Abstract
Formation of long-range axons occurs over multiple stages of morphological maturation. However, the intrinsic transcriptional mechanisms that temporally control different stages of axon projection development are unknown. Here, we addressed this question by studying the formation of mouse serotonin (5-HT) axons, the exemplar of long-range profusely arborized axon architectures. We report that LIM homeodomain factor 1b (Lmx1b)-deficient 5-HT neurons fail to generate axonal projections to the forebrain and spinal cord. Stage-specific targeting demonstrates that Lmx1b is required at successive stages to control 5-HT axon primary outgrowth, selective routing, and terminal arborization. We show a Lmx1b→Pet1 regulatory cascade is temporally required for 5-HT arborization and upregulation of the 5-HT axon arborization gene, Protocadherin-alphac2, during postnatal development of forebrain 5-HT axons. Our findings identify a temporal regulatory mechanism in which a single continuously expressed transcription factor functions at successive stages to orchestrate the progressive development of long-range axon architectures enabling expansive neuromodulation.
Research organism: Mouse
Introduction
Newly generated neurons dramatically transform their morphology to establish mature circuit connectivity. Some neurons, for example interneurons, connect to local circuits and thus need to extend their axons relatively short distances. In contrast, other neuron types, such as those giving rise to neuromodulatory systems, have the capacity to extend extremely long axons to innervate distant target fields. This is well exemplified in the case of serotonin (5-HT) synthesizing neurons. 5-HT modulates the excitability of nearly all neural circuitry in the brain and spinal cord despite being produced in a relatively small population of neurons (Azevedo et al., 2009; Baker et al., 1991; Hornung, 2003). Expansive serotonergic neuromodulation is achieved through the formation of long-range highly diffuse ascending and descending projection pathways that deliver 5-HT throughout the brain and spinal cord for synaptic or non-synaptic interactions with an array of receptors (Hannon and Hoyer, 2008; Steinbusch, 1981). Despite the decades-long knowledge of 5-HT’s importance as a global neuromodulator, it is not understood how these small numbers of neurons develop, over an extended period of morphological maturation, such elaborate axonal architectures.
5-HT neurons initiate axon outgrowth concomitant with their birth and onset of 5-HT synthesis (Hawthorne et al., 2010; Lidov and Molliver, 1982). Classic neuroanatomical studies have defined three successive stages, collectively spanning several weeks, in the formation of 5-HT projection pathways: primary pathway formation during which 5-HT axon outgrowth is initiated, selective pathway routing, and finally postnatal terminal arborization (Lidov and Molliver, 1982). Following primary pathway outgrowth through the medial forebrain bundle (MFB), ascending 5-HT axons originating in the dorsal raphe (DRN), median raphe (MRN) and B9 groups of midbrain/pons 5-HT neurons are selectively routed along pre-existing fiber tracts and reach all forebrain targets at parturition in an unarborized state (Bang et al., 2012; Lidov and Molliver, 1982; Muzerelle et al., 2016). Descending 5-HT axons, originating in the medullary serotonergic clusters, raphe pallidus (RPa), raphe obscurus (ROb), and raphe magnus (RMg), enter the spinal cord via the dorsolateral and ventral funiculi. These primary projections then route medially to invade nearly all lamina of the dorsal and ventral horns as well as the intermediate zone from cervical to sacral levels (Rajaofetra et al., 1989). After the major ascending and descending projection pathways are formed, a final, entirely postnatal, stage of 5-HT projection pathway maturation ensues during which 5-HT axons originating in different anatomically defined sub-regions flourish profuse terminal arbors in complementary and topographically organized patterns (Muzerelle et al., 2016; Ren et al., 2018). Terminal arborization develops at least through the first four postnatal weeks and leaves few, if any, regions of the brain and spinal cord devoid of serotonergic input (Gagnon and Parent, 2014; Hornung, 2003; Lidov and Molliver, 1982; Maddaloni et al., 2017; Steinbusch, 1981). What are the intrinsic mechanisms that govern successive stages in the formation of long-range profusely arborized 5-HT axon projection pathways and how are they temporally coordinated? One possibility is that each stage is governed by different intrinsic regulatory factors. Alternatively, a single continuously expressed intrinsic regulator may act at successive stages to orchestrate progressive morphological maturation of 5-HT pathways.
In contrast to the poor understanding of how 5-HT axonal pathways are formed, there is substantial knowledge of the gene regulatory networks (GRNs) that generate 5-HT neurons and control acquisition of 5-HT transmitter identity (Deneris and Gaspar, 2018). The LIM homeodomain protein, Lmx1b, is a crucial factor in 5-HT GRNs as 5-HT neuron selective targeting of Lmx1b results in the failure to induce Tph2 expression for 5-HT synthesis and Slc6a4 expression for 5-HT reuptake (Zhao et al., 2006). This results in extremely low levels of 5-HT in the adult brain, which is associated with high neonatal mortality and several abnormal behavioral phenotypes including hyperactivity, delayed respiratory maturation, enhanced inflammatory pain sensitivity, deficient opioid analgesia, sleep regulation, and increased contextual fear memories (Dai et al., 2008; Hodges et al., 2009; Zhang et al., 2018; Zhao et al., 2007a; Zhao et al., 2007b).
Lmx1b is a continuously expressed, terminal selector-type factor in 5-HT neurons (Hobert, 2008) raising the possibility that subsequent to its initial role in the induction of 5-HT synthesis and transport it may perform additional stage specific functions in the maturation of serotonergic connectivity. However, stage specific functions of continuously expressed terminal selectors, such as Lmx1b, in postmitotic neuronal morphological maturation are poorly understood (Deneris and Hobert, 2014; Hobert, 2016). Here, we report that lack of Lmx1b results in the failure to build long-range ascending and descending 5-HT axon projection pathways. Using temporal conditional targeting approaches we dissect distinct stage-specific functions for Lmx1b. Our findings show that Lmx1b acts at successive stages to control primary pathway growth rate, selective pathway routing and terminal arborization of 5-HT axons. We identify an ascending-specific Lmx1b-controlled regulatory cascade that regulates selective pathway routing and then switches to control forebrain 5-HT axon arborization through stage specific expression of genes required for arborization. This study demonstrates that a single continuously expressed transcription factor, initially required for induction of 5-HT synthesis and reuptake, subsequently acts at successive stages to build the expansive axon pathway architectures enabling CNS-wide serotonergic neuromodulation.
Results
Lmx1b controls formation of ascending 5-HT projection pathways
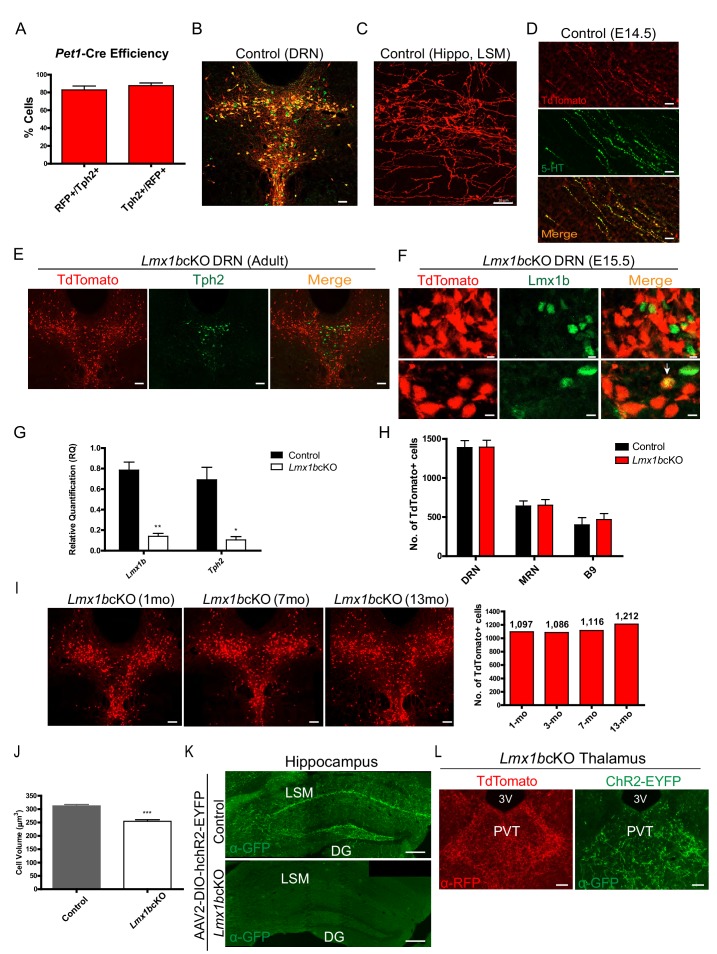
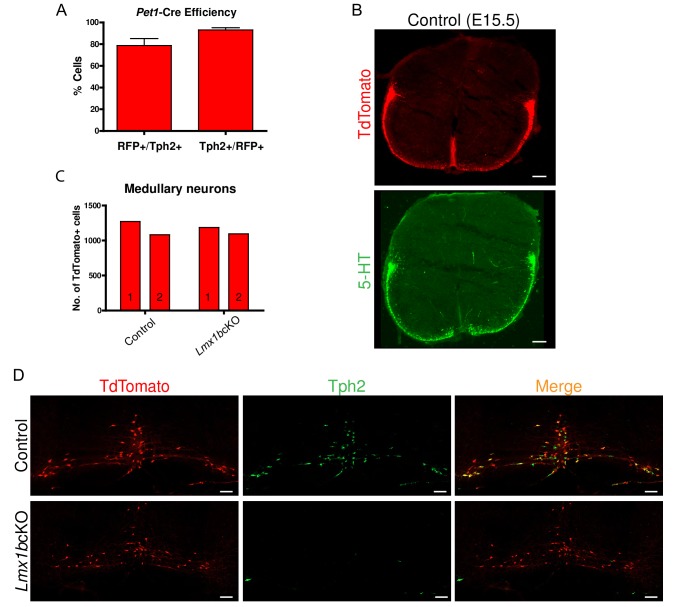
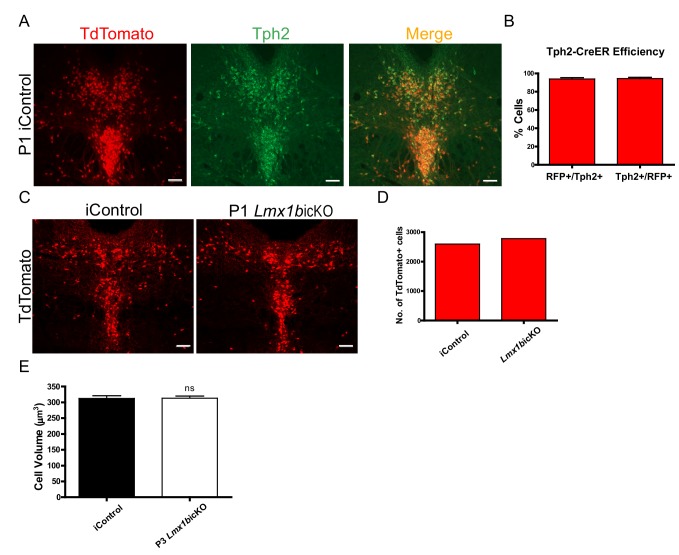
Conditional targeting of Lmx1b with the Pet1-Cre transgene results in loss of endogenous 5-HT neuron markers, Sert, Tph2, and 5-HT at E12.5 (Zhao et al., 2006). Therefore, we generated control (Lmx1b+/+;Pet1-Cre;Ai9) and Lmx1bcKO (Lmx1bfl/fl;Pet1-Cre;Ai9) mice in which the Ai9 reporter allele was used as a surrogate marker to specifically label Pet1+ cell bodies and their axons with red fluorescent protein, TdTomato. In control adult mice, 82% of Tph2+ neurons in the DRN and MRN express TdTomato. Conversely, approximately 88% of TdTomato+ cells co-label with Tph2; the remaining TdTomato+ cells likely express Tph2, but at a level insufficient for detection with standard IHC (Deneris and Gaspar, 2018; Okaty et al., 2015) (Figure 1—figure supplement 1A,B).
TdTomato+ cells were not found outside of the raphe nuclei (Figure 1—figure supplement 1B). TdTomato+ axons in control mice were widely distributed throughout the adult brain and colocalized with 5-HT (Figure 1—figure supplement 1C,D). The pattern of TdTomato+ axons in the control forebrain corresponded closely with the pattern of 5-HT axon distribution previously determined in the rat with an anti-5-HT antibody (Lidov and Molliver, 1982; Steinbusch, 1981). Similarly, TdTomato+ axon distribution in control brains was highly concordant with 5-HT axon projection patterns determined more recently using a mouse line in which GFP was knocked into the Tph2 coding region, thus validating our surrogate marking of 5-HT axons using a soluble reporter (Migliarini et al., 2013).
Abundant numbers of TdTomato+ cell bodies were detected in each of the raphe nuclei of Lmx1bcKO animals (Figure 1—figure supplement 1E). The vast majority of Lmx1bcKO TdTomato+ cells did not co-express Lmx1b or Tph2 (Figure 1—figure supplement 1E,F). Further, RT-qPCR analyses verified severe deficits of Lmx1b and Tph2 mRNAs in flow sorted YFP-labeled Pet1+ neurons in Lmx1bcKO mice, confirming appropriate Lmx1b targeting (Figure 1—figure supplement 1G). Counts of TdTomato+ cell bodies in Lmx1bcKO mice indicated that equivalent numbers were present compared to controls at 3 months and that their numbers remained stable for at least 13 months (Figure 1—figure supplement 1H,I). Lmx1bcKO TdTomato+ cell bodies were located within the normal cytoarchitectural boundaries of the raphe nuclei with a mildly altered distribution and smaller size (Figure 1—figure supplement 1I,J). Together, these data indicate specific fluorescent labeling of 5-HT neurons with Ai9 and efficient Lmx1b knock-down in Lmx1bcKO animals.
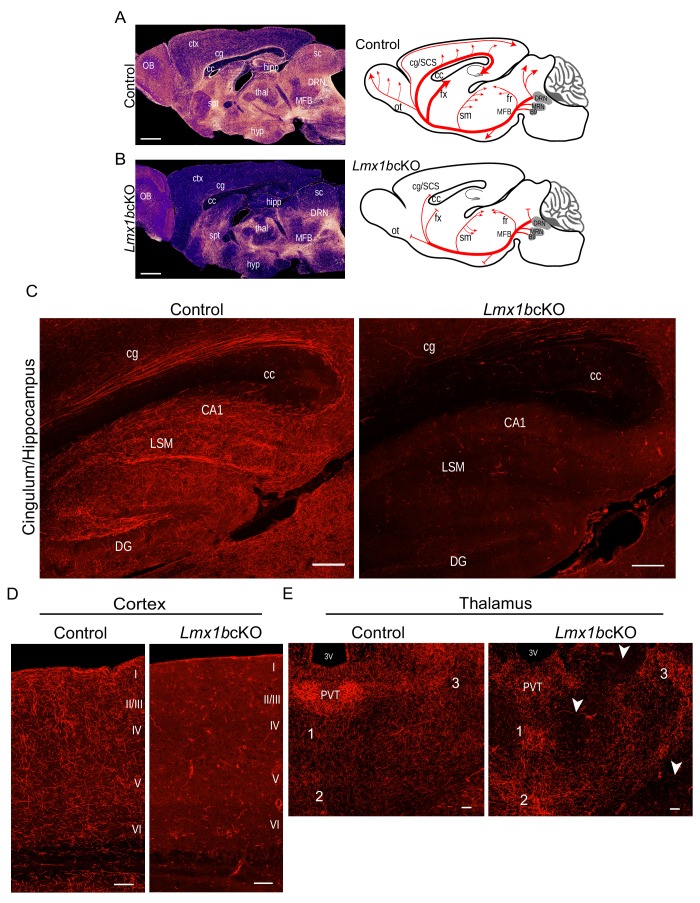
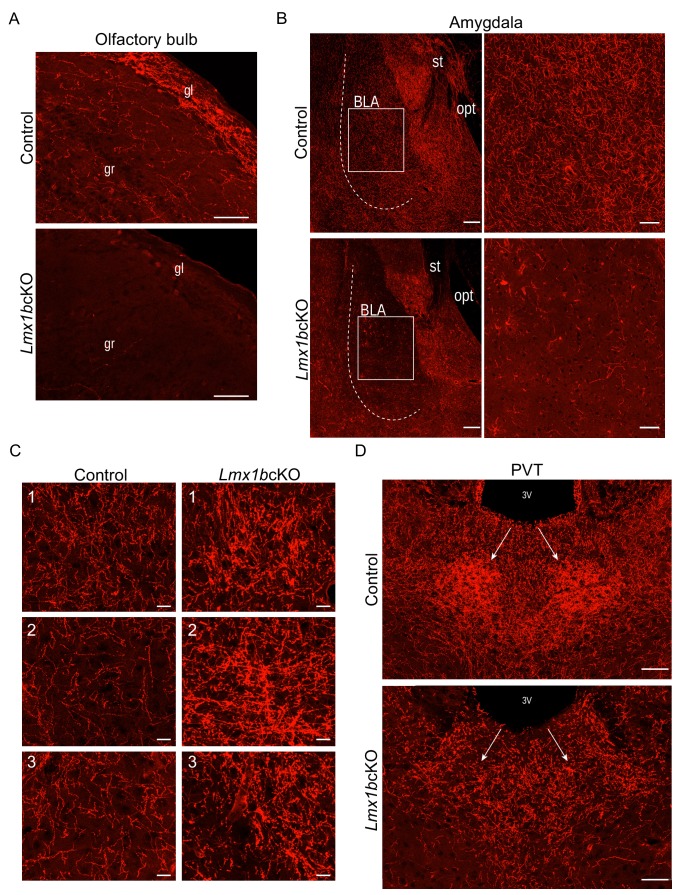
Analysis of TdTomato+ axons in adult Lmx1bcKO mice revealed a dramatically different pattern compared to that in control mice (Figure 1A,B). Although TdTomato+ axons were present in normal density and with proper ascending trajectory within the MFB of Lmx1bcKO mice, TdTomato+ axons were nearly completely missing throughout the forebrain of Lmx1bcKO animals distal to the thalamus (Figure 1B). Indeed, the vast majority of TdTomato+ axons failed to reach various distal fiber tracts including the fimbria-fornix, supracallosal stria, cingulum bundle, and olfactory tract (Figure 1B). Consequently, few if any TdTomato+ axons were present in the olfactory bulb, cortex, amygdala, hippocampus, striatum or many regions of the hypothalamus (Figure 1B–D; Figure 1—figure supplement 2A,B). It is likely that the few TdTomato+ 5-HT axons that did reach the distal forebrain were the result of a small number of 5-HT neurons in which Ai9 expression was activated, but Lmx1b targeting failed (Figure 1—figure supplement 1F).
