Abstract
Background Blood flow restriction (BFR) therapy is an innovative rehabilitative program that enables patients to increase strength at a fraction of the weight typically necessary in endurance exercises. Therefore, we conducted a pilot study evaluating patient outcomes with a BFR therapy program for closed management after a distal radius fracture compared to a traditional rehabilitation protocol.
Literature review A randomized-controlled study was conducted comparing a standardized hand therapy rehabilitation protocol alone to a combined protocol with the use of BFR therapy in patients who were initially treated with closed reduction and short arm cast immobilization for a displaced distal radius fracture between May 1, 2015 and August 1, 2016. BFR therapy was performed with a restrictive tourniquet applied to the upper brachium, performing the same strengthening exercises as the control group but with the restrictive tourniquet in place. Clinical assessment was conducted at 6, 10, and 14 weeks from the date of initial cast immobilization. Outcome measures collected included wrist range of motion; grip strength; pinch strength; visual analogue scale for pain with activity and at rest; patient-rated wrist evaluation (PRWE) scores; and disabilities of the arm, shoulder, and hand scores.
Results Thirteen patients were enrolled and randomized between the BFR ( n = 6) and control ( n = 7) groups. The BFR group noted significantly greater reduction in pain with activity compared to the control group after 8 weeks of therapy (Δ −4.0 vs. −2.3, p = 0.03). Similarly, patients in the BFR group displayed greater reduction in PRWE scores compared to the control group after 8 weeks of BFR therapy (Δ −57.9 vs. 30.8, p = 0.01). The two groups did not demonstrate any significant difference in radiographic outcomes at any time point or throughout the course of the study. All patients tolerated the BFR therapy program and there were no complications.
Clinical relevance The addition of BFR therapy to the rehabilitative program after closed management of a distal radius fracture is safe, well tolerated by patients, without any deleterious effects on radiographic outcomes. This pilot study noted that BFR therapy in patients with nonoperative distal radius fractures may result in a larger reduction in pain with activity and greater improvement in overall self-perceived function.
Keywords: distal radius fracture, blood flow restriction therapy, fracture rehabilitation
Distal radius fractures are a common injury that appears to be increasing in incidence. 1 2 In a 1993 epidemiology study, Larsen and Lauritsen found the incidence of distal radius fractures to be 37 per 10,000 people per year. 1 More recently in a 2013 study, Bonafede et al noted that distal radius fractures accounted for one-sixth of all emergency room visits. 2 Distal radius fractures can result in significant disability in patients, including long-term pain, loss of motion due to prolonged immobilization, and difficulty with daily activities. In fact, 15% of patients sustaining a distal radius fracture will have long-term pain and disability at 20 years follow-up. 3 Patients who may be at risk of poor outcomes and prolonged disability are unable to be identified on the basis of routine indicators such as demographics, fracture type, or type of injury. 3 Therefore, a rehabilitation program to optimize outcomes early during treatment is critical as the greatest degree of recovery occurs within the first 3 to 6 months. 4
However, there are many barriers to achieving success with a rehabilitation program after sustaining a distal radius fracture. Currently, clinical practice guidelines suggest limiting early wrist motion and support active finger range of motion (ROM) following a distal radius fracture. 5 The American College of Sports Medicine (ACSM) has published guidelines for resistance training stating 60 to 100% of one repetition maximum (RM), or the maximum amount of force that can be generated in one maximal contraction, must be performed to achieve type II muscle recruitment and hypertrophy. 6 Improving ROM and strength early in the post-fracture period is limited by weight-bearing restrictions and risk of fracture displacement, making the guidelines set forth by ACSM difficult to follow in the early postoperative period. Furthermore, patients often must attend a prolonged course of therapy to achieve any measurable success in improving strength and ROM.
Blood flow restriction (BFR) therapy is performed by partially occluding venous outflow from an extremity for brief periods via the use of an inflatable cuff while performing resistance exercises which has been shown to stimulate localized cellular and hormonal changes leading to muscular hypertrophy. 7 8 9 It first gained popularity in athletes, where it was found to be a safe method for inducing muscle hypertrophy without the need to perform maximal or submaximal effort, thereby improving strength in a less energy consuming process. 10 11 12 13 14 15 16 Takada et al demonstrated that muscular hypertrophy could be achieved at 20% of RM, thereby decreasing the load needed to achieve these increases in strength. 15 BFR therapy, therefore, emerged as a promising addition to rehabilitation programs after surgery and injury, as they could be used in patients limited by postoperative and post-injury restrictions. This rehabilitative adjunct has since been used with promising results after knee arthroscopy, total knee arthroplasty, and Achilles tendon ruptures to enhance muscle recovery after surgery. 17 18 19 To date, BFR has not been utilized in the upper extremity for post-injury rehabilitation.
