Abstract
Equal access to clinical trial enrollment is important to ensure that findings are generalizable to the broader population. This study aimed to evaluate disparities in enrollment on pediatric oncology clinical trials. We assessed the relationship between patient characteristics and enrollment on COG trial AAML1031 in a cohort of pediatric patients with AML in the Pediatric Health Information System. The associations of enrollment with outcomes were evaluated. Non-Hispanic Black patients, infants, and patients from zip codes with a lower proportion of poverty were less likely to enroll (30% vs. 61%, p =.004; 34% vs. 58%, p =.003;46% vs. 58%, p =.02). On-therapy mortality was similar among enrolled and nonenrolled patients (7.3% vs. 8.9%, p = .47). Differences in early mortality were more pronounced among nonenrolled patients compared to enrolled patients (3.0% vs. 0.5%, p =.03). Understanding the etiology of these disparities will inform strategies to ensure balanced access to clinical trials across patient populations.
Keywords: Clinical trials, enrollment, disparities, leukemia, outcomes research, pediatric oncology, race/ethnicity
Introduction
Much of the progress made in treating pediatric cancer is attributable to collaborative clinical trials conducted through consortia such as the Children’s Oncology Group (COG). Equitable access to and participation in clinical trials for all populations are essential to ensure the generalizability of clinical trial results. In addition to advancing the standard of care for future patients, some studies have suggested that patients who participate in clinical trials experience improved clinical outcomes [1,2].
Historical data from previous pediatric cooperative groups suggest that the majority of patients are enrolled on a trial when treated at a cooperative group institution [3,4] and minorities are proportion-ally represented among them [5,6]. However, other studies suggest lower clinical trial enrollment among Hispanic patients [7,8], Black patients [8], adolescents [4,5,7,8], the uninsured [4], and those who live farther from a tertiary care center [9]. To date, studies that evaluated disparities in COG trial enrollment have been limited to single institutions or have compared estimates of cancer incidence rates by race/ethnicity with trial participation rates. In order to identify true disparities, it is necessary to evaluate study participation within a pediatric cohort representing multiple institutions that offer clinical trials across the United States.
This study leveraged an existing cohort of children treated for new onset acute myeloid leukemia (AML) at tertiary care hospitals contributing to the Pediatric Health Information System database (PHIS) between 2011 to 2014 [10]. The institutions included were all COG centers that opened the most recent Phase-III pediatric AML clinical trial (AAML1031), which randomized patients with de novo AML to receive chemotherapy in combination with bortezomib, a proteasome inhibitor, or standard of care chemotherapy alone. In addition, patients with high allelic ratio FLT3/ITD received a tyrosine kinase inhibitor, sorafenib.
All patients in the cohort received care at an institution where the AAML1031 trial was open. The primary aim was to identify patient- and institution-level factors associated with enrollment on AAML1031. We evaluated the impact of trial participation on inpatient mortality and resource utilization among those treated on clinical trial compared to those treated off study with the same induction chemotherapy. We hypothesized that Black patients, adolescents, patients from impoverished or low education areas [11] and patients with high acuity at presentation were less likely to enroll on AAML1031. We also hypothesized that trial enrollment was associated with decreased inpatient mortality and resource utilization.
Methods
Study population
The study population was derived from an existing unmerged PHIS cohort of children who received standard induction chemotherapy for AML, constructed using a validated process of manual chemotherapy review described elsewhere [12]. The original cohort was additionally restricted to patients treated from the date AAML1031 opened in 2011 through June 2014. Patients who were diagnosed when AAML1031 was not open at their institution and patients with Trisomy 21 were excluded given they were not eligible for the study.
Outcomes of interest
The primary outcome of interest was enrollment on AAML1031. Additional details regarding the AAML1031 trial can be found at ClinicalTrials.gov (Identifier: NCT01371981). A second existing COG-PHIS merged cohort [10] consisting of AAML1031-enrolled patients matched to PHIS based on date of birth, date of diagnosis, treating hospital, and the presence of an ICD-9-CM code for AML (205.xx) was used to determine AAML1031 enrollment status. Patients in the study population who were also included in the AAML1031 COG-PHIS merged cohort were considered ‘enrolled.’ Those who were not identified in the COG-PHIS merge cohort were deemed ‘not enrolled.’
