Abstract
It is 35 years since the first description of arrhythmogenic right ventricular cardiomyopathy (ARVC) and more than 20 years since the first reports establishing desmosomal gene mutations as a major cause of the disease. Early advances in the understanding of the clinical, pathological and genetic architecture of ARVC resulted in consensus diagnostic criteria, which proved to be sensitive but not entirely specific for the disease. In more recent years, clinical and genetic data from families and the recognition of a much broader spectrum of structural disorders affecting both ventricles and associated with a propensity to ventricular arrhythmia have raised many questions about pathogenesis, disease terminology and clinical management. In this paper, we present the conclusions of an expert round table that aimed to summarise the current state of the art in arrhythmogenic cardiomyopathies and to define future research priorities.
Keywords: Arrhythmogenic cardiomyopathy, Arrhythmogenic right ventricular cardiomyopathy, Ventricular arrhythmias
Introduction
In recent years, the clinical paradigm of arrhythmogenic right ventricular cardiomyopathy (ARVC) has moved on from a focus on severe right ventricular disease and malignant arrhythmia to a broader disease spectrum that includes concealed or subclinical phenotypes and biventricular disease. This has led to the development of a new concept–arrhythmogenic cardiomyopathy (AC) – that embraces a confusing array of disease terms and quite different pathologies. In November 2017, a group of international experts met in Athens, Greece, to discuss current knowledge about ACs with the aim of defining future research priorities. The meeting format was that of a moderated round table discussion (edited highlights of which can be viewed at http://www.fondazione-menarini.it/Home/Eventi/International-Closed-Workshop-on-ArrhythmogenicCardiomyopathy/Presentazione). This document summarizes the content of the meeting. A brief summary of each section is presented in Table 1.
Table 1.
Research priorities in arrhythmogenic cardiomyopathy
| Definitions Genetic architecture |
|
| Role of the desmosome |
|
| Role of inflammation |
|
| Sudden cardiac death prediction |
|
| Prospect clinical trials |
|
AC, arrhythmogenic cardiomyopathy; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BB, beta-blocker; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist; S-ICD, subcutaneous implantable cardioverter-defibrillator.
What is arrhythmogenic cardiomyopathy?
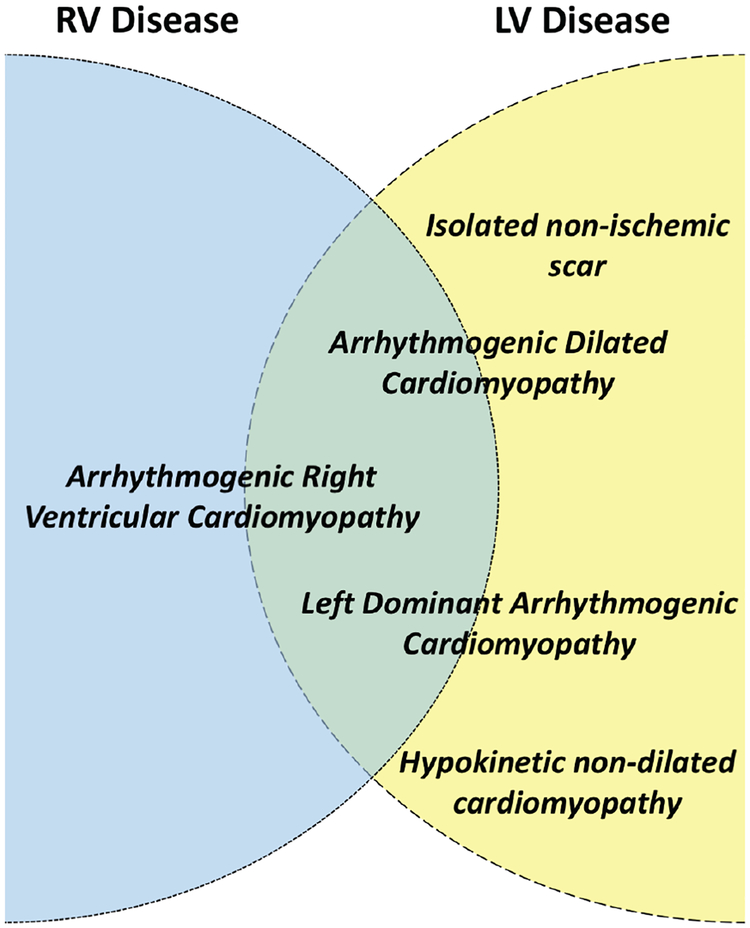
The term ‘arrhythmogenic cardiomyopathy’ is used to describe a family of diseases that feature structural myocardial abnormalities (identified by macroand microscopic pathological examination besides cardiac imaging) and ventricular arrhythmia. In Figure 1, we show how the most commonly used terms in the literature (including ARVC) can be organised by the patterns of ventricular involvement within the rubric of AC.1–3 Inclusion of entities such as hypertrophic and restrictive cardiomyopathy is not considered appropriate as their aetiology and management rarely overlap with the conceptual model of AC as conceived in this paper.
Figure 1.
Ideogram showing current terms that describe different arrhythmogenic cardiomyopathy phenotypes and their possible relationship to left (LV) and right ventricular (RV) disease. By definition, arrhythmogenic RV cardiomyopathy affects predominantly the right ventricle but biventricular forms are frequently seen. Arrhythmogenic dilated cardiomyopathy and left-dominant arrhythmogenic cardiomyopathy describe overlapping entities that mostly affect the left ventricle but RV involvement is also observed. Isolated non-ischaemic scar and hypokinetic non-dilated cardiomyopathy mostly refer to predominantly LV scarring identified using cardiac magnetic resonance imaging.1–3
Accepting that a precise definition for AC has yet to be agreed upon, its diagnosis should follow a systematic approach that builds on the existing ARVC concept. Fundamental aspects include the following:
Arrhythmia: frequent ventricular ectopy, from either ventricle, sustained or non-sustained ventricular tachycardia, or unexplained cardiac arrest are essential manifestations of the AC phenotype.
