Abstract
Context
The incidence of type 1 diabetes (T1D) is increasing worldwide. The quest to understand T1D etiology and how to predict diabetes is ongoing; and, in many ways, those goals intertwine. Although genetic components associate with T1D, not all individuals with T1D have those components, and T1D does not develop in all subjects with those components.
Objective
More robust methods for prediction of T1D are needed. We investigated if high CD4+CD40+ T-cell (Th40) levels can be used as a biomarker.
Methods
Th40 levels were assessed along with other parameters in blood collected from prediabetic subjects in TrialNet.
Results
In prediabetic subjects stratified according to Th40 cell level, patterns paralleled those seen between control subjects and those with T1D. Cytokine patterns were significantly different between those with high Th-40 levels (Th40-high) and those with low levels, and a CD4/CD8 double-positive population was more represented in Th40-high groups. Subjects experiencing impaired glucose tolerance had a significantly higher Th40 level than did control subjects. HLA DR4/DR4 and DQ8/DQ8 were more likely found among Th40-high subjects. Interestingly, HLA DR4/DR4 subjects were significantly older compared with all other subjects, suggesting that this haplotype, together with a high Th40 level, may represent someone in whom T1D will develop after age 30 years, which is reported for 42% of T1D cases.
Conclusion
Considering the differences found in relation to prediabetic Th40 cell level, it may be possible to devise methods that more accurately predict who will proceed toward diabetes and, possibly, indicate prediabetic stage.
Prediabetic subjects were stratified into Th40 cell level groups. Several significant differences emerged between the groups, which parallel patterns found between control subjects and those with T1D.
Type 1 diabetes (T1D) is an autoimmune disease that affects the β-islets of the pancreas, leading to the loss of insulin production and, ultimately, to overt disease signified by hyperglycemia. Although T1D has clear genetic components, the incidence of T1D is increasing much more than genetics would predict (1), and the overall incidence of diabetes in the world is increasing. T1D accounts for 5% to 10% of all cases of diabetes and it is estimated that >150,000 individuals worldwide are diagnosed with the disease annually (2). In recent work, researchers have proposed that human T1D is a relapsing-remitting disease with immunologic and metabolic factors affecting the different phases of progression toward disease, as well as the honeymoon phase, during which the pancreas recovers some of the ability to produce insulin (3, 4). Because the disease is autoimmune, its effects are cumulative. T cells and other immune cells infiltrate the pancreatic islets over time—in some cases, extended periods—and because of this, various stages of a prediabetic state exist. In human subjects, there is no hypothesis, to our knowledge, for why some individuals experience disease onset at <5 years of age and others as late as 45 or even 50 years of age. Determining what the prediabetic stages represent during diabetogenesis will affect eventual treatment options.
Genetic factors, such as the HLA DR3 and DR4, as well as DQ2 and DQ8 haplotypes, are associated with increased risk of T1D developing, but environmental factors also play a role. In T1D, proinsulin peptides form hybrids with other peptides through covalent cross-linking, forming neo-antigens that can be recognized by pathogenic CD4 T cells (5–7). It is likely that factors affected by both the environment and genetics contribute to the risk of disease developing (8). One such factor would be epitope spreading (3), which involves the ability of T cells to alter their receptors such that they can recognize new β-cell autoantigens (8). That process would be influenced by the availability of neo-antigens and immune stimulation (environment) as well as presentation of those new antigens by HLA molecules (genetics) (8). No single factor alone enables prediction of who will develop T1D; therefore, it is essential to identify robust methods to predict who is at high risk for development of T1D. Such methods will necessarily consist of combinations of genetic markers, environmental factors, and immune system inflammatory markers.
Previously, we identified a CD4 T-cell subset, characterized by the expression of CD40 [CD4+CD40+ cells (Th40 cells)], which is significantly (P < 0.0001) expanded in number in peripheral blood of patients with T1D but not of control subjects without autoimmune disease or patients with nonautoimmune type 2 diabetes (T2D) (9). That expansion was present in patients with T1D regardless of HLA haplotype and, in a blinded study, we successfully identified patients with T1D vs control subjects with 95% accuracy (9). Similar to our findings in human patients with T1D, we found an expansion of Th40 cells in the nonobese diabetic (NOD) mouse model of T1D (10–14). Unlike in humans, T1D in NOD mice runs a consistent, predictable course. In those mice, Th40 cells progressively expand in the prediabetic stages (3 to 12 weeks of age) concurrently with establishment of insulitis (10–14). In very young NOD mice, in the preinsulitis stage, peripheral Th40 cells are at numbers equivalent to those in control mice, but expansions already occur in pancreatic lymph nodes (15). Eventually, overt hyperglycemia ensues, at which stage peripheral Th40 cell numbers reach up to 60% compared with ∼10% to 15% in control mice (10, 12). Th40 cells are also necessary and sufficient to transfer T1D from both prediabetic (with present insulitis) and diabetic NOD mice to NOD mice with severe combined immunodeficiency (10, 12, 14). Interestingly, Th40 cells can reactivate recombination activating gene (RAG) 1 and 2, which leads to alteration of the T-cell receptors (TCRs) expressed by these mature, peripheral T cells (16, 17). Such alterations could potentially lead to epitope spreading, thus expanding the TCR repertoire. We recently demonstrated that a peptide that targets the CD40 molecule could prevent development of diabetes and, more importantly, reverse overt hyperglycemia in almost 60% of diabetic mice if treated immediately after reaching hyperglycemic levels (18). Translationally, this would correspond to new onset in human T1D.
Because of these findings, we set out to assess Th40 cell levels in prediabetic (preT1D) subjects enrolled in the TrialNet study. TrialNet recruits subjects based on genetic risk factors (HLA haplotype) as well as being a first-degree relative (FDR) of patients with T1D. Here, we show that when preT1D subjects were stratified according to peripheral blood Th40-cell percentages, several relationships between preT1D subjects with low Th40-cell levels and high Th40-cell (Th40-high) levels paralleled the relationships seen between control subjects and patients with T1D. This type of stratification also revealed significant patterns in cytokine production, CD4/CD8 DP population, and HLA-DR differences between the preT1D groups. Because Th40 cells are readily detected in peripheral blood, these observations suggest a useful biomarker that, in association with other risk factors, may predict diabetes risk.
Materials and Methods
Recruitment of preT1D subjects
All subjects were recruited at the Barbara Davis Center for Diabetes (BDC) and gave consent under Colorado Multiple Institutional Review Board protocol no. 01-384. The TrialNet criteria for inclusion in the preT1D cohort were high-risk HLA and/or being an FDR of a subject with T1D.
Antibody titers and HLA determination
Levels of autoantibodies to β-cell–associated antigens and HLA haplotypes were determined at the BDC Autoantibody/HLA Service Center, which is a Clinical Laboratory Improvement Amendments–certified laboratory and has been designated as a National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases North America Autoantibody/HLA Core Laboratory.
Oral glucose tolerance test
A 2-hour, 75-g oral glucose tolerance test was performed at the BDC clinic. The subjects were asked to arrive at the clinic having fasted for at least 8 hours. A health care provider performed a blood glucose test to determine the fasting glucose level, then administered 8 ounces of a solution containing a total of 75 g of glucose, orally, over 5 minutes. Two hours after ingestion of the glucose solution, another blood glucose test was performed. A blood glucose level at 2 hours of <140 mg/dL was considered normal, a level between 140 and 199 mg/dL was considered impaired glucose tolerance (IGT), and a level ≥200 mg/dL was considered diabetic.
Isolation of peripheral blood mononuclear cells
Blood samples were collected into sodium-heparin vials by venipuncture. The blood was processed within 2 to 6 hours of collection using Ficoll-Paque PLUS (GE Health care, Pittsburgh, PA) density-gradient media according to the manufacturer’s instructions. This yields the following percentages of cells ± SD: 95% ±5% mononucleocytes, 3% ± 2% granulocytes, and 5% ± 2% erythrocytes, and <0.5% of total platelets of the original blood sample remain.
Cell staining and flow cytometry
Peripheral blood mononuclear cells (PBMCs; 150,000 to 500,000 cells) were stained in 100 μL PBS containing 2 mM EDTA and 0.5% BSA (running buffer). Antibodies were added at 0.2 to 0.5 μg each and incubated for 20 to 30 minutes at room temperature. Cells were washed, then fixed in 2% paraformaldehyde. Cells were washed and run on a MACSQuant Analyzer (Miltenyi Biotec, Auburn, CA). Staining was done in the presence of Fc-receptor block (Thermo Fisher Scientific, Waltham, MA). Antibodies were as follows: anti-CD40 (G28-5; produced in-house and conjugated to Alexa-Fluor 405); anti-CD3 (UCHT1; PerCp-Cy5.5), anti-CD4 (RPA-T4; APC-Cy7), and anti-CD8 (OKT8; APC) all from Tonbo Biosciences (San Diego, CA); And anti-CD45RO (UCHL1; PE) from Thermo Fisher Scientific. Data were analyzed using FlowJo analysis software (BD, Ashland, OR). Cells were gated on forward scatter (FSC) vs side scatter (SSC) to gate out debris and dead cells, and then gates were set based on isotype controls. Cells were gated for Th40 cells, CD4+CD40−(CD4hi) T cells, or CD8+ and CD3 expression was confirmed. CD45RO expression was assessed within the gated Th40 and CD4hi T cells.
