Abstract
Innovative technologies, including novel communication and imaging tools, are impacting dermatology in profound ways. A burning question for the field is whether we will retrospectively react to innovations or proactively leverage them to benefit precision medicine. Early detection of melanoma is a dermatologic area particularly poised to benefit from such innovation. This session of the Montagna Symposium on Biology of Skin focused on provocative, potentially disruptive advances, including: crowdsourcing of patient advocacy efforts, rigorous experimental design of public education campaigns, research with mobile phone applications, advanced skin imaging technologies, and the emergence of Artificial Intelligence (AI) as a diagnostic supplement.
INTRODUCTION
The 2017 Montagna Symposium on the Biology of Skin - Precision Dermatology: Next Generation Prevention, Diagnosis, and Treatment - included a session focusing on Melanoma Early Detection: Big Data, Bigger Picture. In this session, technological advances in melanoma early detection were presented to illustrate how large electronic registries, imaging technologies, and artificial intelligence are beginning to be incorporated in science and medicine. Widespread use of internet communication tools, mobile devices, and social media is permitting crowdsourcing of potential study participants as well as initiation of nevus and melanoma image collection. These tools are also being used to feed larger, better data sets into ever-improving neural networks, empowering the development of artificial intelligence platforms. Most of these advances are in previously uncharted territory and will require that we begin to think in broader terms about their rigorous validation and subsequent application and integration into existing health and research systems that may be ill-prepared to transform as quickly as the technology.
Early Detection of Melanoma
Early detection has several advantages over treatment of late-stage cancer. Early detection reduces disfigurement by reducing the size and extent of surgical removal and reduces side effects that late-stage patients experience from systemic therapies. Early detection also prevents long-term side effects that may occur in individuals who would have had to use aggressive systemic therapies, and saves lives of individuals who would never have responded to these treatments. Finally, early detection is likely to reduce the overall care costs because early-stage treatments (eg. simple excision) are less expensive than those used in late-stage disease (Oh et al. 2017).
Of all cancers, melanoma is arguably one of the most straightforward to detect early because most melanomas present on visible areas of the skin and mucosa. This outward-facing property lends itself to a variety of screening opportunities, from whole-population screening programs to molecular and cellular modalities that take advantage of the accessibility of skin for imaging and sampling (Leachman et al. 2016). Skin cancer screening of the general population with a total body skin examination may be safer, easier, and more cost effective than sophisticated screening methods, but this view has been controversial: the United States Preventive Services Task Force (USPSTF) has concluded that “a clear statement cannot be made about the benefit of skin cancer screening for melanoma mortality” due to insufficient evidence (Grade C, Wernli et al. 2015), and little data exists to prove the efficacy of this approach, though the USPSTF does recommend screening of higher risk populations (Johnson et al. 2017).
Results from a skin cancer screening program in Germany are mixed. In the non-randomized Schleswig-Holstein program, which included a public awareness campaign and an 8-hour primary care melanoma curriculum, more than 360,000 residents 21 years and older received total body skin examinations from dermatologists and trained primary care providers (PCP). A review of Cancer Registry data showed that incidence rates were greater and mortality rates dropped an estimated 45% for men and women in Schleswig-Holstein but not in nearby geographical areas during the 10-year study period (Eisemann et al. 2014; Katalinic et al. 2012; Waldmann et al. 2012). Although promising, when the Schleswig-Holstein program was extended nation-wide in Germany, analyses showed mixed results with respect to efficacy, raising significant questions about the validity of the results (Anders et al. 2017; Kaiser et al. 2018; Kornek et al. 2012; Schoffer et al. 2016; Stang et al. 2018; Stang et al. 2016; Stang and Jöckel 2016). Concerns were raised about data integrity, selection bias, and birth-cohort effects as possible causes for the inability to reproduce the results. There were also potentially important differences between the Schleswig-Holstein and German National programs that could account for the failure to replicate results, including a difference in the age of screening (21 and older versus 35 and older), a discontinuation of the public awareness campaign, and a difference in the referral requirements to dermatology.
