA, 20 μL), the sample was vortexed, and allowed to react for 30 min, followed by GC–MS analysis as described in a separate section.
2.4. Mouse experiments
Adult male and female C57Bl/6 mice (Jackson Laboratories) were used for study. The mice were housed in groups of 2 mice per cage under a 12-h light-dark cycle at constant temperature (25 °C) and humidity with ad libitum access to food (Teklad LM-485 Mouse/Rat Sterilizable (#7012) chow) and water in Comparative Medicine at the UNMC, Omaha, NE. All mice were age and weight matched. Five mice (9 months old) received 7 daily injections of cariprazine (0.2 mg/kg; i/p) and five mice (9 months old) received saline. Tissue samples were collected from mice 24 h after the last injection. Trunk blood was collected in BD Microtainer Serum Separator Tube. After 30 min incubation at RT in dark, the tube was spun in table top centrifuge at 6000 g for 90 s and serum collected in separate tube. Samples were frozen and kept at – 80 °C until sterol extraction. Cortex was dissected, frozen in prechilled methyl butane on dry ice, and stored at –80 °C. Half of the cortex was used for sterol analysis and the other half was used for total RNA extraction and qPCR (see supporting information for experimental details and results). Ice-cold lysis buffer (120 mM NaCl, 50 mM HEPES, 1% Igepal) was added to frozen cortex samples and immediately sonicated. The protein was measured using BCA assay (Pierce). The sterol and drug was extracted using a protocol adapted from Xu et al (Xu et al., 2011a,b). and cariprazine was analyzed by LC-MS/MS by the use of the same procedures reported for analysis of aripiprazole (Caloro et al., 2012). All procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals. The use of mice in this study was approved by the Institutional Animal Care and Use Committee of UNMC.
2.5. LC-MS/MS analyses for 13C-sterol profiling
The replacement of natural glucose by 13C6-glucose in the medium provided labeled carbons to be used in the biosynthesis of fresh sterols (Scheme 1). In order to obtain the full isotopic distribution of 13C-labeled sterols in cells we analyzed several human fibroblasts samples by LC/MS/MS in the full scan mode. Samples were injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 mm × 50 mm) with 100% MeOH (0.1% v/v acetic acid) mobile phase for 1.2 min runtime at a flow rate of 500 μL/min. Selective reaction monitoring (SRM) transitions were then set for each possible 7-DHC isotope (13C1-7-DHC, 13C2-7-DHC, 13C3-7-DHC and so on) and the area of each peak was obtained. Once the 13C-sterol profiling was determined, we selected one isotope (13C4-7-DHC, SRM transition 564 → 369) to be used in the analyses and calculated a response factor to account for the sum of every possible isotope, and hence the total amount of isotopically labeled sterol formed. This correction factor was used for every sterol analysis reported in this study.
2.6. LC-MS/MS (SRM) analyses
The sealed plates were placed in an Acquity UPLC system equipped with ANSI-compliant well plate holder coupled to a Thermo Scientific TSQ Quantum mass spectrometer equipped with an APCI source. Then 10 μL was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1mm × 50 mm) with 100% MeOH (0.1% v/v acetic acid) mobile phase for 1.2 min runtime at a flow rate of 500 μL/min. Natural sterols were analyzed by selective reaction monitoring (SRM) using the following transitions: Chol 369 → 369, 7-DHC 560 → 365, desmosterol 592 → 560, lanosterol 634 → 602, with retention times of 0.9, 0.4, 0.3 and 0.3 min, respectively. 13C-labeled sterols were analyzed by selective reaction monitoring (SRM) using the following transitions: 13C-Chol 373 → 373, 13C-7-DHC 564 → 369, 13C-desmosterol 596 → 564, 13C-lanosterol 638 → 606. SRM for the internal standard (ergosterol) was set to 572 → 377. Final sterol numbers are reported as nmol/million cells.
Cariprazine levels were acquired in an Acquity UPLC system coupled to a Thermo Scientific TSQ Quantum mass spectrometer using an ESI source in the positive ion mode. 10 μL of each sample was injected onto the column (Phenomenex Luna Omega C18, 1.6 μm, 100 Å, 2.1 mm × 50 mm) using water (0.1% v/v acetic acid) (solvent A) and acetonitrile (0.1% v/v acetic acid) (solvent B) as mobile phase. The gradient was: 10% to 40% B for 0.5 min; 40% to 95% B for 0.4 min; 95% B for 1.5 min; 95% to 10% B for 0.1 min; 10% B for 0.5 min. Cariprazine was analyzed by selective reaction monitoring (SRM) using the transition 427 → 188. The SRM for the internal standard (d8-aripiprazole) was set to 456 → 293. Final drug levels are reported as ng/mg of protein.
2.7. GC–MS analyses
After the BSTFA addition to each sample, 5 μL was injected onto the column (SPB-5, 0.25 μm, 0.32 mm × 30 m) with a temperature program of 220–300° (5 min) at 20°/min and helium flow rate of 2.0 mL/min. Sterols were analyzed by extracted ion chromatogram (EIC) using the following values: Chol (m/z 458), d7-Chol (m/z 465) and 7-DHD (m/z 349). Final sterol numbers were calculated using d7-Chol as the internal standard. Response factors were calculated for each sterol and the values used to obtain the final amount.
2.8. Residual cholesterol synthesis
Residual cholesterol synthesis was calculated as described previously (Wassif et al., 2005; Korade et al., 2017a,b). Briefly, after determining the “absolute” amount of 13C-cholesterol and 13C-7-DHC in nmol/million cells we used the following equation: [13C-cholesterol/(I3C-7-DHC + 13C-cholesterol)]. For efficient cholesterol biosynthesis, this ratio should be close to 1. Values lower than that suggest a dysfunction in the DHCR7 enzyme.