Intracellular staining for cytokines
Cells were first surface stained as described in the preceding section. Intracellular staining was then performed with the following antibodies: anti-IL-2 (MQ1-18H12; PE), anti-IL-4 (8D4-8; PE-Cy7), anti–IL-6 (MQ2-13A5; PE), anti–IL-10 (JE53-9D7; PE), anti-IL17α (eBio64DEC17; APC), anti-IFNγ (45.B3; FITC), and anti-TNFα (Mab11; PE), all from Thermo Fisher Scientific. Cells were resuspended in 100 μL eBioscience permeabilization buffer (Thermo Fisher Scientific); then antibodies were added at 0.2 to 0.5 μg each and incubated for 30 to 45 minutes at room temperature. Cells were washed and run on a MACSQuant Analyzer (Miltenyi Biotec). Gating was performed as described in the preceding section, but in addition, the CD4−CD40− population from each sample was used as a negative control. This population does not demonstrate any significant intracellular cytokine expression and serves as a built-in control for the actual antibodies used to stain the sample.
Antigen recall assay
Purified PBMCs were sorted on HLA-DR+/IgD+/CD11b+ using microbeads (Miltenyi Biotec) to yield antigen-presenting cells (APCs). The remaining cells were considered T cells. Cells were resuspended in AIM-V medium containing 10% fetal calf serum (Thermo Fisher Scientific). APCs (1 × 105 cells in 100 μL) were plated in a round-bottom, 96-well tissue culture plate. A final concentration of 20 μM peptide antigen (PLP103120, MBP83-102, GAD556-570, GAD271-285, proinsulin peptide, or insulin B9-23) was added. In addition, 20 islets from a patient with diabetes were used as an antigen or, as a control, 2 μL of Pentacel vaccine (reconstituted according to the manufacturer; Sanofi Pasteur, Swiftwater, PA) was added. Cells were incubated for 2 hours at 37°C, then 100 μL of T cells were added at the same ratio present in the particular subject during the sort. That is, if the ratio of APCs to T cells was 1:5, then 5 × 105 T cells were added; if the ratio was 1:3 then 3 × 105 T cells were added, and so on. Incubation was continued overnight, then Brefeldin A (Sigma-Aldrich, St. Louis, MO) was added to a final concentration of 5 μg/mL. Cells were incubated another 4 hours in the presence of Brefeldin A, then the cells were stained for Th40 cells and cytokine content, as described.
Western blot
Cells were sorted using anti-CD4– or anti-CD8–conjugated microbeads, followed by magnetic sort on an AutoMACS (Miltenyi Biotec). Western blot was performed, loading 10 μg of protein per lane of 10% TGX gels and transferring to polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA) (11). Antibodies used for western blot were anti-CD4 (C-18; sc-1140; Santa Cruz Biotechnology, Dallas, TX) and anti-CD8 (PA5-11453; Thermo Fisher Scientific). As an internal standard, the membranes were stripped and stained with Coomassie blue R-250 to visualize all the protein on the membrane.
Results
PreT1D subjects could be stratified by Th40-cell–level SD groups
In previous work, we showed that patients with T1D have significantly elevated levels of Th40 cells in peripheral blood compared with control subjects and that elevation is present regardless of HLA haplotype (9). HLA genes are the most consistent genetic markers for T1D, yet not every subject with an at-risk HLA becomes diabetic (8). Likewise, not all patients with T1D have the high-risk HLAs. Therefore, the Th40-cell level may be a better biomarker for risk of T1D development. We acquired blood samples from preT1D subjects enrolled in the TrialNet study (Table 1). The demographics of those subjects did not differ from the demographics published previously for patients in our study with T1D, T2D, and control subjects (9). From the original data on patients with T1D (9), we created a table of relative Th40-cell levels (Table 2). Using the determined mean and SD of the Th40-cell level from nonautoimmune control subjects, we generated Th40 relative groups by cumulatively adding the control SD to the control mean. The groups were as follows: group 1, any value lower than control mean; group 2, control mean plus ≤1 SD; group 3, control mean plus 1 to 2 SD; group 4, control mean plus 2 to 3 SD; group 5, control mean plus 3 to 4 SD; and group 6, control mean plus >4 SD. On the basis of these groups, we ranked the samples from control subjects, patients with T1D, and patients with T2D (Table 2).
Table 1.
Demographics of PreT1D Subjects
| Sex | No. of Subjects | Age ± SD (y) | Age Range (y) |
|---|---|---|---|
| Female | 48 | 31.8 ± 13.2 | 9–52 |
| Male | 38 | 31.6 ± 16.6 | 7–60 |
Table 2.
Stratification of Control, T1D, T2D, and preT1D Subjects According to Th40 Cell Level
| Subjects | No. of Subjects in Each Cohort | Mean of Th40 Percentage | Group 1: Lower Than Control Mean | Group 2: ≤1 SD (23.3%–29.9%) | Group 3: 1–2 SD (30.0%–36.4%) | Group 4: 2–3 SD (36.5%–43.0%) | Group 5: 3–4 SD (43.1%–49.0%) | Group 6: >4 SD (>49.0%) |
|---|---|---|---|---|---|---|---|---|
| Control | 93 | 23.3 | 43 | 47 | 3 | 0 | 0 | 0 |
| T1D | 85 | 47.8 | 0 | 0 | 3 | 26 | 25 | 31 |
| T2D | 48 | 23.4 | 22 | 26 | 0 | 0 | 0 | 0 |
| preT1D | 86 | 21 | 14 | 21 | 14 | 10 | 6 |
Using the determined mean and SD of the Th40 cell level from nonautoimmune control subjects, Th40 relative groups were created by cumulatively adding the SD to the mean.
As expected, patients with T1D clearly, and almost exclusively, fell into groups 4, 5, and 6. Also as expected, nonautoimmune patients with T2D fell into groups 1 and 2, the same as the control subjects (Table 2). When we applied these groupings to preT1D subjects, 35% were categorized into groups 4, 5, and 6, with more than half of those in groups 5 and 6. Therefore, assuming that preT1D subjects can be divided into those in whom T1D will develop and those in whom it will not, and that those groups will be defined by higher vs lower peripheral blood Th40-cell levels, we studied the relationship between the groups and compared it to the relationship between control subjects and patients with T1D.
Stratified preT1D subjects paralleled several relationships found in control subjects vs patients with T1D
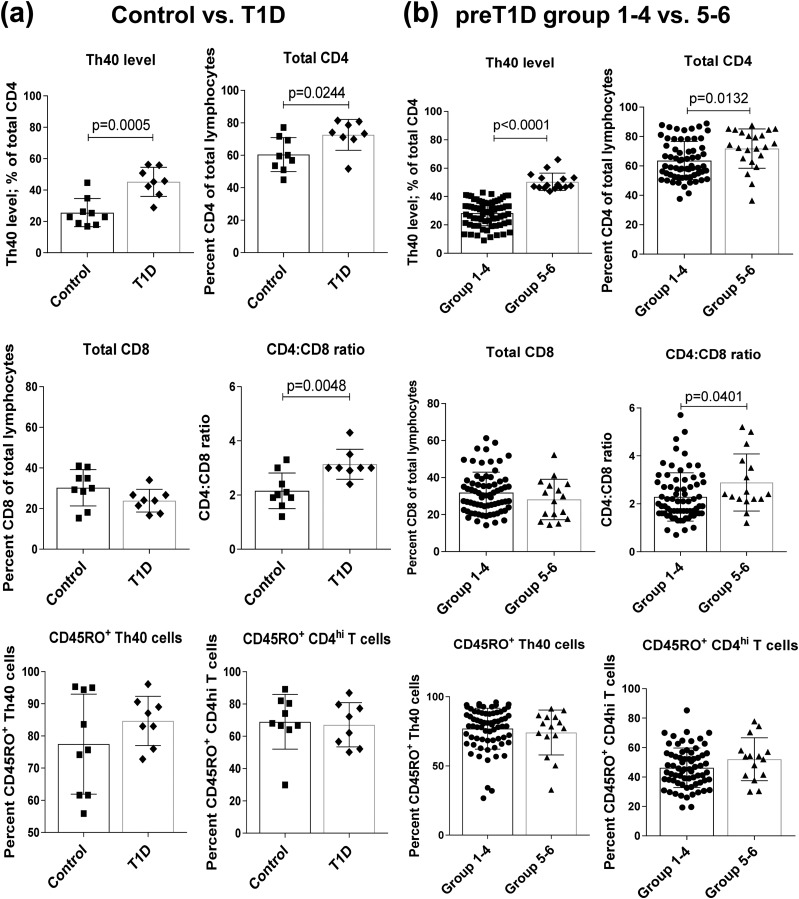
Patients with T1D consistently had a significantly higher level of Th40 cells in peripheral blood compared with control subjects [two-tailed t test, P = 0.0005; Fig. 1(a)], as reported previously (9). Th40 cells often demonstrate a lower surface expression of CD4 (9), whereas the overall expression, surface and intracellular, is similar to that of conventional CD4hi expressors (11). We analyzed CD4 and CD8 levels, taking care not to gate out those with low CD4 expression (CD4lo), and found significant differences between patients with T1D and control subjects in terms of CD4 percentages and ratio of CD4 to CD8 [two-tailed t test, P = 0.0244 and P = 0.0048, respectively; Fig. 1(a)].