Electrical abnormalities: in addition to the classical ECG changes of ARVC, other abnormalities such as conduction disease, atrial arrhythmia, small voltage complexes and the pattern of left ventricular disease provide diagnostic clues to specific aetiologies.
Structural abnormalities: abnormalities of myocardial structure and dysfunction on non-invasive imaging are important but not essential criteria for AC, whereas tissue characterisation with cardiac magnetic resonance imaging, and if necessary nuclear imaging and cardiac histopathology, is essential for the definition of sub-phenotypes.
Heritability: family history (including clinical evaluation of first-degree relatives and at least a three-generation pedigree) is one of the principal diagnostic tools in AC. Genetic analysis is central to the diagnosis in probands and defines carrier status in relatives. Family history of other relevant traits such as premature conduction disease or extra-cardiac manifestations (skin, hair and neuromuscular phenotypes) helps to orient and interpret diagnostic tests.
Phenocopy exclusion: in all cases of AC, it is important to exclude mimics of the AC phenotype such as congenital anomalies, pulmonary hypertension, cardiac sarcoidosis and myocarditis.
What is the genetic architecture of arrhythmogenic cardiomyopathy?
Pathogenic mutations in desmosomal genes (JUP, DSP, PKP2, DSG2, DSC2) account for disease in over 50% of patients with classical ARVC.4–9 Non-desmosomal pathogenic variants are described in DES, LMNA, SCN5A, CDH2, CTNNA3, FLNC, PLN, TGFB3, TMEM43, RYR2, TJP1 and TTN10–21 and reports describe a disproportionately high burden of ventricular arrhythmia in dilated cardiomyopathy caused by pathogenic variants in RBM20, TNNT2, and BAG3.22–24 The possible relation between the main causative genes and the relative involvement of the left and right ventricles are shown in Table 2.4,6,8–16,19,25–31
Table 2.
Observed phenotypes associated with mutations in arrhythmogenic cardiomyopathy-related genes
| Gene | Predominant RV disease | Biventricular disease | Predominant LV disease | Other characteristics | Ref. |
|---|---|---|---|---|---|
| JUP | + | + | − | Cardiocutaneous syndrome | 4 |
| DSP | − | + | + | Cardiocutaneous syndrome | 25 |
| PKP2 | + | + | − | 6,26 | |
| DSG2 | + | + | + | 8,26 | |
| DSC2 | + | + | − | 9,26 | |
| TGFB3 | + | Unknown | Unknown | 27 | |
| TMEM43 | + | + | − | 28 | |
| TTN | + | + | + | 19,29 | |
| DES | − | + | + | 10 | |
| Lamin A/C | − | + | + | Conduction disease | 11 |
| PLN | − | + | + | 16,26 | |
| CTNNA3 | + | + | − | 14 | |
| CDH2 | + | + | − | 13 | |
| SCN5A | − | + | + | Electrical > structural disease | 1 2,29 |
| FLNC | − | + | + | 15 | |
| RBM20 | Unknown | Unknown | + | 30 | |
| BAG3 | Unknown | Unknown | + | 31 |
A literature review identified the spectrum of phenotypes in relation to ventricular disease in patients with carrying mutations in AC-related genes. Presence of disease is marked with ‘+’ and absence with ‘−’.
BAG3, Bcl2-associated athanogene 3; CDH2, cadherin 2; CTTNA3, catenin alpha 3; DES, desmin; DSC2, desmocolin 2; DSG2, desmoglein 2; DSP, desmoplakin; FLNC, filamin C; JUP, plakoglobin; LV, left ventricular; PKP2, plakophillin 2; PLN, phospholamban; RBM20, RNA binding motif protein 20; RV, right ventricular; SCN5A, sodium voltage-gated channel alpha subunit 5; TGFB3, transforming growth factor beta 3; TMEM43, transmembrane protein 43; TTN, titin.
Despite the prominence given to gene mutations in the Task Force diagnostic criteria for ARVC, many pathogenic variants classified as ‘pathogenic’ or ‘likely pathogenic’ are also seen in people without AC. In part, this reflects the challenge of accurately classifying missense variants due to the limitations of current methods of variant calling (functional studies, in-silico methods, etc.). Nevertheless, even within affected families the penetrance of most pathogenic desmosome gene variants is relatively low (approximately 30%), suggesting that disease expression is influenced by other genetic or environmental factors.32 For example, endurance athletes with desmosomal variants have earlier disease onset, are more likely to meet ARVC Task Force criteria, and have lower lifetime-free survival from ventricular tachycardia/fibrillation and heart failure.33
Many questions and challenges remain regarding the genetics of AC. In particular, the increasing number of variants identified by continuously evolving genomic methods has raised the need for more extensive and better curated controls. There is a growing focus on deep intronic variants that alter splicing in genes already known to be associated with the disorder and the use of approaches such as RNA sequencing or assessment of long non-coding RNAs that may improve diagnostic yield. The need for functional assays that can provide quantitative assessment of novel variants and their potential role in disease becomes ever more pressing.
What is the role of the desmosome in arrhythmogenic cardiomyopathy?
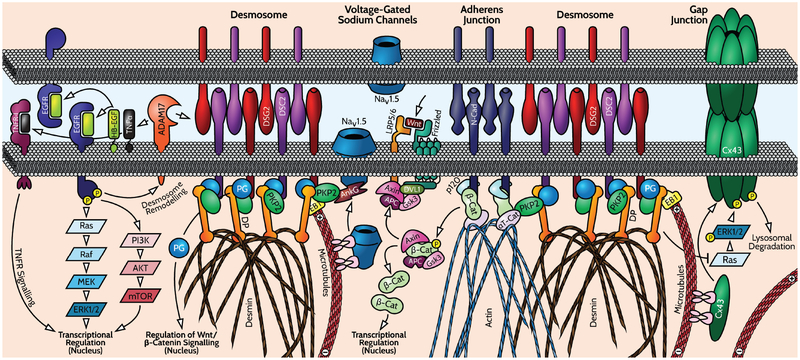
Arrhythmogenic cardiomyopathy is associated with mutations in desmosome genes, and thus it has been assumed that the inability to effectively withstand mechanical stress is a major contributing factor to disease pathogenesis. However, many other functions for desmosomes and their components are emerging and may turn out to have role in the pathogenesis of AC (Figure 2). Postnatally in vertebrates, an intermingling of desmosome and other junction components occurs, creating a specialised form of junctions called ‘area compositae’.34 This mixed junction may provide additional strength to the junction by distributing intermediate filament connections throughout the junctional interface. For instance, desmosomes play critical roles in trafficking and stability of the gap junction protein connexin 4335,36 and other membrane channels including Nav 1.5.37 Thus, impaired desmosome function also leads to loss of efficient electrical coupling and conduction that could be linked to arrhythmias. Desmosomes also help localize the actin-regulatory machinery including Rho-GTPases,38 which might further impact the organisation and function of the adherens junctions associated actin cytoskeleton and contractile apparatus. Desmosome components also regulate multiple other signalling pathways including GSK3beta/Wnt,39,40 p38/TGFbeta41 and Ras/Erk2.42 Coupling with these pathways regulates multiple endpoints in skin and heart, including cell fate, differentiation, and fibrotic gene expression.