Therefore, the purpose of this pilot study is to compare patient and radiographic outcomes after BFR therapy to a traditional rehabilitation protocol after closed management of displaced distal radius fractures.
Materials and Methods
After institutional review board approval was obtained, a prospective, randomized-controlled study was conducted comparing a standardized hand therapy rehabilitation protocol alone (non-BFR group) or combined with BFR therapy (BFR group) in patients who were initially treated with closed reduction and short arm cast immobilization for a displaced distal radius fracture. All patients between the ages of 18 and 65 years treated at a single tertiary referral center by the senior author between May 1, 2015 and August 1, 2016 were enrolled. Exclusion criteria included unacceptable radiographic parameters for continued closed management, concomitant or prior history of injured or altered function in the unaffected upper extremity, active pregnancy, history of a deep vein thrombosis within the last 12 months, a history of upper quadrant lymph node dissection, endothelial dysfunction, self-reported easy bruising, active infection, or current cancer diagnosis.
All patients underwent closed reduction under hematoma block and fiberglass cast application in the emergency department. Weekly surveillance radiographs of the distal radius were obtained at week 1, 2, 3, and 6 after initial closed reduction to identify the presence of ongoing fracture displacement. At week 6, casts were removed and patients were transitioned into a cock-up wrist splint with nonweight bearing restrictions to the injured extremity. Patients were then randomized to the non-BFR or BFR treatment groups determined by a computer-generated randomization program that ensured equal randomization into each study arm. Patients randomized to the non-BFR group underwent a standardized rehabilitation protocol ( Table 1 ), while the patients in the BFR group underwent the same rehabilitation protocol under BFR utilizing a Delfi PTS II portable tourniquet system (Delfi Medical Innovations Inc., Vancouver, Canada, Fig. 1 , Table 1 ). Both treatment groups performed two to three therapy sessions per week for a total of 8 weeks.
Table 1. Standard rehabilitation protocol for all patients enrolled in the study.
| Distal radius fracture rehabilitation program |
|---|
| Four sets of each exercise were performed with 30 repetitions in the initial set, followed by 15 repetitions in each set thereafter |
| Thirty seconds of rest time was given between each set with 1 minute interlude between completions of each exercise |
| Two to three therapy sessions per week for a total of 8 weeks |
| • Passive, active assistive, active ROM of wrist, forearm, hand(all ROM assessed by a plastic goniometer) |
| • Wrist flexion/extension over a foam wedge a |
| • Forearm pronation/supination with arm at size and elbow at 90° a |
| • Pinch strength assessed by the PG-60 pinch gauge a |
| 3-point pinch-exerting force between the thumb and the index/long fingers |
| Lateral pinch-exerting force between the volar aspect of the thumb and the radial aspect of the index finger |
| • Grip strength assessed by the JAMAR Hand Dynamometer a |
Abbreviations: BFR, blood flow restriction; ROM, range of motion.
Exercise performed under a restrictive tourniquet in the BFR group. These strengthening exercises were performed in the control group without the tourniquet system in place.
Fig. 1.
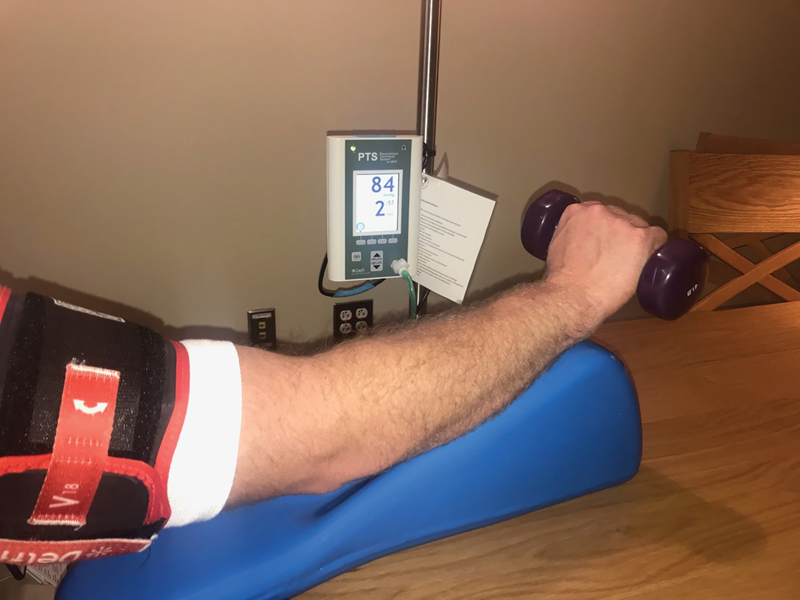
Personalized Tourniquet System (PTS) for Blood Flow Restriction (Delphi Medical, Vancouver VC, Canada).