Figure 1. Lmx1b is required for the formation of ascending 5-HT axon projection pathways.
(A, B) Ascending 5-HT axonal projection system immunolabeled using an anti-RFP antibody to TdTomato in whole sagittal forebrain sections of 3 month old mice displayed by heatmap. Lmx1bcKO TdTomato+ axons were nearly absent in numerous brain regions (B) compared to controls (A) (n = 6, controls; n = 7, Lmx1bcKO adult mice). Right, schematics depicting 5-HT axon trajectories in Lmx1bcKO vs. control brains. Scale bars, 1000 µm. OB, olfactory bulb; ctx, cortex; cg, cingulum; cc, corpus callosum; hipp, hippocampus; spt, septum; hyp, hypothalamus; thal, thalamus; sc, superior colliculus; MFB, medial forebrain bundle; DRN, dorsal raphe nucleus. Schematic (right): ot, olfactory tract; cg/SCS, cingulum/supracallosal stria; fx, fornix; sm, stria medularis; fr, fasciculus retroflexus. (C) Confocal images of TdTomato+ axons in sagittal sections. Lmx1bcKO axons failed to fill cingulum bundles or innervate the hippocampus. Scale bars, 200 µm. cg, cingulum; cc, corpus callosum; LSM, lacunosum moleculare; DG, dentate gyrus; CA1 of hippocampus. (D) Coronal sections of cortex show near complete lack of Lmx1bcKO TdTomato+ axons Scale bars, 50 µm. (E) Coronal view of altered patterns of TdTomato+ axons in Lmx1bcKO thalamus. Arrowheads indicate areas devoid of axons in Lmx1bcKO thalamus. Numbers correspond to areas of axon clumping in Lmx1bcKO thalamus. See Figure 1—figure supplement 2 for high magnification images. Scale bars, 100 µm. PVT, paraventricular nucleus of the thalamus; 3V, third ventricle.
Figure 1—figure supplement 1. Surrogate marking of 5-HT cell bodies and axons and Lmx1b conditional targeting.
Figure 1—figure supplement 2. Lmx1b deficiency disrupts 5-HT axon patterns in the forebrain.
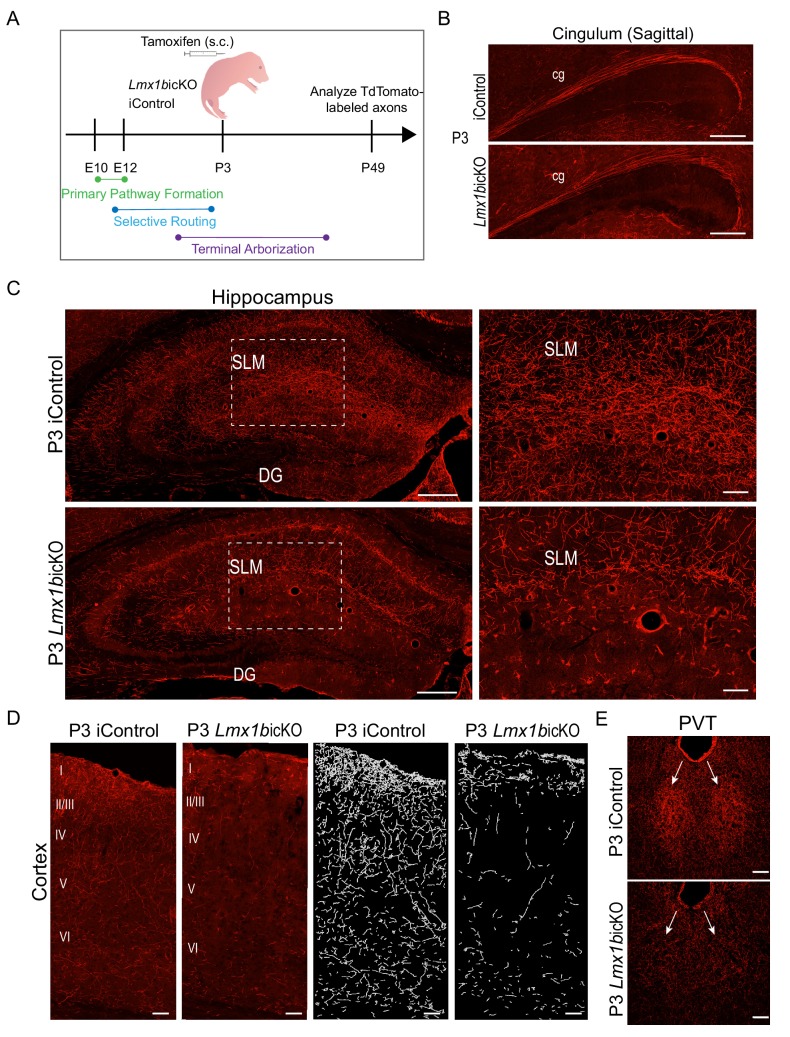
Although Lmx1bcKO TdTomato+ axons were present in the thalamus, the distribution of their terminal arbors was quite different from that in controls (Figure 1B,E). We found aberrant clumping of mutant TdTomato+ terminal arbors in several intralaminar thalamic nuclei (Figure 1E; Figure 1—figure supplement 2C). Many regions were completely devoid of arbors unlike the relatively homogeneously tiled distribution in the control thalamus (Figure 1E, arrowheads). In addition, the conspicuously dense 5-HT arborization that demarcates the paraventricular nucleus of the thalamus (PVT) was completely absent in Lmx1bcKO mice (Figure 1E; Figure 1—figure supplement 2D).
To verify the deficit of TdTomato+ axons in the forebrain of Lmx1bcKO mice, we performed a second method of axon labeling by stereotaxic injection of an AAV2 virus expressing a Cre-dependent membrane bound channelrhodopsin with a YFP tag (rAAV2/Ef1a-DIO-hchR2-EYFP) into the midbrain of adult control and Lmx1bcKO mice. In Lmx1bcKO mice, we found an absence of YFP+ axons in all of the distal areas in which TdTomato+ axons were absent. Moreover, YFP+ axons were colocalized in all forebrain areas with TdTomato+ axons (Figure 1—figure supplement 1K,L).
Lmx1b controls formation of descending 5-HT projection pathways
As Lmx1b is strongly expressed in all medullary raphe 5-HT neurons, we next investigated descending 5-HT axon development. In control animals, 80% of Tph2+ medullary cell bodies expressed TdTomato and 92% of TdTomato+ cell bodies were co-labeled with Tph2 (Figure 2—figure supplement 1A). TdTomato+ axon patterns in control E15.5 embryos corresponded closely with the pattern of developing 5-HT-labeled axons in the ventral and lateral funiculi of the spinal cord, thus indicating specific labeling of descending 5-HT axons with TdTomato (Figure 2—figure supplement 1B). In addition, the pattern of control TdTomato+ axon distribution throughout the embryonic and adult spinal cord corresponded to descending 5-HT axons patterns in gray and white matter described in the rat and mouse with an anti-5-HT antibody (Ballion et al., 2002; Rajaofetra et al., 1989). We confirmed that the number of TdTomato-labeled Pet1+ neurons in the medullary raphe nuclei did not differ between Lmx1bcKO and control mice at 3 months of age (Figure 2—figure supplement 1C). Further, Lmx1bcKO TdTomato+ cells did not express Tph2, confirming targeting of Lmx1b (Figure 2—figure supplement 1D).
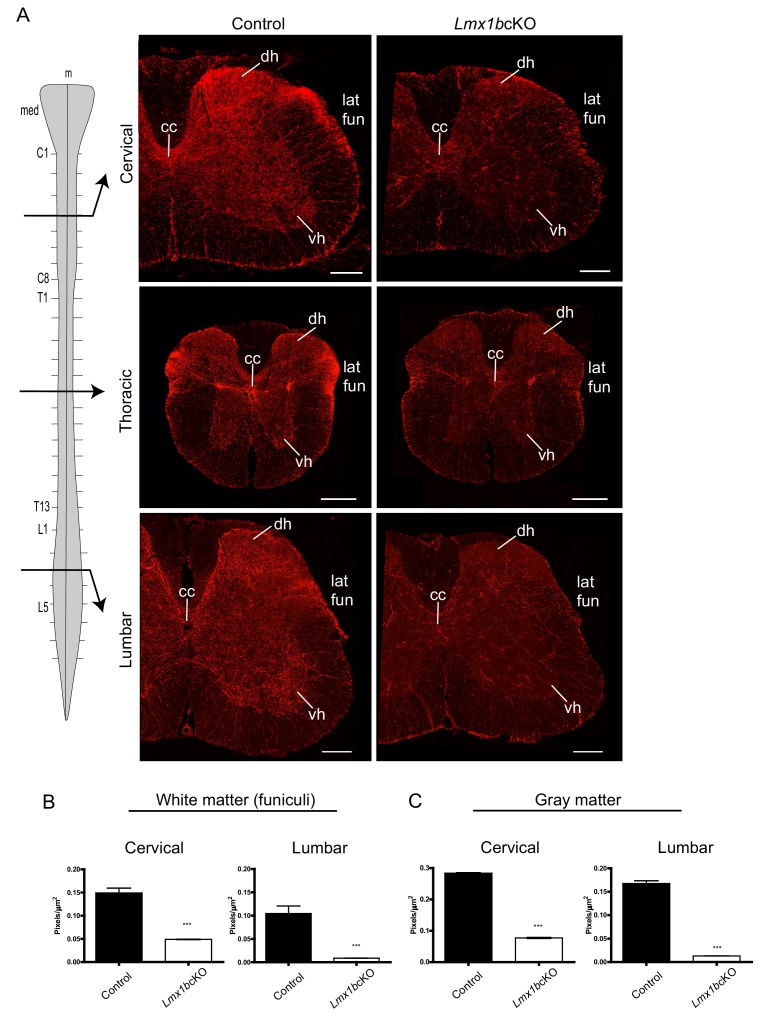
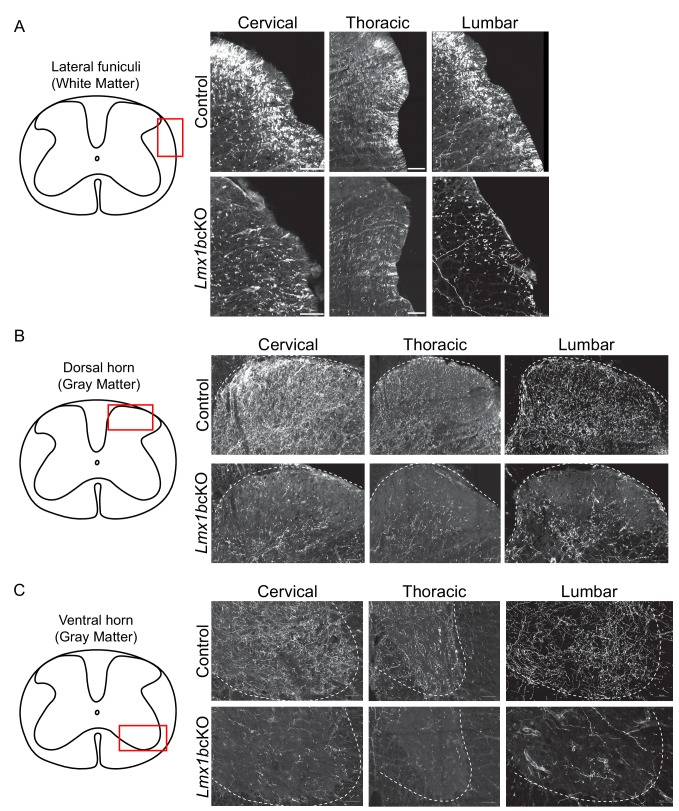
We found a severe lack of TdTomato+ axons in the white matter funiculi, through which 5-HT axon projections normally extend caudally through the spinal cord (Figure 2A; Figure 2—figure supplement 2A). In addition, a severe reduction of Lmx1bcKO TdTomato+ axons was found in gray matter at all levels of the spinal cord (Figure 2A). The normally dense innervation to the dorsal and ventral horns of control spinal cords was dramatically decreased in cervical, thoracic and lumbar levels in Lmx1bcKO mice (Figure 2A; Figure 2—figure supplement 2B,C). At cervical levels there was a 67% deficit of TdTomato+ axons in white matter while at lumbar levels there was a 92% deficit (Figure 2B). Quantitation of TdTomato+ axons in gray matter revealed a progressive cervical to lumbar deficit, 73% and 94%, respectively in Lmx1bcKO cords compared to control cords (Figure 2C). Collectively, our results indicate that Lmx1b deficiency severely disrupts long-range 5-HT axon architecture in the forebrain and spinal cord and that the deficit becomes increasingly profound with increasing distance from 5-HT cell bodies.
Figure 2. Lmx1b is required for the formation of descending 5-HT axon projection pathways.
(A) Coronal sections taken at cervical (C4), thoracic (T6), and lumbar (L3) levels of the spinal cord (diagram, left). Immunolabeling for TdTomato shows Lmx1bcKO axons were severely reduced at every level of the cord in both gray and white matter compared to controls. Scale bars, 200 µm. m, midline; med, medulla; cc, central canal; dh, dorsal horn; vh, ventral horn; lat fun, lateral funiculi. (B, C) Quantification of total TdTomato+ axons (pixels/µm2) in white (B) and gray (C) matter at cervical and lumbar levels (n = 3, control; n = 3 Lmx1bcKO mice). Two-way ANOVA with Welch’s correction, *p<0.05, **p<0.001, and ***p<0.0001. Data are represented as mean ± SEM.
Figure 2—figure supplement 1. Conditional targeting of Lmx1b in the descending 5-HT projection pathway.
Figure 2—figure supplement 2. Progressive deficits of 5-HT axon fibers in Lmx1b deficient spinal cord white and gray matter.