Figure 2.
This schematic shows the complex integration of mechanical and electrochemical signalling at cardiac intercalated discs and highlights the proposed remodelling of desmosomes, gap junctions and ion channels in arrhythmogenic cardiomyopathy. Desmosomes, which are a primary target for arrhythmogenic cardiomyopathy-causing mutations, are distributed in close proximity with adherens junctions, gap junctions (made of connexins) and sodium channels. Both desmosomes and adherens junctions have at their central adhesive core members of the cadherin family of calcium-dependent adhesion molecules [desmoglein 2 (DSG2) and desmocollin 2 (DSC2) in desmosomes, and N-cadherin (N-Cad) in adherens junctions]. Linking these molecules to the desmin intermediate filament and actin cytoskeletons, respectively, are a complex of armadillo and cytoskeletal linker proteins consisting of plakophilin 2 (PKP2), plakoglobin (PG) and the intermediate filament anchoring protein desmoplakin (DP) in desmosomes; and beta-catenin (β-Cat), p120 catenin and the actin binding protein alpha T-catenin (αT-Cat) in adherens junctions. In the heart, alpha-T catenin can interlink with PKP2, providing a structural link between desmosome and adherens junctions that results in inter-mixing and stabilizes the cortical cytoskeleton. Mixed junctions appearing in vertebrates are called the ‘area composita’. Examples of functional interactions at the intercalated discs relevant to arrhythmogenic cardiomyopathy are shown. These include DP-dependent trafficking and stabilization of connexin-43 (Cx43) and associated gap junction communication through(ia) EB1-dependent microtubule stabilization at junctions, and (ii) DP-dependent regulation of Erk1/2-dependent turnover of Cx43 through dampening Ras. In addition, distribution and function of the voltage-gated sodium channel (VGSC) Nav1.5 depends on PKP2 and its associated adaptor protein ankyrin G (AnkG). Conflicting data associate perturbation of Wnt/β-Cat signalling through alterations in PG, β-Cat distribution and Hippo signalling (blocks Wnt), with adipogenic vs. fibrotic cell fate during arrhythmogenic cardiomyopathy development. Desmosomes can themselves be regulated by a combination of the ectodomain sheddase ADAM17 and epidermal growth factor receptor (EGFR) signalling [which is itself regulated via the release of EGFR ligands, such as EGF-like growth factor (HB-EGF) in cardiac muscle, by ADAM17], which cooperate to regulate cleavage of DSG2. This serves to remodel desmosomes and coordinates this remodelling with gene expression changes associated with arrhythmogenic cardiomyopathy. PI3K, phosphoinositide 3-kinase; TNFα, tumour necrosis factor alpha; TNFR, tumour necrosis factor receptor.
The first genetic studies of AC arose from the study of autosomal recessive AC syndromes associated with a skin disease (palmoplantar keratoderma) and woolly hair.4,5,42,43 Better understanding of desmosomal function and disease mechanisms in the skin from AC patients may provide insights into AC pathogenesis and potential therapeutic strategies. Further insights come from studying molecules that regulate desmosomes including the iRhom2/ADAM17 pathway that cleaves membrane bound cytokines, growth factors and desmoglein 2.44–47 Also, there is emerging evidence of links between systemic inflammatory stress and perturbed desmosome function in both heart and skin.48
What is the role of inflammation in the pathogenesis of arrhythmogenic cardiomyopathy?
Evidence from autopsy and endomyocardial biopsy in classic ARVC suggests that cell death is largely the result of apoptosis.49,50 The adipocytes seen on histology appear not to result from cardiomyocyte transdifferentiation,51–54 but are instead derived from interstitial mesenchymal fibroblasts. Cardiomyocyte loss is usually patchy, but can rarely occur as acute segmental pathology with clinical manifestations of angina, ECG ST-segment elevation and release of troponin mimicking myocardial infarction.54
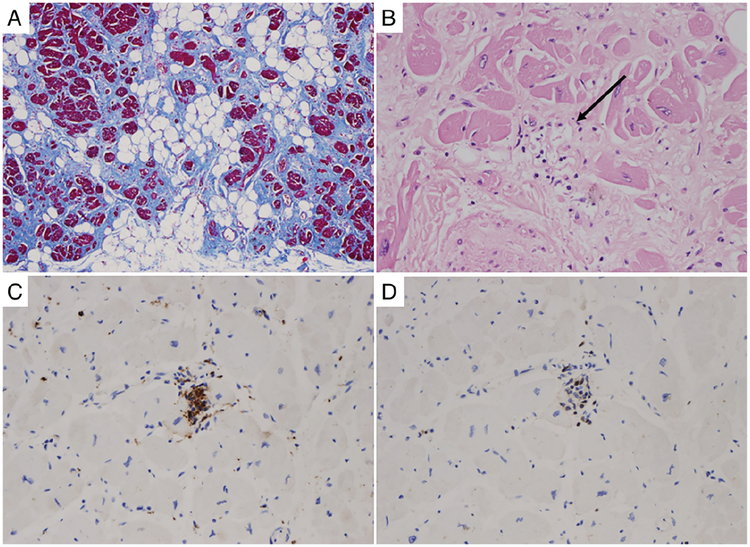
Autopsy studies show that myocardial inflammation in the form of T-cell infiltrates is present in approximately two-thirds of ARVC cases53,55 (Figure 3); the absence of neutrophils rules out ischaemic injury. The search for cardiotropic viruses in the myocardium has yielded conflicting results56,57 with adenovirus, cytomegalovirus, hepatitis C virus, parvovirus and even enterovirus present in some specimens. Whether viruses are by-standers or true pathogenetic viral particles remains an unsolved issue.