Secondary outcomes, for which trial enrollment was evaluated as the exposure, included mortality and resource utilization. Inpatient mortality was identified based on discharge status for each hospitalization. Inpatient death was evaluated at 42 days (early mortality) [13,14] and 9 months (the point at which most patients have completed four chemotherapy courses – on-therapy mortality). ICU-level resources were defined by specific ICD-9-CM procedure codes or billing data considered a priori as markers of organ failure requiring ICU care including need for any of the following therapies: vasopressor support, mechanical ventilation, renal replacement therapy, and leuka-pheresis (versus none) [15].
Utilization of antibiotic, antifungal, antiviral, parenteral nutrition, antiemetics, opiates, antihypertensives, diuretics, blood products, granulocyte colony stimulating factor (G-CSF), and oxygen were determined from billing data and were evaluated individually. The daily-level bills for each resource were summed to obtain the total number of days each resource was utilized. Resource utilization rates were reported as days of use per 100 inpatient days.
Covariates
Patient characteristics including race/ethnicity, age, sex, and insurance type (private, public, or other) were ascertained from PHIS. Race and ethnicity were combined into the following categories: non-Hispanic White, non-Hispanic Black, Hispanic, and other. Patients with ethnicity documented as ‘unknown’ or ‘missing’ were classified as non-Hispanic. PHIS residential zip code at initial AML admission was used to determine zip-level estimates of the proportion of residents below the federal poverty line (zip-based poverty) and the proportion of female residents aged >25 years with less than high school education (zip-based low education) from 2010 U.S. Census data. Zip-based poverty and low education were categorized into quartiles. Based on similarities in magnitude of the association with trial enrollment across quartiles 2–4, zip-based poverty (<4 vs. ≥4%) and zip-based low education (<11 vs. ≥11%) were dichotomized at the first quartile. Institution-level characteristics including racial makeup, urban–rural population mix, payer mix, AML volume, and the hospitalwide mortality rate were also evaluated as covariates.
Statistical analyses
Primary analysis
The primary outcome was enrollment on AAML1031. Baseline distributions of patient and institution-level characteristics were tabulated by enrollment status. Log-binomial regression methods were employed to estimate the crude risk ratios for trial enrollment by patient- and institution-level characteristics. Characteristics that were hypothesized to affect enrollment a priori or those found to be associated with trial enrollment (with a p-value <.05) were included in multivariable models to obtain adjusted RRs. Generalized estimating equations were used to account for nonindependence of observations from the same institution.
Secondary analyses
In secondary analyses, the exposure of interest was enrollment status and the outcomes of interest were as follows: inpatient mortality (at 42 days and 9 months); ICU-level care requirements and total days of ICU-level care (ICU LOS); inpatient days during Induction I (Induction I LOS) and over the course of frontline AML therapy (on-therapy LOS); and resource utilization rates. Dichotomous outcomes were compared between enrolled and non-enrolled patients using log-binomial regression, LOS was compared using linear regression models, resource utilization rates were compared using Poisson regression models with inpatient days as the offset and Pearson scale adjustment to correct for possible overdispersion. A plot of institution-specific rates of enrollment and inpatient mortality was generated to visually assess variability. Post hoc stratification was employed to explore whether the association between race and trial enrollment was modified by region or poverty. All analyses were performed using SAS, and p < .05 was considered statistically significant.