The War on Melanoma: Oregon’s Modifications on the German Experience
An evolving model for population-specific skin cancer screening, the War on Melanoma™ (WoM, https://www.ohsu.edu/xd/health/services/dermatology/war-on-melanoma/), is a comprehensive strategy for attacking melanoma in Oregon (and eventually beyond) and has attempted to resolve several of the criticisms of the Schleswig-Holstein-based German Program (Waldmann et al. 2012). For example, it has retained an education and public awareness campaign component, while also developing objective baseline metrics that will be followed longitudinally throughout the program (e.g., melanoma incidence and mortality, tumor depth, stage at diagnosis, obstacles to early detection that are experienced by melanoma patients, and cost of care). Given the rapid improvement in treatment of melanoma, it will be critical to determine if any reductions in mortality are due to the program or improved therapeutic outcome, so control states without access to the program will be used as comparators. In addition, the WoM is organized and stratified by population risk with the intent to apply different screening strategies to the different populations. It involves several population and participant groups and a variety of projects and is driven both by top-down and bottom-up efforts. Figure 1 shows the main ideas of different population types, different provider types, different screening and outreach methods by risk population, different empowerment methods by provider type, and the ideas that population classes are not uniformly sized nor are the lesions of risk classes seen with the same frequency by different provider types.
Figure 1.
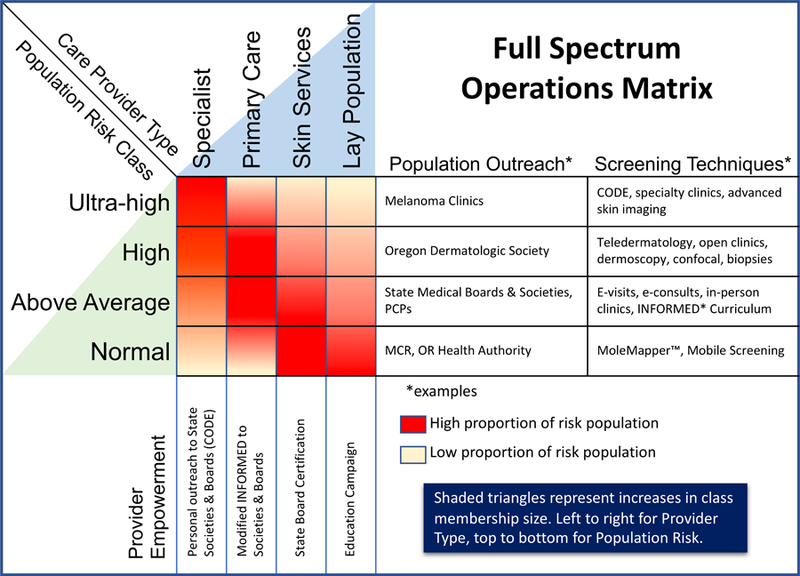
War on Melanoma™ Full Spectrum Operations Matrix. This shows different outreach and screening examples by risk class and different empowerment methods by provider type. Different provider types see different proportions of the various risk populations as represented with the heat map matrix. Provider and risk population classes are not the same size as represented by the shaded triangles.
Oregon, though seemingly an unlikely location to host a WoM, ranks sixth in overall incidence of melanoma in the nation according to the CDC, making it an ideal place to wage the war. In addition, Oregon has a population size and ethnic distribution similar in many ways to Schlesswig-Holstein and a state cancer registry. The Center for Health Systems Effectiveness at Oregon Health & Science University will also facilitate baseline and follow-up measures of melanoma prognostic indicators and cost-effectiveness of melanoma screening. Together, these features make Oregon an excellent location for a pilot test of an early detection program.