Figure 3.
Myocardial histology from a 31-year-old male competitive athlete who died suddenly as first manifestation of arrhythmogenic cardiomyopathy. Autopsy revealed biventricular arrhythmogenic cardiomyopathy and evidence of myocardial inflammation: (A) subepicardial fibrofatty replacement of the left ventricular free wall (Trichrome stain); (B) abnormal cardiomyocytes with dysmorphic nuclei, replacement fibrosis and inflammatory infiltrates with myocytolysis (arrow); (C) immunotyping for macrophages (CD68 antibody); (D) immunotyping for T lymphocytes (CD3 antibody).
Immunohistochemistry has shown a reduced signal for plakoglobin at the intercalated disc,58 but other studies indicate that a reduced desmosomal protein signal is found also in patients with giant cell myocarditis and sarcoidosis.59 The latter is a peculiar form of chronic myocarditis that can mimic AC.60
It remains unclear if myocarditis in AC is a primary phenomenon or reactive to spontaneous cardiomyocyte death. The hypothesis that a genetically61 vulnerable myocardium may predispose to myocarditis has been advanced62 and local myocardial production of selected cytokines and alterations in the balance between circulating pro-inflammatory and anti-inflammatory cytokines in patients with AC has been demonstrated.59 In one study, cytokines implicated in granulomatous inflammation promoted rapid intracellular translocation of junctional plakoglobin in cultured neonatal rat ventricular myocytes, suggesting that inflammatory mediators might play a role in AC, even in the absence of infiltrating inflammatory cells.59 Furthermore, the increasing understanding of the interplay between immune response and exercise might provide further insights in the mechanisms of exercise promoted disease progression in AC.61
How can sudden cardiac death prediction be improved in arrhythmogenic cardiomyopathy?
Most evidence suggests that in AC caused by desmosomal mutations, the degree of structural remodelling is a key determinant of ventricular arrhythmia. Data on arrhythmia risk in non-desmosomal forms of AC are still emerging, but there are already compelling findings for some genetic subtypes. For example, registry data in AC caused by LMNA mutations suggest that male sex, non-sustained ventricular tachycardia, reduced ejection fraction and non-missense mutations define the patient at risk of ventricular tachycardia/fibrillation.63 Other genetic causes of AC are less well studied, but data suggest that for mutations in TMEM43, RBM20, the founder R14del in phospholamban and FLNC, sudden death risk relates to the severity of structural and functional abnormalities, although anecdotal reports and familial evaluation suggest that sudden death can be the initial presenting feature.16,30 Several novel disease biomarkers that might aid risk prediction are under study, such as testosterone, BIN-1, soluble ST2 and anti-desmoglein-2 autoantibodies.64–67 However, these have not been compared with conventional risk factors. The dynamic nature of the disease and inflammatory hot phases may promote a temporary increased vulnerability to ventricular fibrillation. Anti-inflammatory agents may provide a means for reducing arrhythmic risk but require sensitive and specific biomarkers of disease activity and therapeutic response.
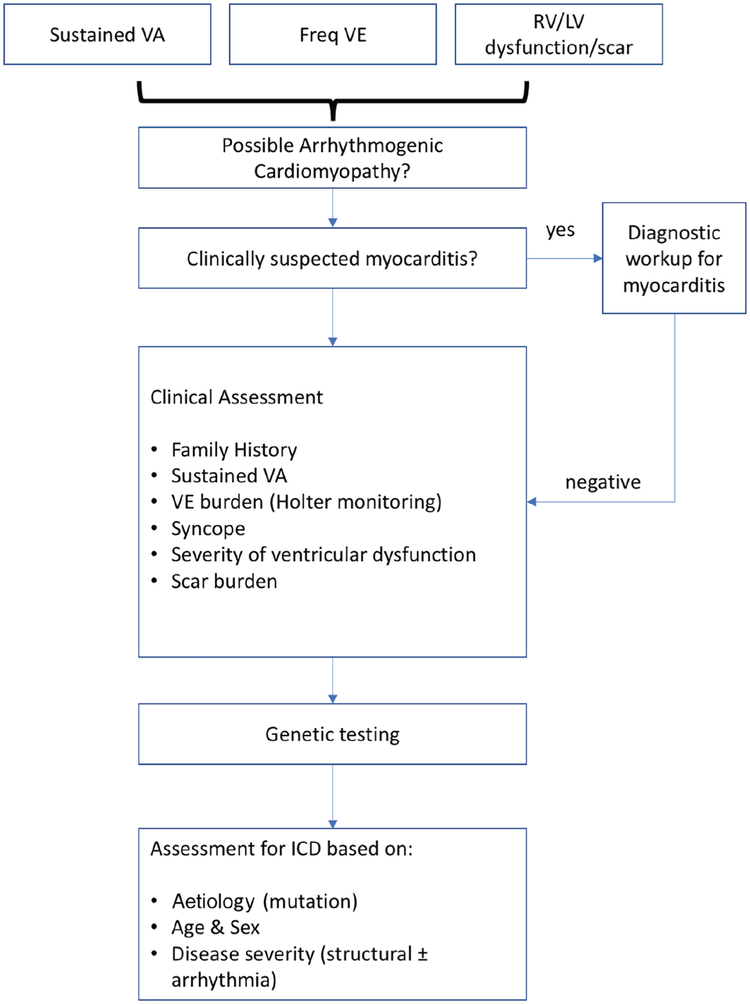
The diversity of causes for AC, together with phenotypic variability and age-related penetrance of genetic mutations, means that risk assessment needs to be tailored to aetiology and stage of disease (Figure 4). For patients fulfilling current Task Force criteria for ARVC, high- and low-risk cohorts have been identified, but there are many individuals who fall into an intermediate category for whom clear guidance on management is lacking. Moreover, most approaches to risk estimation are based on a relatively crude assessment of relative risk rather than absolute risk prediction. The challenge of optimal risk modelling might be addressed through the integration of large deeply phenotyped and genotyped cohorts with sufficient follow-up to permit development and validation of risk models that integrate genetic, ECG, electrophysiological and imaging data.