Results
Patient characteristics
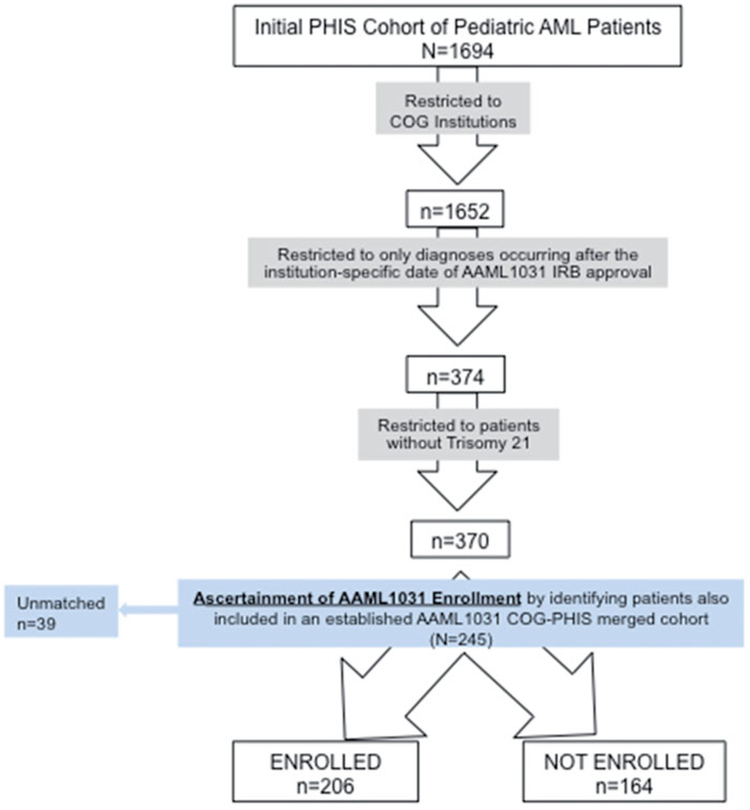
Of the 370 patients in the unmerged PHIS AML cohort, 206 (55%) were identified as enrolled on AAML1031 and 164 (45%) were identified as not enrolled. Thirtynine patients in the COG-PHIS merged cohort were not identified in the PHIS AML cohort. Figure 1 illustrates the flow diagram of cohort identification and ascertainment of enrollment status. Patients were predominately non-Hispanic White (53%) and did not present with evidence of organ failure (90%). There were differences in distributions of race, age, and acuity at presentation between those enrolled and not enrolled (as shown in Table 1).
Figure 1.
Cohort identification and matching.
Table 1.
Patient characteristics by trial enrollment status.
| Total N = 370 (%) | Enrolled N= 206 (%) | Not enrolled N = 164 (%) | |
|---|---|---|---|
| Race/Ethnicity | |||
| Non-Hispanic White | 198 (53) | 121 (59) | 77 (47) |
| Non-Hispanic Black | 48 (13) | 15 (7) | 33 (20) |
| Hispanic | 72 (19) | 43 (21) | 29 (18) |
| Other | 52 (14) | 27 (13) | 25 (15) |
| Sex | |||
| Female | 180 (49) | 98 (48) | 82 (50) |
| Male | 190 (51) | 108 (52) | 82 (50) |
| Age, years | |||
| 0–1 | 42 (12) | 15 (7) | 27 (17) |
| 1–5 | 87 (23) | 54 (26) | 33 (20) |
| 5–10 | 69 (18) | 37 (18) | 32 (19) |
| 10–15 | 83 (22) | 52 (25) | 31 (19) |
| >15 | 89 (24) | 48 (23) | 41 (25) |
| Insurance type | |||
| Private | 168 (45) | 94 (46) | 74 (44) |
| Public | 178 (48) | 97 (47) | 81 (49) |
| Other | 24 (6) | 15 (7) | 9 (5) |
| Acuity at Presentation | |||
| No ICU | 330 (90) | 187 (91) | 143 (87) |
| 1 system | 24 (6) | 14 (6.1) | 10 (6.8) |
| 2 or more systems | 16 (4) | 5 (2.4) | 11 (6.7) |
| Region | |||
| South | 124 (34) | 62 (30) | 62 (38) |
| Midwest | 98 (26) | 50 (24) | 48 (28) |
| Northeast | 50 (14) | 31 (15) | 19 (12) |
| West | 98 (26) | 63 (31) | 35 (21) |
| Zip-based Poverty | |||
| <4% population below FPL | 81 (22) | 37 (18) | 44 (28) |
| ≥4% population below FPL | 281 (73) | 165 (82) | 116 (73) |
| Zip-based Low Education | |||
| <11% without HS diploma | 91 (25) | 44 (22) | 47 (29) |
| ≥11% without HS diploma | 275 (75) | 158 (78) | 113 (71) |
| Year | |||
| 2011 | 40 (11) | 21 (10) | 19 (11) |
| 2012 | 134 (36) | 82 (40) | 52 (31) |
| 2013 | 140 (38) | 68 (33) | 72 (44) |
| 2014 | 56 (15) | 35 (17) | 21 (13) |
Predictors of trial enrollment
In multivariable analysis (displayed in Table 2), non-Hispanic Black patients were less likely to enroll on trial compared to non-Hispanic White patients (aRR 0.51, 95% CI 0.36, 0.73). Patients from zip codes with the lowest proportion of families in poverty (<4% below the federal poverty line - FPL) were also less likely to enroll than those from zip codes with higher concentrations of poverty (aRR 0.70, 95% CI 0.55,0.88). However, the association between zip-based poverty and trial enrollment appeared to vary by race/ethnicity (Supplemental Table 1). Enrollment rates were similar across noninfant age categories (53–63%), while infants had lower enrollment (34%, aRR 0.68, 95% CI 0.47, 0.98). Patients with multisystem organ failure at presentation were enrolled less frequently than patients who did not have any evidence of ICU-level organ failure at presentation (31% v. 56%, aRR 0. 64, 95% CI 0.31, 1.30) as hypothesized, but this association was not statistically significant.