The BFR therapy was performed with a restrictive tourniquet applied to the upper brachium of the involved extremity. Each patient's maximal limb occlusion pressure was measured at the initial visit by noting the pressure at which Doppler signal was lost over the radial artery. Thereafter, each patient performed the strengthening exercises with the cuff inflated at 50% of the maximal limb occlusion pressure. The tourniquet remained inflated through the completion of the strengthening exercises and rest periods with a maximal tourniquet inflation time of 30 minutes.
Clinical assessment was conducted at 6 weeks, 10 weeks, and 14 weeks from the date of initial closed reduction for a total of 8 weeks in occupational therapy. Patients discontinued their wrist brace at 12 weeks after initiation of cast immobilization and were permitted to bear weight as tolerated at that time. Outcome measures included wrist ROM including flexion, extension, and pronosupination as assessed by a handheld manual goniometer (Patterson Medical Supply Inc. Warrenville, IL); grip strength as gauged by a JAMAR Hand Dynamometer (Lafayette Instrument, Lafayette, IN); pinch strength measured by the PG-60 Pinch gauge (B&L Engineering, Sana Ana, CA); pain with activity and at rest based on the visual analogue scale; patient-rated wrist evaluation (PRWE) scores; and disabilities of the arm, shoulder and hand (DASH) scores. 20 21 Paired continuous variables were analyzed using a two-way analysis of variance.
Thirteen patients were enrolled, randomized between the two treatment groups, and completed the study. The majority of patients had a distal radius fracture from a low energy mechanism including nine falls from standing height, one hyperextension injury while weightlifting, and one fall from 15 feet. Six patients were randomized to the BFR group and seven patients into the non-BFR group. Among the patients there were six men and seven women with a mean age of 46 years at the time of injury (range: 27–69 years), demographics are shown in Table 2 . All patients in the BFR group were able to perform the BFR treatment sessions without early termination from pain or discomfort.
Table 2. Demographics of the 13 patients within the BFR group and control group.
| Demographics | |||
|---|---|---|---|
| BFR | Control | p -Value | |
| Age | 51.3 | 41.0 | 0.93 |
| Male (%) | 42.8 | 50.0 | 0.07 |
| RHD (%) | 100.0 | 85.7 | 0.93 |
| Dom hand involved (%) | 33.3 | 71.4 | 0.74 |
| Total therapy visits | 17.7 | 16.4 | 0.36 |
| AO fracture classification | |||
| Extra-articular | 33.3% | 57.1% | 0.63 |
| Partial articular | 33.3% | 28.6% | |
| Complete articular | 33.3% | 14.3% | |
Abbreviations: BFR, blood flow restriction; RHD, right hand dominant.
Results
There was no significant difference in pain with activity between groups at each time point. However, when comparing the magnitude of change over the course of the 8 weeks therapy protocol, the BFR group demonstrated a statistically significant greater reduction in pain over the 8 weeks therapy program when compared to the control group ( p = 0.03, Table 3 ).
Table 3. Pain at rest and pain with activity noted by patients throughout the 8 weeks rehabilitation program.
| Comparison of pain scores | ||||||
|---|---|---|---|---|---|---|
| Time in therapy (wk) | Pain at rest | Pain with activity | ||||
| BFR | Control | p -Value | BFR | Control | p -Value | |
| 0 | 3.0 | 1.3 | 0.14 | 5.5 | 4.4 | 0.29 |
| 4 | 1.5 | 0.1 | 0.07 | 3.7 | 2.0 | 0.054 |
| 8 | 0.2 | 0.3 | 0.74 | 1.5 | 2.1 | 0.53 |
| Mean interval change (0–8 wk) | 2.8 | 1 | 0.08 a | 4.0 | 2.3 | 0.03 a |
Abbreviation: BFR, blood flow restriction.
p -Value when comparing the magnitude of change of the BFR to the control group over the entire 8 weeks rehabilitation protocol.