Figure 4.
Suggested flow chart for the general management of ventricular arrhythmia in arrhythmogenic cardiomyopathies. The pathways for management are critically dependent on aetiology as well as the clinical profile of individual patients. ICD, implantable cardioverter-defibrillator; LV, left ventricular; RV, right ventricular; VA, ventricular arrhythmia; VE, ventricular ectopy.
What are the prospects for trials in arrhythmogenic cardiomyopathy?
Prospective treatment trials focused on novel therapeutic approaches for the treatment of AC are lacking. This reflects the challenges for conventional trial design in diseases that are rare, heterogeneous in presentation, associated with prominent arrhythmia, and slowly progressive. Hard endpoints such as mortality are difficult to achieve in uncommon disorders with long natural histories. Therefore, it is of utmost importance to define subsets of patients with a sufficiently high likelihood of meeting clinically meaningful surrogate endpoints within the duration of a trial, for example progression of structural or electrical characteristics. The endpoint for trials targeting cardiac arrhythmias might include time to the first episode of sustained ventricular tachycardia/fibrillation or premature ventricular complex frequency, as these appear to predict the risk of sustained ventricular arrhythmias. Trials targeting heart failure endpoints are challenging due to slow and variable disease progression.68,69 Serial evaluation of structural myocardial abnormalities is difficult due to the limitations of conventional echocardiography in assessing the right ventricle and because the presence of an implantable cardioverter-defibrillator in many patients precludes serial magnetic resonance imaging. For these reasons, it is likely that trials in this area will require other biomarkers of ventricular impairment and remodelling such as natriuretic peptides and circulating markers of fibrosis.
Specific interventions that have potential to reduce life-threatening ventricular arrhythmias include pharmacologic agents such as beta-blockers, class 1 and 3 anti-arrhythmic agents such as flecainide, sotalol, tikosyn and amiodarone, endocardial and/or epicardial catheter ablation, and sympathetic denervation. As vigorous exercise seems to be factor for disease progression in ARVC and possibly in other forms of AC such as laminopathy,70–73 clinical trials to determine whether exercise restriction can prevent development of ARVC are warranted.
Pharmacologic agents that could directly target the underlying disease mechanism in the various forms of AC are under investigation. For example, a small molecule inhibitor of GSK3β, a major regulator of Wnt/β-catenin signalling identified in an unbiased chemical screen in a zebrafish model of ARVC,74 has been shown to dramatically reduce arrhythmias, myocardial damage and exercise-induced injury in mouse models of ARVC.74,75 However, effective drug therapy will require chronic administration, and long-term use of Wnt agonists may have unacceptable off-target effects, including increased risk of developing cancer. Thus, a potential future strategy to develop a truly mechanism-based drug therapy will be to identify pathways and targets downstream of GSK3β that are driving the cardiomyopathic disease phenotype.
In animal studies, loss of functional lamin proteins produces strong cellular stress signals that activate the p38 mitogen-activated protein kinase (MAPK) pathway which in turn causes decreased contractility, cardiomyocyte apoptosis, cardiomyocyte hypertrophy, and increased brain natriuretic peptide expression.76 ARRY-797 is an oral administered selective p38 MAPK inhibitor that reverses cardiac dysfunction in an animal model of LMNA and studies are now underway in patients with dilated cardiomyopathy caused by LMNA mutations (ClinicalTrials.gov Identifier: ).
Conclusions
Since the first detailed clinical description of ARVC, dramatic advances have been made regarding its pathogenesis, diagnosis and management. This progress was made possible by the development of single centre registries working alone or in partnership and also by the efforts of dedicated basic research laboratories. With the recognition of AC as a broad spectrum of disease, the need for national and international collaborative efforts is even greater. We hope this document will contribute to this endeavour.
Funding
This project was an independent initiative supported by the Fondazione Internazionale Menarini, which had no part in designing or approving the agenda. St. Bartholomew’s Hospital, the Amsterdam UMC and the Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padua-Azienda Ospedaliera are members of ERN GUARD-HEART (European Reference Network for Rare and Complex Diseases of the Heart; http://guardheart.ern-net.eu), which endorsed the meeting.
Conflict of interest: H.C. reports grants and personal fees from Boston Scientifiic, and personal fees from Medtronic, St Jude Medical, during the conduct of the study. D.P.J. reports grants from Menarini Foundation, during the conduct of the study; personal fees from Alnylam, GlaxoSmithKline, Pfizer, outside the submitted work. H.T. reports personal fees from Abbott, Cooltech, outside the submitted work. All other authors have nothing to disclose.