3. Results
3.1. Cariprazine and AY9944 increase 7-DHC and 7-DHD levels in cultured Neuro2a cells
The effect of dozens of small molecules on sterol metabolism has been evaluated(Kim et al., 2016; Korade et al., 2016) in Neuro2a cells, a mouse neuroblastoma cell line that has proven to be valuable for this purpose. For exploratory comparison experiments, aripiprazole, AY9944, brexpiprazole and cariprazine were incubated for two days at 50 nM in cultured Neuro2a and levels of cholesterol (Fig. 2A) and 7-DHC (Fig. 2B) were determined. At the end of the experiment, Hoechst dye was added and the total number of cells was counted in a Molecular Device plate reader. The treatment with all of the concentrations tested did not affect the total number of cells in culture (data not shown). Neuro2a cholesterol (nmol/million cells) was not affected by incubation with either aripiprazole or brexpiprazole, but levels were significantly decreased upon incubation of cells with either cariprazine or AY9944 (Fig. 2A). 7-DHC levels, on the other hand, were significantly elevated after incubation of Neuro2a with all four compounds (Fig. 2B), brexpiprazole having a small but measurable effect on 7-DHC while cariprazine and AY9944 treatments resulted in 7-DHC levels that were higher than those of cholesterol. These results suggest that such compounds impair cholesterol biosynthesis by inhibiting the DHCR7 enzyme.
Fig. 2.
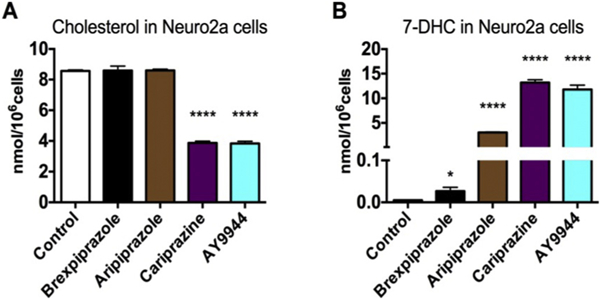
Cariprazine decreases cholesterol (A) and increases 7-DHC (B) in Neuro2a cells. The effects of cariprazine and brexpiprazole on sterol synthesis were compared to other compounds that inhibit DHCR7. 50 nM of each compound was added to the cell culture medium and cells were exposed in lipid-deficient medium for 48 h. *p < 0.05 and ****p < 0.001 relative to the control conditions.
Evaluation of the dose dependence on sterol homeostasis in Neuro2a for both cariprazine and AY9944 indicated a modest decrease in cholesterol levels for concentrations of both compounds up to 10 nM but concentrations of these compounds above 1 nM gave a significant increase in both 7-DHC and 7-DHD, see Table 1. In addition, these compounds led to a modest dose-dependent decrease in desmosterol, with AY9944 affecting these levels at 100 pM and cariprazine at concentrations in excess of 1 nM. Our conclusion is that cariprazine and AY9944 have qualitatively similar effects on sterol homeostasis in Neuro2a cells, where they act principally by inhibiting the DHCR7 enzyme.
Table 1.
Comparison of the Effects of Cariprazine and AY9944 on Sterol Levels in Neuro2a.a
| Cariprazine (nM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cariprazine | 0 (DMSO) | 0.1 | 0.5 | 1.0 | 5.0 | 10.0 | 25.0 | 50.0 |
| Cholesterol | 6.40 ± 0.39 | 7.26 ± 0.34 | 6.14 ± 0.15 | 6.05 ± 0.52 | 5.58 ± 0.41 | 3.69 ± 0.39 | 3.58 ± 0.31 | 3.62 ± 0.58 |
| 7-DHC | 0.07 ± 0.01 | 0.12 ± 0.02 | 0.19 ± 0.03 | 1.31 ± 0.14 | 4.80 ± 0.97 | 8.01 ± 0.89 | 7.41 0.60 | 7.68 ± 0.61 |
| 7-DHD | 0.13 ± 0.02 | 0.16 ± 0.04 | 0.25 ± 0.01 | 2.54 ± 0.51 | 7.50 ± 1.20 | 8.50 ± 0.41 | 8.29 ± 0.77 | 9.05 ± 0.80 |
| Desmosterol | 5.09 ± 0.60 | 5.22 ± 0.14 | 4.54 ± 0.07 | 3.50 ± 0.16 | 2.66 ± 0.55 | 1.57 ± 0.12 | 1.33 ± 0.09 | 1.51 ± 0.14 |
| Lanosterol | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| AY9944 (nM) | ||||||||
| AY9944 | 0 (DMSO) | 0.1 | 0.5 | 1.0 | 5.0 | l0.0 | 25.0 | 50.0 |
| Cholesterol | 6.40 ± 0.39 | 4.44 ± 0.35 | 4.32 ± 0.31 | 4.79 ± 0.50 | 4.51 ± 0.07 | 4.07 ± 1.10 | 3.33 ± 0.07 | 3.56 ± 0.23 |
| 7-DHC | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.05 | 1.09 ± 0.14 | 3.72 ± 0.63 | 8.48 ± 0.49 | 7.96 ± 1.86 | 9.35 ± 0.57 |
| 7-DHD | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.03 | 0.80 ± 0.12 | 4.35 ± 0.57 | 6.02 ± 0.58 | 6.05 ± 0.38 | 7.54 ± 0.44 |
| Desmosterol | 5.09 ± 0.60 | 1.12 ± 0.11 | 1.24 ± 0.10 | 1.30 ± 0.18 | 1.67 ± 0.16 | 1.49 ± 0.05 | 1.41 ± 0.03 | 1.56 ± 0.14 |
| Lanosterol | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 |
Neuro2a cells were incubated for 48h in sterol free medium in the absence or presence of different nM concentrations of cariprazine and AY9944. Sterol values correspond to the mean ± SE of 3 replicates in nmol/million cells. Numbers marked in bold and italics are significantly different from controls.