Grass-Roots Level Engagement of Melanoma Patients and Skin Care Service Professionals
One of the major “bottom-up” efforts has been the creation of a Melanoma Community Registry. The WoM began recruiting an “army” of Oregonians in June 2014, and this IRB-approved repository (the Melanoma Community Registry, IRB#10561) contains almost 8,000 research participants. These volunteers are committed to fighting melanoma through education, activism, and by participating in research surveys and other crowdsourced studies and trials. Importantly, part of the WoM program is a “teach the teachers” curriculum that includes lay-level material with accompanying competency-testing and pre-prepared, uniform teaching materials for those individuals who become WoM teachers. Formal validation of the curriculum began with focus sessions and testing at the 2018 WoM May Awareness Event in Portland, will continue throughout 2018, and has an official campaign planned to launch in May 2019. Similar tailored educational programs for skin-related service industries are in preparation for massage therapists, hairdressers, cosmetologists, body artists, midwives, etc. A formal partnership with the Oregon Health Authority has been developed that coordinates release of educational materials to all licensed groups. In this way the WoM has leveraged the interest of the community, provided standardized materials, and will expand the reach across the state substantially. Metrics such as the number of completed education modules, the number of training sessions that teachers perform and where they are held, and baseline knowledge level in a representative statewide population are expected to provide quantifiable data regarding uptake and effectiveness of the program.
Top-Down Identification of Suspicious Lesions Statewide by Engagement of Medical Professionals
The WoM program for medical professionals builds upon the INFORMED curriculum (INternet curriculum FOR Melanoma Early Detection) that has demonstrated popularity (Jiang et al. 2017) and shown an increase in diagnostic skills in users (Eide et al. 2013) but has not resulted in an increased rate of dermatology referrals or skin surgeries in Western Pennsylvania or the Veterans Affairs Palo Alto Health Care System (Swetter et al. 2017; Weinstock et al. 2016). The WoM provider curriculum will be made available online, will be shared across the Oregon Health & Science University’s (OHSU’s) PCP networks, and will be presented at OHSU-sponsored CME events. Metrics to evaluate uptake and efficacy will include the number of individuals who participated in CME courses, the location of their practices, the number of CME presentations made (and their location), and baseline knowledge testing on the diagnostic features of melanoma.
Risk-Stratified, Population-Based Melanoma Screening Strategy
The risk of melanoma has been estimated in a variety of populations, including the general population, various phenotypic groups (e.g., individuals with lightly colored skin or hair, red hair, numerous freckles, numerous or atypical nevi, or who have had substantial ultraviolet radiation damage; see Johnson et al. 2017 for review), and in individuals with a personal and/or family history of melanoma and those who carry a mutation in a melanoma predisposing gene. The WoM will target each population to enhance early detection using different methodology. In the general population, with risk estimates for melanoma incidence on the order of 1 in 100, the strategy will include a public awareness campaign, the use of social media, recommendations for tracking lesions with a free MoleMapper™ mobile phone app (see below), use of skin-related service professionals to help monitor clients’ skin, and recommendations to all health care providers to perform opportunistic screening when performing examination for other reasons. In populations at higher risk, a recommendation for a basic skin screening will be added, and in individuals identified with suspicious lesions and/or a personal or family history of melanoma, other strategies will be employed. These strategies range from routine total body skin examination in a specialty clinic setting to the use of advanced technologies, such as in vivo confocal reflectance microscopy, or genetic testing. By applying a tiered approach, using safe, inexpensive, and readily available technology for general populations and reserving more sophisticated, labor-intensive, and expensive strategies on the appropriate high-risk populations, it is hoped that the outcome can be improved.