Delayed primary pathway formation and aborted selective pathway routing in Lmx1bcKO mice
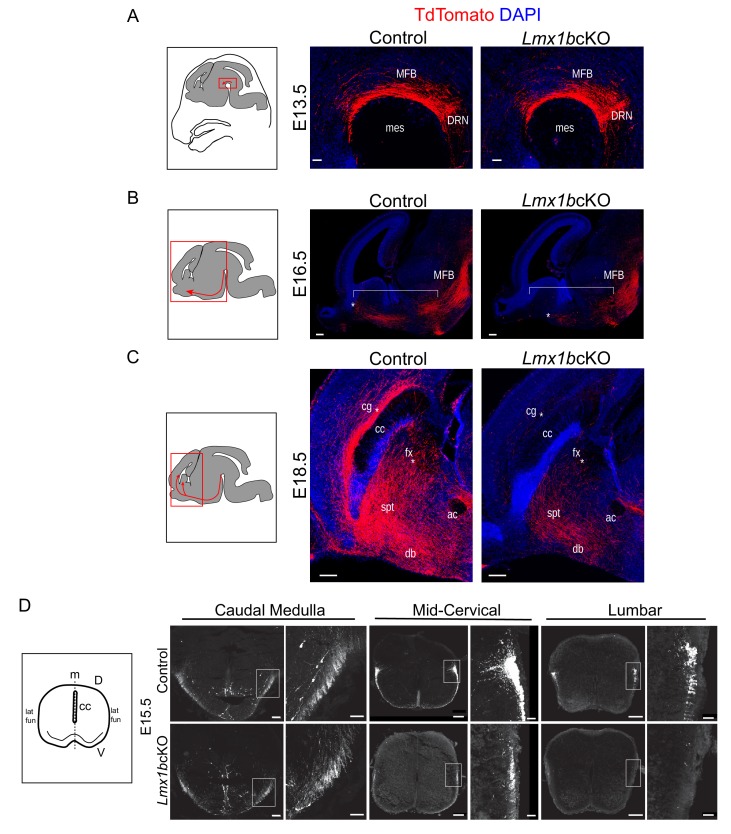
We next followed the development of Lmx1bcKO TdTomato+ axons to distinguish among several possible explanations for the dramatic defects in ascending and descending 5-HT axon pathways: i, disrupted primary pathway formation; ii, failure to selectively route axons through pre-existing tracts; iii, abnormal axon trajectories with subsequent failure to extend, iv, normal pathway development followed by dieback. We first investigated TdTomato+ axons at E13.5 when primary pathway formation is underway and Lmx1b conditional targeting has occurred. At this stage, TdTomato+ axon outgrowth and trajectory in Lmx1bcKO mice were normal as they coursed through the MFB over the mesencephalic flexure (Figure 3A). At E16.5, however, Lmx1bcKO TdTomato+ axons exhibited a clear failure to extend as far rostrally as control axons, suggesting either aborted or delayed axon outgrowth along the more rostral portions of the MFB during late stage primary pathway formation (Figure 3B). At E18.5, the density of control TdTomato+ axons was maximal throughout the MFB and TdTomato+ axons were present as far rostral as the septum and diagonal band, where 5-HT axons turn dorsally to navigate through the cingulum, supracallosal stria, and fimbria-fornix (Figure 3C). Lmx1bcKO TdTomato+ axons now appeared at normal density throughout the MFB, thus supporting delayed axon outgrowth at E16.5 (data not shown). However, at this stage, most Lmx1bcKO TdTomato+ axons failed to turn dorsally and remained stalled within the MFB. A smaller number of Lmx1bcKO TdTomato+ axons did turn dorsally away from the MFB to pass through the septum and diagonal band (Figure 3C). Virtually all Lmx1bcKO axons failed to extend and selectively route into the cingulum and fimbria-fornix, thus failing to reach the cortex and hippocampus. Lmx1bcKO TdTomato+ axons still did not exhibit ectopic trajectories. Further examination in one-month old Lmx1bcKO animals revealed a similar deficit of TdTomato+ axons in distal forebrain tracts (data not shown). These findings suggest that conditional targeting of Lmx1b at E12.5 results in delayed primary pathway formation followed by a profound failure of ascending axons to selectively route into pre-existing fiber tracts.
Figure 3. Initial axon outgrowth is delayed and selective pathway routing fails in Lmx1b deficient 5-HT neurons.
(A–C) Immunolabeled TdTomato+ ascending axons in sagittal slices at different embryonic stages. Diagrams (left) show area of image (red box) presented for each time point. Arrows indicate direction of growing axons. E13.5 Lmx1bcKO axons exhibited similar ascending trajectories and densities as controls (A). E16.5 Lmx1bcKO axons did not extend as far (asterisk) and were less abundant (under bracket) compared to control axons (B). E18.5 Lmx1bcKO axons failed to fill multiple axon tracts (cg, fx; asterisks) compared to controls (C). Scale bars, 50 µm (A), 200 µm (B,C). DRN, dorsal raphe nucleus; MFB, medial forebrain bundle; mes, mesencephalic flexure; cg, cingulum bundle; fx, fornix; ac, anterior commissure; spt, septum; db, diagonal band; cc, corpus callosum. (D) Diagram (left) depicting coronal section of an embryonic spinal cord. TdTomato+ descending axons at E15.5 in control vs Lmx1bcKO embryos. Lmx1bcKO axons exit caudal medulla similar to controls but were severely reduced in funiculi at lower levels of the cord (mid-cervical and lumbar). Boxed region of lateral funiculi enlarged to the right of each image. Scale bars, 100 µm (low magnification), 50 µm (high magnification-medulla), 20 µm (high magnification- cervical/lumbar insets). Lat fun, lateral funiculi; cc, central canal; m, midline; D, dorsal; V, ventral.
Analysis of descending TdTomato+ fibers at E15.5 revealed similar initial axon outgrowth through the caudal medulla to the upper cervical spinal cord from Lmx1bcKO and control medullary 5-HT cell bodies (Figure 3D). Although Lmx1bcKO TdTomato+ axons appropriately entered the lateral and ventral funiculi of the cervical spinal cord, greatly reduced densities were evident beginning at mid-cervical levels (Figure 3D). At lumbar levels, Lmx1bcKO TdTomato+ axons were nearly undetectable in the lateral and ventral funiculi (Figure 3D). These results indicate that TdTomato+ axons were not able to extend into the funiculi, which caused a severe and progressive cervical to lumbar deficit of 5-HT innervation in the adult Lmx1bcKO spinal cord (Figures 2 and 3D).
Lmx1b acts temporally to control 5-HT axon selective pathways
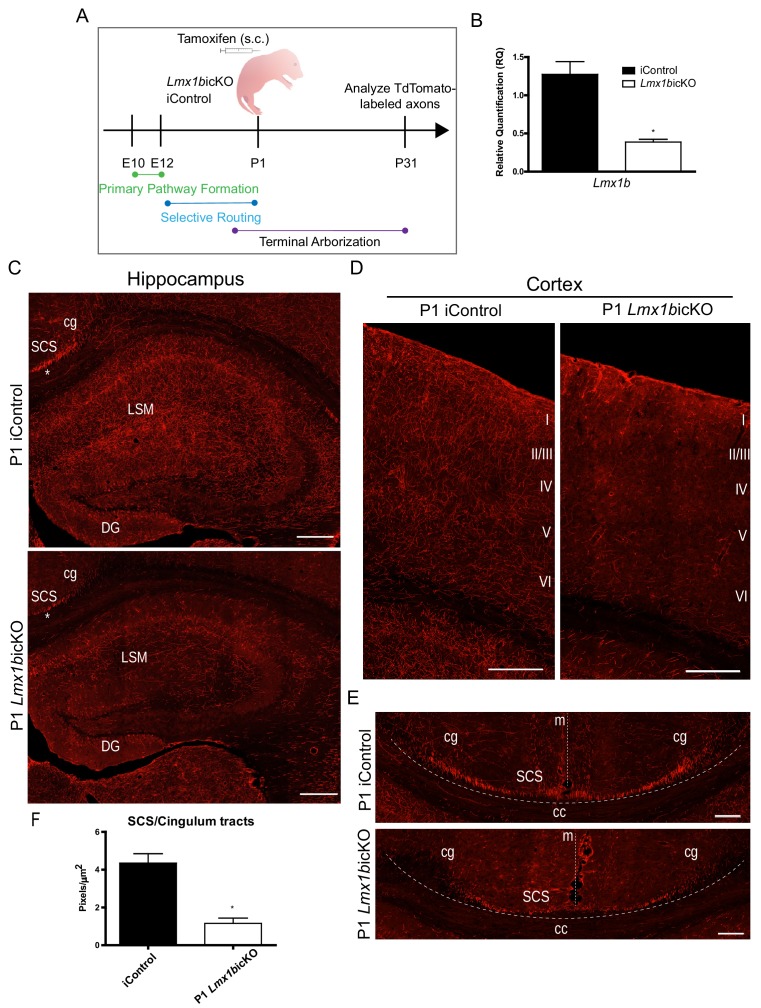
As formation of 5-HT axon projection pathways occurs over several weeks of embryonic to early postnatal neural maturation we next sought to determine whether Lmx1b is temporally required for 5-HT axon pathway formation. To address this question, we developed a tamoxifen inducible targeting strategy (Figure 4A) to knock-down Lmx1b at different early postnatal timepoints. Thus, we generated Lmx1bicKO (Lmx1bfl/fl; Tph2-CreER; Ai9) and iControl (Lmx1b+/+; Tph2-CreER; Ai9) mice. Subcutaneous delivery of tamoxifen into iControl pups resulted in approximately 94% of Tph2+ neurons co-labeled with TdTomato in the DRN/MRN/B9 (Figure 4—figure supplement 1A,B). Conversely, 94% of TdTomato+ cells were co-labeled with Tph2 (Figure 4—figure supplement 1B). As with the Pet1-Cre driver, the Tph2-CreER activated TdTomato+ cells that are Tph2- evidently express Tph2 at a low level (Deneris and Gaspar, 2018; Okaty et al., 2015). RT-qPCR analysis of flow sorted TdTomato+ cells verified that Lmx1b mRNA was significantly reduced in Lmx1bicKO mice (Figure 4B). Equivalent numbers of TdTomato-labeled cell bodies were generated in Lmx1bicKO versus iControl mice after injection of tamoxifen (Figure 4—figure supplement 1C,D). In contrast to Lmx1bcKO TdTomato+ cells, cell body size and distribution of tamoxifen treated Lmx1bicKO TdTomato+ cells were not different from that of iControl TdTomato+ cells (Figure 4—figure supplement 1C,E).
Figure 4. Lmx1b is temporally required for 5-HT projection pathway formation.
(A) Schematic of tamoxifen-inducible approach to target Lmx1b at postnatal day (P)1. (B) RT-qPCR of flow sorted TdTomato+ neurons from postnatal targeted mice (n = 3, iControl; n = 4, Lmx1bicKO mice). Unpaired t-test with Welch's correction, *p<0.05. Data are represented as mean ± SEM. (C) Coronal sections of P1 targeted Lmx1bicKO hippocampus compared to iControls analyzed at P31. *, incomplete formation of SCS and cingulum in P1 targeted Lmx1bicKO brain. Scale bars, 200 µm. (D) Coronal sections of P1 targeted Lmx1bicKO cortex compared to iControls analyzed at P31. Scale bars, 200 µm. (E) Coronal sections at level of corpus callosum showing incomplete formation of major 5-HT axon routes, SCS and cingulum, in P1 targeted Lmx1bicKO forebrain compared to iControls (above dotted line). Scale bars, 100 µm. cg, cingulum; SCS, supracallosal stria; cc, corpus callosum; m, midline. (F) Quantification of axons within SCS and cingulum tracts (n = 3, iControl; n = 3, Lmx1bicKO mice). Unpaired t-test with Welch's correction, p=0.0112. Data are represented as mean ± SEM.
Figure 4—figure supplement 1. Efficiency of postnatal tamoxifen inducible targeting of Lmx1b.
We analyzed TdTomato+ axons in P1 targeted Lmx1bicKO mice four weeks after tamoxifen delivery, when 5-HT axon pathways have fully matured (Lidov and Molliver, 1982; Maddaloni et al., 2017). We found a dramatic deficit in TdTomato+ arbors throughout the hippocampus in Lmx1bicKO mice compared to controls (Figure 4C). In addition, TdTomato+ arbors were severely reduced in all layers of the cortex with the most medial layers almost completely devoid of arbors in Lmx1bicKO mice (Figure 4D). These results clearly demonstrate that Lmx1b is temporally required for postnatal development of 5-HT terminal axons. However, we noticed that in addition to reduced 5-HT arbors in the hippocampus and cortex, TdTomato labeling within the supracallosal stria and cingulum, major routes to the hippocampus and cortex, were consistently less intense in P1 targeted Lmx1bicKO mice compared to controls (Figure 4C, asterisk). Indeed, further detailed examination revealed that these major 5-HT routes had not yet fully formed (Figure 4E). Quantification of TdTomato+ axons within the cingulum and supracallosal stria tracts confirmed a significant decrease in P1 targeted Lmx1bicKO mice compared to controls (Figure 4F). These results indicate that i, long-range 5-HT axon routing is continuing to develop in the early neonatal period and ii, Lmx1b is required at this stage for completion of 5-HT axon selective routing.
Lmx1b switches function to control terminal arborization
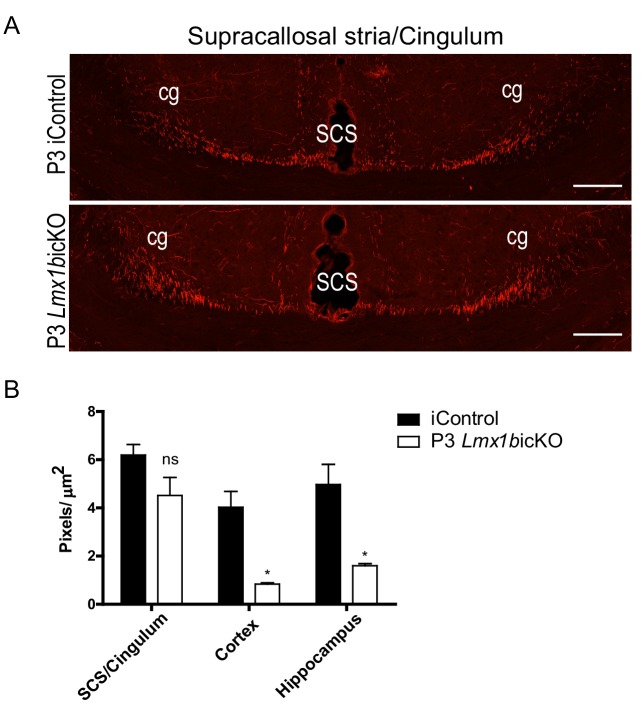
We next targeted Lmx1bicKO mice at P3 to further investigate temporal requirements for Lmx1b (Figure 5A). Importantly, in contrast to findings obtained in P1 targeted mice, we found that the density of TdTomato-labeled fibers in the supracallosal stria was now similar in P3 targeted Lmx1bicKO and iControl mice (Figure 5—figure supplement 1A). Furthermore, TdTomato-labeled fibers in the cingulum, one of the longest of 5-HT forebrain tracts, was now comparable in P3 targeted Lmx1bicKO and iControl mice (Figure 5B). Quantification of the cingulum and supracallosal stria confirmed no significant difference between P3 targeted Lmx1bicKO and iControl mice (Figure 5—figure supplement 1B). These findings indicate that 5-HT axon routing was complete in P3 targeted Lmx1bicKO mice.
Figure 5. Lmx1b temporally controls postnatal 5-HT terminal arborization.
(A) Schematic of tamoxifen inducible targeting of Lmx1b at postnatal day (P)3. (B) Sagittal view of cingulum shows fully formed long-range axon routes in P3 targeted Lmx1bicKO mice compared to iControls. Scale bars, 200 µm. (C) Coronal sections of hippocampus in P3 targeted Lmx1bicKO mice compared to iControls. Dashed boxed region: higher magnification image at right highlighting reduced TdTomato+ axons in Lmx1bicKO SLM. Scale bars, 200 µm (low magnification), 50 µm (high magnification). SLM, stratum lacunosum moleculare; DG, dentate gyrus. (D) Coronal sections of cortex of P3 targeted Lmx1bicKO mice compared to iControls. Imaris tracing; right panels. Scale bars, 100 µm. (E) Decreased TdTomato+ arbors detected in P3 targeted Lmx1bicKO PVT compared to iControls (arrows). Scale bars, 50 µm.
Figure 5—figure supplement 1. P3 targeted Lmx1bicKO mice display normal 5-HT axon routing but decreased 5-HT terminal arbors.
Despite fully developed 5-HT selective pathway routes in the forebrain, TdTomato+ arbors were significantly decreased throughout the hippocampus (Figure 5C; Figure 5—figure supplement 1B). A majority of remaining arbors detected within the hippocampus of P3 targeted Lmx1bicKO mice were found in the molecular layer (SLM), which are the first 5-HT arbors to appear in the hippocampus during rat development (Lidov and Molliver, 1982). TdTomato+ arbors were also severely decreased in all cortical layers of the P3 targeted Lmx1bicKO brain including in layer I and VI where axons of passage also exist (Figure 5D) (Lidov and Molliver, 1982). Quantification of the cortex confirmed a significant decrease of TdTomato+ axon arbors in P3 targeted Lmx1bicKO compared to iControls (Figure 5—figure supplement 1B). It is important to note that many 5-HT terminal arbors have already been generated in the forebrain at P3 (Lidov and Molliver, 1982; Maddaloni et al., 2017). Thus, Lmx1b targeting at P3 results in the failure of new arbors to form while leaving previously generated arbors intact.
Interestingly, arborization deficits were also found within the thalamus of P3 targeted Lmx1bicKO mice. Similar to what we observed in Lmx1bcKO mice, the normally dense arborization within the PVT was absent in P3 targeted Lmx1bicKO mice, rendering the PVT indiscernible (Figure 5E). Together, these results reveal a successive stage of continuous Lmx1b function during which it switches to control terminal arborization of forebrain 5-HT axons.