References
- 1.Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucifora G, Muser D, Masci PG, Barison A, Rebellato L, Piccoli G, Daleffe E, Toniolo M, Zanuttini D, Facchin D, Lombardi M, Proclemer A. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: a magnetic resonance imaging study. Circ Arrhythm Electrophysiol 2014;7:456–462. [DOI] [PubMed] [Google Scholar]
- 3.Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Bohm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–1858. [DOI] [PubMed] [Google Scholar]
- 4.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000;355:2119–2124. [DOI] [PubMed] [Google Scholar]
- 5.Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 2000;9:2761–2766. [DOI] [PubMed] [Google Scholar]
- 6.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 2004;36:1162–1164. [DOI] [PubMed] [Google Scholar]
- 7.Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet 2006;79:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 2006;113:1171–1179. [DOI] [PubMed] [Google Scholar]
- 9.Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, Basson CT, Lerman BB, Sasse-Klaassen S, Thierfelder L, MacRae CA, Gerull B. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 2006;79:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tintelen JP, Van Gelder IC, Asimaki A, Suurmeijer AJ, Wiesfeld AC, Jongbloed JD, van den Wijngaard A, Kuks JB, van Spaendonck-Zwarts KY, Notermans N, Boven L, van den Heuvel F, Veenstra-Knol HE, Saffitz JE, Hofstra RM, van den Berg MP. Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the DES gene. Heart Rhythm 2009;6:1574–1583. [DOI] [PubMed] [Google Scholar]
- 11.Quarta G, Syrris P, Ashworth M, Jenkins S, Zuborne Alapi K, Morgan J, Muir A, Pantazis A, McKenna WJ, Elliott PM. Mutations in the lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2012;33:1128–1136. [DOI] [PubMed] [Google Scholar]
- 12.Te Riele AS, Agullo-Pascual E, James CA, Leo-Macias A, Cerrone M, Zhang M, Lin X, Lin B, Sobreira NL, Amat-Alarcon N, Marsman RF, Murray B, Tichnell C, van der Heijden JF, Dooijes D, van Veen TA, Tandri H, Fowler SJ, Hauer RN, Tomaselli G, van den Berg MP, Taylor MR, Brun F, Sinagra G, Wilde AA, Mestroni L, Bezzina CR, Calkins H, Peter van Tintelen J, Bu L, Delmar M, Judge DP. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc Res 2017;113:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayosi BM, Fish M, Shaboodien G, Mastantuono E, Kraus S, Wieland T, Kotta MC, Chin A, Laing N, Ntusi NB, Chong M, Horsfall C, Pimstone SN, Gentilini D, Parati G, Strom TM, Meitinger T, Pare G, Schwartz PJ, Crotti L. Identification of cadherin 2 (CDH2) mutations in arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2017;10:e001605. [DOI] [PubMed] [Google Scholar]
- 14.van Hengel J, Calore M, Bauce B, Dazzo E, Mazzotti E, De Bortoli M, Lorenzon A, Li Mura IE, Beffagna G, Rigato I, Vleeschouwers M, Tyberghein K, Hulpiau P, van Hamme E, Zaglia T, Corrado D, Basso C, Thiene G, Daliento L, Nava A, van Roy F, Rampazzo A. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2013;34:201–210. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz-Genga MF, Cuenca S, Dal Ferro M, Zorio E, Salgado-Aranda R, Climent V, Padron-Barthe L, Duro-Aguado I, Jimenez-Jaimez J, Hidalgo-Olivares VM, Garcia-Campo E, Lanzillo C, Suarez-Mier MP, Yonath H, Marcos-Alonso S, Ochoa JP, Santome JL, Garcia-Giustiniani D, Rodriguez-Garrido JL, Dominguez F, Merlo M, Palomino J, Pena ML, Trujillo JP, Martin-Vila A, Stolfo D, Molina P, Lara-Pezzi E, Calvo-Iglesias FE, Nof E, Calo L, Barriales-Villa R, Gimeno-Blanes JR, Arad M, Garcia-Pavia P, Monserrat L. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol 2016;68:2440–2451. [DOI] [PubMed] [Google Scholar]
- 16.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck-Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP, van Tintelen JP. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail 2012;14:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beffagna G, Occhi G, Nava A, Vitiello L, Ditadi A, Basso C, Bauce B, Carraro G, Thiene G, Towbin JA, Danieli GA, Rampazzo A. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res 2005;65:366–373. [DOI] [PubMed] [Google Scholar]
- 18.Haywood AF, Merner ND, Hodgkinson KA, Houston J, Syrris P, Booth V, Connors S, Pantazis A, Quarta G, Elliott P, McKenna W, Young TL. Recurrent missense mutations in TMEM43 (ARVD5) due to founder effects cause arrhythmogenic cardiomyopathies in the UK and Canada. Eur Heart J 2013;34:1002–1011. [DOI] [PubMed] [Google Scholar]
- 19.Taylor M, Graw S, Sinagra G, Barnes C, Slavov D, Brun F, Pinamonti B, Salcedo EE, Sauer W, Pyxaras S, Anderson B, Simon B, Bogomolovas J, Labeit S, Granzier H, Mestroni L. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation 2011;124:876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet 2001;10:189–194. [DOI] [PubMed] [Google Scholar]
- 21.De Bortoli M, Postma AV, Poloni G, Calore M, Minervini G, Mazzotti E, Rigato I, Ebert M, Lorenzon A, Vazza G, Cipriani A, Bariani R, Perazzolo Marra M, Husser D, Thiene G, Daliento L, Corrado D, Basso C, Tosatto SCE, Bauce B, van Tintelen JP, Rampazzo A. Whole-exome sequencing identifies pathogenic variants in TJP1 gene associated with arrhythmogenic cardiomyopathy. Circ Genom Precis Med 2018;11:e002123. [DOI] [PubMed] [Google Scholar]
- 22.van den Hoogenhof MM, Beqqali A, Amin AS, van der Made I, Aufiero S, Khan MA, Schumacher CA, Jansweijer JA, van Spaendonck-Zwarts KY, Remme CA, Backs J, Verkerk AO, Baartscheer A, Pinto YM, Creemers EE. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation 2018;138:1330–1342. [DOI] [PubMed] [Google Scholar]