Table 2.
Univariable and multivariable analyses of predictors for enrollment.
| Enrolled, N= 206 (%) | Unadjusted RR (95% CI) | Adjusted RR* (95% CI) | p-value | |
|---|---|---|---|---|
| Race/Ethnicity | ||||
| White Non-Hispanic | 61 | ref | ref | |
| Black Non-Hispanic | 30 | 0.53 (0.38, 0.74) | 0.51 (0.36, 0.73) | .002 |
| Hispanic | 60 | 0.98 (0.78, 1.23) | 0.90 (0.74, 1.10) | .29 |
| Other | 51 | 0.80 (0.61, 1.05) | 0.79 (0.60, 1.40) | .09 |
| Sex | ||||
| Female | 53 | ref | ||
| Male | 56 | 1.04 (0.86, 1.24) | ||
| Age, years | ||||
| 0–1 | 34 | 0.66 (0.46, 0.95) | 0.68 (0.47, 0.98) | .04 |
| 1–15 | 60 | ref | ref | |
| >15+ | 53 | 0.91 (0.78, 1.06) | 0.92 (0.81, 1.05) | .21 |
| Insurance type | ||||
| Private | 55 | ref | ||
| Public | 54 | 0.97 (0.85, 1.09) | ||
| Other | 62 | 1.20 (0.96, 1.51) | ||
| Acuity at Presentation | ||||
| No ICU | 56 | ref | ||
| 1 system | 57 | 0.92 (0.60, 1.40) | 1.15 (0.87, 1.51) | .33 |
| 2 or more systems | 31 | 0.59 (0.28, 1.21) | 0.64 (0.31, 1.30) | .22 |
| Region | ||||
| South | 49 | ref | ||
| Midwest | 51 | 1.13 (0.71, 1.77) | ||
| Northeast | 60 | 1.33 (0.96, 1.77) | ||
| West | 64 | 1.31 (0.95, 1.81) | ||
| Zip-based Poverty | ||||
| <4% population below FPL | 46 | 0.77 (0.61, 0.97) | 0.70 (0.55, 0.88) | .02 |
| ≥4% population below FPL | 58 | ref | ref | |
| Zip-based Low Education | ||||
| <11% without HS diploma | 48 | ref | ||
| ≥11% without HS diploma | 58 | 1.25 (0.99, 1.59) | ||
| Year | ||||
| 2011 | 53 | 0.90 (0.64, 1.27) | 0.88 (0.64, 1.20) | .43 |
| 2012 | 61 | 0.96 (0.76, 1.21) | 0.94 (0.77, 1.15) | .55 |
| 2013 | 48 | 0.80 (0.66, 0.97) | 0.80 (0.67, 0.96) | .02 |
| 2014 | 61 | ref | ref |
Adjusted for race/ethnicity, age, acuity at presentation, poverty, and year.
There was considerable variability in enrollment rates by institution (Supplemental Figure 1, left panel). However, none of the institution-level characteristics explored (including racial makeup, urban-rural population mix, payer mix, AML volume, and the hospital-wide mortality rate – Supplemental Table 2) accounted for variability in trial enrollment.
Mortality and resource utilization
While early inpatient mortality was rare (2.9% overall), it was lower among patients enrolled on AAML1031 compared to those not enrolled (0.5% vs. 3.0%, crude RR 0.14, 95% CI 0.02, 0.79). This difference in early mortality explained the marginally lower on-therapy mortality among enrolled relative to nonenrolled patients (7.3% vs. 8.9%, aRR 0.77, 95% CI 0.41, 1.58). No statistically significant or clinically meaningful difference in ICU-level resource utilization or any length of stay measure was observed.