Targeting of 5-HT synthesis does not impair formation of forebrain and spinal cord 5-HT arbors
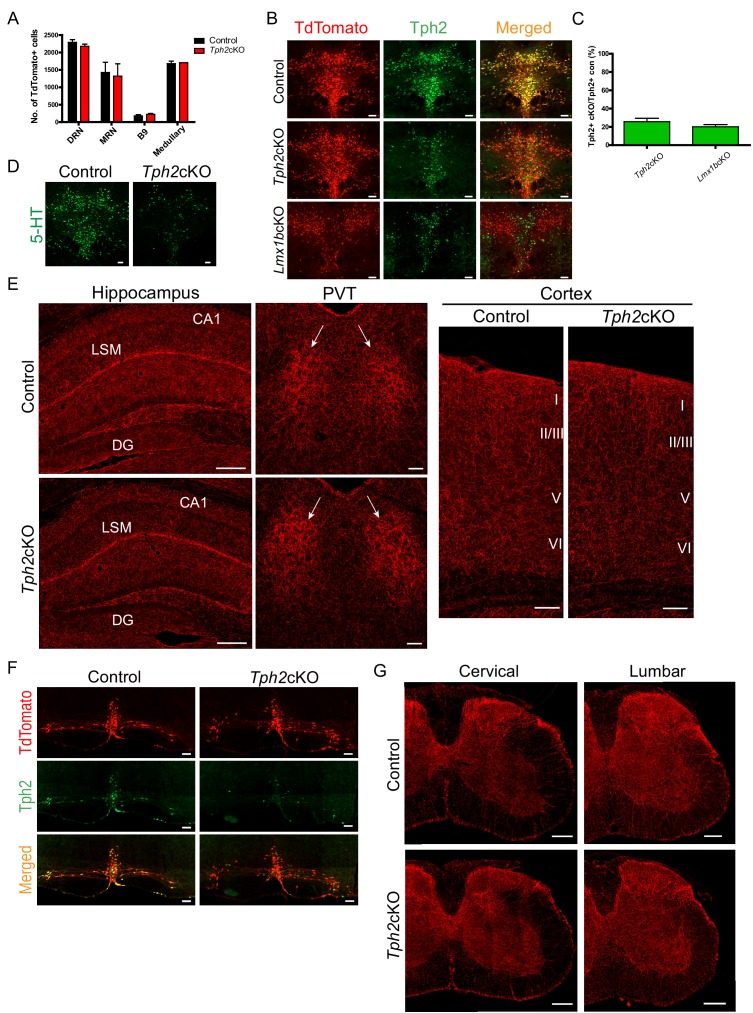
Lack of 5-HT itself has been reported to affect development of forebrain 5-HT terminal arbor patterns (Migliarini et al., 2013). Therefore, we next investigated whether the arborization defects present in Lmx1b deficient mice resulted from reduced Tph2 expression and consequently reduced 5-HT levels. To specifically knock down Tph2 to a level comparable to that in Lmx1bcKO mice, we generated Tph2cKO (Tph2fl/fl;Pet1-Cre;Ai9) and control (Tph2+/+;Pet1-Cre;Ai9) mice. The numbers of TdTomato+ cells in each raphe nuclei did not differ in Tph2cKO and control mice (Figure 6A). In the DRN/MRN/B9 nuclei, we found a 74% reduction of Tph2+ neurons in Tph2cKO compared to the 79% reduction of Tph2+ neurons in Lmx1bcKO mice (Figure 6B,C). Additionally, we confirmed loss of 5-HT itself in Tph2cKO mice (Figure 6D).
Figure 6. Specific targeting of 5-HT synthesis does not alter 5-HT arborization patterns.
(A) Counts of TdTomato+ cells in each raphe nucleus of Tph2cKO mice did not differ from controls (n = 2 mice/genotype). Data are represented as mean ± SEM. (B) Comparable Tph2 knock-down in Tph2cKO and Lmx1bcKO mice. Scale bars, 100 µm. (C) Cell counts of residual Tph2+ neurons in Tph2cKO and Lmx1bcKO mice expressed as a percentage (n = 2 mice/genotype). Data are represented as mean ± SEM. (D) Immunolabeling shows 5-HT was severely reduced in Tph2cKO mice. Scale bars, 100 µm. (E) Coronal forebrain sections showing no deficits of TdTomato+ axon densities in Tph2cKO hippocampus, PVT, and cortex (n = 3 mice/genotype). LSM, lacunosum moleculare; DG, dentate gyrus; CA1 of hippocampus. Scale bars, 100 µm (PVT, cortex); 200 µm (hippocampus). (F) Co-immunolabeling for Tph2 and TdTomato in medullary neurons. Tph2 expression was severely reduced in medullary neurons of Tph2cKO mice. Scale bars, 50 µm. (G) No deficits of TdTomato+ axons were present throughout the Tph2cKO spinal cord (n = 3 mice/genotype). Scale bars, 200 µm.
We analyzed TdTomato+ terminal arbors throughout the forebrain in adult Tph2cKO mice. We did not find differences in Tph2cKO versus control TdTomato-labeled arbor patterns anywhere in the forebrain including the hippocampus, cortex, and the PVT (Figure 6E). Further, despite loss of Tph2 in medullary neurons of Tph2cKO mice (Figure 6F), analysis of the spinal cord from cervical to lumbar levels revealed no differences in axon densities compared to control mice (Figure 6G). Collectively, these results indicate that the loss of Tph2 and thus reduced levels of 5-HT in Lmx1bcKO or Lmx1bicKO mice did not contribute to either the routing or arborization defects. Evidently, Tph2 and 5-HT levels need to be reduced to a greater extent than we achieved in either our Lmx1bcKO or our Tph2cKO mice to generate the 5-HT arborization defects observed in mice engineered to completely eliminate Tph2 function and brain 5-HT (Migliarini et al., 2013).
Lmx1b controlled axon-related transcriptomes
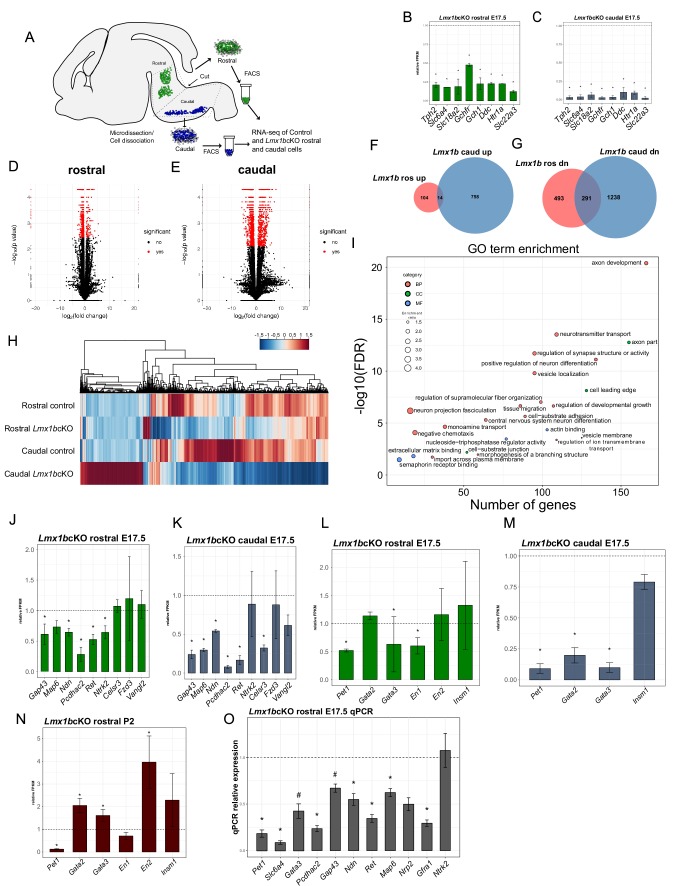
Genome-wide analysis of Lmx1b controlled serotonergic transcriptomes has not been performed and consequently only a few serotonergic genes are known Lmx1b targets (Zhao et al., 2006). We performed RNA-sequencing (RNA-seq) on flow sorted rostral hindbrain 5-HT neurons, which give rise to the ascending system, and caudal hindbrain 5-HT neurons, which give rise to the descending system, obtained from Lmx1bcKO and control E17.5 embryos (Figure 7A). Differential expression analysis revealed that the known Lmx1b target genes, Tph2, Slc18a2, Slc6a4, SCG II, Ctr, were significantly decreased in our E17.5 rostral and caudal Lmx1bcKO datasets. Moreover, we found significantly decreased expression of other 5-HT pathway genes, Ddc, Slc22a3, Htr1a, Gch1, Gchfr, in both rostral and caudal Lmx1bcKO sorted 5-HT neurons that were previously unknown Lmx1b targets (Figure 7B,C).
Figure 7. Ascending and descending Lmx1b regulated transcriptomes.
(A) Schematic for dissection of E17.5 brain to isolate rostral and caudal 5-HT neurons genetically labeled by Pet1-EYFP. After dissection, EYFP+ neurons were flow-sorted separately and prepared for RNA-sequencing (n = 3, controls; n = 2 Lmx1bcKO embryos). (B) Relative expression level of 5-HT pathway genes in rostral control versus Lmx1bcKO 5-HT neurons. Control gene expression levels were normalized to one. * indicates FDR ≤ 0.05. Data are represented as mean ± SEM. (C) Relative expression level of 5-HT pathway genes in caudal control versus Lmx1bcKO 5-HT neurons. Control gene expression levels were normalized to one. * indicates FDR ≤ 0.05. Data are represented as mean ± SEM. (D) Volcano plot for rostral control versus Lmx1bcKO differential expression. Significantly altered genes are in red with ≥log2(1.5X) and FDR ≤ 0.05. (E) Volcano plot for caudal control versus Lmx1bcKO differential expression. Significantly altered genes are in red with ≥log2(1.5X) and FDR ≤ 0.05. (F) Venn diagram of genes upregulated in rostral and caudal Lmx1bcKO 5-HT neurons. (G) Venn diagram of genes downregulated in rostral and caudal Lmx1bcKO 5-HT neurons. (H) Heatmap of differentially-expressed genes in rostral and caudal Lmx1bcKO 5-HT neurons. (I) GO term enrichment of Lmx1b regulated genes. BP = biological process, CC = cellular component, MF = molecular function. GO terms were enriched with FDR ≤ 0.05. (J) Relative expression (FPKMs) of known 5-HT neuron axon-related genes in rostral Lmx1bcKO 5-HT neurons. Data are represented as mean ± SEM. (K) Relative expression (FPKMs) of known 5-HT neuron axon-related genes in caudal Lmx1bcKO 5-HT neurons. Data are represented as mean ± SEM. (L) Relative expression (FPKMs) of 5-HT GRN transcription factors in rostral Lmx1bcKO 5-HT neurons. Data are represented as mean ± SEM. (M) Relative expression (FPKMs) of 5-HT GRN transcription factors in caudal Lmx1bcKO 5-HT neurons. Data are represented as mean ± SEM. (N) Relative expression (FPKMs) of 5-HT GRN transcription factors in flow sorted TdTomato+ rostral Lmx1bcKO 5-HT neurons at postnatal day 2 (n = 3, controls; n = 4, Lmx1bcKO mice). Data are represented as mean ± SEM. (O) RT-qPCR verification of 5-HT GRN transcription factors and known axon-related genes from flow sorted rostral YFP+ Lmx1bcKO 5-HT neurons relative to control levels (n = 4 mice/genotype). * indicates p-value≤0.05, # indicates p<0.1, t-test with Welch’s correction. Data are represented as mean ± SEM.
In addition to these changes, expression of many other genes was altered in the rostral and caudal sorted neurons (Figure 7D,E). In rostral Lmx1bcKO neurons, expression of 784 genes were significantly decreased, while 118 genes showed significantly increased expression (Figure 7F,G). In caudal Lmx1bcKO neurons, expression of 1529 genes were significantly decreased and 772 were significantly increased (Figure 7F,G). Although there were many genes commonly regulated by Lmx1b, many more were uniquely regulated by Lmx1b in rostral versus caudal 5-HT neurons (Figure 7F–H).
Over-representation analysis of Gene Ontology (GO) terms from rostral and caudal Lmx1b-regulated 5-HT neuron genes revealed a pattern of strong enrichment for genes involved in axon development and morphogenesis (Figure 7I). In fact, the top enriched GO term, axon development, represents over 150 genes with a Benjamini-Hochberg (BH) adjusted p-value of 0. Many other axon-related terms included axon part, cell leading edge, regulation of supramolecular fiber organization, neuron projection fasciculation, tissue migration, cell-substrate adhesion, negative chemotaxis, morphogenesis of a branching structure, cell leading edge, extracellular matrix binding, and semaphorin receptor binding were enriched at FDR ≤ 0.05. Grouping the genes from all of these axon-related categories together produced a dataset of 422 genes regulated by Lmx1b in rostral and/or caudal 5-HT neurons that have known roles in axon development or function (Supplementary file 1). Sixty-six of these downstream genes were significantly altered in both the ascending and descending 5-HT subsystems; 71 genes were uniquely altered in the ascending subsystem while 285 were uniquely altered in the descending subsystem. This analysis suggests that Lmx1b coordinates distinct axonal regulatory programs and transcriptomes to build the two divergent axonal subsystems.
Previous studies have implicated several effector genes in the growth and patterning of 5-HT axons (Chen et al., 2017; Donovan et al., 2002; Fournet et al., 2010; Katori et al., 2017; Lee et al., 2005). In most cases, however, is it not known whether these genes perform cell intrinsic functions in 5-HT neurons. Examination of our rostral and caudal RNA-seq datasets revealed significantly decreased expression of most but not all of these genes (Figure 7J,K). We validated the expression changes of these genes with RT-qPCR from flow-sorted E17.5 rostral 5-HT neurons (Figure 7O). Together, these expression studies suggest Lmx1b controls ascending and descending 5-HT projection pathways in part through Gap43, Pcdhac2, Ndn, Ret, Ntrk2, Map6 (STOP), and Celsr3 but that potentially hundreds of other functionally diverse Lmx1b-controlled genes are likely involved in the formation of 5-HT projection pathways (Figure 7J,K,O; Supplementary file 1).
An ascending-specific axonal Lmx1b→Pet1 regulatory cascade
The regulatory interactions among transcription factors in the 5-HT GRN are poorly understood but are likely to be important for the development of 5-HT neurons. We reasoned that further study of these interactions might illuminate the regulatory mechanisms through which Lmx1b temporally controls formation of ascending and descending 5-HT axon pathways. Our whole genome expression analyses revealed complex effects of Lmx1b deficiency on the expression of other regulatory factors in the 5-HT GRN (Figure 7L–O). Decreased Pet1 expression was the most consistent and persistent change among the regulatory factors in the rostral and caudal 5-HT GRNs after conditional targeting of Lmx1b, which raised the possibility that an Lmx1b→Pet1 regulatory cascade acts in the formation of 5-HT axons.
To investigate this idea, we generated Pet1cKO (Pet1fl/fl;Pet1-Cre;Ai9) and control (Pet1+/+;Pet1-Cre;Ai9) mice (Liu et al., 2010). We imagined three alternative experimental outcomes: i, Pet1cKO fully phenocopies the axon defects found in Lmx1bcKO supporting an exclusive Lmx1b→Pet1 regulatory cascade in 5-HT axon formation, ii, 5-HT axon projection pathways are intact in Pet1cKO mice and therefore the Lmx1b→Pet1 regulatory path is not operational in 5-HT axon development or iii, Pet1cKO incompletely phenocopies Lmx1bcKO axon defects suggesting that the Lmx1b→Pet1 cascade operates in parallel with other Lmx1b orchestrated regulatory programs.