Similarly, patients in the BFR group had a significant reduction in PRWE scores compared to the non-BFR group over the course of the 8 weeks rehabilitation program ( p = 0.01) despite no significant differences between groups when directly compared at each time point. ( Table 4 ). Overall, both groups demonstrated a reduction in PRWE from their initial visit to 8 weeks after therapy as shown in Table 4 .
Table 4. PRWE scores during the course of therapy in the BFR and control groups.
| Comparison of PRWE scores | |||
|---|---|---|---|
| Time in therapy (wk) | PRWE score | p -Value | |
| BFR | Control | ||
| 0 | 66.8 | 47.8 | 0.11 |
| 4 | 31.1 | 30.2 | 0.95 |
| 8 | 8.9 | 17.0 | 0.31 |
| Mean interval change (0–8 wk) | 57.9 | 30.8 | 0.01 a |
Abbreviations: BFR, blood flow restriction; PRWE, patient-rated wrist evaluation.
Note: The BFR group demonstrated a significant reduction from 0 to 8 wk in PRWE score compared to the control group.
p -Value when comparing the magnitude of change of the BFR to the control group over the entire 8 wks rehabilitation protocol.
Despite improvement in wrist and forearm ROM throughout the course of the study, there were no statistically significant differences between groups at any time point or in magnitude of change throughout the 8 weeks rehabilitation program ( Table 5 ). Similarly, both groups demonstrated improvement in their DASH scores, grip strength, 3-point pinch, and lateral pinch during the length of the study. However, there was no statistically significant difference at final follow-up or over the course of therapy between groups with regard to these variables ( Table 6 ). There were no complications and all patients returned to their previous occupations at the same level of pre-injury function.
Table 5. Wrist and forearm ROM at each occupational therapy follow-up visit.
| Comparison of ROM | ||||||
|---|---|---|---|---|---|---|
| Time in therapy (wk) | Wrist ROM (flexion/extension arc) | p -Value | Forearm ROM (supination/pronation arc) | p -Value | ||
| BFR | Control | BFR | Control | |||
| 0 | 71.7° | 72.9° | 0.93 | 136.7° | 125.7° | 0.67 |
| 4 | 101.7° | 106.4° | 0.74 | 161.7° | 159.3° | 0.83 |
| 8 | 117.5° | 123.6° | 0.64 | 174.2° | 170.0° | 0.46 |
| Mean interval change (0–8 wk) | 45.8° | 50.7° | 0.65 a | 37.5° | 44.3° | 0.68 a |
Abbreviations: BFR, blood flow restriction; ROM, range of motion.
p -Value when comparing the magnitude of change of the BFR to the control group over the entire 8-week rehabilitation protocol.
Table 6. No difference was seen between groups in terms of DASH scores, grip strength, 3-pt pinch, or lateral pinch at any time point.
| Comparison of clinical outcome measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time in therapy (wk) | DASH score | p -Value | Grip strength | p -Value | 3-pt pinch | p -Value | Lateral pinch | p -Value | ||||
| BFR | Control | BFR | Control | BFR | Control | BFR | Control | |||||
| 0 | 51.3 | 44.1 | 0.52 | 24.2 | 26.9 | 0.76 | 7.3 | 7.6 | 0.92 | 12.8 | 11.1 | 0.63 |
| 4 | 26.7 | 25.5 | 0.86 | 39 | 42.9 | 0.95 | 12.5 | 10.4 | 0.38 | 15.3 | 14.9 | 0.87 |
| 8 | 8.8 | 20.1 | 0.18 | 56.2 | 55.1 | 0.85 | 14.3 | 12.3 | 0.34 | 17.0 | 16.6 | 0.89 |
| Mean interval change (0–8 wk) |
42.5 | 24.0 | 0.56 a | 32.0 | 28.2 | 0.65 a | 7.0 | 4.7 | 0.27 a | 4.2 | 5.5 | 0.07 a |
Abbreviations: BFR, blood flow restriction; DASH, Disabilities of the Arm, Shoulder and Hand Scores.
p-Value when comparing the magnitude of change of the BFR to the control group over the entire 8-week rehabilitation protocol.