Comparisons of resources utilized for supportive care in patients enrolled versus those not enrolled on AAML1031 are displayed in Table 3. After adjusting for race/ethnicity and zip-based poverty, enrolled patients received significantly fewer days of parenteral nutrition (8 v. 15 days of use per 100 hospital days, p = .04), diuretics (6 v. 10 days of use per 100 hospital days, p = .01), and antifungals (71 v. 79 days of use per 100 hospital days, p = .003) than those not enrolled.
Table 3.
Univariable and multivariable analysis of enrollment effect on mortality, icu utilization, length of stay and resource utilization.
| Enrolled | Not Enrolled | Unadjusted comparisona (95% CI) | Adjusted comparisona (95% CI)b | p-value | |
|---|---|---|---|---|---|
| Early Mortality (<42 days) | 1 (0.5%) | 5 (3.0%) | 0.14 (0.02, 0.79) | NE | .03c |
| On therapy Mortality (9 mo) | 15 (7.3%) | 15 (8.9%) | 0.76 (0.38, 1.52) | 0.77 (0.41, 1.44) | .40 |
| ICU (yes/no) | 79 (38%) | 61 (36%) | 1.07 (0.81, 1.39) | 1.17 (0.89, 1.52) | .25 |
| Length of Stay | |||||
| Induction I LOSd | 30 (27, 35) | 29 (26, 38) | 0.57 (−6.72, 7.86) | 0.43 (−7.63, 8.51) | .92 |
| On therapy LOSd | 117 (97, 141) | 112 (82, 146) | 5.50 (−3.89, 14.89) | 5.73 (−3.15, 14.61) | .21 |
| ICU LOSe | 3.75 (SD 10) | 4.29 (SD 9.5) | −0.59 (−2.38, 1.19) | −0.12 (−1.98, 1.74) | .90 |
| Resource Utilizationf | |||||
| Antibiotics (5 subclasses) | 100 (96, 105) | 97 (92, 102) | 1.02 (0.97, 1.08) | 1.02 (0.97, 1.09) | .43 |
| Antifungals (3 subclasses) | 71 (68, 75) | 79 (75, 83) | 0.90 (0.85, 0.95) | 0.91 (0.86, 0.97) | .003 |
| Amphotericin | 6 (5, 8) | 8 (6, 10) | 0.82 (0.57, 1.21) | 0.87 (0.59, 1.26) | .46 |
| Echinocandin | 20 (17, 24) | 21 (17, 25) | 1.15 (0.91, 1.45) | 1.19 (0.92, 1.54) | .19 |
| Azole | 45 (41, 49) | 50 (46, 55) | 0.81 (0.74, 0.89) | 0.82 (0.73, 0.91) | .0003 |
| Antivirals | 15 (12, 19) | 17 (13, 22) | 0.85 (0.63, 1.16) | 0.85 (0.59, 1.23) | .40 |
| Antiemetics | 56 (53, 58) | 55 (53, 58) | 1.03 (0.96, 1.10) | 1.05 (0.99, 1.11) | .07 |
| Parenteral Nutrition | 8 (6, 10) | 15 (12, 18) | 0.65 (0.47, 0.91) | 0.72 (0.53, 0.98) | .04 |
| Opiates | 20 (18, 22) | 22 (19, 25) | 0.90 (0.75, 1.08) | 0.90 (0.76, 1.06) | .19 |
| Antihypertensives | 14 (10, 18) | 15 (11, 20) | 0.85 (0.53, 1.34) | 1.05 (0.61, 1.82) | .85 |
| Diuretics | 6 (5, 7) | 10 (8, 12) | 0.61 (0.47, 0.80) | 0.65 (0.46, 0.92) | .01 |
| Oxygen Therapy | 4 (3, 5) | 4 (3, 5) | 0.97 (0.68, 1.37) | 1.00 (0.72, 1.38) | .99 |
| Blood Products | 30 (27, 31) | 30 (28,3 2) | 0.97 (0.89, 1.06) | 0.99 (0.9, 1.08) | .83 |
| G-CSF | 5 (4, 6) | 5 (4, 7) | 0.89 (0.58, 1.36) | 0.89 (0.60, 1.32) | .57 |
Risk ratio for morality, ICU; rate ratio for resource use, and mean difference for lengths of stay; Not enrolled is the reference group.
Adjusted for race/ethnicity, age, and poverty.
p-value for unadjusted comparison as adjusted model had to few events to converge.
Median, interquartile range.
Mean, standard deviation (SD).
Days of use per 100 inpatient days.