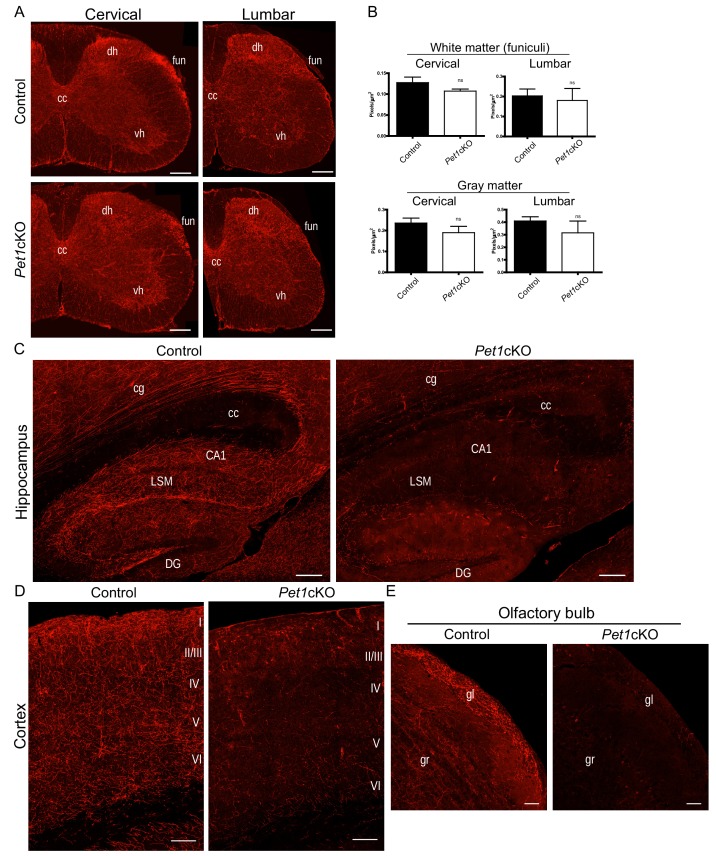
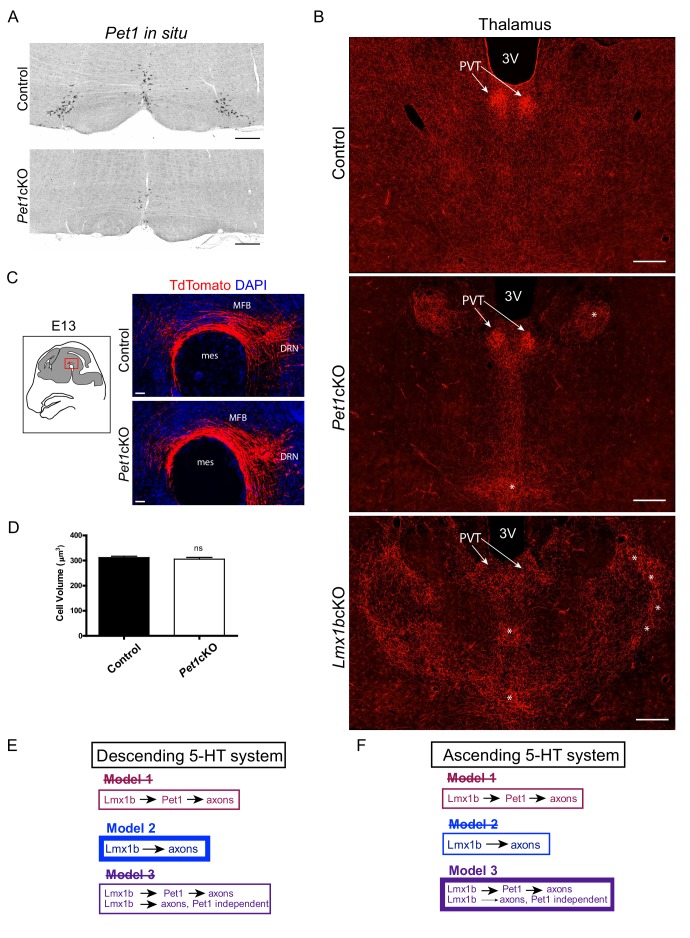
We first examined TdTomato+ axon patterns in adult Pet1cKO versus control spinal cords. We confirmed Pet1 transcript loss in medullary 5-HT neurons by in situ hybridization (ISH) (Figure 8—figure supplement 1A). TdTomato+ axon patterns from cervical to lumbar levels of the spinal cord were similar in Pet1cKO and control mice (Figure 8A). Quantitation of total TdTomato+ axons in gray and white matter revealed no significant difference between Pet1cKO and control mice (Figure 8B). These findings indicate that Pet1, and consequently the hypothetical Lmx1b→Pet1 cascade, is not required for the formation of descending 5-HT axon pathways (Figure 8—figure supplement 1E).
Figure 8. Distinct transcription factor requirements in the formation of ascending and descending 5-HT projection pathways.
(A) TdTomato+ axon innervation in Pet1cKO vs control spinal cords in 3 month old mice. Coronal semi-section views of cervical and lumbar levels. Scale bars, 200 µm. cc, central canal; dh, dorsal horn; vh, ventral horn; fun, funiculi. (B) Quantification of TdTomato+ axons (pixels/µm2) in cervical and lumbar spinal cords (n = 3, controls; n = 3, Pet1cKO animals; Two-way ANOVA; white matter: cervical p=0.1372; lumbar p=0.6764; gray matter: cervical p=0.4440; lumbar p=0.1995). Data are represented as mean ± SEM. (C) Decreased TdTomato+ arbors detected in Pet1cKO hippocampus compared to controls at 3 months of age. Scale bars, 200 µm, sagittal view. (D) Decreased TdTomato+ arbors detected in Pet1cKO cortex compared to controls at 3 months of age. Scale bars, 100 µm, coronal view. (E) Decreased TdTomato+ arbors detected in Pet1cKO olfactory bulb compared to controls at 3 months of age. Scale bars, 50 µm, sagittal view. cg, cingulum; cc, corpus callosum; LSM, lacunosum moleculare; DG, dentate gyrus; CA1 of hippocampus; gr, granule layer; gl, glomerular layer.
Figure 8—figure supplement 1. Pet1cKO and Lmx1bcKO mice exhibit distinct axon defects in thalamus.
Figure 8—figure supplement 2. DKO and Pet1-/- analyses.
We next investigated the Pet1cKO forebrain and found in striking contrast to the spinal cord that few if any TdTomato+ axons were present in the olfactory bulb, cortex, amygdala, hippocampus, striatum and many regions of the hypothalamus in Pet1cKO forebrains (Figure 8C–E). Furthermore, the pattern of TdTomato+ axons in the thalamus of Pet1cKO mice was severely disrupted with notable patches of clumped fibers and other areas with few, if any, fibers (Figure 8—figure supplement 1B). Similar to Lmx1bcKO mice, TdTomato+ axons were present in normal density and with proper ascending trajectory within the MFB of Pet1cKO mice (Figure 8—figure supplement 1C). These findings clearly demonstrate a requirement for Pet1 in forebrain 5-HT pathway formation and thus distinct transcription factor requirements in the generation of ascending and descending 5-HT axon projection pathways.
Although Pet1 conditional targeting largely phenocopies the forebrain 5-HT axon defects found in Lmx1bcKO mice, we noticed distinctly different patterns of TdTomato+ arbors in the Pet1cKO vs. Lmx1bcKO thalamus (Figure 8—figure supplement 1B). The Pet1cKO thalamus lacked TdTomato+ axons in lateral regions while in other thalamic regions we found abnormal clumping of Pet1cKO TdTomato+ axons (Figure 8—figure supplement 1B). Further, in contrast to the failure of Lmx1bcKO axons to demarcate the PVT, Pet1cKO TdTomato+ axons were properly patterned in this region (Figure 8—figure supplement 1B). This suggests Lmx1b may act independently of Pet1 in this specific highly discrete target field. Interestingly, early Pet1 deficiency did not result in reduced cell body size thus demonstrating that reduced cell body size did not contribute to the axon defects in Lmx1bcKO mice (Figure 8—figure supplement 1D). The robust similarities and yet distinct differences in TdTomato+ axon patterns in Pet1cKO compared to Lmx1bcKO forebrain support a model in which Lmx1b→Pet1 is the main regulatory program but that additional minor Lmx1b- or Pet1-orchestrated regulatory pathways operate in building ascending 5-HT axonal architectures (Figure 8—figure supplement 1F).
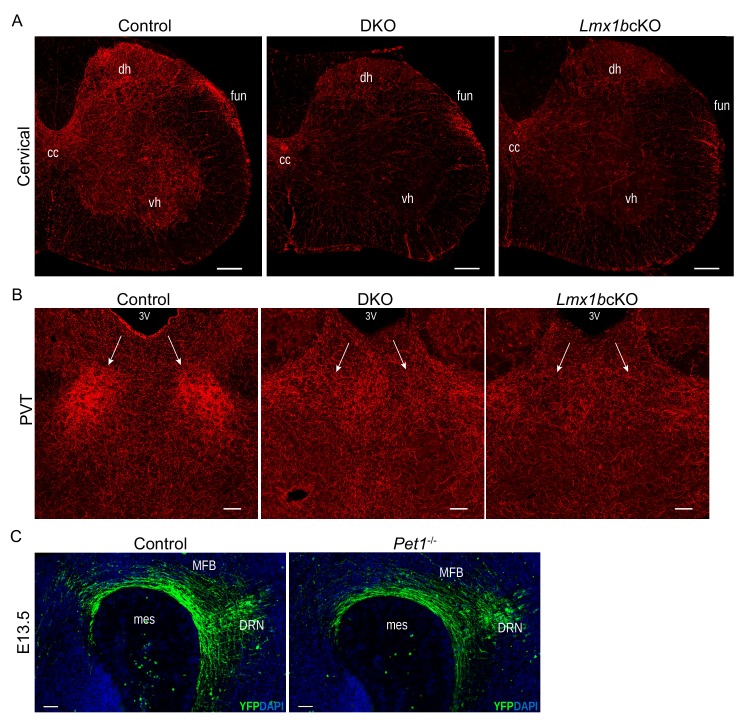
We investigated whether Lmx1b and Pet1 compensate for one another in the formation of 5-HT axons, by examining double-targeted mice (DKO: Lmx1bfl/fl; Pet1fl/fl;Pet1-Cre;Ai9) and controls (Lmx1b+/+; Pet1+/+;Pet1-Cre;Ai9). Analysis of DKO spinal cords revealed deficits in TdTomato+ axons patterns to similar levels as Lmx1bcKO spinal cord, further confirming Pet1 does not play a role in descending 5-HT axon development (Figure 8—figure supplement 2A). In the forebrain, we did not find a more extreme deficit in TdTomato+ axons in DKO mice compared to Lmx1bcKO mice. The DKO thalamus mirrored the Lmx1bcKO thalamus in the specific clumping of arbors and inability to properly pattern arbors in the PVT (Figure 8—figure supplement 2B).
To investigate whether the initial 1–2 days of Pet1 expression, not targeted by Pet1-Cre in Pet1cKO mice is required for primary ascending pathway formation, we analyzed Pet1-/-;Pet1-YFP mice in which the Pet1-YFP transgene labels control and mutant Pet1+ cell bodies and their axons with YFP (Hawthorne et al., 2010). We found comparable initial 5-HT primary axon outgrowth in Pet1-/- and control 5-HT neuron cell bodies at E13.5 (Figure 8—figure supplement 2C).
Lmx1b acts through Pet1 to temporally control postnatal stage-specific gene expression and forebrain arborization
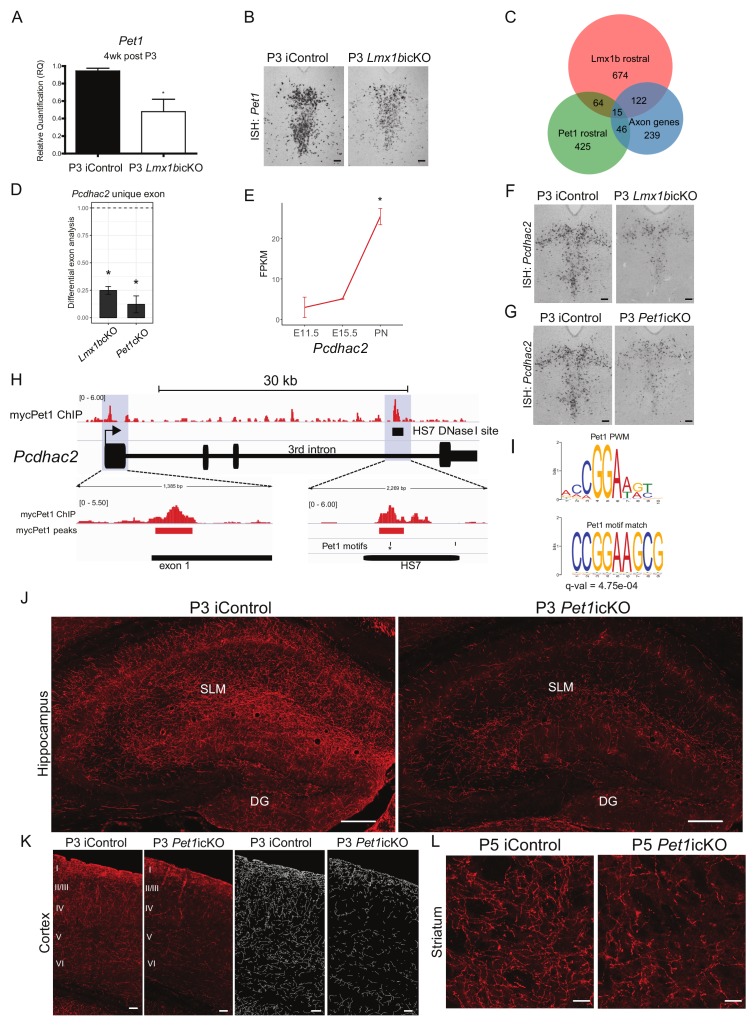
Stage-specific regulatory functions suggest potential stage-specific control of gene expression to fulfill successive steps in the morphological maturation of axon projection pathways. Thus, we next sought to determine whether the Lmx1b→Pet1 cascade temporally regulates expression of axon-related genes that are required at specific stages of 5-HT pathway formation. We found that Lmx1b continues to control Pet1 postnatally as Pet1 transcript levels were decreased in P3 targeted Lmx1bicKO mice (Figure 9A,B). Next, we used RNA-sequencing to find common Lmx1b and Pet1 targets. Comparing rostral Lmx1b and Pet1 regulated genes, we found 82 regulated genes present in both datasets (Figure 9C). By intersecting rostral Lmx1b and Pet1 regulated genes with the set of Lmx1b-regulated axon-related genes (Supplementary file 1), we found 15 co-regulated genes (Figure 9C). Interestingly, one of these coregulated genes is Pcdhac2. Extensive studies of Pcdhac2 have demonstrated its key intrinsic role in the formation and patterning 5-HT axon arbors (Chen et al., 2017; Katori et al., 2009; Katori et al., 2017). In particular, Pcdhac2 deficient mice exhibit a lack of forebrain 5-HT arbors and severe clumping of remaining arbors, thus raising a functional link to Lmx1b and Pet1.
Figure 9. An ascending specific Lmx1b→Pet1 cascade controls stage specific 5-HT gene expression and postnatal terminal arborization.
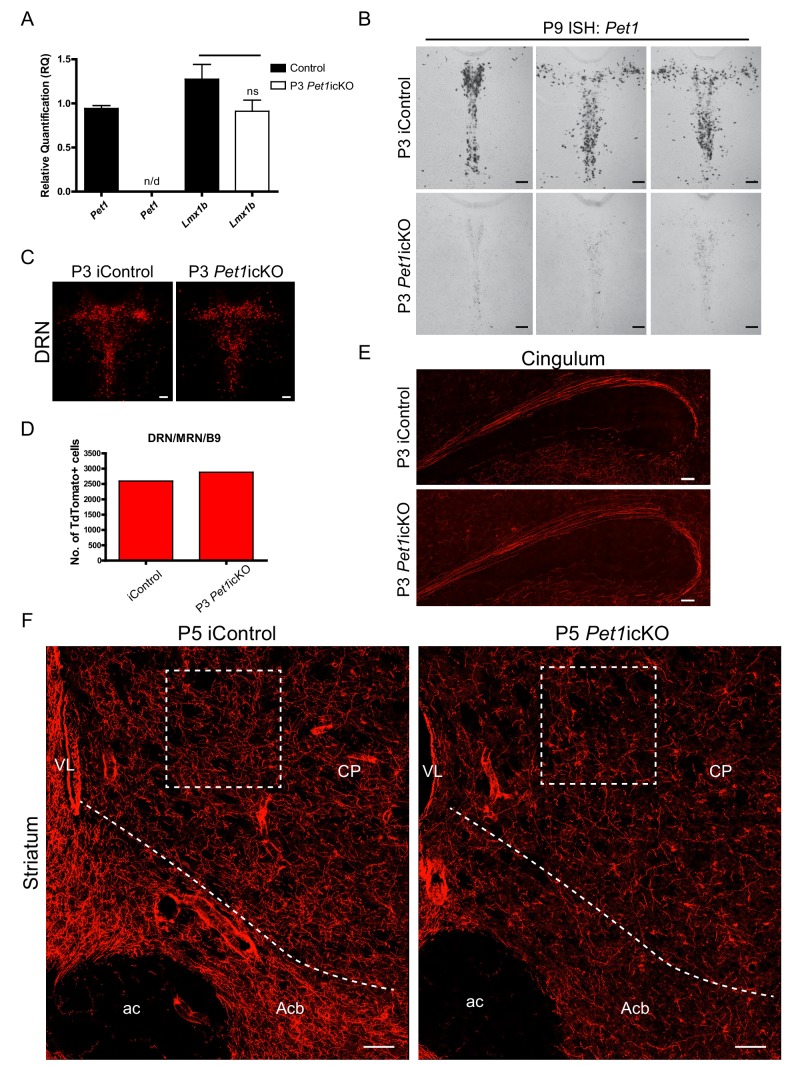
(A) RT-qPCR analysis of Pet1 expression in flow sorted TdTomato+ neurons 4 weeks post P3 tamoxifen treatment (n = 3, iControl; n = 4, P3 Lmx1bicKO mice). Unpaired t-test with Welch's correction, *p<0.05. Data are represented as mean ± SEM. (B) Pet1 in situ hybridization in P3 targeted Lmx1bicKO mice. Scale bars, 100 µm. (C) Venn diagram showing overlap of rostral Lmx1b and Pet1 regulated genes and the axon-related gene dataset. Lmx1b rostral: genes controlled by Lmx1b in rostral 5-HT neurons; Pet1 rostral: genes controlled by Pet1 in rostral 5-HT neurons; Axon genes: Lmx1b regulated rostral and caudal axon-related genes. (D) Relative expression of the unique Pcdhac2 exon in rostral Lmx1bcKO and Pet1cKO 5-HT neurons. * indicates FDR ≤ 0.05. Data are represented as mean ± SEM. (E) Developmental expression profile of Pcdhac2 in 5-HT neurons from E11.5 to early postnatal (PN). * indicates FDR ≤ 0.05. Data are represented as mean ± SEM. (F) Pcdhac2 in situ hybridization at postnatal day 14 in P3 targeted Lmx1bicKO mice. Representative image from n = 3 mice/genotype. Scale bars, 100 µm. (G) Pcdhac2 in situ hybridization at postnatal day 14 in P3 targeted Pet1icKO mice. ISH experiments done in parallel, iControl section is the same section in (F) and (G) to compare icKOs at the same tissue level. Representative image from n = 3 Pet1icKO mice. Scale bars, 100 µm. (H) Visualization of the Pcdhac2 gene locus showing mycPet1 ChIP peaks at the TSS and 3rd intron (shaded) and matches to the known Pet1 motif. Zoomed regions of the significant mycPet1 binding sites are shown at the bottom. (I) The mycPet1 binding region located within the 3rd Pcdhac2 intron DNAse I hypersensitivity site (HS7) contains a significant match to the known Pet1 position-weight matrix. TOMTOM q-value = 4.75×10−04. (J) Decreased TdTomato+ arbors detected in P3 targeted Pet1icKO hippocampus compared to iControl mice. Scale bars, 200 µm. (K) Decreased TdTomato+ arbors detected in all layers of P3 targeted Pet1icKO cortex (Imaris tracing; right panels) compared to iControl mice. Scale bars, 50 µm. (L) Decreased TdTomato+ arbors in striatum of P5 targeted Pet1icKO mice. Scale bars, 20 µm. See also Figure 9—figure supplement 1F.