The DHCR7 enzyme catalyzes the reduction of 7-DHC and 7-DHD into cholesterol and desmosterol, respectively. An inhibition of this enzyme should result in an increase in both the 7-DHC/cholesterol and 7-DHD/desmosterol ratios and Fig. 3 shows that these ratios do increase as a function of either cariprazine or AY9944 concentrations, further suggesting an inhibition at the DHCR7 step. The LC50 values found are in the range of 3–5 nM, highlighting the potency of such drugs on the sterols involved in the ultimate steps of cholesterol biosynthesis.
Fig. 3.
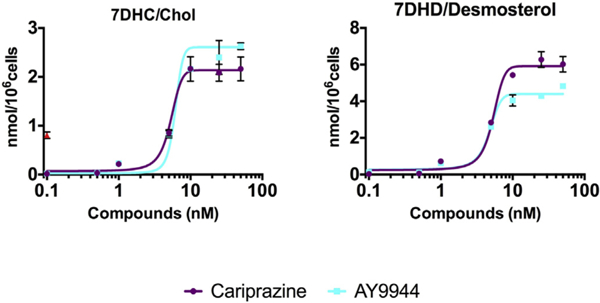
Cariprazine and AY9944 are potent inhibitors of the DHCR7 enzyme. Both compounds increase 7-DHC/cholesterol and 7-DHC/Desmosterol ratios in cultured Neuro2a cells.
3.2. Cariprazine and AY9944 inhibit de novo cholesterol synthesis in human fibroblasts
In order to further elucidate the mechanism of action of these compounds, their inhibitory effects on de novo cholesterol synthesis was investigated by replacing 12C6-glucose in the incubation medium with 13C6-glucose. Cultured human fibroblasts take up the labeled carbohydrate and convert it to acetate and, subsequently, 13C-labeled 7-DHC and cholesterol (Fig. 4A). Introduction of 13C-labeled glucose at the same time as the antipsychotic medications permitted an evaluation of only those sterols biosynthesized when drug was present, i.e. the 13C-labeled sterols. While 13C-labeled acetate has precedent for studies in sterol biosynthesis,(Giera et al., 2007) the use of 13C-labeled glucose permits replication of our normal incubation conditions without modification by the addition of acetate, be it either 13C or 2H-modified (Wassif et al., 2005; Giera et al., 2007).
Fig. 4.
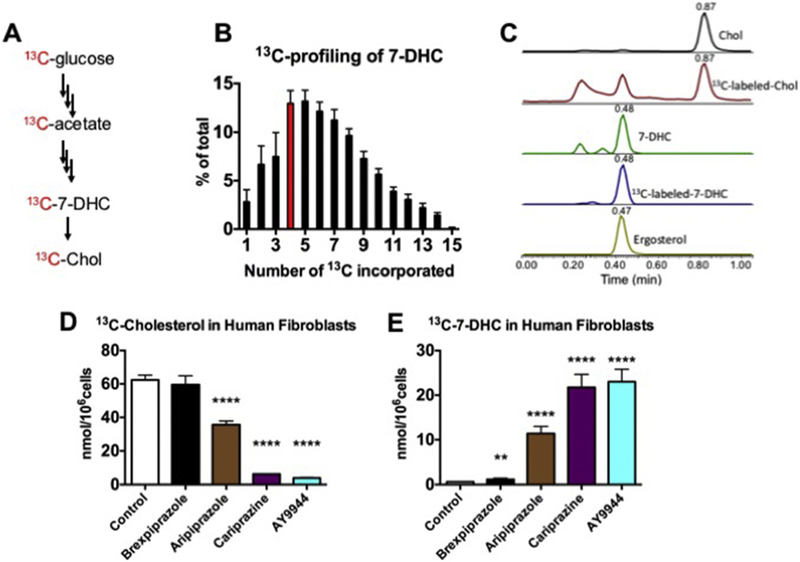
Antipsychotics affect sterol biosynthesis in cultured human fibroblasts. Panel A shows a simplified scheme illustrating the conversion of 13C-glucose to 13C-cholesterol. Panel B shows the distribution of 13C in de novo synthesized 7-DHC; highlighted in red is the m/z (M + 4) species that was chosen to monitor the labeled 7-DHC levels in cells. Panel C shows a typical chromatogram of natural and 13C-labeled sterols and the internal standard. Ergosterol. Panel D shows the effects of 50 nM brexpiprazole, aripiprazole, cariprazine and AY9944 on 13C-cholesterol formed over 7 days in control human fibroblasts. Panel E shows13C-7DHC formed in the experiments described in Panel D.
This labeling approach provides 13C that can be incorporated at any of the 27 atoms in the cholesterol molecule. The mass spectrometry analysis of sterols makes use of an SRM protocol that scans for all possible m/z transitions for the incorporation of 1 to 27 13C isotopes in the 7-DHC and cholesterol molecules (see the Experimental Section for details). Of those 27 possibilities, we detected up to 15 isotopic labeled carbons incorporated in the sterol backbone after 7 days of incubation of fibroblasts in the presence of 13C6-glucose (Fig. 4B). For these experiments, ergosterol was used as a standard added during workup since it forms PTAD adducts in chemistry that parallels that of 7-DHC. A typical chromatogram of the analysis of unlabeled and 13C-labeled sterols as well as the internal standard is shown in Fig. 4C. With the 13C-profiling determined (Fig. 4B), we selected the signal for the SRM corresponding to M + 4 highlighted in red in the Figure and calculated a response factor to account for sterol having all incorporations of 13C up to 15 atoms.