MoleMapper™: A Personal, Free, Quantitative Mole-Tracking Tool
MoleMapper™ is a free app that helps users to photographically track changes to skin lesions. Although MoleMapper™ does not triage participants or provide a diagnostic readout at this time, it does allow users to optionally consent to participate in a research project that makes image data available for data analysis challenges. It was first released in October 2015 as the Apple ResearchKit app focused on melanoma and has been continuously improved since then. The app has three primary goals: to provide a mole-tracking tool to individuals, to help facilitate health care visits, and to enable research. MoleMapper™ empowers the general population to effectively track changes in their moles, both visually and quantitatively. By including a reference coin of known size in photographs, the size of a lesion can be determined, and changes can be tracked over time. A downstream benefit of using MoleMapper™ is that having the images available at the time of a clinical visit can facilitate a more data-based assessment of a lesion’s risk of being a melanoma. A further source of information for providers is a visual history of the lesion. MoleMapper™ gives patients the ability to provide a monthly sequence of images so the provider can detect if and how it has changed over time.
Finally, MoleMapper™ is a tool that is enabling crowd-sourced research. Since at least the early 1990s, the computer science community has been attempting to detect melanomas in images (Cascinelli et al. 1992; Green et al. 1991; Shao and Grams 1994). Many premature claims were made during this time (Alcon et al. 2009; Blum et al. 2004; Gola Isasi et al. 2011; Husemann et al. 1997), usually by assessing performance on very small data sets. Recent progress has been encouraging because it uses Deep Learning algorithms that have been proven successful in other domains and because data set sizes are larger (Codella et al. 2017; Esteva et al. 2017).
Unfortunately, the largest data sets are still closed and research restricted to a small number of institutions. Additionally, work is still based on single images of a lesion and presumes high quality image inputs to systems, which may not always be true. It remains unclear whether images collected by a lay person on a mobile phone will be of sufficient quality to allow image diagnostics. The MoleMapper™ project aims to address both of these issues. By making a curated data set public, any qualified research group in projects related to lesion detection and classification can engage. The study will also provide a richer data set consisting of longitudinal images of lesions, peer lesions from the same participant, and images of non-lesional skin. We believe these three forms of context will enhance the ability to detect not whether a lesion has become melanoma, but whether it is likely to become melanoma.
Non-Invasive Diagnostic Imaging Technologies to Improve Early Detection
Skin tumor diagnosis can be difficult due to the diverse clinical presentation of cutaneous lesions. In order to correctly identify an early melanoma, the use of dermoscopy, also known as dermatoscopy or epiluminescence microscopy, has been shown to significantly increase the sensitivity and specificity of diagnosis when compared with traditional naked-eye examination (Kittler et al. 2002; Vestergaard et al. 2008). Over the last decade, it has become a standard of initial screening in the United States. A dermatoscope uses a magnifier (typically x10 to x20) with both polarized and non-polarized light source that allows inspection of pigmented and non-pigmented skin structures without obstruction by skin surface reflection. In dermoscopically equivocal cases, lesions may be excised when further cytological information is required to rule out malignancy. The efficacy of benign tumor differentiation from melanoma can be measured by the number needed to excise ratio (NNE), for which dermoscopy ranges between 8.7 to 29.4, according to the level of expertise and clinical setting (Argenziano et al. 2014; Argenziano et al. 2012).
For those patients who have been initially screened with handheld dermoscopy and identified as high-risk, digital dermoscopy may be of benefit to document their pigmented skin lesions. This technique originally was termed videodermoscopy and has of late been termed sequential digital dermoscopy. The digital dermoscopy images can be used for follow up, enabling an objective follow-up of skin lesions (moles) that may be of concern on initial presentation, allowing for identification of architectural changes in dermoscopy images over time, and precise triage of skin lesions for further biopsy, if needed (Stanganelli et al. 2015).