Discussion
We found that 55% of eligible patients in our cohort of children and adolescents treated at tertiary care children’s hospitals enrolled on COG study AAML1031. Consistent with our hypothesis, non-Hispanic Black patients were less likely to enroll compared to non-Hispanic White patients; however, adolescents and patients from low SES were equally or more likely to enroll, which was not consistent with our initial hypotheses. Trial enrollment was associated with decreased early inpatient mortality and utilization of several specific resources, including azoles, TPN, and diuretics.
The enrollment rate of 55% is consistent with previous estimates of pediatric trial enrollment, which range from 40–60% of eligible patients treated at pediatric cancer centers [8,9]. Studies of trial enrollment in adults show lower enrollment amongst Black patients [16], but some have failed to confirm this finding [17]. We found that among those eligible for the most recent pediatric COG AML trial, non-Hispanic Black patients were 49% less likely to be enrolled on study compared to non-Hispanic White patients after accounting for potential confounding factors. Notably, acuity at presentation did not have a substantial effect on the association between race and trial enrollment. The etiology of low Black patient clinical trial enrollment is likely multifactorial. The contribution of skepticism and negative attitudes among the Black community regarding experimental therapies, as well as the impact of religious beliefs, continues to be debated [18]. In addition, data suggest that providers may be less likely to offer clinical trial enrollment to Black patients [19].
Racial disparities in trial enrollment are particularly concerning in light of previously documented racial dis-parities in AML outcomes [20,21]. Low rates of trial enrollment amongst non-Hispanic Black patients suggest inequitable access to potential benefits, such as ‘trial effects’ due to investigational agents and ‘inclusion benefits’ derived from differences in protocol-specified supportive care. Furthermore, low participation among non-Hispanic Black patients limits generalizability of the trial’s results to this population. As a group with lower overall survival, advances observed on clinical trials may be less applicable to non-Hispanic Black patients and may contribute to ongoing survival disparities.
Reported rates of clinical trial participation among Hispanic patients relative to non-Hispanic White patients vary between studies. For example, Hispanic patients were more likely to enroll than non-Hispanic White patients at one institution and less likely to enroll at another institution [7,22]. Reasons for these reported differences across studies may be due to factors such as heterogeneity of the Hispanic population by region or heterogeneity of institutional resources and approaches to enrollment. We found that Hispanic patients had the same rates of trial enrollment as non-Hispanic White patients and when stratified by region, Hispanic enrollment did not vary substantially, except in the Northeast, where there were very few Hispanic patients (Supplemental Table 1). Regional variation observed in unadjusted analyses (with the Western region most likely to enroll patients) resolved in multivariable analysis suggestive of confounding due to the racial/ethnic makeup of different regions. Of note, Hispanic patients may be under-identified in our study given that ethnicity is not consistently specified in PHIS. However, they represent 19% of the overall study population consistent with national estimates.
In contrast to our hypothesis, we found that patients from zip codes with a low rate of poverty (<4%) were less likely to enroll on AAML1031. This differs from previous literature, which has generally shown that low income adults are less likely to participate in clinical trials [23–25] and that pediatric patients from low-income areas have no differences in enrollment [9]. Differences have previously been attributed to patient concerns about paying for clinical trial treatment, which may be less of a concern in pediatric cancer patients who often have secondary Medicaid to cover out-of-pocket expenses. In exploratory stratified analyses, we observed White and Black patients from low poverty areas have a similar enrollment to the overall population at 52% and 50% respectively, while Hispanic patients and those in the other race category have notably low enrollment at 25% and 27% (as shown in Supplemental Table 1). While not statistically significant, these exploratory analyses suggest that the difference in enrollment among patients from low poverty neighborhoods may be only observed in select subgroups of patients. Of note, insurance type (public v. private) did not have any association with trial enrollment.
There is great interest in trial enrollment amongst adolescents and young adults (AYA) given their well-documented worse outcomes. Several previous studies have examined enrollment across pediatric and adult centers, finding adolescents and young adults are less likely to enroll relative to pediatric patients [26]. Even among those treated exclusively at pediatric facilities, AYA patients have been shown to be less likely to enroll than their younger counterparts [27]. Our study suggests that the AYA population had similar rates of enrollment to non-infant patients when treated at a COG center, contrary to the previous literature and our hypothesis. Of note, our data also suggest that infants less than 1 year of age were less likely to enroll on study in contrast to previous work, which has generally incorporated infants with other children under age 5 years and concluded that they are overrepresented on clinical trials [26,28,29].