Figure 1.
Stratified preT1D subjects parallel relationships found between control subjects and patients with T1D. (a) PBMCs from nonautoimmune control subjects and patients with long-standing T1D were stained to determine Th40-cell levels, total CD4 and CD8 levels, and CD45RO levels in the CD4lo and CD4hi and run-in flow cytometry. Cells were gated on FSC and SSC for live cells, then gates were set on the basis of isotype controls. (b) PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 4 were compared with subjects in groups 5 and 6 by staining and gating as in (a). Significant differences reported in both sets of graphs were calculated by two-tailed t test.
Because almost all control subjects fell into groups 1, 2, or 3 and almost all patients with T1D fell into groups 4, 5, or 6 (Table 2), we compared preT1D subjects in groups 1 through 3 with those in groups 4 through 6. Because grouping was based on Th40-cell level, the Th40-cell level means were necessarily significantly different; but when analyzing other parameters, we found no other significant differences when grouping this way. Therefore, we applied a more stringent grouping, comparing preT1D subjects in groups 1 through 4 to those in groups 5 and 6. Again, the Th40-cell levels were necessarily significantly different [two-tailed t test, P < 0.0001; Fig. 1(b)].
The CD4 T-cell content of total lymphocytes in groups 5 and 6 was significantly greater than that of groups 1 through 4 [two-tailed t test, P = 0.0132; Fig. 1(b)], paralleling the relationship between patients with T1D and control subjects [Fig. 1(a)]. When the same analysis was done for total CD8 T-cell content, no significant difference was found between control subjects and patients with T1D or preT1D groups 1 through 4 and groups 5 and 6, although there was a trend toward a lower level in patients with T1D as well as in preT1D groups 5 and 6 [Fig. 1(a) and 1(b)]. We then compared the ratio of CD4 to CD8 and found that preT1D groups 5and 6 had a significantly higher ratio than groups 1 through 4 [two-tailed t test, P = 0.0401; Fig. 1(b)], again paralleling the relationship between patients with T1D and control subjects. Th40 cells tend to express lower cell surface levels of CD4 (CD4lo) (9), which has long been known to be associated with an activated T-cell state (19). T1D is also suggested to be due to the persistence of memory T cells that attack β-islet cells (20). Therefore, we examined the memory marker CD45RO in CD4lo and CD4hi T cells. We did not discover any significant difference between control subjects and patients with T1D or preT1D groups 1 through 4 and groups 5 and 6 [Fig. 1(a) and 1(b)].
Stratifying preT1D subjects according to Th40 cell levels revealed differences in cytokine production potential
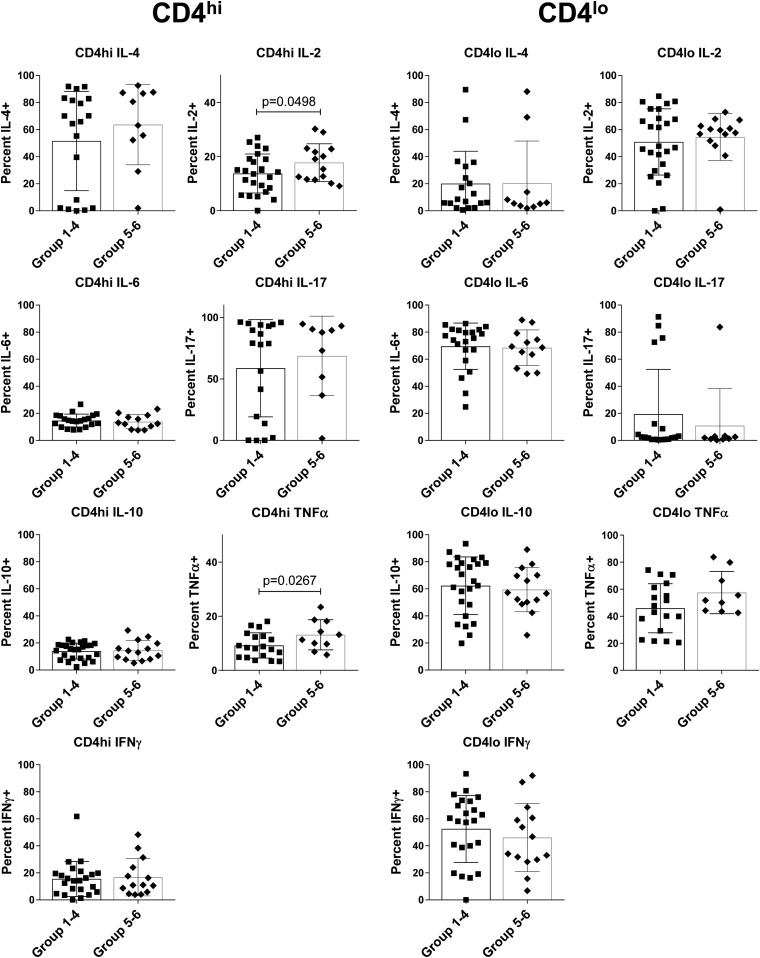
Given the autoimmune nature of T1D, an underlying, ongoing inflammation may not only drive the disease but also cause its fulmination. Therefore, we examined both baseline cytokine expression and antigen-recall cytokine expression by intracellular staining in a subgroup of the preT1D subjects. For the baseline cytokine, we examined PBMCs immediately after isolation. Because Th40 cells generally express a lower level of CD4 on their surface, we gated on CD4lo and CD4hi T cells in preT1D groups 1 through 4 and groups 5 and 6, and analyzed intracellular cytokine levels (Fig. 2). Few differences were detected, but CD4hi T cells from groups 5 and 6 expressed more IL-2 and TNFα than did CD4hi T cells from groups 1 through 4 (two-tailed t test, P = 0.0498 and P = 0.0267, respectively; Fig. 2).
Figure 2.
Conventional CD4 T cells from stratified preT1D subjects demonstrate a difference in basal cytokine levels. PBMCs were stained for CD4, CD3, and CD40, as well as for intracellular cytokines. Cells were gated on FSC and SSC for live cells then on CD4lo and CD4hi on the basis of isotype control, and CD3 expression was confirmed. PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 4 were compared with subjects in groups 5 and 6. Intracellular cytokine was measured by using the CD4−CD40− cells in each sample as a built-in negative control. Percentages of IL-2, IL-4, IL-6, IL-10, IL-17, IFNγ, and TNFα content in CD4hi (left panel) and CD4lo (right panel) are shown. Significant differences were calculated by one-tailed t test.