The effect of 50 nM aripiprazole, brexpiprazole, cariprazine and AY9944 on de novo biosynthesis of cholesterol and 7-DHC in human fibroblasts is shown in Fig. 4D and E. Brexpiprazole did not appreciably change levels of 13C-labeled cholesterol measured but a small, statistically significant increase in 13C-labeled 7-DHC was observed in treatments with this compound. Of the compounds tested, cariprazine and AY9944 had the greatest effect on the formation of both 13C-labeled cholesterol and 13C-labeled 7-DHC, an almost complete inhibition of cholesterol production was observed for both compounds at the concentrations tested (50 nM), with levels of 13C-labeled 7-DHC exceeding those of labeled cholesterol under the conditions of these experiments. Aripiprazole’s effect on sterol biosynthesis was greater than that of brexpiprazole but it did not appear to be as potent an inhibitor of DHCR7 as either cariprazine or AY9944. The 13C-glucose labeling approach was also applied to studies in the A549 cell line, a comparison of the effects of all compounds on the formation of labeled sterols in these cells is presented in Supporting Information, Fig. S2. The potency order on sterol homeostasis in A549 cells was similar to that determined in Neuro2a and human fibroblasts: AY9944 ~ cariprazine > aripiprazole > brexpiprazole.
Determination of de novo sterol biosynthesis of 7-DHC and cholesterol by the use of the 13C-label permitted the assessment of the Residual Cholesterol Synthesis (RCS), a parameter that has proven to be useful in the evaluation of the efficiency of the transformation of 7-DHC to cholesterol in human fibroblasts (Wassif et al., 2005). RCS is defined as 13C-cholesterol/(13C-7-DHC + 13C-cholesterol), the fraction of 7-DHC that is converted to cholesterol during de novo synthesis. For control fibroblasts, RCS is close to 1.0 while the value of this parameter can be as low as 0.02 in fibroblasts from a severely affected individual with SLOS or as high as 0.6 in a mildly affected individual.
Since cariprazine and AY9944 were the most potent compounds we assayed in Neuro2a, A549 and human fibroblasts, the scope of study of these compounds was expanded to include an evaluation of the dose dependency of de novo cholesterol synthesis of human fibroblasts and an assessment of the RCS dose-response. Fig. 5 presents the dose dependent effect of AY9944 and cariprazine on RCS by AY9944 and cariprazine from 0.1 to 50 nM in human fibroblasts. As was the case for the dose response for AY9944 and cariprazine on endogenous sterols in Neuro2a cells, the LC50 values found for RCS in human fibroblasts were in the 3–5 nM range, both compounds are potent inhibitors of human DHCR7. As a comparison, we determined the RCS for one SLOS fibroblast cell line (RCS 0.10) using this method. The levels of 13C-sterols determined (in nmol/million cells) for these incubations are presented in Supporting Informations.
Fig. 5.
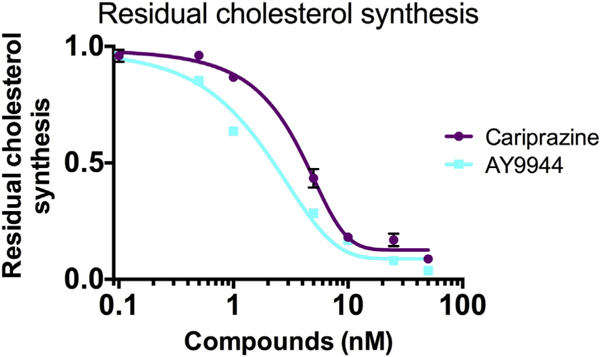
Cariprazine and AY9944 affect the residual cholesterol synthesis (RCS) of control human fibroblasts in a dose-dependent manner. At 5–10 nM of both compounds, the RCS values determined in control cells are comparable to those of values determined for several SLOS cell lines, see Reference 28.
3.3. 2,3-Dichlorophenyl-piperazine is a potent inhibitor of the DHCR7 enzyme
In contrast to cariprazine, brexpiprazole showed a minimal but measurable inhibitory effect on the DHCR7 enzyme. In point of fact, comparison of the chemical structures of the compounds provides clues about the source of DHCR7 activity in the compounds. Aripiprazole, brexpiprazole and cariprazine each have piperazines linked to an aromatic substructure on one nitrogen, label N1 in Fig. 2 with an alkyl linker on N2. Aripiprazole and cariprazine have a common N1 2,3-di-chlorophenyl substituent, shown in red in the Figure, that appears to be the decisive active substructure of the molecules. Brexpiprazole and aripiprazole, on the other hand, have identical N2 substitution (shown in green in Fig. 2), a dihydroquinolin-2-one moiety that apparently contributes little to the overall DHCR7 inhibitory activity.
To explore structure-activity relationships further, 1-(2,3-di-chlorophenyl) piperazine (2,3-DCPP), the core substructure present in aripiprazole and cariprazine, was assayed for its effect on sterol bio-synthesis in Neuro2a cells. As shown in Figs. 6, 2, 3-DCPP shows activity that is comparable to cariprazine, with substantial increases in 7-DHC levels observed at concentrations as low as 5 nM. In contrast m-CPP, the core arylpiperazine structure in trazodone (see Fig. 1), has essentially no effect on sterol levels in Neuro2a cells at the concentrations tested. Trazodone is a highly prescribe psychoactive drug that leads to increased levels of 7-DHC in cell culture and in plasmas of individuals taking this medication (Hall et al., 2013; Kim et al., 2016). The effect of aripiprazole and trazodone on 7-DHC levels in cell culture parallels the effect of their core piperazine substructures, aripiprazole and its core substructure 2,3-DCPP are more potent than trazodone and m-CPP. The activity differences between the meta-chloro and 2,3-di-chlorophenyl piperazines are not trivially explained by the presence of an ortho chloro substituent in aripiprazole and 2,3-DCPP, since ortho-chlorophenyl and ortho-fluorophenyl piperazines do not affect 7-DHC levels in Neuro2a at concentrations up to 50 nM (data not shown).