- 23.Fiset C, Giles WR. Cardiac troponin T mutations promote life-threatening arrhythmias. J Clin Invest 2008;118:3845–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman AM, Gordon J, Wang J, Song J, Zhang XQ, Myers VD, Tilley DG, Gao E, Hoffman NE, Tomar D, Madesh M, Rabinowitz J, Koch WJ, Su F, Khalili K, Cheung JY. BAG3 regulates contractility and Ca2+ homeostasis in adult mouse ventricular myocytes. J Mol Cell Cardiol 2016;92:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 2002;71:1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, Murray B, te Riele AS, van den Berg MP, Bikker H, Atsma DE, de Groot NM, Houweling AC, van der Heijden JF, Russell SD, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Calkins H, Hauer RN. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–855. [DOI] [PubMed] [Google Scholar]
- 27.Rampazzo A, Nava A, Danieli GA, Buja G, Daliento L, Fasoli G, Scognamiglio R, Corrado D, Thiene G. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet 1994;3:959–962. [DOI] [PubMed] [Google Scholar]
- 28.Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet 2008;82:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas J, Frese KS, Peil B, Kloos W, Keller A, Nietsch R, Feng Z, Muller S, Kayvanpour E, Vogel B, Sedaghat-Hamedani F, Lim WK, Zhao X, Fradkin D, Kohler D, Fischer S, Franke J, Marquart S, Barb I, Li DT, Amr A, Ehlermann P, Mereles D, Weis T, Hassel S, Kremer A, King V, Wirsz E, Isnard R, Komajda M, Serio A, Grasso M, Syrris P, Wicks E, Plagnol V, Lopes L, Gadgaard T, Eiskjaer H, Jorgensen M, Garcia-Giustiniani D, Ortiz-Genga M, Crespo-Leiro MG, Deprez RH, Christiaans I, van Rijsingen IA, Wilde AA, Waldenstrom A, Bolognesi M, Bellazzi R, Morner S, Bermejo JL, Monserrat L, Villard E, Mogensen J, Pinto YM, Charron P, Elliott P, Arbustini E, Katus HA, Meder B. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J 2015;36:1123–1135a. [DOI] [PubMed] [Google Scholar]
- 30.Refaat MM, Lubitz SA, Makino S, Islam Z, Frangiskakis JM, Mehdi H, Gutmann R, Zhang ML, Bloom HL, MacRae CA, Dudley SC, Shalaby AA, Weiss R, McNamara DM, London B, Ellinor PT. Genetic variation in the alternative splicing regulator RBM20 is associated with dilated cardiomyopathy. Heart Rhythm 2012;9:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toro R, Perez-Serra A, Campuzano O, Moncayo-Arlandi J, Allegue C, Iglesias A, Mangas A, Brugada R. Familial dilated cardiomyopathy caused by a novel frameshift in the BAG3 gene. PLoS One 2016;11:e0158730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalal D, James C, Devanagondi R, Tichnell C, Tucker A, Prakasa K, Spevak PJ, Bluemke DA, Abraham T, Russell SD, Calkins H, Judge DP. Penetrance of mutations in plakophilin-2 among families with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2006;48:1416–1424. [DOI] [PubMed] [Google Scholar]
- 33.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calore M, Lorenzon A, De Bortoli M, Poloni G, Rampazzo A. Arrhythmogenic cardiomyopathy: a disease of intercalated discs. Cell Tissue Res 2015;360:491–500. [DOI] [PubMed] [Google Scholar]
- 35.Patel DM, Dubash AD, Kreitzer G, Green KJ. Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin-EB1 interactions. J Cell Biol 2014;206:779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kam CY, Dubash AD, Magistrati E, Polo S, Satchell KJF, Sheikh F, Lampe PD, Green KJ. Desmoplakin maintains gap junctions by inhibiting Ras/MAPK and lysosomal degradation of connexin-43. J Cell Biol 2018;217:3219–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, plakophilin-2, and connexin 43 at the cardiac intercalated disc. Circ Res 2011;109:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spindler V, Waschke J. Role of Rho GTPases in desmosomal adhesion and pemphigus pathogenesis. Ann Anat 2011;193:177–180. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 2006;116:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliodori A, Beffagna G, Marchetto G, Fornetto C, Vanzi F, Toppo S, Facchinello N, Santimaria M, Vettori A, Rizzo S, Della Barbera M, Pilichou K, Argenton F, Thiene G, Tiso N, Basso C. Loss of cardiac Wnt/beta-catenin signalling in desmoplakin-deficient AC8 zebrafish models is rescuable by genetic and pharmacological intervention. Cardiovasc Res 2018;114:1082–1097. [DOI] [PubMed] [Google Scholar]
- 41.Dubash AD, Kam CY, Aguado BA, Patel DM, Delmar M, Shea LD, Green KJ. Plakophilin-2 loss promotes TGF-beta1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol 2016;212:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta -catenin signaling. J Biol Chem 2002;277:10512–10522. [DOI] [PubMed] [Google Scholar]
- 43.Maruthappu T, Posafalvi A, Castelletti S, Delaney PJ, Syrris P, O’Toole EA, Green KJ, Elliott PM, Lambiase PD, Tinker A, McKenna WJ, Kelsell DP. Loss of function desmoplakin I and II mutations underlie dominant arrhythmogenic cardiomyopathy with a hair and skin phenotype. Br J Dermatol 2019;180:1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooke MA, Etheridge SL, Kaplan N, Simpson C, O’Toole EA, Ishida-Yamamoto A, Marches O, Getsios S, Kelsell DP. iRHOM2-dependent regulation of ADAM17 in cutaneous disease and epidermal barrier function. Hum Mol Genet 2014;23:4064–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaydon DC, Biancheri P, Di WL, Plagnol V, Cabral RM, Brooke MA, van Heel DA, Ruschendorf F, Toynbee M, Walne A, O’Toole EA, Martin JE, Lindley K, Vulliamy T, Abrams DJ, MacDonald TT, Harper JI, Kelsell DP. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med 2011;365: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 46.Maruthappu T, Chikh A, Fell B, Delaney PJ, Brooke MA, Levet C, Moncada-Pazos A, Ishida-Yamamoto A, Blaydon D, Waseem A, Leigh IM, Freeman M, Kelsell DP. Rhomboid family member 2 regulates cytoskeletal stress-associated keratin 16. Nat Commun 2017;8:14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, Spiegel R, Eytan O, Geller S, Peleg S, Shomron N, Goh CS, Wilson NJ, Smith FJ, Pohler E, Simpson MA, McLean WH, Irvine AD, Horowitz M, McGrath JA, Green KJ, Sprecher E. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 2013;45:1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paller AS, Czarnowicki T, Renert-Yuval Y, Holland K, Huynh T, Sadlier M, McAleer MA, Tran G, Geddes GC, Irvine AD, Guttman-Yassky E. The spectrum of manifestations in desmoplakin gene (DSP) spectrin repeat 6 domain mutations: immunophenotyping and response to ustekinumab. J Am Acad Dermatol 2018;78:498–505.e2. [DOI] [PubMed] [Google Scholar]