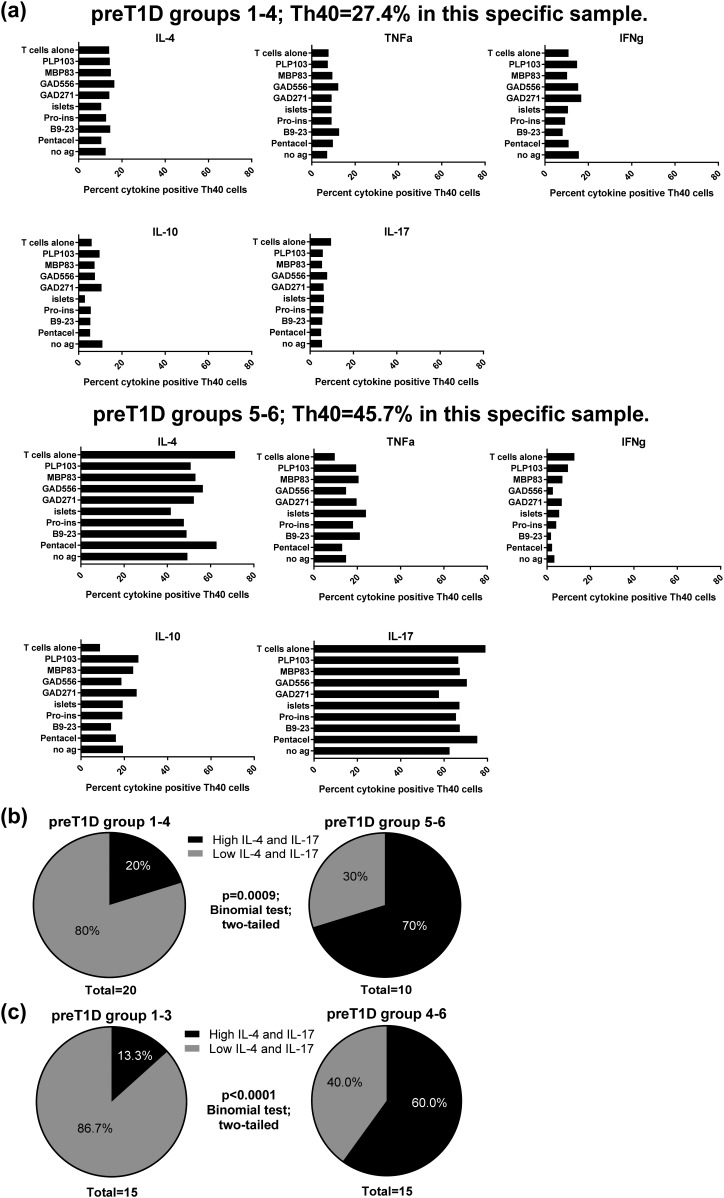
We then performed antigen recall assays and graphed the intracellular expression levels of IL-4, TNFα, IFNγ, IL-10, and IL-17, in CD4lo cells from preT1D groups 1 through 4 and groups 5 and 6. Interestingly, a pattern emerged where 70% of the subjects in preT1D groups 5 and 6 had high levels of intracellular IL-4 and IL-17 regardless of antigen or the presence of APCs [Fig. 3(a) and 3(b)]. This was true for only 20% of the subjects in preT1D groups 1 through 4 [Fig. 3(a) and 3(b)]. The difference between the two groups was significant (two-tailed binomial test, P = 0.0009). If we set the cutoff at 2 SD instead (groups 1 through 3 vs groups 4 through 6), the significant difference was still present (two-tailed binomial test, P < 0.0001; Fig. 3(c)]
Figure 3.
Stratification of preT1D subjects revealed a different inducible cytokine profile in subjects with high Th40-cell levels. PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 4 were compared with subjects in groups 5 and 6. T cells were incubated with antigen-loaded APCs overnight, then Brefeldin A was added. Cells were stained for CD4, CD3, and CD40, as well as for intracellular cytokines. Cells were gated on FSC and SSC for live cells then on Th40 cells on the basis of isotype control, and CD3 expression was confirmed. Intracellular cytokines were measured using isotype control and the CD4−CD40− cells in each sample as a built-in negative control. Percentages of IL-4, IL-10, IL-17, IFNγ, and TNFα content were graphed for each subject, creating a cytokine profile. (a) Representative cytokine profiles from subjects in groups 1 through 4 (top panels) and groups 5 and 6 (bottom panels). (b) Pie charts representing the subjects with high IL-4/IL-17 vs low IL-4/IL-17 profiles in preT1D groups 1 through 4 (left) and groups 5 and 6 (right). (c) Pie charts representing the subjects with high IL-4/IL-17 vs low IL-4/IL-17 profiles in preT1D groups 1 through 3 (left) and groups 4 through 6 (right). Significant differences in the data presented in (b) and (c) were calculated by two-tailed binomial test. ag, antigen.
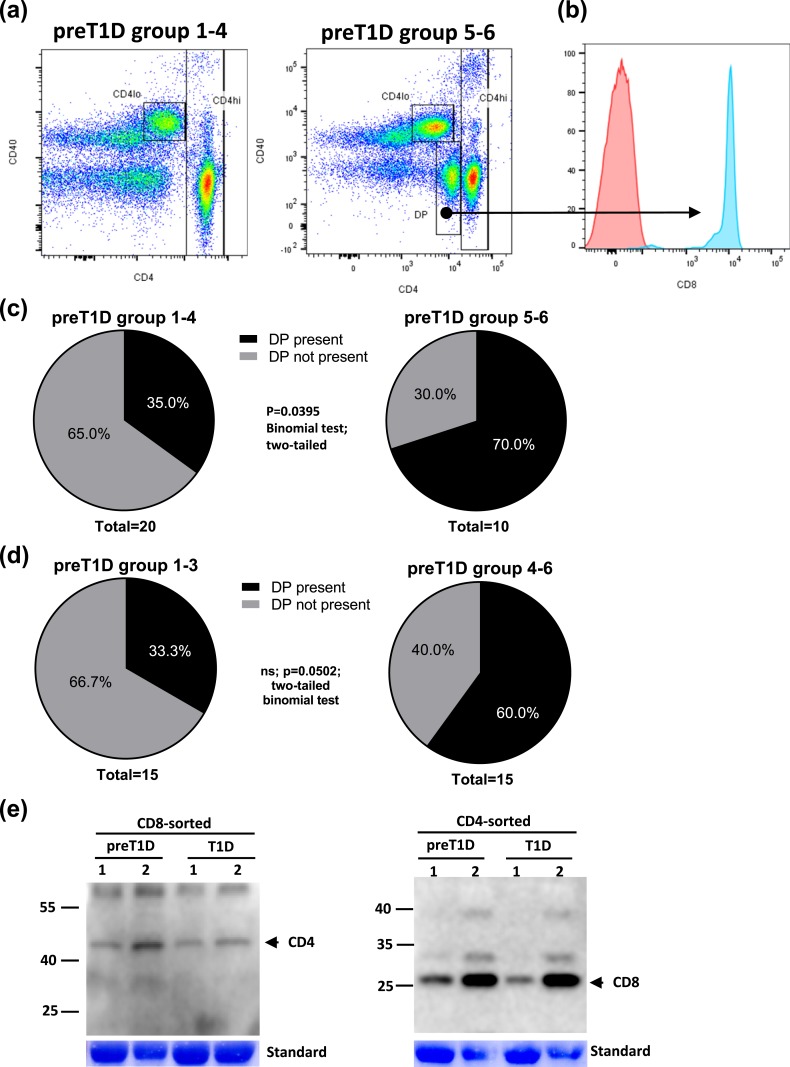
The presence of a CD4/CD8 DP population was more common in preT1D groups 5 and 6
When plotting CD4 vs CD40 in stains of the PBMCs from preT1D subjects, we noticed that in some samples, a CD4-intermediate population was present (Fig. 4). When further analyzing that population, it became clear that it was not only CD4 positive but was also positive for CD8 [Fig. 4(a), right panel, and Fig. 4(b)]. Interestingly, that DP population was significantly more prevalent in the preT1D groups 5 and 6 than in groups 1 through 4 [two-tailed binomial test, P = 0.0395; Fig. 4(c)]. If the cutoff was set to 2 SD (groups 1 through 3 vs 5 and 6), the significant difference was no longer apparent [two-tailed binomial test, P = 0.0502; Fig. 4(d)]. Further confirming double positivity, we sorted CD8 or CD4 T cells from PBMCs from preT1D subjects and patients with T1D that stained positive for that population and performed western blots for the molecule not sorted on (i.e., if sorted on CD4, western blotting was done for CD8 and vice versa) [Fig. 4(e)]. Very clearly, cells sorted on CD8 then probed for CD4 demonstrated CD4 protein expression, and cells sorted on CD4 demonstrated CD8 expression [Fig. 4(e)], demonstrating that the double-positive population was not an artifact of staining in flow cytometry.
Figure 4.
Stratified preT1D groups demonstrated differences in the presence of CD4/CD8 DP cells. PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 4 were compared with subjects in groups 5 and 6. PBMCs were stained for CD4, CD3, CD8, and CD40. Cells were gated on FSC and SSC for live cells, then CD4 and CD40 were plotted and gated on the basis of isotype controls, and CD3 expression was confirmed. (a) Representative CD4/CD40 dot plots from groups 1 through 4 (left) and groups 5 and 6 (right). (b) Histogram depicting CD8 content in the CD4 intermediate population [arrow from dot plot in (a)] from groups 5 and 6. (c) Pie charts representing the number of subjects with and without DP population in groups 1 through 4 (left) and groups 5 and 6 (right). (d) Pie charts representing the number of subjects with and without DP population in groups 1 through 3 (left) and groups 4 through 6 (right). (e) PBMCs from two preT1D samples and two long-standing T1D samples were assayed in western blots for CD4 (left) and CD8 (right) after sorting on CD8 and CD4, respectively, was performed. Significant differences in the data presented in (c) and (d) were calculated by two-tailed binomial test. ns, not significant.