Given the observed variation in enrollment between institutions, we evaluated institution-level characteristics that might explain these differences, including AML patient volume and payer mix. However, these characteristics failed to explain the observed variation. Previous data suggest institutional variation in enrollment is driven by variation at the provider-level [30,31]. We were unable assess variability in enrollment relative to provider-specific variables as data on treating providers is not available in PHIS.
In light of insufficient and conflicting evidence for the impact of trial participation on clinical outcomes [32,33], mortality and resource utilization were evaluated. Given the infrequent nature of deaths, this study is underpowered to detect a statistically significant effect and the multivariable model for early morality did not converge. However the magnitude of the inverse association between trial enrollment and early mortality raises concern that the sickest patients are not being enrolled on clinical trials, further substantiating the concern that trial data underestimate early mortality [34]. Because multisystem organ failure is also rare, we were not able account for the effect of confounding by presentation acuity on the relationship between trial enrollment and early mortality. Thus, it remains unclear if early mortality in those not enrolled is due to a trial effect or selection of a less acute subpopulation for enrollment. Patients treated off study received more parenteral nutrition, diuretics, and antifungal medications. However, on-therapy mortality is comparable between those treated on and off study. Although our data suggest that when treated similarly at institutions with open trials patients both on and off study have similar outcomes, the question of whether enrollment translates to longer-term outcome benefits is an area that requires additional investigation.
Our study is strengthened by the use of a nationally representative set of children’s hospitals as many previous studies have either employed single center institutional datasets or used SEER, which is limited to a subset of geographic areas. Previous studies demonstrate different rates of trial enrollment depending on the cancer type and trial type (therapeutic versus supportive care) [5,7]. Thus, our results may be less generalizable outside the setting of pediatric AML or therapeutic clinical trials.
In identifying patients from the COG-PHIS merge within the unmerged PHIS AML cohort, we were unable to match 15% (demographics shown in Supplemental Table 3), raising the risk of misclassification of the primary outcome and the exposure for secondary analyses. More likely, the missing patients are not included in the PHIS AML cohort; this could be due to a variety of reasons, including transfer in from a non-PHIS institution, lack of administration of standard induction chemotherapy due to complications, or inaccurate billing for chemotherapy. If those who enrolled were more likely to be included in the study population, rates of trial enrollment would be overestimated. No details regarding enrollment onto other AML trials aside from AAML1031 were available.
Other limitations include lack of clinical details about a patient’s leukemia. As a result, the definition of acuity of presentation relies on resource use rather than clinical information. Only inpatient deaths were captured, so deaths outside of PHIS institutions would not be reflected. SES data were derived from patient zip code and are thus only available for relatively broad geographic areas around a patient’s address rather than at the individual level, raising potential for misclassification. Still very few pediatric studies have evaluated any SES factors, even at the zip code level.
The etiology of the observed disparities in enrollment could have been due to providers not offering study participation or families declining participation despite the opportunity. In order to further study the drivers of the differences observed in enrollment by race and SES, tracking when trials are offered and the circumstances that prevent them from being offered is necessary. Understanding when trial participation is declined and the rationale for nonparticipation through prospective data collection is equally important. Capturing the socio-demographic characteristics of patients, family members, and providers involved as well as the details of the circumstances, such as time of day and setting in which the trial is offered, would provide more granular details which would aid in the design of approaches to improve recruitment of diverse pediatric patients and lessen existing disparities.
Supplementary Material
Funding
The research group receives support from Dr. Aplencʹs NIH R01CA165277. Dr. Winestoneʹs effort was supported by the Abramson Cancer Center’s Paul Calabresi Career Development Award for Clinical Oncology [K12CA076931]. Her research is also supported by a Young Investigator Award from Alexʹs Lemonade Stand Foundation.
Footnotes
Supplemental data for this article can be accessed here.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2019.1574002.