The two groups did not demonstrate any difference in radial height, radial inclination, ulnar variance, tear drop angle, articular step-off, or volar tilt at any time point thus indicating no interval displacement with the therapy protocol initiated at 6 weeks post-cast immobilization ( Table 7 ). Additionally, there was no statistically significant changes in radiographic parameters in the BFR group between 6 weeks radiographs, prior to initiation of BFR, and terminal follow-up radiographs ( Table 7 ). All fractures were noted to have bridging trabecular bone across the fracture site at 3 months after initial cast immobilization.
Table 7. Radiographic parameters were measured at the time of each clinical follow-up.
| Comparison of radiographic parameters | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Radial height (mm) | p -Value | Radial inclination (°) | p -Value | Ulnar variance (mm) | p -Value | Tear drop angle (°) | p -Value | Articular diastasis (mm) | p -Value | Volar tilt (°) | p -Value | ||||||
| BFR | Control | BFR | Control | BFR | Control | BFR | Control | BFR | Control | BFR | Control | |||||||
| Injury | 8.8 | 9.7 | 0.47 | 20.3 | 20.3 | 0.97 | 0.6 | 1.1 | 0.63 | 59.4 | 57.3 | 0.73 | 0.4 | 0.4 | 0.83 | –1.3 | 4.9 | 0.30 |
| Post reduction | 10.8 | 10.8 | 0.99 | 23.1 | 22.2 | 0.64 | 0.4 | 0.3 | 0.92 | 68.6 | 62.8 | 0.34 | 0.3 | 0.2 | 0.52 | 7.3 | 0.0 | 0.24 |
| Clinic visit 1–3 wk | 10.7 | 10.6 | 0.96 | 22.0 | 22.1 | 0.86 | –1.0 | –0.1 | 0.75 | 68.7 | 69.3 | 0.98 | 0.2 | 0.3 | 0.85 | 7.8 | 5.5 | 0.65 |
| Clinic visit 5–9 wk | 10.7 | 10.6 | 0.79 | 22.0 | 22.8 | 0.55 | 0.5 | 0.7 | 0.96 | 68.5 | 68.9 | 0.88 | 0.0 | 0.2 | 0.93 | 7.2 | 5.0 | 0.74 |
| Terminal clinic visit >10 wk | 10.4 | 10.8 | 0.84 | 22.2 | 22.2 | 0.97 | 2.4 | 0.7 | 0.31 | 68.5 | 68.4 | 0.92 | 0.3 | 0.1 | 0.74 | 6.8 | 2.6 | 0.43 |
| BFR 6 wk X-rays vs. terminal f/u | 0.81 | 0.95 | 0.12 | 0.99 | 0.41 | 0.93 | ||||||||||||
Abbreviations: BFR, blood flow restriction; f/u, follow-up.
Note: There were no statistical differences noted between the BFR and control group for any time point as well as in the BFR group before initiation of BFR and at terminal follow-up.
Discussion
Distal radius fractures present a growing epidemic within the United States accounting for one-sixth of all emergency department visits and the most common long bone fracture. 2 22 Furthermore, a 17% increase in the incidence of this fracture has occurred over a 40-year period. 23 Returning patients to previous level of function remains a primary goal, yet distal radius fractures remain a predominant source of decreased school and work attendance, declining independence, and long-lasting disability. 24 Among 9,000 women, those with a history of a distal radius fracture were noted to have a significant decline in function when compared to those women without a previous fracture. 25 Pain has been shown to be a positive predictor of disability 2 years following a distal radius fracture. 26 Previous studies indicate that patients have ongoing pain, decreased strength, loss of ROM, and disability after sustaining a distal radius fracture. 27 28 29 30
BFR training first emerged as a valuable asset to increase muscular strength and endurance via muscular hypertrophy in athletes. 10 11 12 13 14 15 16 Since that time its indications have been expanded, and it has been utilized as an adjunct in postoperative rehabilitation. Muscle training under BFR increases strength and hypertrophy at lower loads than traditional rehabilitation methods. 15 31 32 33 Tennent et al demonstrated statistically significant increases in thigh circumference, knee strength, and functional outcome scores in patients undergoing BFR after knee arthroscopy when compared to traditional therapy. 17 Therapy under BFR has since been used in cases of Achilles tendon rupture with marked improvements in both strength and power. 19 There have been no prior studies reporting on the utility of BFR in the upper extremity after a fracture.