Th40 level did not correlate with age or antibody status
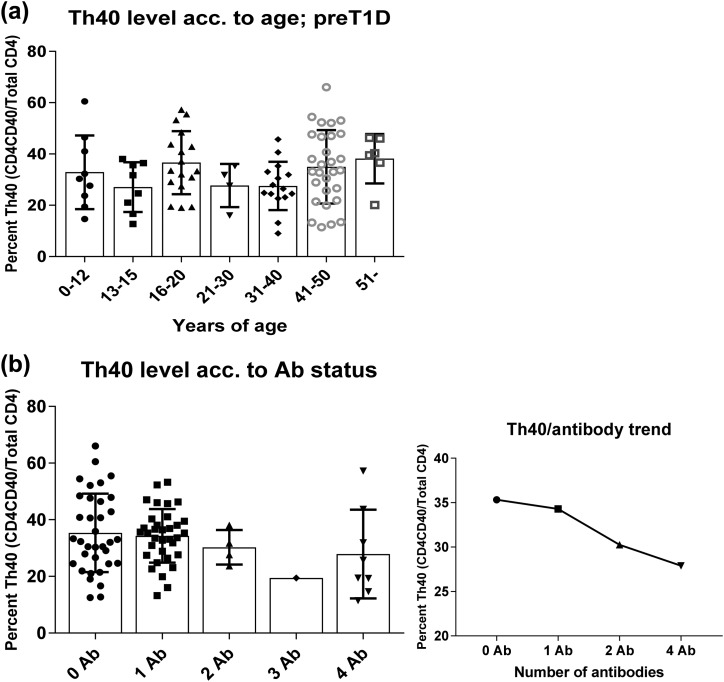
Previously we demonstrated that Th40 cell level does not correlate with age (9). Those data were from the general population, control subjects, and patients with T1D without any further discrimination. In the current study, we performed the same analysis on the more narrowly defined cohort of subjects enrolled in TrialNet because of known risk factors, HLA, and/or FDR of a patient with T1D. Similar to what we found before, there was no significant difference in Th40-cell levels between different age groups, and a highly variable range was observed in each age group [Fig. 5(a)].
Figure 5.
Th40 cell levels in preT1D subjects did not correlate with age or Ab status. PBMCs from preT1D subjects were stained for CD4, CD3, and CD40. Cells were gated on FSC and SSC for live cells, then CD4/CD40 was plotted and gated on the basis of isotype controls, and CD3 expression confirmed. Th40-cell levels of preT1D subjects were plotted according to (a) age groups or (b) number of Abs (left panel, all subjects; right panel, mean Th40-cell–level trend). acc, according.
The presence of islet autoantibodies (Abs) in the serum of preT1D subjects is considered a risk factor for developing T1D (21). Therefore, we analyzed Th40-cell levels on the basis of Ab status among the preT1D subjects and found no significant difference [Fig. 5(b), left panel]. In fact, the Th40-cell–level range was quite broad for the none, one, and four Ab groups [ranges: 12% to 66%, 13% to 53%, and 11% to 57%, respectively; Fig. 5(b), left panel]. In subjects with two Abs, the range was more narrow, 24% to 38%, but there were only four subjects in this group [Fig. 5(b), left panel]. In addition, we only had one subject with three Abs. Regardless of the range, there was a trend toward a lower level of Th40 cells as the number of Abs increased [Fig. 5(b), right panel].
PreT1D subjects with IGT had higher levels of peripheral blood Th40 cells than did control subjects
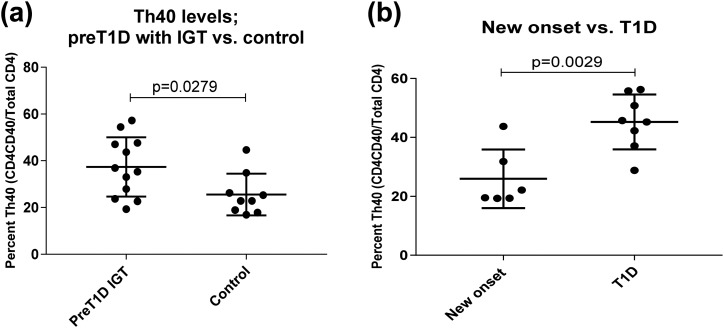
IGT precedes overt hyperglycemia. At the time oral glucose tolerance tests were performed, we acquired blood samples from the preT1D subjects for Th40-cell–level determination. Analysis of peripheral blood Th40-cell levels revealed that subjects experiencing IGT had significantly higher levels of Th40 cells compared with control subjects (two-tailed t test, P = 0.0279; Fig. 6(a)].
Figure 6.
Patients with new-onset T1D had lower Th40-cell levels than did patients with long-standing T1D, and preT1D subjects experiencing IGT had higher Th40-cell levels than did control subjects. PBMCs from patients with new-onset T1D, long-standing T1D, preT1D subjects experiencing IGT, and control subjects were stained for CD4, CD3, and CD40. Cells were gated on FSC and SSC for live cells, then CD4/CD40 was plotted and gated on the basis of isotype controls, and CD3 expression confirmed. (a) Th40-cell levels in patients with new-onset T1D compared with those with long-standing T1D. (b) Th40-cell levels in preT1D subjects experiencing IGT compared with those in control subjects. Significant differences in the data presented in (a) and (b) were calculated by two-tailed t test.
Patients with new-onset T1D experienced a dip in peripheral blood Th40-cell level
Many patients with new-onset T1D experience a honeymoon period during which they require less insulin and have better glycemic control (4). The honeymoon phase is thought to be influenced by metabolic and immunological factors and is regarded as one of many phases of remission that occur during the disease (4). We acquired several samples from subjects who had recent (within 6 months) onset of T1D. Analysis of the peripheral blood Th40-cell levels revealed that this group typically had significantly lower levels of Th40 cells than did patients with long-standing T1D (two-tailed t test, P = 0.0029; Fig. 6(b)].
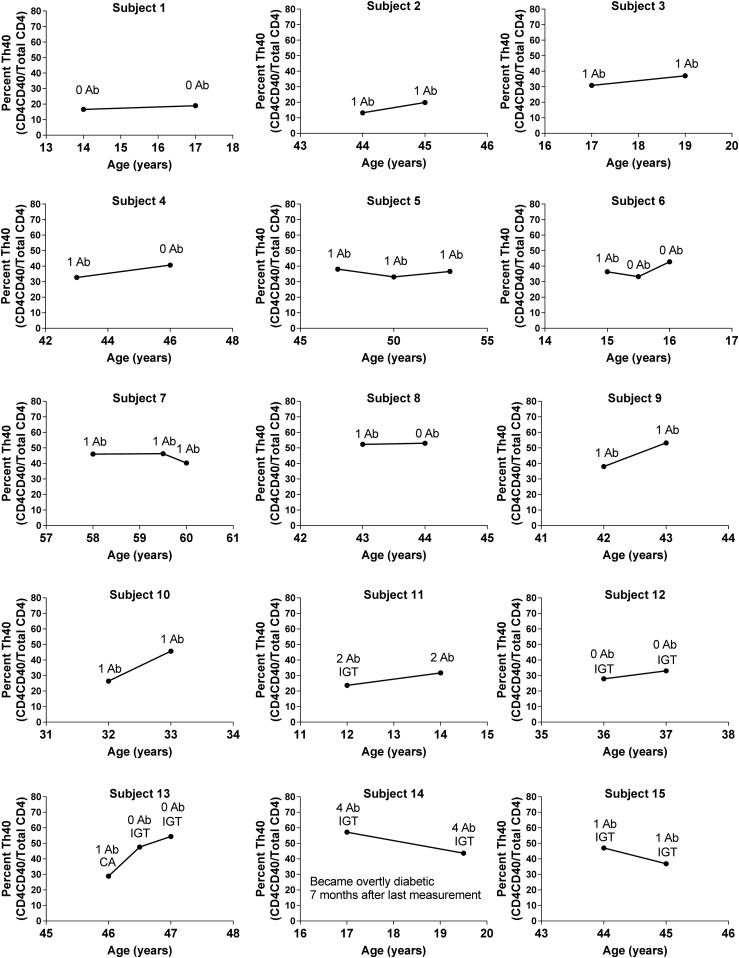
Can trends be discerned that may predict when a preT1D subject will become overtly hyperglycemic?
