Growing evidence supports the “developmental origins of the health and disease” hypothesis, which links stressors and epigenetic mechanisms within the in utero environment to the propensity of the offspring to develop disease states later in life. In particular, early adult-onset hypertension has been linked to early-life insults.1 Epidemiologic studies support the notion that maternal malnutrition, both under and over-nutrition, during gestation and lactation has lifelong consequences on adult offspring’s health. For example, the Dutch Hunger Winter Famine study found that offspring exposed to maternal famine develop adult diseases including hypertension.2 Several nutritional risks, such as vitamin D deficiency, short-term breastfeeding, undernutrition, gestational diabetes mellitus, and excessive gestational weight gain, have been established to have a causal relationship between the maternal nutrition status and hypertension in offspring.1
Maternal over-nutrition and associated maternal metabolic and cardiovascular abnormalities are increasing throughout the world, in large part, due to increased consumption of fructose-rich foods and drinks.3–5 To this point, fructose, a naturally occurring monosaccharide, is present in fruits, honey, fruit juices, and most root vegetables.3–5 However, fructose is also a component of high-fructose corn syrup, which is an integral constituent of many beverages, processed fruits, condiments, frozen desserts, jams, jellies, dry mix beverages, flavored water, carbonated beverages, sports and energy drinks, frozen desserts, jams, jellies, breakfast cereals, baked goods and confectioneries.3–5 It is increasingly recognized that high dietary fructose intake, in the form of high-fructose corn syrup, is a major contributor to the increasing prevalence of obesity and has adverse effect on health outcomes. Recent reports suggest that consumption of high fructose diets by mothers during pregnancy and lactation induces renal programming leading to hypertension in adult offspring.3–6 In a systematic meta-analysis of human studies, fructose consumption was positively associated with increased fasting blood sugar, elevated triglycerides, and elevated systolic blood pressure.6 Studies in Sprague-Dawley rats and C57BL/6J mice have shown that high fructose feeding alters fetal and offspring metabolism and promotes fetal programming for subsequent hypertension in adult life4, 5,7 High salt and high fat diets further exacerbate programmed hypertension in the offspring of mothers consuming a high fructose diet.5 The mechanisms by which high maternal fructose consumption leads to programmed hypertension include excessive oxidative stress, activation of the renin angiotensin aldosterone system (RAAS), increased sympathetic nerve activity, dysregulation of nutrient-sensing signals, increased uric acid, impaired endothelium-dependent relaxation, abnormal gut microbiota composition which are abnormalities impacted by sexual dimorphism (Fig 1).1, 4–6 Several nutritional insults including a low protein diet,8 high-fat diet,1 and high-fructose diet9 during pregnancy and lactation lead to predisposition toward dysregulation of RAAS in the offspring.3–5 Related to this, high maternal fructose intake has been found to induce programmed hypertension in 12-week-old offspring of both sexes,9 which was associated with increased gene expression angiotensin II receptor (Agtr) 1b, renin (Ren), and Ace while decreasing Mas1 expression.9 Other nutritional factors such as protein deprivation in pregnant dams also induced an increase of Agtr protein expression in regions of the brain in their off spring.8 Further, administration of a Ren antagonist increased Ace2 and Mas protein levels in female kidneys exposed to high maternal fructose intake, suggesting a protective effect of inhibition of the RAAS cascade in hypertension programmed by maternal high fructose intake.9 Activation of nutrient-sensing signaling pathways, such as AMP-activated protein kinase and peroxisome proliferator-activated receptors, increases in uric acid and oxidative stress, and attenuation of the nitric oxide pathway, are also involved in RAAS activation-related hypertension of developmental origin (in adult offspring) induced by maternal and postnatal high fructose intake (Fig 1).
Fig 1.
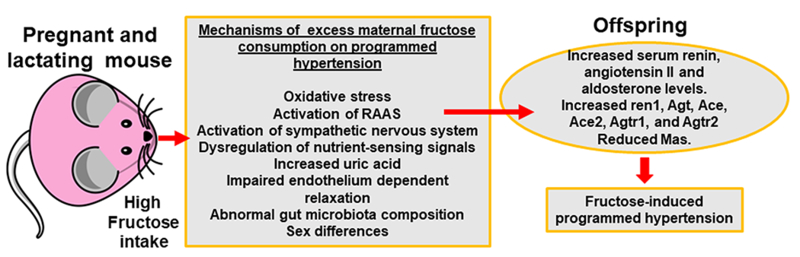
The mechanisms of maternal high fructose consumption on activation of renin angiotensin-aldosterone system (RAAS) and programmed hypertension.
In concert with RAAS activation and resultant programmed hypertension, maternal high fructose consumption also induces abnormal expression of renal sodium transporters, excessive oxidative stress, inflammation, and renal fibrosis in adult offspring. In this issue of Hypertension, Seong et al3 investigated whether maternal exposure to high fructose induces hypertension together with RAAS activation, renal tissue oxidative stress, inflammation, and tissue fibrosis in successive multiple generations. Importantly, the results of these basic research studies highlight the increasing risk for hypertension in offspring of dams subjected to high fructose intake. Feeding a 20% fructose diet in female mice during pregnancy and the subsequent lactation period induced complex temporal responses with elevated systolic blood pressure evident in multiple generations of adult offspring (at 8 months). Development of hypertension paralleled progressive RAAS activation, renal oxidative stress, inflammation and tissue remodeling. Specifically, maternal exposure to high fructose significantly increased blood pressure in the 1st and 2nd generation offspring along with increased serum Ren, angiotensin II and aldosterone levels, as well as abnormal mRNA expression for Ren1, angiotensinogen, Ace, Ace2, Agtr1, Agtr2 and Mas. Further, maternal exposure to high fructose also induced abnormal renal mRNA expression of sodium transporters Slc9a3 Slc12a1, Slc12a3, and Scnn1b in multiple-generations of the adult offspring. Renal tissue inflammation, oxidative stress and fibrosis in the offspring were associated with pro-inflammatory/fibrotic factors including elevated expression of interleukin 6, nuclear factor-κB, nicotinamide adenine dinucleotide phosphate oxidase (NOX) 3, NOX4 and transforming growth factor β1.