Fig. 6.
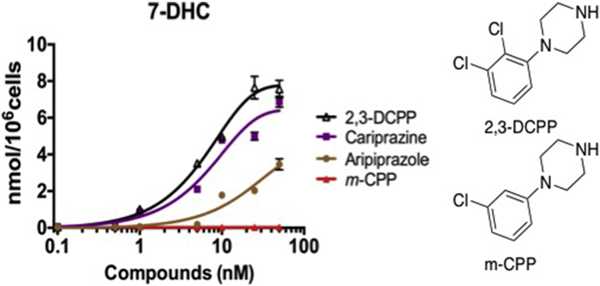
Chloropiperazine Inhibitors of DHCR7. Assays for 7-DHC in Neuro2a. Cells were incubated with each compound in lipid-deficient medium for 48 h.
3.4. Cariprazine treatment of mice alters cholesterol biosynthesis
Cariprazine is approved for the treatment of schizophrenia and acute manic or mixed episodes associated with bipolar I disorder (Agai-Csongor et al., 2012; Veselinovic et al., 2013; Findlay et al., 2017) It is a dopamine D2/D3 receptor partial agonist(McCormick et al., 2010; Kiss et al., 2012; Ellenbroek and Cesura, 2015) and in a mouse model of depression, it produced antidepressant-like activity (Duric et al., 2017).We tested if cariprazine, used in a dose previously reported in mice and equivalent to a typical human dose, has any effect on sterol levels in the brain and serum. After seven daily injections of cariprazine and 24 h after the last injection, samples of the blood and brain were collected for analyses. Sterols were measured in the cortex (Fig. 7A–C) and compared to vehicle treated mice, the cariprazine treated mice had greatly elevated levels of 7-DHC and 8-DHC. Desmosterol levels were found to be decreased in cariprazine treated mice compared to controls both in cortex and serum, all serum data is presented in Supporting Information. In addition to sterols, samples were analyzed for cariprazine. While the drug was not detected in vehicle treated mice, it was present in the brain tissue from drug treated mice (Fig. 7D).
Fig. 7.
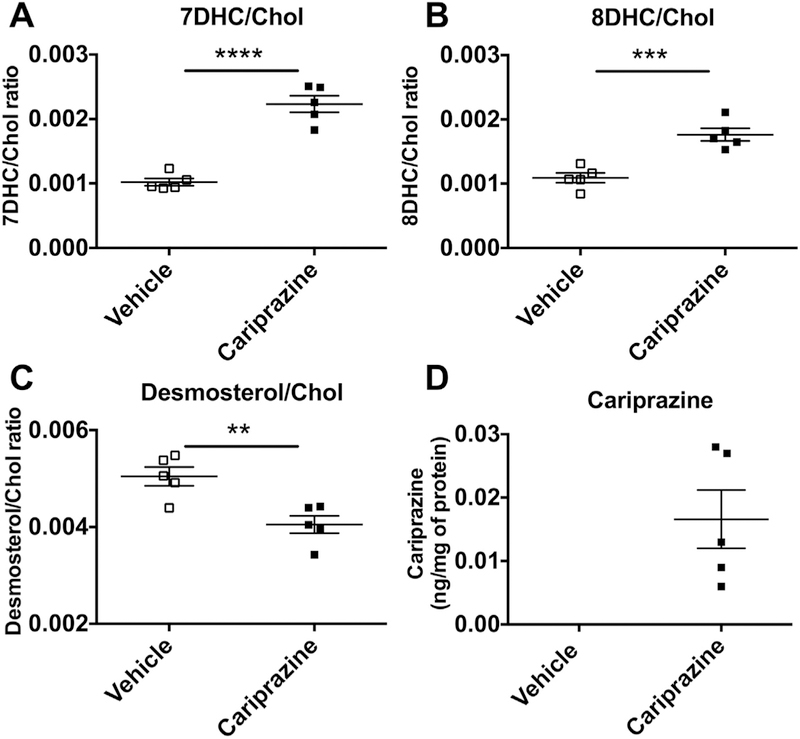
Cariprazine affects sterol levels in mouse brain and serum. 7-DHC, 8-DHC and desmosterol levels were altered in the brain tissue of mice injected with cariprazine (panels A-C). Cariprazine was found in the brain of mice injected with the drug (panel D). Cholesterol levels correspond to: 333.6 ± 17.3 nmol/million cells (vehicle) and 313.9 ± 12.3 nmol/million cells (cariprazine).
4. Discussion
Mutations in the gene that encodes the DHCR7 enzyme are associated with the disorder SLOS, which is characterized biochemically by elevated levels of 7-DHC and lower than normal levels of cholesterol. Protein adducts of 7-DHC-derived oxysterols have been shown to be elevated in cellular models of SLOS(Windsor et al., 2013) and the oxysterols themselves are toxic to cells (Korade et al., 2010). A recent report indicates that 7-DHC derived oxysterols inhibit the Hedgehog component Smoothened, providing a rationale for diminished Hedgehog pathway activity in SLOS (Sever et al., 2016). What seems clear from many of the recent studies is that 7-DHC and its oxidation products are likely to be important mediators of SLOS pathophysiology (Xu et al., 2010, 2011a,b, 2013).