Reflectance confocal microscopy (RCM) is a novel technique enabling in vivo examination of the skin at cellular-level resolution with an excellent diagnostic accuracy. This procedure is painless, takes an average of 5 minutes, and offers an alternative to traditional skin biopsies. In the past decade RCM use in clinical practice has been shown to further improve early skin cancer diagnosis and help to significantly reduce the number of unnecessary excisions, with reduction of the NNE down to 6.25 (Guitera et al. 2012; Guitera et al. 2010; Guitera et al. 2009; Pellacani et al. 2016). The reflective confocal microscope works by highlighting a single point in the skin and allows frontal visualization (horizontal plane) of the skin with a resolution at the cellular level (0.5–1.0 μm in the transverse dimension and 4–5 μm in the axial dimension). The contrast in RCM images is based on the difference in the reflectance of the tissue depending on the chemical and molecular structures. The commercially used RCM Vivascope 1500 (Caliber Imaging and Diagnostics, Inc., Rochester, New York, USA) uses a laser diode with a near infrared wavelength of 830 nm, which permits the imaging depth to 300 μm, providing sufficient visual information to differentiate benign skin lesions from keratinocyte carcinoma and melanoma without damaging tissue (Cinotti et al.; Łudzik et al. 2016; Witkowski et al. 2017). With the recent adoption of CPT codes for RCM in the United States, this technique shows a promising future and is gaining traction to help streamline precision screening of skin cancer in clinical settings.
Using Deep Learning and Artificial Intelligence to Improve Melanoma Early Detection
In traditional computer programming, data inputs are processed by hand-crafted programs to generate outputs. Machine learning, in contrast, takes data inputs and outputs to train computer-generated programs. These computer-generated programs can then take new inputs and infer outputs. This approach makes predictive systems far more robust and generalizable than hand-crafted programs (Goodfellow et al. 2016). Neural networks, a type of machine learning algorithm in which data is processed sequentially through a series of layers, have progressed significantly in recent years, achieving unprecedented milestones across a number of key artificial intelligence (AI) tasks ranging from computer vision tasks to natural language processing to game playing. These strides have been contingent on the aggregation of large-scale datasets with which to train these algorithms (Krizhevsky et al. 2017).
Convolutional neural networks (CNNs), a type of neural network algorithm that has proven effective for tasks involving images, have achieved human-level accuracy at the task of object recognition (Shin et al. 2016). In this task, a computer must identify the class of an object displayed in an image. To do so, the network is typically first trained on a large corpus of images (input data) and object labels (outputs), containing around 1,000 object classes and over 1,000 different example images per class. This first step, called pre-training, embeds into the network many of the natural statistics of images: the presence of horizontal and vertical lines, naturally-occurring color schemes, etc. In the second step, called fine-tuning, this network is further trained on the final object classes of interest, typically with less data than during pre-training.
CNNs, applied in dermatology, offer the unique opportunity to improve the diagnostic accuracy and early detection of malignancies. It has been shown that a CNN pre-trained on the ImageNet dataset, then further fine-tuned on ~130,000 images of various skin lesions, matched the performance of board-certified dermatologists at discerning benign from malignant neoplasms using photographic and dermoscopic images (Esteva et al. 2017). In this study, ~24 dermatologists were tested for their ability to discriminate between benign and malignant pigmented lesions (melanomas, nevi), as well as benign and malignant epidermal lesions (basal and squamous cell carcinomas, seborrheic keratosis). In each case, the algorithm performed on par with all tested experts. Similar results were recently reported by (Haenssle et al. 2018).
Such an algorithm, deployed on mobile devices, could assist a variety of healthcare practitioners at identifying malignancies early, when survival probability is significantly higher (Zouridakis et al. 2015). The algorithm is similarly compatible with total body scanners and can be used to identify concerning lesions amongst a myriad of benign ones, potentially helping patients with basal cell nevus carcinoma syndrome or equivalently challenging cases.
CONCLUSION
The early detection of melanoma is a laudable goal from the perspective of saving lives, reducing morbidity, and perhaps reducing health care costs. However, it is also a field that is ripe for the implementation of novel tools, applied in effective ways, if rigorous processes are put in place. The tools and processes that are evolving for the purpose of early detection of melanoma have the potential to guide similar development in other areas of medicine.