Ongoing pain after distal radius fracture remains difficult to treat. Approximately 16% of patients report continued pain at 1 year after distal radius fracture. 28 29 30 Patients who continue to have pain at 1 year follow-up can be expected to have persistent pain in the long-term, even up to 20 years despite functional improvements. 3 34 Additionally, a study by Mehta et al noted that patients with a PRWE pain subscale score of 35 or higher were eight times more likely to complain of chronic upper extremity pain than patients with lower pain scores. 29 Swart et al reported that postoperative pain was a significant predictor of disability in 190 patients with distal radius fractures. 26 In a prospective cohort study of 129 patients with distal radius fractures, patients experienced the most difficulty and pain with the activity of carrying weight. 35 With the introduction of BFR in our study, a statistically significant difference was noted in the magnitude of reduction in pain scores during activity over the course of the 8 weeks therapy program compared to the non-BFR cohort. The results observed with the addition of BFR may be due to the systemic effects of BFR, which can result in both local and systemic changes. 32 33 Patients undergoing BFR training of the upper extremity were noted to have increased muscle sympathetic nerve activity within the muscle being tested as well as at sites remote to the occluded extremity. 36 This may help to explain the disparity in pain improvement with activity between groups secondary to stress induced analgesia when the sympathetic nervous system response is more robust. 37
Functional outcome scores, particularly the PRWE, have long been used to determine disability following distal radius fractures. 20 The PRWE represents a validated outcome score specifically evaluating the wrist and distal radius fractures. 38 39 The maximal functional improvement and most change in PRWE score after sustaining a distal radius fracture are usually achieved by 3 months to 1 year, despite continued pain in some patients. 3 4 28 40 The PRWE scores at 1 year were found to be predictive of long-term scores. 3 In a survey of patients in the United Kingdom with distal radius fractures treated by either operative or nonoperative means, 95% (83 of 87) reported residual disability, 16% (14 of 87) of which reported marked disability altering their daily life. 28 Similarly, MacDermid et al prospectively followed 129 patients with operatively and nonoperatively treated distal radius fractures with serial PRWE evaluations and noted at 1 year, 41% of patients had no difficulty with specific activities and only 54% could report no difficulty with their usual activities. 35 In our study, the addition of BFR to the standardized therapy program produced significant improvements in PRWE scores when comparing the magnitude of change over the course of the 8 weeks occupational therapy program ( p = 0.01). It is postulated that if maximal functional improvement can be attained between 3 months to 1 year after sustaining a distal radius fracture, then it may lead to less long-term disability. 3 4
Both groups illustrated improvement in DASH scores, ROM, grip strength, 3-point, and lateral pinch. However, at final therapy follow, no significant benefits were noted in patients with or without BFR in terms of these variables. Nonetheless, it does appear to be safe to use in fracture rehabilitation as all patients were able to tolerate the BFR therapy sessions without complications. In addition, there were no statistically significant differences in radiographic parameters between the BFR and control groups between the 6 weeks radiographs prior to initiation of BFR and radiographic parameters at terminal follow-up indicating fracture stability despite the BFR rehabilitation program.
This study represents the first of its kind evaluating the utility of BFR therapy in distal radius fractures via a prospective, randomized control trial. All patients underwent a standardized treatment and therapy protocol by a single surgeon and a single occupational therapist, assuring consistency. However, there were several limitations to our study. First, we were unable to blind patients to the use of BFR, and this may have resulted in patients experiencing a placebo effect. Furthermore, there may be confounding variables at play that could have altered our results including patient motivation, secondary gain, or missed therapy visits. Lastly, this study was underpowered with a small sample size which may serve to limit its external validity thus stressing the importance of additional studies with a larger sample size.
Ultimately, BFR therapy can be safely employed in the nonoperative treatment of a distal radius fracture without deleterious effects to the patient. The addition of BFR to the rehabilitation protocol may result in a quicker reduction in pain with activity and improvement in overall function more quickly which may translate to less long-term disability. This proof of concept study illustrates that the addition of BFR is well tolerated by patients and no early complications were noted. Therefore, larger studies are required to determine the efficacy and utility of adding this modality to a rehabilitation protocol in the upper extremity after a sustaining a distal radius fracture or other hand and wrist injuries.
Footnotes
Conflict of Interest None declared.