This study3 provides new insights into the role of maternal exposure to high fructose in RAAS activation and development of programmed hypertension which extends into multiple generations of adult offspring in rodents. These results, obtained in mice, are translationally relevant as an increase in fructose consumption over the past 50 years in human subjects has been significantly linked to a rise in obesity, metabolic disorders, and related cardiovascular and renal diseases. These data3 highlight the role for maternal high dietary fructose intake in increasing the risk of development of hypertension in multigenerational offspring through activation of RAAS, increased vasocontractile peptides, renal sodium transporters, oxidative stress and inflammation. The present study is consistent with and extend previous studies in which maternal and post‐weaning high‐fructose diets induced hypertension of developmental origin.4–6 However, there are several questions that need to be further investigated and clarified. Firstly, adverse effects of fructose feeding appear to depend on both the amount and duration of fructose consumption. In Sprague-Dawley rats, maternal consumption of a 60% fructose diet caused an increase of blood pressure in offspring at 12 weeks.4 In C57BL/6J mice, maternal 10% fructose intake was shown to increase mean arterial blood pressure at 12 month of age in the offspring.7 In the current study the offspring were studied at 8 month of age, which is equivalent to middle aged humans. Provision of a strong rationale for the maternal 20% high fructose intake and the 8 month determination of blood pressure values in the offspring would have been instructive. Meanwhile, recent studies also suggest that sex differences exist in the developmental programming of hypertension, and that males are more prone to hypertension than females prior to menopause.10 The current study does not identify fundamental sex-specific differences in RAAS activation, sodium transporters, oxidative stress factors, and inflammation induced by maternal high fructose intake in the multigenerational offspring. To help clarify these issues use of next generation gene sequencing and blood pressure measurements by telemetry would enhance the impact of relating to maternal diet effects on offspring.
In conclusion, the results of this study in rodents3 show an important role of maternal exposure to high fructose during pregnancy and lactation in development of multigenerational RAAS activation and associated hypertension. Indeed, reductions in maternal fructose consumption may be a novel recommendation and therapeutic strategy for preventing multigenerational hypertension and associated cardiovascular and renal diseases. Additional studies including in humans are warranted to more definitively understand the relative role of high fructose consumption in maternal diet- induced cardio-renal metabolic abnormalities and vascular stiffness in conjunction with hypertension.
Acknowledgments
Sources of Funding
Dr. Jia received funding from the American Diabetes Association (Innovative Basic Science Award #1-17-IBS-201). Dr. Sowers received funding from NIH (R01 HL73101-01A and R01 HL107910-01) and from Veterans Affairs Merit System (2I01BX001981-05A1). Dr. Hill received funding from NIH (RO1HL085119).
Footnotes
Disclosures: No potential conflicts of interest relevant to this article were reported.
References
- 1.Tain YL, Lin YJ, Sheen JM, Yu HR, Tiao MM, Chen CC, Tsai CC, Huang LT and Hsu CN. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein AD, Zybert PA, van der Pal-de Bruin K and Lumey LH. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–65. [DOI] [PubMed] [Google Scholar]
- 3.Seong HY, Cho HM, Kim M and Kim I. Maternal high-fructose intake induces multigenerational activation of the renin-angiotensin-aldosterone system. Hypertension. 2019; In Press. [DOI] [PubMed] [Google Scholar]
- 4.Tain YL, Leu S, Wu KL, Lee WC and Chan JY. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: roles of nitric oxide and arachidonic acid metabolites. J Pineal Res. 2014;57:80–9. [DOI] [PubMed] [Google Scholar]
- 5.Tain YL, Lee WC, Leu S, Wu K and Chan J. High salt exacerbates programmed hypertension in maternal fructose-fed male offspring. Nutr Metab Cardiovasc Dis. 2015;25:1146–51. [DOI] [PubMed] [Google Scholar]
- 6.Kelishadi R, Mansourian M and Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30:503–10. [DOI] [PubMed] [Google Scholar]
- 7.Saad AF, Dickerson J, Kechichian TB, Yin H, Gamble P, Salazar A, Patrikeev I, Motamedi M, Saade GR and Costantine MM. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am J Obstet Gynecol. 2016;215:378 e1-6. [DOI] [PubMed] [Google Scholar]
- 8.Woods LL, Ingelfinger JR, Nyengaard JR and Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–7. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CN, Wu KL, Lee WC, Leu S, Chan JY and Tain YL. Aliskiren Administration during Early Postnatal Life Sex-Specifically Alleviates Hypertension Programmed by Maternal High Fructose Consumption. Front Physiol. 2016;7:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojeda NB, Intapad S and Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf). 2014;210:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