For some preT1D subjects, we performed longitudinal analysis on Th40-cell status. When viewing the graphs of these subjects’ Th40-cell levels over time, several patterns emerged (Fig. 7). Some had levels comparable to that of control subjects and remained stable at those levels, and some had higher Th40-cell levels but remained stable at those levels. Several subjects started at a low to intermediate Th40-cell level, but the level increased sharply over time, whereas others started at a high level but that decreased sharply over time. In the last group, one subject was diagnosed with T1D seven months after the last Th40-cell measurement. That subject also was positive for four β-cell antigen antibodies. This is interesting in the light of the aforementioned data, which demonstrate that four Ab-positive subjects trended toward a lower level of peripheral blood Th40 cells and that patients with new-onset T1D had lower levels of peripheral blood Th40 cells compared with patients with long-standing T1D. Several subjects in the latter two groups (i.e., sharp increase or decrease) experienced IGT at the time of sample collection. Therefore, it is possible that subjects whose Th40-cell levels expand sharply over time and then experience a sharp decrease are those who should be considered at highest risk of imminent hyperglycemia.
Figure 7.
Different patterns of Th40-cell levels emerged from longitudinal studies of preT1D subjects. PBMCs from preT1D subjects were stained for CD4, CD3, and CD40. Cells were gated on FSC and SSC for live cells, then CD4/CD40 was plotted and gated on the basis of isotype controls, and CD3 expression confirmed. Th40-cell levels over time were plotted for each patient who had been tested more than once. Ab status and any IGT are noted for each time point.
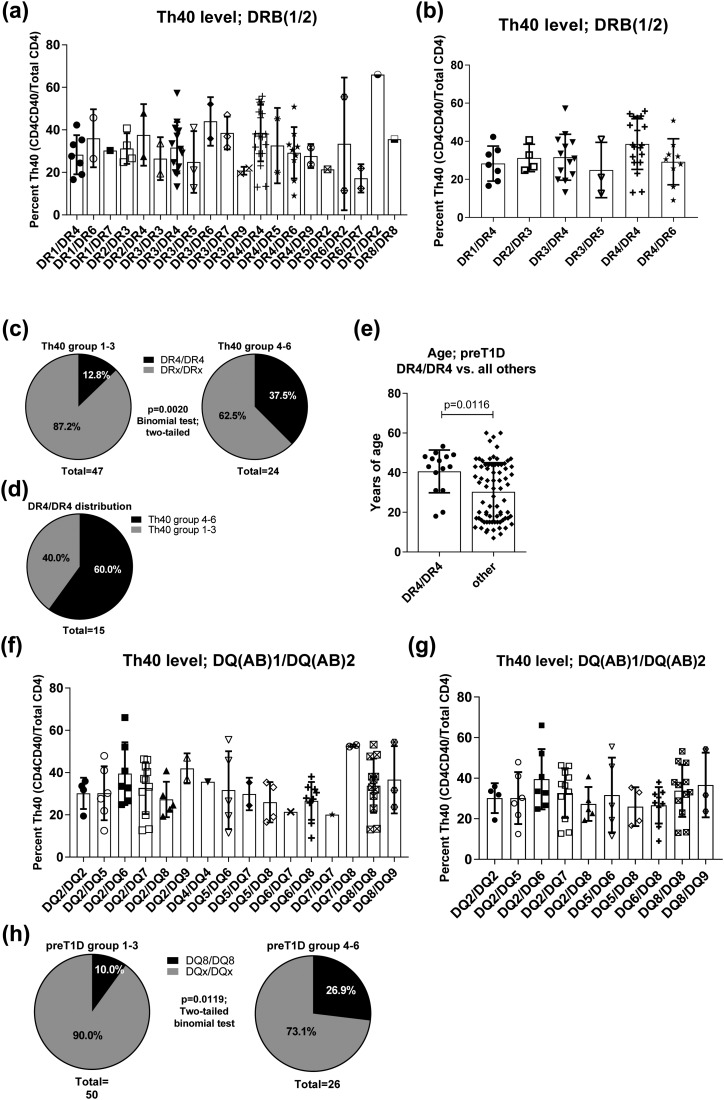
PreT1D subjects with HLA DR4-DR4 haplotype were more likely to be found in Th40 level groups 4 through 6
Previously we demonstrated that peripheral blood Th40 cell level in diagnosed long-term T1D subjects does not correlate with HLA haplotype (9). However, this was done on the general population, comparing control and T1D subjects. Here, however, the subjects were recruited on the basis of diabetes-associated HLAs or being a first-degree relative of a T1D subject. We performed HLA haplotype analysis on the preT1D samples. Plotting all subjects with known HLA-DRB haplotype, it was clear that there was a wide representation of haplotypes but with no significant difference in Th40-cell percentage between the haplotype groups [Fig. 8(a)]. However, several haplotypes were represented by only one or two preT1D subjects. Therefore, we removed those from the analysis to better discern whether any differences were present among the remaining, better-represented groups [Fig. 8(b)]. Again, no significant difference in Th40-cell level relative to HLA-DRB was found, but there was a trend toward a higher Th40-cell level in the DR4/DR4 group. Therefore, we divided the subjects depicted in Fig. 8(a) into Th40-cell–level groups on the basis of the groupings in Table 2 and tallied the representation of subjects with DR4/DR4 in those groups. We found that 37.5% of the subjects in preT1D groups 4 through 6 were DR4/DR4, whereas only 12.8% in the preT1D groups 1 through 3 had that haplotype [two-tailed binomial test, P = 0.0020; Fig. 8(c)]. When considering only subjects with the DR4/DR4 haplotype and graphing their representation in Th40-cell–level groups 1 through 3 and 4 through 6, they were clearly more represented in groups 4 through 6 [Fig. 8(d)]. Interestingly, when we plotted the age of the DR4/DR4 subjects against all other haplotype combinations, the DR4/DR4 subjects were significantly older than the other subjects [mean age, 40.6 vs 30.3 years, respectively; two-tailed t test, P = 0.0116; Fig. 8(e)].
Figure 8.
HLA DR4/DR4 and DQ8/DQ8 were more prevalent in preT1D subjects with higher Th40-cell levels. Th40-cell levels in preT1D subjects were plotted according to HLA-DR haplotypes for (a) all subjects or (b) HLA-DR haplotypes represented by more than two subjects. (c) PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 3 (left) were compared with subjects in groups 4 through 6 (right) for the representation of HLA DR4/DR4 vs all other DR-haplotypes (DRx/DRx). (d) Distribution of DR4/DR4 subjects only among preT1D groups 1 through 3 and 4 through 6. (e) Graph depicting the age of DR4/DR4 subjects compared with all other haplotype subjects. (f) Th40-cell level according to HLA-DQ haplotype in all subjects or (g) HLA-DQ haplotypes represented by more than two subjects. (h) PreT1D subjects were stratified into groups as shown in Table 2, then subjects in groups 1 through 3 (left) were compared with subjects in groups 4 through 6 (right) for the representation of HLA DQ8/DQ8 vs all other DQ-haplotypes (DQx/DQx). Significant differences in the data presented in (c) and (h) were calculated by two-tailed binomial test and in (e) by two-tailed t test.
PreT1D subjects with HLA DQ8/DQ8 haplotype were more likely to be found in Th40-cell–level groups 4 through 6
Like HLA DR3 and DR4, HLA DQ2 and DQ8 are strongly associated with T1D (22). We performed a similar analysis on HLA-DQ as we did for HLA-DRB. Again, there was a wide representation of haplotypes but no significant difference in Th40-cell level [Fig. 8(f)]. When haplotypes represented by only one or two subjects were removed from the analysis, there was still no significant difference [Fig. 8(g)]. However, when we divided the subjects depicted in Fig. 8(f) into Th40-cell–level groups on the basis of the groupings in Table 2 and tallied the representation of subjects with DQ8/DQ8 in those groups, DQ8/DQ8 was clearly more represented in the preT1D groups 4 through 6 than in groups 1 through 3 [two-tailed binomial test, P = 0.0119; Fig. 8(h)].
Discussion
The pursuit of methods for predicting who will become diabetic is ongoing on many fronts. Obviously, if such a prediction was accurate, it could also be possible to intervene before overt disease onsets. We previously discovered that peripheral blood Th40-cell levels are significantly higher in patients with T1D compared with healthy control subjects (9). We have also demonstrated, in the NOD mouse model of T1D, that an expansion of Th40 cells precedes overt hyperglycemia and that isolated Th40 cells from diabetic and prediabetic NOD mice are necessary and sufficient to adoptively transfer T1D (10–14). These findings made it enticing to examine human preT1D subjects to determine if they parallel the control and T1D findings and if the Th40-cell level can be combined with other risk factors to form a more powerful method of predicting who may become diabetic.