ACKNOWLEDGEMENTS
This review arises from NIAMS/NIA support for the Montagna Symposium on the Biology of Skin (R13 AR009431). Additionally, we would like to thank OHSU Knight Cancer Institute (P30 CA069533) and the Department of Dermatology for support of the WoM Community Registry and MoleMapper, as well as the participants in the registry.
Abbreviations Used:
- WoM
War on Melanoma
- PCP
primary care provider
- RCM
reflectance confocal microscopy
- AI
artificial intelligence
- CNN
Convolutional neural network
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Alcon JF, Ciuhu C, Kate W ten, Heinrich A, Uzunbajakava N, Krekels G, et al. Automatic Imaging System With Decision Support for Inspection of Pigmented Skin Lesions and Melanoma Diagnosis. IEEE J. Sel. Top. Signal Process 2009;3(1):14–25 [Google Scholar]
- Anders MP, Fengler S, Volkmer B, Greinert R, Breitbart EW. Nationwide skin cancer screening in Germany: Evaluation of the training program. Int. J. Dermatol 2017;56(10):1046–51 [DOI] [PubMed] [Google Scholar]
- Argenziano G, Cerroni L, Zalaudek I, Staibano S, Hofmann-Wellenhof R, Arpaia N, et al. Accuracy in melanoma detection: A 10-year multicenter survey. J. Am. Acad. Dermatol 2012;67(1):54–59.e1 [DOI] [PubMed] [Google Scholar]
- Argenziano G, Moscarella E, Annetta A, Battarra VC, Brunetti B, Buligan C, et al. Melanoma detection in Italian pigmented lesion clinics. G. Ital. Dermatol. Venereol 2014;149(2):161–7 [PubMed] [Google Scholar]
- Blum A, Luedtke H, Ellwanger U, Schwabe R, Rassner G, Garbe C. Digital image analysis for diagnosis of cutaneous melanoma. Development of a highly effective computer algorithm based on analysis of 837 melanocytic lesions. Br. J. Dermatol 2004;151(5):1029–38 [DOI] [PubMed] [Google Scholar]
- Cascinelli N, Ferrario M, Bufalino R, Zurrida S, Galimberti V, Mascheroni L, et al. Results obtained by using a computerized image analysis system designed as an aid to diagnosis of cutaneous melanoma. Melanoma Res 1992;2(3):163–70 [DOI] [PubMed] [Google Scholar]
- Cinotti E, Labeille B, Debarbieux S, Carrera C, Lacarrubba F, Witkowski AM, et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J. Eur. Acad. Dermatol. Venereol 2018; 32:1284–1291. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jdv.14791 [DOI] [PubMed] [Google Scholar]
- Codella NCF, Nguyen QB, Pankanti S, Gutman DA, Helba B, Halpern AC, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J. Res. Dev 2017;61(4):5:1–5:15 [Google Scholar]
- Eide MJ, Asgari MM, Fletcher SW, Geller AC, Halpern AC, Shaikh WR, et al. Effects on Skills and Practice from a Web-Based Skin Cancer Course for Primary Care Providers. J. Am. Board Fam. Med 2013;26(6):648–57 [DOI] [PubMed] [Google Scholar]
- Eisemann N, Waldmann A, Geller AC, Weinstock MA, Volkmer B, Greinert R, et al. Non-Melanoma Skin Cancer Incidence and Impact of Skin Cancer Screening on Incidence. J. Invest. Dermatol 2014;134(1):43–50 [DOI] [PubMed] [Google Scholar]
- Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542(7639):115–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola Isasi A, García Zapirain B, Méndez Zorrilla A. Melanomas non-invasive diagnosis application based on the ABCD rule and pattern recognition image processing algorithms. Comput. Biol. Med 2011;41(9):742–55 [DOI] [PubMed] [Google Scholar]
- Goodfellow I, Bengio Y, Courville A. Deep Learning. Cambridge, Massachusetts: The MIT Press; 2016. [Google Scholar]