References
- 1.Larsen C F, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(05):911–916. doi: 10.1093/ije/22.5.911. [DOI] [PubMed] [Google Scholar]
- 2.Bonafede M, Espindle D, Bower A G. The direct and indirect costs of long bone fractures in a working age US population. J Med Econ. 2013;16(01):169–178. doi: 10.3111/13696998.2012.737391. [DOI] [PubMed] [Google Scholar]
- 3.Lalone E, MacDermid J, Grewal R, King G. Patient reported pain and disability following a distal radius fracture: a prospective study. Open Orthop J. 2017;11:589–599. doi: 10.2174/1874325001711010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDermid J C, Roth J H, McMurtry R. Predictors of time lost from work following a distal radius fracture. J Occup Rehabil. 2007;17(01):47–62. doi: 10.1007/s10926-007-9069-0. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Orthopedic Surgeons. Guidelines on treatment of distal radius fractures. Available at:http://www.aaos.org/guidelines/. Accessed March 21, 2018
- 6.American College of Sports Medicine.American College of Sports Medicine position stand. Progression models in resistance training for healthy adults Med Sci Sports Exerc 20094103687–708. [DOI] [PubMed] [Google Scholar]
- 7.Loenneke J P, Kim D, Fahs C A et al. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve. 2015;51(05):713–721. doi: 10.1002/mus.24448. [DOI] [PubMed] [Google Scholar]
- 8.Loenneke J P, Wilson J M, Marín P J, Zourdos M C, Bemben M G. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(05):1849–1859. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 9.Pearson S J, Hussain S R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45(02):187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 10.Cook S B, Clark B C, Ploutz-Snyder L L. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc. 2007;39(10):1708–1713. doi: 10.1249/mss.0b013e31812383d6. [DOI] [PubMed] [Google Scholar]
- 11.Iida H, Kurano M, Takano H et al. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol. 2007;100(03):275–285. doi: 10.1007/s00421-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 12.Loenneke J P, Kearney M L, Thrower A D, Collins S, Pujol T J. The acute response of practical occlusion in the knee extensors. J Strength Cond Res. 2010;24(10):2831–2834. doi: 10.1519/JSC.0b013e3181f0ac3a. [DOI] [PubMed] [Google Scholar]
- 13.Loenneke J P, Wilson G J, Wilson J M. A mechanistic approach to blood flow occlusion. Int J Sports Med. 2010;31(01):1–4. doi: 10.1055/s-0029-1239499. [DOI] [PubMed] [Google Scholar]
- 14.Pope Z K, Willardson J M, Schoenfeld B J. Exercise and blood flow restriction. J Strength Cond Res. 2013;27(10):2914–2926. doi: 10.1519/JSC.0b013e3182874721. [DOI] [PubMed] [Google Scholar]
- 15.Takada S, Okita K, Suga T et al. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J Appl Physiol (1985) 2012;113(02):199–205. doi: 10.1152/japplphysiol.00149.2012. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka T, Farley R S, Caputo J L. Occlusion training increases muscular strength in division IA football players. J Strength Cond Res. 2012;26(09):2523–2529. doi: 10.1519/JSC.0b013e31823f2b0e. [DOI] [PubMed] [Google Scholar]
- 17.Tennent D J, Hylden C M, Johnson A E, Burns T C, Wilken J M, Owens J G. Blood flow restriction training after knee arthroscopy: a randomized controlled pilot study. Clin J Sport Med. 2017;27(03):245–252. doi: 10.1097/JSM.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 18.Gaunder C L, Hawkinson M P, Tennent D J, Tubb C C.Occlusion training: pilot study for postoperative lower extremity rehabilitation following primary total knee arthroplasty US Army Med Dep J 2017(2-17):39–43. [PubMed] [Google Scholar]
- 19.Yow B G, Tennent D J, Dowd T C, Loenneke J P, Owens J G.Blood flow restriction training after Achilles tendon rupture J Foot Ankle Surg 20185703635–638.; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.MacDermid J C. Development of a scale for patient rating of wrist pain and disability. J Hand Ther. 1996;9(02):178–183. doi: 10.1016/s0894-1130(96)80076-7. [DOI] [PubMed] [Google Scholar]
- 21.Hudak P L, Amadio P C, Bombardier C; The Upper Extremity Collaborative Group (UECG).Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected] Am J Ind Med 19962906602–608. [DOI] [PubMed] [Google Scholar]