The importance of 7-DHC and its derived oxysterols in SLOS suggests that other conditions that lead to elevated levels of this sterol may pose neurodevelopmental threats. Human exposures to small molecule inhibitors of DHCR7 are of concern, particularly during pregnancy, since inhibition of this enzyme causes elevations in 7-DHC. Indeed, a pharmacological rodent model of SLOS that has been used extensively relies on the exposure of pregnant rats to controlled doses of AY9944 during fetal development followed by post-natal exposure of the pups to this compound (Fliesler and Bretillon, 2010). The data presented here confirm the effect of AY9944 on DHCR7 in Neuro2a cells and they show a similar effect on sterol levels in A549 cells and human fibroblasts. In our experiments we used a low dose of AY9944 (nM) since it has been shown previously that, in addition to DHCR7, high doses of AY9944 inhibit other enzymes in the cholesterol biosynthesis pathway (Giera et al., 2007, 2008). At concentrations used in the current study there was no effect of AY9944 on Δ7Δ8 Isomerase (not shown).
We conclude from the results shown in Figs. 2–5 that cariprazine has an effect on sterol levels in each of the cultured cells examined that approaches that of AY9944, the teratogen used in the chemically-induced SLOS model. This seems particularly noteworthy since the increase in endogenous levels of 7-DHC is the core biochemical perturbation found in SLOS and the AY9944 SLOS model. The levels of 7-DHD found in Neuro2a cells were comparable to those of 7-DHC after treatment with either cariprazine or AY9944 and the efficacy of cariprazine on DHCR7 is essentially equivalent to AY9944, as shown in Fig. 3 where the ratio of 7-DHC/cholesterol and 7-DHD/desmosterol indicate comparable activities. While 7-DHD and its derived oxysterols have not been the focus of studies as extensive as the examinations of 7-DHC and its oxysterols, it seems likely that 7-DHD will be highly reactive and its oxidized derivatives will have activities that are of comparable importance (Xu et al., 2009; Lamberson et al., 2017).
Wassif and collaborators determined the residual cholesterol synthesis (RCS) values in fibroblasts from SLOS patients by carrying out incubations of these cells in deuterated water and measuring the relative yield of deuterated 7-DHC and cholesterol formed (Wassif et al., 2005). RCS values (labeled Chol/(labeled Chol + labeled 7-DHC)) determined by this method were close to 1.0 for control fibroblasts, in the range of 0.95 to 0.99 for parents of SLOS patients who were carriers of a SLOS mutation and 0.65 to 0.02 for SLOS patients (from mild to severe cases). We modified the RCS method, using 13C-labeled glucose as the source of isotope, and RCS values that we determined by this approach were comparable to those reported by Wassif for fibroblasts from control, SLOS carriers and SLOS donors. In point of fact, the RCS method seems particularly well-suited for studies of inhibitors of cholesterol biosynthesis since the determination of levels of isotopically labeled sterols can be used to assay only that biosynthesis that occurs when inhibitor is present.
Fig. 5 shows that 5–10 nM of either cariprazine or AY9944 induces a sterol profile and RCS value similar to a severe SLOS cell line. Thus, incubation of a control fibroblast with 10 nM cariprazine or AY9944 yields an RCS of < 0.2 for biosynthesis in the presence of the compound. An RCS of 0.2 corresponds to a value found in a fibroblast from a SLOS individual who would be classified in the moderate to severe category (Wassif et al., 2005). According to VRAYLAR’s prescribing information (www.accessdata.fda.gov), plasma levels of cariprazine or its active metabolites can reach 120 nM, a concentration that has a consequential effect on cellular sterol levels and RCS. Furthermore, a link was established between these cell culture experiments and an animal study in which mice were treated i.p. with the drug. The cell culture studies clearly translate in the in vivo model.
Antipsychotics like aripiprazole, cariprazine and trazodone are cleared primarily by hepatic metabolism, one pathway being CYP3A4 N-dealkylation of the drugs leading to 2,3-DCPP and m-CPP. The increased potency of the metabolite 2,3-DCPP compared to the parent aripiprazole is noteworthy, given the fact that 2,3-DCPP can constitute up to 40% of the parent drug steady-state plasma concentrations in rat brain (Caccia, 2011). While there is no information about the in vivo formation of 2,3-DCPP from cariprazine, its formation by N-deal-kylation seems probable. In addition, the principal cariprazine metabolites that have been identified, the N-desmethyl and di-desmethyl derivatives, seem likely to be potent DHCR7 inhibitors themselves.
A recent review showed that pharmaceuticals known to modulate sterol levels (especially 7-DHC and cholesterol) can have dramatic effects in fetal development, leading to a wide spectrum of negative pregnancy outcomes (Boland and Tatonetti, 2016). The fact that mutations in the DHCR7 gene (SLOS) as well as the use of AY9944 during pregnancy (a SLOS model) are associated with fetal developmental problems, leads one to the conclusion that compounds elevating 7-DHC and lowering cholesterol during developmental stages of life should be avoided. Furthermore, prescription medications that contain the N-halophenyl-piperazine moiety or are metabolized to this core structure, cariprazine being a particularly potent member of this group, should draw particular attention for use during development. It is, however, worth noting that there is an extensive history of use of aripiprazole (as well as other antipsychotics) by adult individuals, where its improvement in the quality of life has been shown. Perturbation of the balance between 7-DHC and cholesterol is apparently not as important in adults as it is in the developmental phases of life. Type 2 antipsychotic medications play an important role in therapy and our data suggest only additional cautions, particularly with newly approved pharmaceuticals.