- Green A, Martin N, McKenzie G, Pfitzner J, Quintarelli F, Thomas BW, et al. Computer image analysis of pigmented skin lesions. Melanoma Res 1991;1(4):231–6 [DOI] [PubMed] [Google Scholar]
- Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In Vivo Confocal Microscopy for Diagnosis of Melanoma and Basal Cell Carcinoma Using a Two-Step Method: Analysis of 710 Consecutive Clinically Equivocal Cases. J. Invest. Dermatol 2012;132(10):2386–94 [DOI] [PubMed] [Google Scholar]
- Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li L-XL, Bassoli S, et al. The Impact of In Vivo Reflectance Confocal Microscopy on the Diagnostic Accuracy of Lentigo Maligna and Equivocal Pigmented and Nonpigmented Macules of the Face. J. Invest. Dermatol 2010;130(8):2080–91 [DOI] [PubMed] [Google Scholar]
- Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In Vivo Reflectance Confocal Microscopy Enhances Secondary Evaluation of Melanocytic Lesions. J. Invest. Dermatol 2009;129(1):131–8 [DOI] [PubMed] [Google Scholar]
- Haenssle HA, Fink C, Schneiderbauer R, Toberer F, Buhl T, Blum A, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann. Oncol Available from: https://academic.oup.com/annonc/advance-article/doi/10.1093/annonc/mdy166/5004443 [DOI] [PubMed] [Google Scholar]
- Husemann R, Tölg S, Seelen WV, Altmeyer P, Frosch PJ, Stücker M, et al. Computerised Diagnosis of Skin Cancer Using Neural Networks. Skin Cancer UV Radiat Springer, Berlin, Heidelberg; 1997. [cited 2018 Apr 23]. p. 1052–63 Available from: https://link.springer.com/chapter/10.1007/978-3-642-60771-4_121 [Google Scholar]
- Jiang AJ, Eide MJ, Alexander GL, Altschuler A, Asgari MM, Geller AC, et al. Providers’ Experiences with a Melanoma Web-Based Course: a Discussion on Barriers and Intentions. J. Cancer Educ 2017;32(2):272–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MM, Leachman SA, Aspinwall LG, Cranmer LD, Curiel-Lewandrowski C, Sondak VK, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag 2017;4(1):13–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur. J. Health Econ 2018;19(3):355–67 [DOI] [PubMed] [Google Scholar]
- Katalinic A, Waldmann A, Weinstock MA, Geller AC, Eisemann N, Greinert R, et al. Does skin cancer screening save lives? Cancer 2012;118(21):5395–402 [DOI] [PubMed] [Google Scholar]
- Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol 2002;3(3):159–65 [DOI] [PubMed] [Google Scholar]
- Kornek T, Schäfer I, Reusch M, Blome C, Herberger K, Beikert FC, et al. Routine Skin Cancer Screening in Germany: Four Years of Experience from the Dermatologists’ Perspective. Dermatology 2012;225(4):289–93 [DOI] [PubMed] [Google Scholar]
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. Commun ACM 2017;60(6):84–90 [Google Scholar]
- Leachman SA, Cassidy PB, Chen SC, Curiel C, Geller A, Gareau D, et al. Methods of Melanoma Detection. Melanoma Springer, Cham; 2016. [cited 2018 Apr 23]. p. 51–105 Available from: https://link.springer.com/chapter/10.1007/978-3-319-22539-5_3 [DOI] [PubMed] [Google Scholar]
- Łudzik J, Witkowski AM, Roterman-Konieczna I, Bassoli S, Farnetani F, Pellacani G. Improving Diagnostic Accuracy of Dermoscopically Equivocal Pink Cutaneous Lesions with Reflectance Confocal Microscopy in Telemedicine Settings: Double Reader Concordance Evaluation of 316 Cases. PLOS ONE 2016;11(9):e0162495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A, Tran DM, McDowell LC, Keyvani D, Barcelon JA, Merino O, et al. Cost-Effectiveness of Nivolumab-Ipilimumab Combination Therapy Compared with Monotherapy for First-Line Treatment of Metastatic Melanoma in the United States. J. Manag. Care Spec. Pharm 2017;23(6):653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellacani G, Witkowski A, Cesinaro AM, Losi A, Colombo GL, Campagna A, et al. Cost–benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J. Eur. Acad. Dermatol. Venereol 2016;30(3):413–9 [DOI] [PubMed] [Google Scholar]