- 22.Kilgore M L, Morrisey M A, Becker D J et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999-2005. J Bone Miner Res. 2009;24(12):2050–2055. doi: 10.1359/jbmr.090523. [DOI] [PubMed] [Google Scholar]
- 23.Melton L J, III, Amadio P C, Crowson C S, O'Fallon W M. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(04):341–348. doi: 10.1007/s001980050073. [DOI] [PubMed] [Google Scholar]
- 24.Nellans K W, Kowalski E, Chung K C. The epidemiology of distal radius fractures. Hand Clin. 2012;28(02):113–125. doi: 10.1016/j.hcl.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards B J, Song J, Dunlop D D, Fink H A, Cauley J A. Functional decline after incident wrist fractures--study of osteoporotic fractures: prospective cohort study. BMJ. 2010;341:c3324. doi: 10.1136/bmj.c3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swart E, Nellans K, Rosenwasser M. The effects of pain, supination, and grip strength on patient-rated disability after operatively treated distal radius fractures. J Hand Surg Am. 2012;37(05):957–962. doi: 10.1016/j.jhsa.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Ploegmakers J, The B, Wang A, Brutty M, Ackland T . Supination and pronation strength deficits persist at 2–4 years after treatment of distal radius fractures. Hand Surg. 2015;20(03):430–434. doi: 10.1142/S0218810415500355. [DOI] [PubMed] [Google Scholar]
- 28.Moore C M, Leonardi-Bee J. The prevalence of pain and disability one year post fracture of the distal radius in a UK population: a cross sectional survey. BMC Musculoskelet Disord. 2008;9:129. doi: 10.1186/1471-2474-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta S, MacDermid J, Tremblay M. The implications of chronic pain models for rehabilitation of distal radius fracture. Hand Ther. 2011;16(01):2–11. [Google Scholar]
- 30.Dewan N, MacDermid J C, Packham T.Role of a self-efficacy-based model of intervention: the LEARN approach in rehabilitation of distal radius fracture Crit Rev Phys Rehabil Med 201325(3–4):241–259. [Google Scholar]
- 31.Abe T, Kearns C F, Manso Filho H C, Sato Y, McKeever K H. Muscle, tendon, and somatotropin responses to the restriction of muscle blood flow induced by KAATSU-walk training. Equine Vet J Suppl. 2006;36(36):345–348. doi: 10.1111/j.2042-3306.2006.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 32.Abe T, Kearns C F, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol (1985) 2006;100(05):1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 33.Burgomaster K A, Moore D R, Schofield L M, Phillips S M, Sale D G, Gibala M J. Resistance training with vascular occlusion: metabolic adaptations in human muscle. Med Sci Sports Exerc. 2003;35(07):1203–1208. doi: 10.1249/01.MSS.0000074458.71025.71. [DOI] [PubMed] [Google Scholar]
- 34.Ydreborg K, Engstrand C, Steinvall I, Larsson E L. Hand function, experienced pain, and disability after distal radius fracture. Am J Occup Ther. 2015;69(01):6.90129003E9. doi: 10.5014/ajot.2015.013102. [DOI] [PubMed] [Google Scholar]
- 35.MacDermid J C, Roth J H, Richards R S. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord. 2003;4(24):24. doi: 10.1186/1471-2474-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito M, Iwase S, Hachiya T. Resistance exercise training enhances sympathetic nerve activity during fatigue-inducing isometric handgrip trials. Eur J Appl Physiol. 2009;105(02):225–234. doi: 10.1007/s00421-008-0893-5. [DOI] [PubMed] [Google Scholar]
- 37.Butler R K, Finn D P. Stress-induced analgesia. Prog Neurobiol. 2009;88(03):184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 38.MacDermid J C, Turgeon T, Richards R S, Beadle M, Roth J H. Patient rating of wrist pain and disability: a reliable and valid measurement tool. J Orthop Trauma. 1998;12(08):577–586. doi: 10.1097/00005131-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Paranaíba V F, Santos J BGD, Raduan Neto J, Moraes V Y, Belotti J C, Faloppa F. PRWE application in distal radius fracture: comparison and correlation with established outcomes. Rev Bras Ortop. 2017;52(03):278–283. doi: 10.1016/j.rboe.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobos P, Lalone E A, Grewal R, MacDermid J C. Recovery, age, and gender effects on hand dexterity after a distal radius fracture. A 1-year prospective cohort study. J Hand Ther. 2018;31(04):465–471. doi: 10.1016/j.jht.2017.08.002. [DOI] [PubMed] [Google Scholar]