In summary, our findings suggest that cariprazine greatly increases 7-dehydrosterols while it decreases cholesterol levels in cultured cells. The levels of 7-DHC and 7-DHD in control fibroblasts treated with < 10 nM cariprazine were found to be comparable to those found in fibroblasts obtained from SLOS patients. The structural element that affects DHCR7 activity appears to be the dichlorophenyl-piperazine substructure in aripiprazole, cariprazine and 2,3-DCPP. These observations suggest a cautionary note for commercial products that bear this core structural unit.
Supplementary Material
Funding
This work was supported by The National Institutes of Health, NICHD HD064727 (NAP), NIEHS ES024133 (NAP/ZK) and NIMH MH110636 (KM and NAP). The authors thank Dr. Bruce Carter of Vanderbilt University for allowing the use of his cell culture facilities.
Abbreviations:
- DHCR7
dehydrocholesterol reductase 7
- DHCR24
dehydrocholesterol reductase 24
- 7-DHC7
dehydrocholesterol
- 8-DHC8
dehydrocholesterol
- Des
desmosterol
- Lan
lanosterol
- Chol
cholesterol
- SLOS
Smith-Lemli-Opitz syndrome
- PTAD
4-Phenyl-1,2,4-triazoline-3,5-dione
- BSTFA
N,O-bis(trimethylsilyl)trifluoroacetamide
- APCI
atmospheric pressure chemical ionization
- SRM
selected reaction monitoring
- AY9944
trans-1,4-bis(2-chloro-benzylaminomethyl)
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- GC/MS
gas chromatography – mass spectrometry
- NIH
National Institute of Health
- FDA
US Food and Drug Administration
- MeOH
methanol
- TIC
total ion current
- FBS
fetal bovine serum
- DMEM
Dulbecco’s Modified Eagle Medium
- PBS
phosphate buffered saline
- RCS
residual cholesterol synthesis
- DMSO
dimethylsulfoxide
- HF
human fibroblasts
- m-CPP
meta-chlorophenyl piperazine
- 2,3-DCPP
1-(2,3-dichlorophenyl)piperazine
Footnotes
Conflict of interest
None declared.
Appendix A. Supplementary data
Effect of different concentrations of cariprazine and AY9944 on human fibroblasts 13C-sterols (Fig. S1); 13C-sterols and Residual Cholesterol Synthesis in A549 cells lines in the presence and absence of the antipsychotics (Fig. S2); cariprazine affects serum sterol levels of mice injected with the drug (Fig. S3); method description for RNA extraction and qPCR analysis; references used for RNA extraction and qPCR; mRNA levels of hmgcr and dhcr7 in the cortex of animals injected with either vehicle or cariprazine; and purity of tested compounds. Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2018.04.029.
References
- Agai-Csongor E, Domany G, Nogradi K, Galambos J, Vago I, Keseru GM, Greiner I, Laszlovszky I, Gere A, Schmidt E, Kiss B, Vastag M, Tihanyi K, Saghy K, Laszy J, Gyertyan I, Zajer-Balazs M, Gemesi L, Kapas M, Szombathelyi Z, 2012. Discovery of cariprazine (RGH-188): a novel antipsychotic acting on dopamine D3/D2 receptors. Bioorg. Med. Chem. Lett. 22, 3437–3440. [DOI] [PubMed] [Google Scholar]
- Boland MR, Tatonetti NP, 2016. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: a systematic review. Pharmacogenom J. 16, 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia S, 2011. Pharmacokinetics and metabolism update for some recent anti-psychotics. Expert Opin. Drug Metabol. Toxicol. 7, 829–846. [DOI] [PubMed] [Google Scholar]
- Caloro M, Lionetto L, Cuomo I, Simonetti A, Pucci D, De Persis S, Casolla B, Kotzalidis GD, Sciarretta A, De Filippis S, Simmaco M, Girardi P, 2012. An improved simple LC-MS/MS method for the measurement of serum aripiprazole and its major metabolite. J. Pharm. Biomed. Anal. 62, 135–139. [DOI] [PubMed] [Google Scholar]
- Canfran-Duque A, Casado ME, Pastor O, Sanchez-Wandelmer J, de la Pena G, Lerma M, Mariscal P, Bracher F, Lasuncion MA, Busto R, 2013. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J. Lipid Res. 54, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12, 105–112. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, 2004. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45, 1375–1397. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Franklin T, Lepack A, Adham N, Kiss B, Gyertyan I, Duman RS, 2017. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int. J. Neuropsychopharmacol. 20, 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B, Cesura A, 2015. Antipsychotics and the dopamine-serotonin connection. Top. Med. Chem. 13, 1–50. [Google Scholar]
- Findlay LJ, El-Mallakh PL, El-Mallakh RS, 2017. Cariprazine for the treatment of bipolar disorder. Perspect. Psychiatr. Care. 53, 148–155. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Bretillon L, 2010. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Peachey NS, Richards MJ, Nagel BA, Vaughan DK, 2004. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 122, 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Hoffmann G, Schwall A, Brock RL, Aramaki S, Sweetman L, Nyhan WL, Brandt IK, Wappner RS, Lehnert W, 1990. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J. Lipid Res. 55, 329–337. [PubMed] [Google Scholar]
- Giera M, Plossl F, Bracher F, 2007. Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post-squalene pathway. Steroids 72, 633–642. [DOI] [PubMed] [Google Scholar]
- Giera M, Renard D, Plossl F, Bracher F, 2008. Lathosterol side chain amides: a new class of human lathosterol oxidase inhibitors. Steroids 73, 299–308. [DOI] [PubMed] [Google Scholar]
- Hall P, Michels V, Gavrilov D, Matern D, Oglesbee D, Raymond K, Rinaldo P, Tortorelli S, 2013. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith–Lemli–Opitz Syndrome. Mol. Gen. Metab. 1–3. [DOI] [PubMed] [Google Scholar]