- Schoffer O, Schülein S, Arand G, Arnholdt H, Baaske D, Bargou RC, et al. Tumour stage distribution and survival of malignant melanoma in Germany 2002–2011. BMC Cancer 2016;16:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Grams RR. A proposed computer diagnostic system for malignant melanoma (CDSMM). J. Med. Syst 1994;18(2):85–96 [DOI] [PubMed] [Google Scholar]
- Shin HC, Roth HR, Gao M, Lu L, Xu Z, Nogues I, et al. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016;35(5):1285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A, Garbe C, Autier P, Jöckel K-H. The many unanswered questions related to the German skin cancer screening programme. Eur. J. Cancer 2016;64:83–8 [DOI] [PubMed] [Google Scholar]
- Stang A, Jöckel K-H. Does skin cancer screening save lives? A detailed analysis of mortality time trends in Schleswig-Holstein and Germany. Cancer 2016;122(3):432–7 [DOI] [PubMed] [Google Scholar]
- Stang A, Jöckel K-H, Heidinger O. Skin cancer rates in North Rhine-Westphalia, Germany before and after the introduction of the nationwide skin cancer screening program (2000–2015). Eur. J. Epidemiol 2018;33(3):303–12 [DOI] [PubMed] [Google Scholar]
- Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br. J. Dermatol 2015;172(2):365–71 [DOI] [PubMed] [Google Scholar]
- Swetter SM, Chang J, Shaub AR, Weinstock MA, Lewis ET, Asch SM. Primary Care–Based Skin Cancer Screening in a Veterans Affairs Health Care System. JAMA Dermatol 2017;153(8):797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br. J. Dermatol 2008;159(3):669–76 [DOI] [PubMed] [Google Scholar]
- Waldmann A, Nolte S, Weinstock MA, Breitbart EW, Eisemann N, Geller AC, et al. Skin cancer screening participation and impact on melanoma incidence in Germany – an observational study on incidence trends in regions with and without population-based screening. Br. J. Cancer 2012;106(5):970–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock MA, Ferris LK, Saul MI, Geller AC, Risica PM, Siegel JA, et al. Downstream consequences of melanoma screening in a community practice setting: First results. Cancer 2016;122(20):3152–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernli KJ, Henrikson NB, Morrison CC, Nguyen M, Pocobelli G, Whitlock EP. Draft Evidence Review: Skin Cancer: Screening - US Preventive Services Task Force [Internet] 2015. [cited 2018 Apr 23]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Witkowski AM, Łudzik J, Arginelli F, Bassoli S, Benati E, Casari A, et al. Improving diagnostic sensitivity of combined dermoscopy and reflectance confocal microscopy imaging through double reader concordance evaluation in telemedicine settings: A retrospective study of 1000 equivocal cases. PLOS ONE 2017;12(11):e0187748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouridakis G, Wadhawan T, Situ N, Hu R, Yuan X, Lancaster K, et al. Melanoma and Other Skin Lesion Detection Using Smart Handheld Devices. Mob. Health Technol Humana Press, New York, NY; 2015. [cited 2018 Jun 20]. p. 459–96 Available from: https://link.springer.com/protocol/10.1007/978-1-4939-2172-0_30 [DOI] [PubMed] [Google Scholar]