Figure 9—figure supplement 1. Lmx1b→Pet1 cascade acts postnatally to control 5-HT terminal arborization.
Since Pcdhac2 is in the Pcdh alpha gene cluster and shares 3 (out of 4) exons with all other Pcdh alpha genes, we performed differential exon expression analysis with the Lmx1b and Pet1 RNA-seq datasets. By testing the only unique exon for Pcdhac2, we found that Pcdhac2 was significantly regulated by Lmx1b and Pet1 at E17.5 (Figure 9D). In contrast, Pcdhac2 expression was not significantly regulated by Pet1 at earlier stages (Wyler et al., 2016). Furthermore, our time-series RNA-seq analyses (Wyler et al., 2016) showed a significant and dramatic upregulation of Pcdhac2 only at the onset of the arborization stage (Figure 9E). Thus, Pcdhac2 expression is precisely controlled at the stage in which it is required for morphological maturation of 5-HT neurons.
To determine whether Lmx1b was required to control the postnatal upregulation of Pcdhac2, we treated Lmx1bicKO mice with tamoxifen at postnatal day three and analyzed Pcdhac2 expression by ISH at P14, when arborization is profusely developing. Indeed, we found substantially reduced expression of Pcdhac2 in P3 Lmx1bicKO mice (Figure 9F). To further probe whether the Lmx1b→Pet1 regulatory cascade is required for upregulation of Pcdhac2 during arborization, we generated Pet1icKO (Pet1fl/fl; Tph2-CreER; Ai9) mice. We first verified that Pet1 was effectively targeted in P3 Pet1icKO mice by RT-qPCR of flow sorted TdTomato+ cells as well as ISH for Pet1 (Figure 9—figure supplement 1A,B). We confirmed that similar numbers of TdTomato+ neurons were activated in P3 Pet1icKO mice (Figure 9—figure supplement 1C,D) and that long-range routes were filled compared to controls (Figure 9—figure supplement 1E). We found that Pet1 did not regulate Lmx1b at this stage (Figure 9—figure supplement 1A). Interestingly, we found that Pet1 was also required for the dramatic postnatal upregulation of Pcdhac2 expression (Figure 9G). These findings show that the Lmx1b→Pet1 regulatory cascade acts during the arborization stage to temporally control Pcdhac2 upregulation.
To determine if Pcdhac2 is a direct target of Lmx1b→Pet1, we analyzed our previously published ChIP-seq datasets (Wyler et al., 2016) for mycPet1 binding sites within the Pcdhac2 locus. Notably, we identified only two significant mycPet1 ChIP-seq peaks throughout the entire 250 kb Pcdha locus (Figure 9H). One peak was located at the 5’ end of the unique Pcdhac2 first exon. The second peak was located precisely within the third-intronic DNase I hypersensitivity site, HS7, which marks a well characterized transcriptional enhancer known to regulate midbrain expression of the Pcdha gene isoform (Kehayova et al., 2011; Ribich et al., 2006) (Figure 9H). Although the mycPet1 peak at the TSS of Pcdhac2 does not contain a match to the known Pet1 binding motif, the second peak within HS7 does contain a significant match to the Pet1 motif (Wei et al., 2010) (Figure 9I).
To determine whether Lmx1b→Pet1 cascade acts postnatally to control terminal arborization we analyzed the forebrains of P3 targeted Pet1icKO mice. Analyses performed at P49 revealed a severe deficit of 5-HT arbors in the hippocampus and cortex of P3 targeted Pet1icKO mice, comparable to the deficit found in P3 targeted Lmx1bicKO mice (Figures 5C, 5D; Figures 9J, 9K). The timing of 5-HT terminal arborization varies widely in different regions of the early postnatal forebrain (Lidov and Molliver, 1982; Maddaloni et al., 2017). Thus, we next sought to determine whether the Lmx1b→Pet1 cascade continues to control arborization in forebrain regions that are late to develop their mature axon patterns. To investigate this, we administered a single dose of tamoxifen to Pet1icKO pups at P5. We focused our subsequent analyses on the striatum, which is one of last regions of the forebrain to develop mature 5-HT arborization patterns (Lidov and Molliver, 1982). Interestingly, we found a notable deficit in the 5-HT arbors throughout the striatum (Figure 9L; Figure 9—figure supplement 1F). Together, our findings demonstrate that the Lmx1b→Pet1 cascade operates continually over an extended postnatal period to control stage-specific gene expression and to generate both early and late 5-HT terminal arbors patterns in different forebrain regions.
Discussion
The process of long-range axon pathway formation occurs in temporally defined stages over an extended period during which successive morphological events occur (Fame et al., 2011; Lidov and Molliver, 1982; Shirasaki and Pfaff, 2002). Many regulatory factors have been implicated in the intrinsic control (Santiago and Bashaw, 2014) of axon outgrowth, target selection, and terminal arborization (Arlotta et al., 2005; Chen et al., 2005; Galazo et al., 2016; Livet et al., 2002; Srivatsa et al., 2015). What is not understood are the intrinsic programs that operate temporally to progressively control the prolonged, multistage process of long-range axon projection pathway formation (Paolino et al., 2018). Our findings uncover a temporal regulatory strategy through which a continuously expressed transcription factor, Lmx1b, operates at successive stages to control progressive steps in the postmitotic morphological maturation of long-range highly diffuse axonal projection pathways. Thus, Lmx1b through its continuous expression not only controls the capacity for 5-HT synthesis and reuptake (Zhao et al., 2006), but also the formation of long range profusely arborized projection pathways that enable delivery of the transmitter throughout the CNS.
Our findings suggest Lmx1b acts to successively control 5-HT axon primary outgrowth, selective routing, and terminal arborization. We uncovered a delay in primary 5-HT axon outgrowth between E16.5 to E18.5 in Lmx1bcKO mice. However, initiation of primary axon outgrowth toward the forebrain or spinal cord was not disrupted in either Lmx1bcKO, Pet1cKO, or Pet1-/- mice. As 5-HT axonogenesis occurs concomitant with the onset of 5-HT synthesis in newborn 5-HT neurons (Hawthorne et al., 2010), perhaps upstream regulatory programs operating at the progenitor stage control initial axon outgrowth from newborn 5-HT neurons (Briscoe et al., 1999; Jacob et al., 2007; Pattyn et al., 2004).
The profound defect in the subsequent selective 5-HT axon routing through the cingulum, SCS, and fornix suggests a failure of intrinsic growth extension beyond selective routing choice points. In support of this, we never observed ectopic 5-HT axon trajectories. Yet, we leave open the possibility that axon guidance defects contribute to the failure of 5-HT axons to selectively extend through proper routes. 5-HT axons normally turn dorsally through the septal area at E18.5, which might constitute a critical choice point in building expansive axon 5-HT architectures. In contrast, the vast majority of mutant 5-HT axons fail to make the dorsal turn and then fail to continue into selective tracts. In support of a possible guidance defect, our RNA-seq studies indicated that Lmx1b controls expression of a large number of genes encoding guidance receptors, guidance receptor ligands, and adhesion molecules.
Transcription factors function within GRNs as elucidated in the specification and differentiation of various types of neurons (Deneris and Gaspar, 2018; Guillemot, 2007; Shirasaki and Pfaff, 2002). How transcription factors temporally interact within networks to progressively generate precise connectivity patterns is poorly understood (Paolino et al., 2018). A focus of our experiments was to determine whether Lmx1b functions in the context of the 5-HT GRN to control progressive development of 5-HT axonal pathways. We uncovered a temporal requirement for Lmx1b in maintaining Pet1 expression during the early postnatal stage of 5-HT pathway formation. This finding together with postnatal temporal targeting of Pet1 revealed the Lmx1b→Pet1 regulatory cascade acts stage specifically to control selective pathway routing and arborization of forebrain 5-HT axons. Despite the requirement for Pet1 in the acquisition of medullary 5-HT neuron-type identity (Hendricks et al., 2003; Kiyasova et al., 2011), it was surprising to find that Pet1 was not required for routing and arborization of descending 5-HT axons, thus revealing distinct transcription factor requirements in the generation of ascending and descending 5-HT axon pathways. Perhaps, Lmx1b operates with other continuously expressed serotonergic transcription factors in a regulatory cascade to control descending 5-HT axonal pathways. Gata3 is an interesting candidate as it functions more prominently in the medullary 5-HT neurons that generate the descending subsystem than 5-HT neurons that give rise to the ascending subsystem (Pattyn et al., 2004; van Doorninck et al., 1999).
The hundreds of axon related genes controlled by Lmx1b in the ascending and descending subsystems suggests that mis-expression of scores of functionally diverse effector genes accounts for the axon pathway defects we have reported. One gene of note is Gap43. Telencephalic commissures of Gap43 null mice fail to form, including the hippocampal commissure and the corpus callosum (Shen et al., 2002). This may largely explain the 5-HT neuron axonal projection deficits reported in the Gap43 null forebrain (Donovan et al., 2002). Although Gap43 is expressed in 5-HT neurons, it is not yet known whether it plays an intrinsic role in 5-HT axon development (Bendotti et al., 1991). Our findings highlight the potential importance of an intrinsic Lmx1b→Pet1→Gap43→5-HT axon regulatory path given that Gap43 expression is reduced in both Lmx1bcKO and Pet1cKO mice. It will be interesting to investigate this putative path in 5-HT conditionally targeted Gap43 mice to determine whether it accounts for the selective routing defects present in Lmx1bcKO and Pet1cKO mice.
The stage specific regulatory roles we have uncovered suggests Lmx1b may temporally control certain genes to fulfill stage-specific events in the morphological maturation of 5-HT neurons. In support of this idea, our findings reveal that Pcdhac2, a key intrinsic effector of 5-HT terminal arbor growth and patterning (Chen et al., 2017; Katori et al., 2009; Katori et al., 2017), is dynamically regulated by the ascending Lmx1b→Pet1 cascade. Tamoxifen-inducible targeting of Lmx1b and Pet1 revealed a temporal requirement for Lmx1b and Pet1 in the postnatal upregulation of Pcdhac2. Notably, our ChIP-seq datasets revealed Pet1 occupancy at the Pcdhac2-specific promoter and HS7 suggesting Lmx1b→Pet1 directly controls upregulation of Pcdhac2 during the postnatal arborization stage. Perhaps Pet1 occupancy at HS7 facilitates cohesin-mediated looping from HS7 to the Pcdhac2 promoter thus accounting for Pet1 occupancy at this promoter in the absence of a high affinity Pet1 binding motif (Guo et al., 2012).
Deficient expression of a single stage-specifically expressed target gene such as Pcdhac2 likely does not account for the severe and complex multi-stage defects in long-range 5-HT axon pathway formation reported here. However, our studies do serve to illustrate the concept that Lmx1b and Pet1 are critical terminal selectors of neuronal morphology and likely do so by dynamically regulating downstream genes that are required at specific stages for the progressive morphological maturation of 5-HT neurons. Perhaps subsets of Lmx1b controlled genes act in different 5-HT neuron subtypes and at distinct stages to control the specific routes of 5-HT axons to diverse forebrain and spinal cord targets.
We speculate that our findings illustrate a possible general mechanism through which continuously expressed terminal selectors build long-range diffuse axon pathways. In addition to 5-HT neurons, noradrenergic, dopaminergic, and histaminergic neurons also generate expansive and highly arborized axonal architectures (Björklund and Dunnett, 2007; Haas et al., 2008; Moore and Bloom, 1979). Lmx1b plays a critical role in dopaminergic (DA) neuron development. However, in contrast to 5-HT neurons, Lmx1b is co-expressed in postmitotic DA neurons with its paralog, Lmx1a, up to about 2 months of age (Laguna et al., 2015). These two LIM HD factors play compensatory roles in mesDA neuron specification and differentiation (Laguna et al., 2015; Yan et al., 2011). Simultaneous DA targeting of Lmx1a and Lmx1b beginning at E14 resulted in diminished DA axon outgrowth to the dorsal striatum while DA axon projections to the ventral striatum and other DA axon targets throughout the forebrain remained intact (Chabrat et al., 2017). These findings suggest that temporal control of axon routing and arborization of the DA mesostriatal, mesolimbic and mesocortical projection pathways may be controlled by different continuously acting terminal selector transcription factors expressed in DA neurons.