Clearly, when we stratified the preT1D subjects into Th40-cell–level groups, it became evident that several relationships that are present between control subjects and patients with T1D were paralleled by the preT1D groups 1 through 4 vs preT1D groups 5 and 6. There was a significantly higher level of total CD4 T cells in the peripheral blood of patients with T1D and preT1D subjects in groups 5 and 6 compared with control and preT1D groups 1 through 4, respectively. To our knowledge, an increased CD4 T-cell level in patients with T1D has not been reported previously. This is possibly because many investigators tend to gate out the population that is surface CD4lo (9) but has overall, surface and intracellular, CD4 expression similar to that of conventional CD4hi T cells (11). It is not clear from the data here why there would be an expansion in the CD4 T-cell compartment, but we have demonstrated in the NOD mouse model of T1D that CD40 stimulation expands the Th40-cell population (23). Therefore, we speculate that the expansion noted here might be CD40 driven. There was also a nonsignificant trend toward a lower percentage of CD8 T cells in the T1D and preT1D groups 5 and 6. It is possible that this is not truly a decrease in absolute CD8 T-cell numbers but rather a reflection of the expansion of CD4 T cells, which would cause a decrease in the percentage of CD8 T cells when considering total lymphocytes, as done here. Certainly, the ratio of CD4 to CD8 was significantly different in control vs T1D and in preT1D groups 1 through 4 vs 5 and 6. At any rate, it appears that those subjects with the highest peripheral blood Th40-cell levels are more like patients with T1D immunologically. It is important to note that although the differences in the level of CD4 T cells and the CD4-to-CD8 ratio between the preT1D groups were statistically significant, those observations by themselves are not relevant as biomarkers, because there is a lot of overlap between the groups. However, in addition to other predisposing criteria, these kinds of observations may add value when attempting to elucidate who is at greater risk. An important caveat is that our cohort is from the TrialNet study and therefore the subjects have met the criteria for enrollment (HLA haplotype and/or FDR). That means that in this group, there is already a genetic risk factor from the start of our analysis. Indeed, we did find that HLA DR4/DR4 as well as DQ8/DQ8 haplotypes, known to be associated with increased T1D risk (8, 9), were significantly more represented in the preT1D groups 4 through 6 than in the preT1D groups 1 through 3.
In subjects with the highest Th40-cell levels (preT1D groups 5 and 6), the CD4hi cells produced higher baseline levels of intracellular IL-2 and TNFα. It is possible that this is due to the underlying, ongoing inflammation that may drive the progression of disease development.
We have previously demonstrated that portions of Th40 cells from patients with T1D proliferate in response to islet autoantigens (9). Here, we performed antigen recall assays but with cytokine production as an outcome. Interestingly, when T cells from preT1D groups 5 and 6 were cultured in those assays, the Th40 cells were more likely to produce high levels of IL-4 and IL-17 simultaneously. This profile was present regardless of the presence of APCs and antigen, suggesting that those Th40 cells are poised to produce cytokines without any stimulus other than being removed from the subject and being cultured, which, in itself, could constitute a stimulus. It is difficult to interpret what such a cytokine expression profile would mean immunologically, because this was done by intracellular staining and essentially is a snapshot in time of what the cells are producing or containing. Several possible scenarios exist where the cells produce and secrete many cytokines before Brefeldin A addition, which would not be detected intracellularly at the time of assaying, or where the cells produce cytokines temporally and, therefore, cytokines that are not produced in the 4-hour Brefeldin A treatment window will not be detected. Nonetheless, the difference is present and may merit additional analysis to determine what cytokines are secreted and, possibly more important, why the cells from those subjects are so poised to produce cytokines. Certainly, the role of the cytokine environment in T1D remains poorly understood but has moved beyond the Th1/Th2 paradigm (24). The high IL-17 content found in Th40 cells in the current study may point toward a potential role for IL-17 in preT1D. Several other studies have also found elevated IL-17 in patients with T1D (24). It is highly likely that the total composition and level of all cytokines in the microenvironment will matter. To examine this is an almost insurmountable task when studying humans, given that we only have access to blood samples most of the time. Therefore, studying the microenvironment cytokines and the relationship to peripheral blood cytokine production potential in mice may help translate the findings in human blood samples.
We have shown in the NOD mouse model that Th40 cells can reactivate recombinases RAG1 and RAG2 when CD40 stimulated (16, 17) and that there is a bias in TCR expression in Th40 cells in that model (10). Other investigators have demonstrated clonal expansions in patients with T1D who are reactive to insulin (25), although it is not clear that those cells were Th40 cells. Although we did not have enough sample to expand on those studies here, it will be interesting in to determine if subjects from the different preT1D groups have clonal expansions recognizing islet autoantigens and whether specific TCRs are involved.
Peripheral, CD4+CD8+ DP cells have been described before (26, 27). Interestingly, DP cells are elevated in patients with rheumatoid arthritis, another autoimmune disease, and are present in the rheumatoid synovium (27). DP cells in rheumatoid arthritis express IL-21, IL-4, and IFNγ and are thought to contribute to the pathogenesis of that disease (27). In this study, we show the presence of CD4 CD8 DP cells. Typically, DP cells are considered immature, such as those that arise during thymic T-cell development. In NOD mice, it was postulated that DP cells escape thymic negative selection and migrate to the periphery (28). These DP cells in preT1D may represent such a population. It would be interesting to determine if DP cells are capable of responding to antigen presented on either MHCI or MHCII, or, given the correct microenvironment, both MHCI and MHCII simultaneously. Would such capabilities increase the chances of activating T cells and essentially poise such T cells to be fast responders to presented antigens? This could also help better explain the involvement of CD8 T cells in the disease. We did not have sufficient sample to perform a more detailed analysis of the DP cells, but considering that they were more likely to be present in the Th40-high group, this may be an important observation.
The finding that subjects experiencing IGT have a significantly higher peripheral blood levels of Th40 cells than did control subjects is very promising. This is a direct indication that the disease progression in the NOD mouse model, where Th40 cells expand in the prediabetic stages, parallels that of humans (10–14). We also found that patients with new-onset T1D had a lower Th40 level than did patients with long-standing T1D, and there was a trend in subjects with more β-cell associated autoantibodies toward a lower level of Th40 cells. This may seem counterintuitive but is interesting because many patients with T1D experience a honeymoon soon after onset of overt hyperglycemia, during which they require less insulin and experience better metabolic control of their blood glucose (4). Therefore, the following scenario is a possibility: (1) A subject at high risk of T1D has a high peripheral blood Th40-cell level and one or two autoantibodies. (2) As the subject progresses toward disease onset, Th40 cells migrate into the affected tissue(s) (i.e., pancreatic β-islets) in larger numbers, essentially lowering the Th40-cell level in peripheral blood. During this time, the subject has a higher number of autoantibodies. (3) The disease fulminates due to the heavy autoimmune attack and hyperglycemia onsets. This is when the current availability of antigen may have dwindled and, therefore, the immune attack subsides, leading to the honeymoon. (4) Th40 cells, which are long lived and poised for survival (11, 12, 23), return to the circulation from the pancreas. Those returning cells, as well as Th40 cells that remained in the circulation, are better poised to be activated than the same cells from a nonautoimmune prone subject. Therefore, those cells begin epitope spreading and eventually rebound to high levels in peripheral blood, because of the recognition of new antigens. Th40 cells are especially interesting in such epitope spreading because they can reactivate RAG1 and RAG2 with subsequent TCR alterations (16, 17).
Considering these data, it may be possible to follow disease progression in subjects who demonstrate several risk factors, such as autoantibodies, IGT, and HLA haplotype, together with a high level of Th40 cells. When a dip in the Th40-cell level is observed, overt hyperglycemia may be imminent. It would be interesting to follow subjects during overt T1D as well to determine if dips in peripheral blood Th40-cell levels occur throughout the disease and whether such dips are associated with the onset of complications of T1D followed by a honeymoon in those symptoms.
Recently, it was shown that a surprising proportion, 42%, of T1D cases are diagnosed in patients older than 30 years (29). In their study on the predictive properties of HLA DR3 and DR4 in the UK population Thomas et al.(30) demonstrated that although DR3/DR4 carriers have an increased risk of T1D relative to DR3/DR3 and DR4/DR4 (4.2% vs 1.2% and 3.5%, respectively) in children and young adults, DR4/DR4 specifically predisposes carriers to develop T1D after the age of 30 years. Interestingly, in the data presented here, we found that DR4/DR4 haplotypes are more likely to be found in preT1D groups 4 through 6 (with elevated Th40-cell levels) and that their average age was significantly older than that of all other subjects with any other HLA-DR haplotype. Therefore, these findings merit follow-up to determine whether T1D is more likely to develop in those subjects after the age of 30 years. It would also be interesting to discern whether an elevated Th40-cell level in association with DR4/DR4 or DQ8/DQ8 as well as two or more autoantibodies is more predictive of T1D than any of those criteria alone.