- 49.Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996;335:1190–1196. [DOI] [PubMed] [Google Scholar]
- 50.Valente M, Calabrese F, Thiene G, Angelini A, Basso C, Nava A, Rossi L. In vivo evidence of apoptosis in arrhythmogenic right ventricular cardiomyopathy. Am J Pathol 1998;152:479–484. [PMC free article] [PubMed] [Google Scholar]
- 51.d’Amati G, di Gioia CR, Giordano C, Gallo P. Myocyte transdifferentiation: a possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch Pathol Lab Med 2000;124:287–290. [DOI] [PubMed] [Google Scholar]
- 52.Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, Tan HL, Valente M, Nava A, Wilde AA, Moorman AF, Thiene G, Bezzina CR. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med 2009;206:1787–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 1996;94:983–991. [DOI] [PubMed] [Google Scholar]
- 54.Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G, Malacrida S, Settimo L, Danieli G, Thiene G, Nava A. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J 2005;26:1666–1675. [DOI] [PubMed] [Google Scholar]
- 55.Thiene G, Corrado D, Nava A, Rossi L, Poletti A, Boffa GM, Daliento L, Pennelli N. Right ventricular cardiomyopathy: is there evidence of an inflammatory aetiology? Eur Heart J 1991;12 Suppl D:22–25. [DOI] [PubMed] [Google Scholar]
- 56.Bowles NE, Ni J, Marcus F, Towbin JA. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2002;39:892–895. [DOI] [PubMed] [Google Scholar]
- 57.Calabrese F, Basso C, Carturan E, Valente M, Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovasc Pathol 2006;15:11–17. [DOI] [PubMed] [Google Scholar]
- 58.Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2009;360:1075–1084. [DOI] [PubMed] [Google Scholar]
- 59.Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, Cooper LT, Wilber DJ, Marcus FI, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, Stevenson WG, McKenna WJ, Gautam S, Remick DG, Calkins H, Saffitz JE. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasaiwala SC, Finn C, Delpriore J, Leya F, Gagermeier J, Akar JG, Santucci P, Dajani K, Bova D, Picken MM, Basso C, Marcus F, Wilber DJ. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol 2009;20:473–476. [DOI] [PubMed] [Google Scholar]
- 61.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campuzano O, Fernandez-Falgueras A, Sarquella-Brugada G, Sanchez O, Cesar S, Mademont I, Allegue C, Mates J, Perez-Serra A, Coll M, Alcalde M, Iglesias A, Tiron C, Gallego MA, Ferrer-Costa C, Hospital A, Escribano C, Dasi C, Borondo JC, Castella J, Arbelo E, Medallo J, Brugada J, Brugada R. A genetically vulnerable myocardium may predispose to myocarditis. J Am Coll Cardiol 2015;66:2913–2914. [DOI] [PubMed] [Google Scholar]
- 63.van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP, Pilotto A, Pasotti M, Jenkins S, Rowland C, Aslam U, Wilde AA, Perrot A, Pankuweit S, Zwinderman AH, Charron P, Pinto YM. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers: a European cohort study. J Am Coll Cardiol 2012;59:493–500. [DOI] [PubMed] [Google Scholar]
- 64.Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, Luscher TF, Brunckhorst C, Chen HS, Duru F. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J 2017;38:1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong TT, Cogswell R, James CA, Kang G, Pullinger CR, Malloy MJ, Kane JP, Wojciak J, Calkins H, Scheinman MM, Tseng ZH, Ganz P, De Marco T, Judge DP, Shaw RM. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2012;9:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broch K, Leren IS, Saberniak J, Ueland T, Edvardsen T, Gullestad L, Haugaa KH. Soluble ST2 is associated with disease severity in arrhythmogenic right ventricular cardiomyopathy. Biomarkers 2017;22:367–371. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee D, Fatah M, Akdis D, Spears DA, Koopmann TT, Mittal K, Rafiq MA, Cattanach BM, Zhao Q, Healey JS, Ackerman MJ, Bos JM, Sun Y, Maynes JT, Brunckhorst C, Medeiros-Domingo A, Duru F, Saguner AM, Hamilton RM. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur Heart J 2018;39:3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilotra NA, Bhonsale A, James CA, Te Riele ASJ, Murray B, Tichnell C, Sawant A, Ong CS, Judge DP, Russell SD, Calkins H, Tedford RJ. Heart failure is common and under-recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail 2017;10:e003819. [DOI] [PubMed] [Google Scholar]
- 69.Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, Loh P, Russell SD, Velthuis BK, Judge DP, Dooijes D, Tedford RJ, van der Heijden JF, Tandri H, Hauer RN, Abraham TP, Doevendans PA, Te Riele AS, Cramer MJ. Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiol 2017;2:293–302. [DOI] [PubMed] [Google Scholar]
- 70.Sawant AC, Calkins H. Sports in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy and desmosomal mutations. Herz 2015;40: 402–409. [DOI] [PubMed] [Google Scholar]
- 71.Sawant AC, Te Riele AS, Tichnell C, Murray B, Bhonsale A, Tandri H, Judge DP, Calkins H, James CA. Safety of American Heart Association-recommended minimum exercise for desmosomal mutation carriers. Heart Rhythm 2016;13:199–207. [DOI] [PubMed] [Google Scholar]
- 72.Ruwald AC, Marcus F, Estes NA 3rd, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cattin ME, Ferry A, Vignaud A, Mougenot N, Jacquet A, Wahbi K, Bertrand AT, Bonne G. Mutation in lamin A/C sensitizes the myocardium to exercise-induced mechanical stress but has no effect on skeletal muscles in mouse. Neuromuscul Disord 2016;26:490–499. [DOI] [PubMed] [Google Scholar]
- 74.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 2014;6:240ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP. Central role for GSK3beta in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016;1:85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, Worman HJ. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet 2012;21: 4325–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]