- Horling A, Müller C, Barthel R, Bracher F, Imming P, 2012. A new class of selective and potent 7-dehydrocholesterol reductase inhibitors. J. Med. Chem. 55, 7614–7622. [DOI] [PubMed] [Google Scholar]
- Kanungo S, Soares N, He M, Steiner RD, 2013. Sterol metabolism disorders and neurodevelopment—an update. Dev. Dis. Res. Rev. 17, 197–210. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC, 2000. The Smith-Lemli-Opitz syndrome. J. Med. Gen. 37, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Korade Z, Tallman KA, Liu W, Weaver CD, Mirnics K, Porter NA, 2016. Inhibitors of 7-Dehydrocholesterol reductase: screening of a collection of pharmacologically active compounds in Neuro2a cells. Chem. Res. Toxicol. 29, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I, Kelemen O, Keri S, 2012. Decreased peripheral expression of neuregulin 1 in high-risk individuals who later converted to psychosis. Schizophr. Res. 135, 198–199. [DOI] [PubMed] [Google Scholar]
- Kolf-Clauw M, Chevy F, Wolf C, Siliart B, Citadelle D, Roux C, 1996. Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: a rat model for Smith-Lemli-Opitz syndrome. Teratology 54, 115–125. [DOI] [PubMed] [Google Scholar]
- Korade Z, Xu L, Shelton R, Porter NA, 2010. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 51, 3259–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kim HY, Tallman KA, Liu W, Koczok K, Balogh I, Xu L, Mirnics K, Porter NA, 2016. The effect of small molecules on sterol homeostasis: measuring 7-dehydrocholesterol in Dhcr7-deficient Neuro2a cells and human fibroblasts. J. Med. Chem. 59, 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Genaro-Mattos TC, Tallman KA, Liu W, Garbett KA, Koczok K, Balogh I, Mirnics K, Porter NA, 2017a. Vulnerability of DHCR7 + /– mutation carriers to aripiprazole and trazodone exposure. J. Lipid Res. 58, 2139–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Liu W, Warren EB, Armstrong K, Porter NA, Konradi C, 2017b. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 187, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberson CR, Muchalski H, McDuffee KB, Tallman KA, Xu L, Porter NA, 2017. Propagation rate constants for the peroxidation of sterols on the biosynthetic pathway to cholesterol. Chem. Phys. Lipids 2017 (Part B), 51–58 (Epub Feb 05). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA, 2010. The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology 35, 1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes WD, 2011. Biosynthesis of cholesterol and other sterols. Chem. Rev. 111, 6423–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, Herman GE, 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52, 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Dupuis R, Horvath C, Talbot JN, 1980. Teratogenic effect of an inhibitor of cholesterol synthesis (AY 9944) in rats: correlation with maternal cholesterolemia. J. Nutr. 110, 2310–2312. [DOI] [PubMed] [Google Scholar]
- Sanchez-Wandelmer J, Davalos A, de la Pena G, Cano S, Giera M, Canfran-Duque A, Bracher F, Martin-Hidalgo A, Fernandez-Hernando C, Lasuncion MA, Busto R, 2010. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience 167, 143–153. [DOI] [PubMed] [Google Scholar]
- Sever N, Mann RK, Xu L, Snell WJ, Hernandez-Lara CI, Porter NA, Beachy PA, 2016. Endogenous B-ring oxysterols inhibit the Hedgehog component Smoothened in a manner distinct from cyclopamine or side-chain oxysterols. Proc. Natl. Acad. Sci. U. S. A. 113 (Epub May 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, LemIi L, Opitz JM, 1964. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 64, 210–217. [DOI] [PubMed] [Google Scholar]
- Veselinovic T, Paulzen M, Grunder G, 2013. Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert. Rev. Neurother. 13, 1141–1159. [DOI] [PubMed] [Google Scholar]
- Wassif CA, Krakowiak PA, Wright BS, Gewandter JS, Sterner AL, Javitt N, Yergey AL, Porter FD, 2005. Residual cholesterol synthesis and simvastatin induction of cholesterol synthesis in Smith-Lemli-Opitz syndrome fibroblasts. Mol. Gen. Metab. 85, 96–107. [DOI] [PubMed] [Google Scholar]
- Windsor K, Genaro-Mattos TC, Kim HY, Liu W, Tallman KA, Miyamoto S, Korade Z, Porter NA, 2013. Probing lipid-protein adduction with alkynyl surrogates: application to Smith-Lemli-Opitz syndrome. J. Lipid Res. 54, 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch-Baumgartner M, Schwentner I, Gruber M, Benlian P, Bertranpetit J, Bieth E, Chevy F, Clusellas N, Estivill X, Gasparini G, Giros M, Kelley RI, Krajewska-Walasek M, Menzel J, Miettinen T, Ogorelkova M, Rossi M, Scala I, Schinzel A, Schmidt K, Schonitzer D, Seemanova E, Sperling K, Syrrou M, Talmud PJ, Wollnik B, Krawczak M, Labuda D, Utermann G, 2008. Age and origin of major Smith-Lemli-Opitz syndrome (SLOS) mutations in European populations. J. Med. Gen. 45, 200–209. [DOI] [PubMed] [Google Scholar]
- Xu L, Davis TA, Porter NA, 2009. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Porter NA, 2010. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132, 2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Dale A Rosado J, Liu W, Lamberson CR, Porter NA, 2011a. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52, 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA, 2011b. Novel oxysterols observed in tissues and fluids of AY9944-treated rats - a model for Smith-Lemli-Opitz Syndrome. J. Lipid Res. 52, 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Rosado DA Jr., Mirnics K, Porter NA, 2013. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 54, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