References
- [1].Strahlendorf C, Pole JD, Barber R, et al. Enrolling children with acute lymphoblastic leukaemia on a clinical trial improves event-free survival: a population-based study. Br J Cancer. 2018;118:744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stiller CA, Draper GJ. Treatment centre size, entry to trials, and survival in acute lymphoblastic leukaemia. Arch Dis Child. 1989;64:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J. Natl. Cancer Inst. 1996;88:812–816. [DOI] [PubMed] [Google Scholar]
- [4].Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29:4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Krailo MD, Bernstein L, Sullivan-Halley J, et al. Patterns of enrollment on cooperative group studies. An analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer. 1993;71: 3325–3330. [DOI] [PubMed] [Google Scholar]
- [6].Bleyer WA, Tejeda HA, Murphy SB, et al. Equal participation of minority patients in U.S. national pediatric cancer clinical trials. J Pediatr Hematol Oncol. 1997; 19:423–427. [DOI] [PubMed] [Google Scholar]
- [7].Aristizabal P, Singer J, Cooper R, et al. Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Pediatr Blood Cancer. 2015;62:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lund MJ, Eliason MT, Haight AE, et al. Racial/ethnic diversity in children’s oncology clinical trials: ten years later. Cancer. 2009;115:3808–3816. [DOI] [PubMed] [Google Scholar]
- [9].Pole JD, Barber R, Bergeron R-É , et al. Most children with cancer are not enrolled on a clinical trial in Canada: a population-based study. BMC Cancer. 2017; 17:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aplenc R, Fisher BT, Huang YS, et al. Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: a report from the Children Oncology Group. Pharmacoepidemiol Drug Saf. 2012;21:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miller VA, Nelson RM. Factors related to voluntary parental decision-making in pediatric oncology. Pediatrics. 2012;129:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kavcic M, Fisher BT, Torp K, et al. Assembly of a cohort of children treated for acute myeloid leukemia at free-standing children’s hospitals in the United States using an administrative database. Pediatr Blood Cancer. 2013;60:508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Creutzig U, Zimmermann M, Reinhardt D, et al. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22: 4384–4393. [DOI] [PubMed] [Google Scholar]
- [14].Cheng S, Pole JD, Sung L. Early deaths in pediatric acute leukemia: a population-based study. Leuk Lymphoma. 2014;55:1518–1522. [DOI] [PubMed] [Google Scholar]
- [15].Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr. Crit Care Med. 2014;15:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- [17].Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rivers D, August EM, Sehovic I, et al. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp Clin Trials. 2013;35:13–32. [DOI] [PubMed] [Google Scholar]
- [19].Durant RW, Wenzel JA, Scarinci IC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer. 2014;120:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Winestone LE, Getz KD, Miller TP, et al. The role of acuity of illness at presentation in early mortality in black children with acute myeloid leukemia. Am J Hematol. 2017;92:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gramatges MM, Deshpande A, Lupo PJ. Ethnic disparities relative to disease features and outcomes in children with acute myeloid leukemia. Pediatr Blood Cancer. 2017;64:e26487. [DOI] [PubMed] [Google Scholar]
- [23].Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gross CP, Filardo G, Mayne ST, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005; 103:483–491. [DOI] [PubMed] [Google Scholar]
- [25].Sateren WB, Trimble EL, Abrams J, et al. How socio-demographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20: 2109–2117. [DOI] [PubMed] [Google Scholar]
- [26].Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21: 366–373. [DOI] [PubMed] [Google Scholar]
- [27].Thomas SM, Malvar J, Tran H, et al. A prospective, observational cohort study comparing cancer clinical trial availability and enrollment between early adolescents/young adults and children. Cancer. 2018;124: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shochat SJ, Fremgen AM, Murphy SB, et al. Childhood cancer: patterns of protocol participation in a national survey. CA Cancer J Clin. 2001;51:119–130. [DOI] [PubMed] [Google Scholar]
- [29].Liu L, Krailo M, Reaman GH, et al. L, Surveillance EaERCCLG. Childhood cancer patients’ access to cooperative group cancer programs: a population-based study. Cancer. 2003;97:1339–1345. [DOI] [PubMed] [Google Scholar]
- [30].Jacobs SR, Weiner BJ, Reeve BB, et al. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clinical Trials. 2014;11: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Somkin CP, Ackerson L, Husson G, et al. Effect of medical oncologists’ attitudes on accrual to clinical trials in a community setting. J Oncol Pract. 2013;9: e275–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363:263–270. [DOI] [PubMed] [Google Scholar]
- [33].Abu-Hejleh T, Chrischilles EA, Halfdanarson TR, et al. The Effect of Receiving Treatment Within a Clinical Trial Setting on Survival and Quality of Care Perception in Advanced Stage Non-Small Cell Lung Cancer. Am J Clin Oncol. 2016;39: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Green AL, Furutani E, Ribeiro KB, et al. Death within 1 month of diagnosis in childhood cancer: an analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.