In addition to their tremendous intrinsic capacity for long-range axonal growth and arborization during development, 5-HT neurons are noted for their rare ability to sprout and regenerate axons after injury (Hawthorne et al., 2011; Jin et al., 2016). Given Lmx1b’s continuous expression into adulthood and crucial function in the formation of 5-HT projection pathways an intriguing line of investigation will be to determine whether Lmx1b transcriptionally powers 5-HT neuron’s intrinsic potential for axon regrowth following injury in adult brain or spinal cord, which may suggest ways to harness that power for development of new repair strategies.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference |
Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mus musculus) | Lmx1b | NA | MGI:1100513 | |
| Gene (M. musculus) | Pet1 | NA | MGI:2449712 | |
| Gene (M. musculus) | Tph2 | NA | MGI:2651811 | |
| Genetic reagent (M. musculus) | Pet1-Cre | PMID:16251278 | RRID:MGI:4837211 | Evan Deneris (Case Western Reserve University) |
| Genetic reagent (M. musculus) | RosaTom | Jackson Laboratory | Stock #: 007909; RRID:MGI:4436851 | Hongkui Zeng (Allen Institute for Brain Science) |
| Genetic reagent (M. musculus) | Tph2-CreERT2 | Jackson Laboratory | Stock #: 016584; RRID:IMSR_JAX:016584 | Bernd Gloss (NIEHS) |
| Genetic reagent (M. musculus) | Lmx1bflox | PMID:17151281 | RRID:MGI:3810753 | Zhou-Feng Chen (Washington University) |
| Genetic reagent (M. musculus) | Pet1flox | PMID:20818386 | RRID:MGI:4837213 | Evan Deneris (Case Western Reserve University) |
| Genetic reagent (M. musculus) | Tph2flox | PMID:24972638 | RRID:IMSR_JAX:027590 | Zhou-Feng Chen (Washington University) |
| Genetic reagent (M. musculus) | Pet1-/- | PMID:12546819 | MGI:2449922 | Evan Deneris (Case Western Reserve University) |
| Genetic reagent (M. musculus) | Pet1-YFP | PMID:16251278 | n/a | Evan Deneris (Case Western Reserve University) |
| Antibody | anti-RFP (rabbit polyclonal) | Rockland | Rockland: p/n 600-401-379; RRID:AB_2209751 | (1:200) |
| Antibody | anti-GFP (rabbit polyclonal) | Invitrogen | Invitrogen: A6455; RRID:AB_221570 | (1:200) |
| Antibody | anti-5-HT (rabbit polyclonal) | Immunostar | Immunostar: 20080; RRID:AB_1624670 | (1:200) |
| Antibody | anti-Tph2 (rabbit polyclonal) | Millipore | Millipore: ABN60; RRID:AB_10806898 | (1:500) |
| Antibody | anti-Lmx1b (rabbit polyclonal) | Suleiman et al., 2007-gift | n/a | (1:200) |
| Antibody | anti-RFP (mouse monoclonal) | Abcam | Abcam: ab65856; RRID:AB_1141717 | (1:200) |
| Antibody | anti-RFP (mouse monoclonal) | Rockland | Rockland: p/n 200-301-379; RRID:AB_2611063 | (1:200) |
| Antibody | Alexa 488- or 594- secondaries | Invitrogen | (1:500) | |
| Genetic reagent (Virus) | rAAv2/Ef1a-DIO-hchR2 (H134R)-EYFP | UNC GTC Vector Core | Lot# AV4378K | |
| Sequence-based reagent | Pcdhac2 in situ primer: F: 5’ AGCCACCTCTATCAGCTACCG 3’ | this paper | ||
| Sequence-based reagent | Pcdhac2 in situ primer: R: 5’ AGAATTAACCCTCACTAAAGGGCTCATTTTGAGAGCCAGCATCA 3’ | this paper | ||
| Sequence-based reagent | Pet1 in situ primers: F:5’CCAGTGACCAATCCCATCCTC3’ | PMID:26843655 | ||
| Commercial assay or kit | Transcriptor First Strand cDNA Synthesis Kit | Roche | REF 4896866001 | |
| Commercial assay or kit | PerfeCTa PreAmp SuperMix | QuantoBio | Cat. No. 95146–040 | |
| Commercial assay or kit | PerfeCTa FastMix II ROX mastermix | QuantaBio | Cat. No. 95119–012 | |
| Commercial assay or kit | RNA Clean and ConcentratorTM-5 kit | Zymo Research | Catalog Nos. R1015 and R1016 | |
| Chemical compound, drug | Tamoxifen | Sigma-Aldrich | CAS Number: 10540-29-1 | 10 mg/mL stock in corn oil |
| Other | 35 μm filter | BD biosciences | Cat. No. 352235 | |
| Other | Fetal bovine serum (FBS) | Gibco | Cat. No. 16000044 | |
| Other | DNase I | Invitrogen | Cat. No. 18047019 | |
| Other | Trizol LS | Invitrogen | Cat. No. 10296010 | |
| Other | TrypLE | Invitrogen | Cat. No. 12605010 | |
| Other | Leibovitz’s L15 medium | Invitrogen | Cat. No. 11415114 | |
| Commercial assay or kit | Quantifluor RNA system | Promega | Cat. No. E3310 | |
| Commercial assay or kit | Nugen TRIO RNA-seq | Nugen | Cat. No. 0507–08 | |
| Commercial assay or kit | Zymo RNA clean and concentrator | Zymo Research | Cat. No. R1013 | |
| Other | FACS Aria I | Becton Dickinson | ||
| Other | iCyt cell sorter | Sony | ||
| Other | Fragment analyzer | Advanced Analytics | ||
| Other | Nextseq 550 | Illumina | RRID:SCR_016381 | |
| Software, algorithm | FASTQC | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ | RRID:SCR_014583 | |
| Software, algorithm | Trimmomatic | Bolger et al., 2014 | RRID:SCR_011848 | |
| Software, algorithm | Cufflinks (v2.2.2) | Trapnell et al., 2010 | RRID:SCR_014597 | |
| Software, algorithm | R (v. 3.5) | http://www.r-project.org | RRID:SCR_001905 | |
| Software, algorithm | Webgestalt | http://www.webgestalt.org | RRID:SCR_006786 | |
| Software, algorithm | Biovenn | http://www.biovenn.nl | ||
| Software, algorithm | DEXSeq | Anders et al., 2012 | RRID:SCR_012823 | |
| Software, algorithm | Tophat2 (v2.1.1) | Kim et al., 2013 | RRID:SCR_013035 | |
| Other | Mus musculus genome | Ensembl, v. 96 | RRID:SCR_002344 | |
| Other | Mus musculus genome | UCSC, mm10 | RRID:SCR_005780 | |
| Software, algorithm | featureCounts | Rsubread | RRID:SCR_012919 | |
| Software, algorithm | ENCODE Transcription Factor ChIP-seq analysis pipeline | https://github.com/ENCODE-DCC/chip-seq-pipeline | RRID:SCR_015482 | |
| Software, algorithm | Burroughs-Wheeler aligner (BWA) | http://bio-bwa.sourceforge.net/ | RRID:SCR_010910 | |
| Software, algorithm | MACS2 | Zhang et al., 2008 | RRID:SCR_013291 | |
| Software, algorithm | Imaris 7.4.2 | Bitplane, Zurich, Switzerland | Surfaces or Filament Tracer tool |
Animals
All procedures were approved by the Institutional Animal Care and Use Committees of Case Western Reserve University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed on male and female mice using age-matched and sex-matched controls in triplicate unless otherwise noted. Adult mice between the ages 2.5 to 3.5 months were used unless otherwise noted. Early conditional knockout mice (designated Lmx1bcKO, Pet1cKO, and Tph2cKO) were generated by using the following genetic mouse lines: Pet1-Cre (original name ePet-Cre) (Scott et al., 2005), Ai9 Rosa TdTomato (RosaTom; Jackson labs) and either Lmx1bfl (Zhao et al., 2006), Pet1fl (Liu et al., 2010), or Tph2fl (Kim et al., 2014). All early conditional knockout mice were compared to non-floxed controls (+/+;Pet1-Cre;Ai9). The following genetic lines were used to generate Lmx1bicKO or Pet1icKO mice for postnatal stage conditional knockout: Tph2-CreERT2 (Jackson lab), Ai9 Rosa TdTomato (RosaTom; Jackson lab) and either Lmx1bfl (Zhao et al., 2006) or Pet1fl (Liu et al., 2010). All postnatal stage conditional knockout mice were compared to non-floxed controls (iControls: +/+;Tph2-CreER; Ai9). Pet1-/- mice (Hendricks et al., 2003) carrying the Pet1-YFP transgene (Scott et al., 2005) were used for embryo studies at E13.5. Tail or ear genomic DNA was used to determine genotypes of all animals. All mice were housed in ventilated cages on a 12 hr light/dark cycles with access to food and water with 2–5 mice per cage.
Embryos and postnatal pups
All embryo experiments at each time point were performed in triplicate unless otherwise noted and compared to littermate controls (Lmx1bfl/+ vs Lmx1bfl/fl; Pet1fl/+ vs Pet1fl/fl; Pet1+/- vs Pet1-/-). Both male and female littermates were used for analysis. Embryonic day 0.5 (E0.5) was determined by presence of a vaginal plug. Postnatal day 0 (P0) was designated by date of birth.
Histology and immunohistochemistry
Adult mice were anesthetized with Avertin (44 mM tribromoethanol, 2.5% tert-amyl alcohol, 0.02 ml/g body weight) and perfused for 2–3 min with cold PBS followed by 20 min cold 4% paraformaldehyde (PFA) in PBS. Brains and/or spinal cords were removed, post-fixed in 4%PFA for 2 hr and placed in 30% sucrose/PBS overnight (O/N) for cryoprotection. E12-E13 embryos were drop fixed in 4% PFA in PBS O/N followed by 30% sucrose incubation O/N. All embryos between age E15-E18 were transcardially perfused with 5 mL of 4% PFA in PBS, incubated in 4% PFA in PBS O/N, and incubated in 30% sucrose O/N. All tissue collected was frozen in Optimal Cutting Temperature (OCT) solution and sectioned on a cryostat at 25 µm. Tissue sections were mounted on SuperFrost Plus slides (Thermo Fisher Scientific) and vacuum dried. Sections were then permeabilized in 0.3% Triton 100X-PBS (PBS-T) for 15 min followed by antigen retrieval in Sodium Citrate buffer for 5 min in the microwave at low power. Sections were blocked with 10% NGS in PBS-T for 1 hr followed by incubation in primary antibody using a rabbit anti-RFP antibody (1:200; p/n 600-401-379, Rockland) at 4 °C O/N. Antigen retrieval step was not used for the following primary antibodies: rabbit anti-GFP (1:200; A6455, Invitrogen), rabbit anti-5-HT (1:200; Immunostar), rabbit anti-Tph2 (1:500; ABN60, Millipore), and rabbit anti-Lmx1b (1:200; Suleiman et al., 2007). For all co-stains with TdTomato a mouse anti-RFP (1:200; ab65856, Abcam) or a mouse anti-RFP (1:200; p/n 200-301-379, Rockland) primary antibody was used. Secondary antibodies were used at 1:500; goat anti-rabbit or mouse, Alexa Fluor 594 or 488 (Invitrogen).
In situ hybridization
In situ hybridization was performed using a standard protocol using a digoxigen-11-UTP labeled antisense RNA probe to detect Pet1 and Pcdhac2 (Roche diagnostics) as described elsewhere (Wyler et al., 2016). Pcdhac2 probe (566 bp) was generated using the following primers: F: 5’ AGCCACCTCTATCAGCTACCG 3’ and R: 5’ AGAATTAACCCTCACTAAAGGGCTCATTTTGAGAGCCAGCATCA 3’. Pet1 probe (513 bp) was generated using primers: F:5’CCAGTGACCAATCCCATCCTC3’ and R: 5’AGAATTAACCCTCACTAAAGGGTTAATGGGGCTGAAAGGGATA3’.
Cell counts
5-HT neurons were identified by Tph2 immunostaining. To calculate the Pet1-Cre efficiency, every 4th section was taken through the entire rostrocaudal extent of the DRN/MRN/B9 and medullary areas of control mice (n = 2 control mice) and RFP+/TPH2+ and TPH2+/RFP+ ratios were calculated. Tph2-CreER efficiency in postnatal tamoxifen injected pups was calculated from sections taken through the entire rostrocaudal extent of the DRN/MRN/B9 and expressed as a ratio of RFP+/TPH2+ and TPH2+/RFP+ cells (n = 4 iControl mice). A computer program that permits blinded manual cell counting was used (Fox and Deneris, 2012) to determine numbers of TdTomato+ cells between control vs Lmx1bcKO, control vs Tph2cKO, and iControl vs Lmx1bicKO or Pet1icKO mice. Every 4th matched section was counted through the entire rostrocaudal extent of the DRN/MRN/B9 or medullary 5-HT system as described. To calculate the percentage of remaining Tph2+ neurons in Lmx1bcKO vs Tph2cKO mice, Tph2+ neurons were counted in every 4th section throughout the DRN/MRN/B9. Tph2+ cell numbers in cKO animals was normalized to the number of Tph2+ cells in control matched sections and expressed as a percentage for each genotype comparison.
Tamoxifen injections
P1 pups (Lmx1bfl/fl;Tph2-CreER;Ai9 and iControls; n = 4 mice/genotype) were injected 1X with 100 µg of tamoxifen (10 mg/mL stock in corn oil). P3 or P5 pups (P3: Lmx1bfl/fl;Tph2-CreER;Ai9, Pet1fl/fl;Tph2-CreER;Ai9, and iControls; n = 3 mice/genotype; P5: Pet1fl/fl;Tph2-CreER;Ai9 and iControls, n = 2 mice/genotype) were injected 1X with 200 µg tamoxifen. All tamoxifen injections were performed subcutaneously at the back of the neck using a 30-gauge needle on a 1 mL syringe. Pups were allowed to sit for a few minutes before returning to mother. Injected mice were taken at either 31 or 49 days of age for analysis as indicated.
Viral injections
Adult animals (Lmx1bfl/fl;Pet1-Cre;Ai9 and Lmx1b+/+;Pet1-Cre;Ai9; n = 2 mice/genotype) were anesthetized with Isoflurane and stereotaxically injected unilaterally at two sites with 1.5 µl and 1 µl rAAv2/Ef1a-DIO-hchR2 (H134R)-EYFP (UNC GTC Vector Core Lot# AV4378K) at X = 0.6 mm, Y = −4.2 mm, Z = −3.2 mm and X = 0.6 mm, Y = −4.2 mm, Z = −4.2 mm relative to Bregma respectively. Animals were treated with a local anesthetic (bupivacaine HCL; Hospira) administered subcutaneously prior to surgery and with analgesic (carprofen 5 mg/Kg; Pfizer) for 3 days following. Holes were drilled through the skull to expose brain, following which a Hamilton syringe was slowly lowered to desired coordinates. Infusion rate was set to 0.1 µl/min with 10 min after each injection to allow diffusion of virus. Animals were returned to group housing following recovery and sacrificed 10 weeks after surgery.
5-HT neuron dissociation and flow cytometry
For RNAseq analyses rostral and caudal E17.5 YFP+ 5-HT neurons were collected from Lmx1bfl/fl;Pet1-EYFP or Pet1fl/fl;Pet1-EYFP (controls) and Lmx1bfl/fl;Pet1-EYFP;Pet1-Cre or Pet1fl/fl;Pet1-EYFP;Pet1-Cre (Lmx1bcKO or Pet1cKO) mice using flow cytometry. To dissociate embryonic 5-HT neurons, hindbrains were initially dissected to separate rostral 5-HT neurons from caudal 5-HT neurons in cold PBS. Tissue was transferred to 1.5 mL ependorf tubes and centrifuged at 1500 rpm for 1 min at 4 °C. PBS was removed and 500 µL 1X TrypLE Express (Gibco) was added to the tissue and incubated at 37 °C for 15 min followed by addition of L-15 media (Gibco). Samples were centrifuged for 1 min at 1500 rpm and washed 3X with PBS. Tissue was then resuspended in 500 µL L15/0.1%BSA/DNase solution and slowly triturated 30X with fire-polished Pasteur pipettes of decreasing bore size until fully suspended. Samples were then filtered, and flow sorted on a FACS Aria I or Sony iCyt cell sorter.
Postnatal 5-HT neuron dissociation was performed in either P2 Lmx1bcKO or 4 week old Lmx1bicKO or Pet1icKO mice. Brains were sliced at 400 µm on a vibratome (Pelco easiSlicer) in continuously bubbling (95%O2; 5%CO2) aCSF solution (3.5 mM KCl, 126 mM NaCl, 20 mM NaHCO3, 20 mM Dextrose, 1.25 mM NaH2PO4, 2 mM CaCl2, 2 mM MgCl2, 50 μm AP-V (Tocris), 20 μm DNQX (Sigma), and 100 nM TTX (Abcam)). Sections containing rostral 5-HT neurons were incubated in 1 mg/mL Protease from Streptomyces griseus (Sigma; P8811) in bubbling aCSF solution for 30min (P2 mice) or 45min (4-week-old mice) at room temperature. Slices were then incubated for 15min in bubbling aCSF alone at RT. TdTomato+ neurons were microdissected from slices in cold aCSF/10%FBS solution. Samples were slowly triturated 30-100X with fire-polished Pasteur pipettes of decreasing bore size until fully suspended. Samples were then filtered, and flow sorted on a FACS ARIA-SORP sorter.
RNA sequencing
Total RNA was isolated from flow-sorted neurons using Trizol LS (Invitrogen) and the RNA Clean and Concentrator-5 kit (Zymo Research). RNA concentration and quality was determined using Quantifluor RNA system (Promega) and Fragment analyzer (Advanced Analytics). Samples were converted to cDNA, depleted of rRNA transcripts, and amplified using the TRIO RNA-seq kit for mouse (Nugen Inc). Single-end sequencing was performed on a Nextseq 550 (Illumina) for 76 cycles. Read quality was assessed using FASTQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and adapters were trimmed using Trimmomatic (Bolger et al., 2014). Filtered and trimmed reads were aligned to the mouse genome (mm10, UCSC) using Tophat2 (v2.1.1) (Kim et al., 2013).