In conclusion, our data support Th40 cells as an important measure of the immune status of subjects at risk for T1D. A high peripheral blood level of Th40 cells could be a common denominator in preT1D subjects that may present with differences in other risk factors. Therefore, even if only one other risk factor is present, once a high Th40-cell level is confirmed, this may constitute a red flag for commencing much closer follow-up. In fact, because we have demonstrated a similar elevation of Th40 cells in patients with multiple sclerosis (31), it is possible that a high Th40-cell level alone is the most telling biomarker for predisposition to autoimmune disease. With the addition of disease-specific biomarkers, such as HLA haplotype, autoantibodies, and first-degree relationship to a patient with a specific autoimmune disease, it may be possible to predict which type of autoimmune disease is at play.
Because the subjects in our studies are part of TrialNet and many are seen regularly, in our future studies we intend to attempt follow-up to determine whether T1D develops in some subjects and if so, which preT1D group they were in in the current study. This would contribute to proof of Th40-cell levels being predictive of disease.
Acknowledgments
Financial Support: This work was supported by grants from the American Diabetes Association (grant 7-13-TS-30) and National Institutes of Health (grants 1R41AI131784-01, 1R42DK115296-01, and 4R42DK115296-02) awarded to D.H.W. FlowJo software was licensed through the University of Colorado Cancer Center Support Grant (P30CA046934) and the Skin Diseases Research Cores Grant (P30AR057212). The islet cells mentioned in “Materials and Methods” (antigen recall assay) were from the Islet Cell Resources Facility at the University of Colorado Health Sciences Center (NCRR grant RR16599).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Ab
autoantibody
- APC
antigen-presenting cell
- BDC
Barbara Davis Center for Diabetes
- CD4hi
CD4+CD40−
- CD4lo
low CD4 expression
- DP
double positive
- FDR
first-degree relative
- FSC
forward scatter
- IGT
impaired glucose tolerance
- NOD
nonobese diabetic
- PBMC
peripheral blood mononuclear cell
- preT1D
prediabetic
- RAG
recombination activating gene
- SSC
side scatter
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Th40
CD4+CD40+ T cell
- Th40-high
high levels of Th40 cells
References and Notes
- 1. Bodin J, Stene LC, Nygaard UC. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? BioMed Res Int. 2015;2015:208947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battaglia M, Anderson MS, Buckner JH, Geyer SM, Gottlieb PA, Kay TWH, Lernmark A, Muller S, Pugliese A, Roep BO, Greenbaum CJ, Peakman M. Understanding and preventing type 1 diabetes through the unique working model of TrialNet [published correction appears in Diabetologia. 2017;60(12):2540] Diabetologia. 2017;60(11):2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7(12):988–994. [DOI] [PubMed] [Google Scholar]
- 4. Aly H, Gottlieb P. The honeymoon phase: intersection of metabolism and immunology. Curr Opin Endocrinol Diabetes Obes. 2009;16(4):286–292. [DOI] [PubMed] [Google Scholar]
- 5. Baker RL, Jamison BL, Wiles TA, Lindsay RS, Barbour G, Bradley B, Delong T, Friedman RS, Nakayama M, Haskins K. CD4 T cells reactive to hybrid insulin peptides are indicators of disease activity in the NOD mouse. Diabetes. 2018;67(9):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, Armstrong M, Powell RL, Reisdorph N, Kumar N, Elso CM, DeNicola M, Bottino R, Powers AC, Harlan DM, Kent SC, Mannering SI, Haskins K. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351(6274):711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiles TA, Delong T, Baker RL, Bradley B, Barbour G, Powell RL, Reisdorph N, Haskins K. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J Autoimmun. 2017;78:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner DH. Of the multiple mechanisms leading to type 1 diabetes, T cell receptor revision may play a prominent role (is type 1 diabetes more than a single disease?). Clin Exp Immunol. 2016;185(3):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waid DM, Wagner RJ, Putnam A, Vaitaitis GM, Pennock ND, Calverley DC, Gottlieb P, Wagner DH Jr. A unique T cell subset described as CD4loCD40+ T cells (TCD40) in human type 1 diabetes. Clin Immunol. 2007;124(2):138–148. [DOI] [PubMed] [Google Scholar]
- 10. Waid DM, Vaitaitis GM, Wagner DH Jr. Peripheral CD4loCD40+ auto-aggressive T cell expansion during insulin-dependent diabetes mellitus. Eur J Immunol. 2004;34(5):1488–1497. [DOI] [PubMed] [Google Scholar]
- 11. Vaitaitis GM, Wagner DH Jr. High distribution of CD40 and TRAF2 in Th40 T cell rafts leads to preferential survival of this auto-aggressive population in autoimmunity. PLoS One. 2008;3(4):e2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waid DM, Vaitaitis GM, Pennock ND, Wagner DH Jr. Disruption of the homeostatic balance between autoaggressive (CD4+CD40+) and regulatory (CD4+CD25+FoxP3+) T cells promotes diabetes. J Leukoc Biol. 2008;84(2):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaitaitis GM, Waid DM, Wagner DH Jr. The expanding role of TNF-receptor super family member CD40 (tnfrsf5) in autoimmune disease: focus on Th40 cells. Curr Immunol Rev. 2010;6(2):130–136. [Google Scholar]
- 14. Wagner DH Jr, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci USA. 2002;99(6):3782–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaitaitis GM, Waid DM, Yussman MG, Wagner DH Jr. CD40-mediated signalling influences trafficking, T-cell receptor expression, and T-cell pathogenesis, in the NOD model of type 1 diabetes. Immunology. 2017;152(2):243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaitaitis GM, Poulin M, Sanderson RJ, Haskins K, Wagner DH Jr. Cutting edge: CD40-induced expression of recombination activating gene (RAG) 1 and RAG2: a mechanism for the generation of autoaggressive T cells in the periphery. J Immunol. 2003;170(7):3455–3459. [DOI] [PubMed] [Google Scholar]
- 17. Vaitaitis GM, Wagner DH Jr. CD40 interacts directly with RAG1 and RAG2 in autoaggressive T cells and Fas prevents CD40-induced RAG expression. Cell Mol Immunol. 2013;10(6):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaitaitis GM, Olmstead MH, Waid DM, Carter JR, Wagner DH Jr. A CD40-targeted peptide controls and reverses type 1 diabetes in NOD mice. Diabetologia. 2014;57(11):2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoxie JA, Matthews DM, Callahan KJ, Cassel DL, Cooper RA. Transient modulation and internalization of T4 antigen induced by phorbol esters. J Immunol. 1986;137(4):1194–1201. [PubMed] [Google Scholar]
- 20. Ehlers MR, Rigby MR. Targeting memory T cells in type 1 diabetes. Curr Diab Rep. 2015;15(11):84. [DOI] [PubMed] [Google Scholar]
- 21. Vehik K, Beam CA, Mahon JL, Schatz DA, Haller MJ, Sosenko JM, Skyler JS, Krischer JP; TrialNet Natural History Study Group. Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care. 2011;34(9):1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Lummel M, van Veelen PA, de Ru AH, Pool J, Nikolic T, Laban S, Joosten A, Drijfhout JW, Gómez-Touriño I, Arif S, Aanstoot HJ, Peakman M, Roep BO. Discovery of a selective islet peptidome presented by the highest-risk HLA-DQ8trans molecule. Diabetes. 2016;65(3):732–741. [DOI] [PubMed] [Google Scholar]
- 23. Vaitaitis GM, Wagner DH Jr. CD40 glycoforms and TNF-receptors 1 and 2 in the formation of CD40 receptor(s) in autoimmunity. Mol Immunol. 2010;47(14):2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker LS, von Herrath M. CD4 T cell differentiation in type 1 diabetes. Clin Exp Immunol. 2016;183(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435(7039):224–228. [DOI] [PubMed] [Google Scholar]
- 26. Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104(2):478–486. [DOI] [PubMed] [Google Scholar]
- 27. Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One. 2014;9(3):e93293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2(11):1025–1031. [DOI] [PubMed] [Google Scholar]
- 29. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas NJM, Jones SE, Weedon M, Oram R, Hattersley A. New insights into HLA in type 1 diabetes from population analysis: DR4 homozygosity specifically predisposes to type 1 diabetes after 30 years. In: Program of the European Association for the Study of Diabetes Annual Meeting; 11–15 September 2017; Lisbon, Portugal. Abstract 49.
- 31. Waid DM, Schreiner T, Vaitaitis G, Carter JR, Corboy JR, Wagner DH Jr. Defining a new biomarker for the autoimmune component of multiple sclerosis: Th40 cells. J Neuroimmunol. 2014;270(1-2):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]