RNA sequencing analysis
Gene expression quantification and differential expression were analyzed using Cufflinks v2.2.2 (Trapnell et al., 2010). In all differential expression comparisons, a gene was called differentially expressed if fold-change was ≥1.5 and false discovery rate (FDR) was ≤5%. Hierarchical clustering of differentially expressed genes in rostral Lmx1bcKO and caudal Lmx1bcKO samples was performed in R (v. 3.5) using row-scaled values with Euclidean distance and complete linkage. The heatmap was plotted with the gplots library. Gene Ontology analysis was performed using Webgestalt (http://www.webgestalt.org), requiring five genes per category and FDR ≤ 5%. Venn diagrams were generated using Biovenn (http://www.biovenn.nl) (Hulsen et al., 2008; Wang et al., 2017). Differential exon expression analysis was performed to test for differences of the unique Pcdhac2 first exon with DEXSeq (Anders et al., 2012). RNA-seq reads were mapped to the Mus musculus genome (Ensembl, v. 96) and reads falling within annotated exons were counted using featureCounts (Rsubread). DEXSeq default settings were used and significant exon usage differences were determined at FDR ≤ 5%.
qPCR
RNA was isolated from YFP+ flow sorted cells in Trizol LS (Invitrogen) using chloroform extraction followed by the RNA Clean and Concentrator-5 kit (Zymo Research). cDNA was then synthesized using equal input RNA with the Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNA was then amplified (8 PCR cycles) using PerfeCTa PreAmp SuperMix (QuantoBio) followed by ExoI digest to remove excess primers. RT-qPCR was performed using PerfeCTa FastMix II ROX mastermix (QuantaBio) with TaqMan probes (Thermofisher Scientific).
Chromatin immunoprecipitation analysis
ChIP-seq data for mycPet1 was obtained from Wyler et al. (2016). The data was re-analyzed using the ENCODE Transcription Factor ChIP-seq analysis pipeline (https://github.com/ENCODE-DCC/chip-seq-pipeline). Reads were mapped to the Mus musculus genome (UCSC mm10) using the Burroughs-Wheeler aligner (BWA) and peaks were called with MACS2 with FDR ≤ 1% (Zhang et al., 2008).
Axon quantification
Spinal cord: Coronal sections of spinal cords were taken at 25 µm. Two sections were taken blindly from each cervical and lumbar spinal segment from each genotype (n = 3, control; n = 3, Lmx1bcKO mice) and (n = 3, control; n = 3, Pet1cKO). Whole gray area or whole white matter area was selected independently, and pixels were quantified using Zeiss 2.3 Image Analysis module. Pixel values were normalized to selected area (µm2) for each section. Two-way ANOVA with Welch’s correction analysis was performed. p values for each comparison are detailed in Fig. Legends.
Forebrain: Coronal sections of hippocampus, cortex, and axon tracts (SCS and cingulum) were taken and TdTomato+ pixels were quantified using Zeiss 2.3 Image Analysis module in P3 targeted iControls (n = 3 non-littermate mice) and P3 Lmx1bicKO mice (n = 3 non-littermate mice). Pixel values were normalized to selected area (µm2). Unpaired t-test with Welch's correction statistical analysis was performed. p values for each comparison are detailed in Fig. Legends.
Cell volume analysis
Thick tissue sections were sliced at 150 µm and cleared using CUBIC clearing technique to increase the optical transparency of sections (Susaki et al., 2015). CUBIC reagent was prepared using urea (25 wt% final concentration), Quadrol (25 wt% final concentration), Triton X-100 (15 wt% final concentration) and dH2O. Tissue sections were incubated in CUBIC reagent at 4°C overnight. To avoid the distortion of tissue by compression of the glass cover slip, Blu-Tack reusable adhesive was used to create a reservoir with a 0.3–0.5 mm depth. The transferred tissue sections were then sealed in CUBIC reagent using a glass cover slip for z-stack confocal imaging of the endogenous TdTomato fluorescence of cell bodies. The 3D neuron images were reconstructed and measured using the Surfaces or Filament Tracer tool in Imaris 7.4.2 (Bitplane, Zurich, Switzerland). Unpaired t-test with Welch's correction statistical analysis was performed. Sample size and p values for each comparison are detailed in Fig. Legends.
Image acquisition and processing
Immuno-stained slides were imaged on an LSM800 confocal microscope (Carl Zeiss). All embryonic forebrain images were captured using a BZ-X700 fluorescence microscope (Keyence) or Olympus Optical BX51 microscope. Global brightness and contrast were edited across whole images equally between genotypes. Whole sagittal sections (Figure 1A,B) were acquired using confocal 10X objective and both control and Lmx1bcKO sections were processed in ImageJ and subjected to equal background subtraction followed by Lookup Table-Fire to enhance visualization of TdTomato+ axon intensities in the forebrain. Axon tracing in Figure 5D and Figure 9K was performed using the Filament Tracer module in Imaris 7.4.2 (Bitplane, Zurich, Switzerland).
Accession codes
All data generated in this study are deposited in NCBI GEO under accession code GSE130514.
Acknowledgements
We thank Lynn Landmesser for key insights and suggestions throughout the course of this study. We thank Polyxeni Philippidou for her many helpful comments on the manuscript. We thank Wen-Cheng Xiong for the use of the BZ-X700 fluorescence microscope. We thank Randy Johnson for Lmx1b floxed mice. We thank Ralph Witzgall for the Lmx1b antibody. We thank Steven Wyler for the pup picture. This research was supported by the Genomics Core Facility and the Flow cytometry core at the CWRU School of Medicine. This research was supported by NIH grants P50 MH096972 and RO1 MH062723 to ESD.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Evan S Deneris, Email: esd@case.edu.
Anne E West, Duke University School of Medicine, United States.
Eve Marder, Brandeis University, United States.
Funding Information
This paper was supported by the following grants:
National Institute of Mental Health P50 MH096972 to Evan S Deneris.
National Institute of Mental Health RO1 MH062723 to Evan S Deneris.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Validation, Investigation, Interpretation of data.
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Writing—original draft, Writing—review and editing, Interpretation of data.
Investigation, Performed stereotaxic injection of rAAV2/Ef1a-DIO-hchR2-EYFP and in situ hybridization.
Investigation, Performed histochemical analysis and imaging of DKO mice.
Investigation, Methodology.
Investigation, Methodology, Performed cell body morphological analyses and measurements, Performed Imaris tracing and imaging.
Writing—review and editing, Interpretation of data.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Writing—review and editing, Interpretation of data.
Ethics
Animal experimentation: All animal procedures used in this study were in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Case Western Reserve University School of Medicine Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3145-01, protocol #: 2014-0044).
Additional files
Axon genes that are also regulated by Pet1 in rostral 5-HT neurons at E17.5 are in bold.
Data availability
Raw ChIP-seq data GEO accession: GSE74315. RNA-seq data generated in this study and ChIP-seq analysis are deposited in NCBI GEO under accession code GSE130514.
The following dataset was generated:
Donovan LJ, Spencer WC, Kitt MM, Eastman BA, Lobur KJ, Jiao K, Silver J, Deneris ES. 2019. Lmx1b is required at multiple stages to build expansive serotonergic axon architectures. NCBI Gene Expression Omnibus. GSE130514
The following previously published dataset was used:
Wyler SC, Spencer WC, Green NH, Rood BD, Crawford L, Craige C, Gresch P, McMahon DG, Beck SG, Deneris ES. 2016. Pet-1 Switches Transcriptional Targets Postnatally to Regulate Maturation of Serotonin Neuron Excitability. NCBI Gene Expression Omnibus. GSE74315
References
- Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Research. 2012;22:2008–2017. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. The Journal of Comparative Neurology. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RG, Törk I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- Ballion B, Branchereau P, Chapron J, Viala D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Developmental Brain Research. 2002;137:81–88. doi: 10.1016/S0165-3806(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. European Journal of Neuroscience. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendotti C, Servadio A, Samanin R. Distribution of GAP-43 mRNA in the brain stem of adult rats as evidenced by in situ hybridization: localization within monoaminergic neurons. The Journal of Neuroscience. 1991;11:600–607. doi: 10.1523/JNEUROSCI.11-03-00600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neurosciences. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Chabrat A, Brisson G, Doucet-Beaupré H, Salesse C, Schaan Profes M, Dovonou A, Akitegetse C, Charest J, Lemstra S, Côté D, Pasterkamp RJ, Abrudan MI, Metzakopian E, Ang SL, Lévesque M. Transcriptional repression of Plxnc1 by Lmx1a and Lmx1b directs topographic dopaminergic circuit formation. Nature Communications. 2017;8:933. doi: 10.1038/s41467-017-01042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. PNAS. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WV, Nwakeze CL, Denny CA, O'Keeffe S, Rieger MA, Mountoufaris G, Kirner A, Dougherty JD, Hen R, Wu Q, Maniatis T. Pcdhαc2 is required for axonal tiling and assembly of serotonergic circuitries in mice. Science. 2017;356:406–411. doi: 10.1126/science.aal3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, Huang Y, Xu TL, Ding YQ, Xu L. Enhanced contextual fear memory in central serotonin-deficient mice. PNAS. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris E, Gaspar P. Serotonin neuron development: shaping molecular and structural identities. Wiley Interdisciplinary Reviews: Developmental Biology. 2018;7:e301. doi: 10.1002/wdev.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Hobert O. Maintenance of postmitotic neuronal cell identity. Nature Neuroscience. 2014;17:899–907. doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan SL, Mamounas LA, Andrews AM, Blue ME, McCasland JS. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. The Journal of Neuroscience. 2002;22:3543–3552. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends in Neurosciences. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet V, Jany M, Fabre V, Chali F, Orsal D, Schweitzer A, Andrieux A, Messanvi F, Giros B, Hamon M, Lanfumey L, Deloulme JC, Martres MP. The deletion of the microtubule-associated STOP protein affects the serotonergic mouse brain network. Journal of Neurochemistry. 2010;115:1579–1594. doi: 10.1111/j.1471-4159.2010.07064.x. [DOI] [PubMed] [Google Scholar]
- Fox SR, Deneris ES. Engrailed is required in maturing serotonin neurons to regulate the cytoarchitecture and survival of the dorsal raphe nucleus. Journal of Neuroscience. 2012;32:7832–7842. doi: 10.1523/JNEUROSCI.5829-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D, Parent M. Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLOS ONE. 2014;9:e87709. doi: 10.1371/journal.pone.0087709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazo MJ, Emsley JG, Macklis JD. Corticothalamic projection neuron development beyond subtype specification: fog2 and intersectional controls regulate intraclass neuronal diversity. Neuron. 2016;91:90–106. doi: 10.1016/j.neuron.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- Guo Y, Monahan K, Wu H, Gertz J, Varley KE, Li W, Myers RM, Maniatis T, Wu Q. CTCF/cohesin-mediated DNA looping is required for protocadherin α promoter choice. PNAS. 2012;109:21081–21086. doi: 10.1073/pnas.1219280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiological Reviews. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioural Brain Research. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hawthorne AL, Wylie CJ, Landmesser LT, Deneris ES, Silver J. Serotonergic neurons migrate radially through the neuroepithelium by dynamin-mediated somal translocation. Journal of Neuroscience. 2010;30:420–430. doi: 10.1523/JNEUROSCI.2333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, Silver J. The unusual response of serotonergic neurons after CNS injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. Journal of Neuroscience. 2011;31:5605–5616. doi: 10.1523/JNEUROSCI.6663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/S0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. PNAS. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. Terminal selectors of neuronal identity. Current Topics in Developmental Biology. 2016;116:455–475. doi: 10.1016/bs.ctdb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. Journal of Neuroscience. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. Journal of Chemical Neuroanatomy. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional venn diagrams. BMC Genomics. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Ferri AL, Milton C, Prin F, Pla P, Lin W, Gavalas A, Ang SL, Briscoe J. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nature Neuroscience. 2007;10:1433–1439. doi: 10.1038/nn1985. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dougherty SE, Wood K, Sun L, Cudmore RH, Abdalla A, Kannan G, Pletnikov M, Hashemi P, Linden DJ. Regrowth of serotonin axons in the adult mouse brain following injury. Neuron. 2016;91:748–762. doi: 10.1016/j.neuron.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain Areas. Journal of Neuroscience. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori S, Noguchi-Katori Y, Okayama A, Kawamura Y, Luo W, Sakimura K, Hirabayashi T, Iwasato T, Yagi T. Protocadherin-αC2 is required for diffuse projections of serotonergic axons. Scientific Reports. 2017;7:15908. doi: 10.1038/s41598-017-16120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehayova P, Monahan K, Chen W, Maniatis T. Regulatory elements required for the activation and repression of the protocadherin-alpha gene cluster. PNAS. 2011;108:17195–17200. doi: 10.1073/pnas.1114357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kim A, Zhao ZQ, Liu XY, Chen ZF. Postnatal maintenance of the 5-Ht1a-Pet1 autoregulatory loop by serotonin in the raphe nuclei of the brainstem. Molecular Brain. 2014;7:48. doi: 10.1186/1756-6606-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. Journal of Neuroscience. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna A, Schintu N, Nobre A, Alvarsson A, Volakakis N, Jacobsen JK, Gómez-Galán M, Sopova E, Joodmardi E, Yoshitake T, Deng Q, Kehr J, Ericson J, Svenningsson P, Shupliakov O, Perlmann T. Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson's disease. Nature Neuroscience. 2015;18:826–835. doi: 10.1038/nn.4004. [DOI] [PubMed] [Google Scholar]
- Lee S, Walker CL, Karten B, Kuny SL, Tennese AA, O'Neill MA, Wevrick R. Essential role for the Prader-Willi syndrome protein necdin in axonal outgrowth. Human Molecular Genetics. 2005;14:627–637. doi: 10.1093/hmg/ddi059. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Research Bulletin. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nature Neuroscience. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/S0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- Maddaloni G, Bertero A, Pratelli M, Barsotti N, Boonstra A, Giorgi A, Migliarini S, Pasqualetti M. Development of serotonergic fibers in the Post-Natal mouse brain. Frontiers in Cellular Neuroscience. 2017;11 doi: 10.3389/fncel.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Molecular Psychiatry. 2013;18:1106–1118. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annual Review of Neuroscience. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Structure and Function. 2016;221:535–561. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, Kim JC, Cook MN, Dymecki SM. Multi-Scale molecular deconstruction of the serotonin neuron system. Neuron. 2015;88:774–791. doi: 10.1016/j.neuron.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino A, Fenlon LR, Suárez R, Richards LJ. Transcriptional control of long-range cortical projections. Current Opinion in Neurobiology. 2018;53:57–65. doi: 10.1016/j.conb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nature Neuroscience. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Sandillon F, Geffard M, Privat A. Pre- and post-natal ontogeny of serotonergic projections to the rat spinal cord. Journal of Neuroscience Research. 1989;22:305–321. doi: 10.1002/jnr.490220311. [DOI] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, Luo L. Anatomically defined and functionally distinct dorsal raphe serotonin Sub-systems. Cell. 2018;175:472–487. doi: 10.1016/j.cell.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribich S, Tasic B, Maniatis T. Identification of long-range regulatory elements in the protocadherin-alpha gene cluster. PNAS. 2006;103:19719–19724. doi: 10.1073/pnas.0609445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Bashaw GJ. Transcription factors and effectors that regulate neuronal morphology. Development. 2014;141:4667–4680. doi: 10.1242/dev.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. PNAS. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. The Journal of Neuroscience. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annual Review of Neuroscience. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Srivatsa S, Parthasarathy S, Molnár Z, Tarabykin V. Sip1 downstream effector ninein controls neocortical axonal growth, ipsilateral branching, and microtubule growth and stability. Neuron. 2015;85:998–1012. doi: 10.1016/j.neuron.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Suleiman H, Heudobler D, Raschta AS, Zhao Y, Zhao Q, Hertting I, Vitzthum H, Moeller MJ, Holzman LB, Rachel R, Johnson R, Westphal H, Rascle A, Witzgall R. The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Developmental Biology. 2007;304:701–712. doi: 10.1016/j.ydbio.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nature Protocols. 2015;10:1709–1727. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. The Journal of Neuroscience. 1999;19:RC12. doi: 10.1523/JNEUROSCI.19-12-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Research. 2017;45:W130–W137. doi: 10.1093/nar/gkx356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GH, Badis G, Berger MF, Kivioja T, Palin K, Enge M, Bonke M, Jolma A, Varjosalo M, Gehrke AR, Yan J, Talukder S, Turunen M, Taipale M, Stunnenberg HG, Ukkonen E, Hughes TR, Bulyk ML, Taipale J. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. The EMBO Journal. 2010;29:2147–2160. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler SC, Spencer WC, Green NH, Rood BD, Crawford L, Craige C, Gresch P, McMahon DG, Beck SG, Deneris E. Pet-1 switches transcriptional targets postnatally to regulate maturation of serotonin neuron excitability. The Journal of Neuroscience. 2016;36:1758–1774. doi: 10.1523/JNEUROSCI.3798-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CH, Levesque M, Claxton S, Johnson RL, Ang SL. Lmx1a and lmx1b function cooperatively to regulate proliferation, specification, and differentiation of midbrain dopaminergic progenitors. Journal of Neuroscience. 2011;31:12413–12425. doi: 10.1523/JNEUROSCI.1077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome Biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yan H, Luo Y, Huang Z, Rao Y. Thermoregulation-Independent regulation of sleep by serotonin revealed in mice defective in serotonin synthesis. Molecular Pharmacology. 2018;93:657–664. doi: 10.1124/mol.117.111229. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. Journal of Neuroscience. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW, Chen ZF. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. Journal of Neuroscience. 2007a;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RW, Chen ZF. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. PNAS. 2007b;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]