Abstract
Background
Acute heart failure (HF) patients with renal insufficiency and risk factors for diuretic resistance may be most likely to derive incremental improvement in congestion with addition of spironolactone.
Methods
The ATHENA-HF trial randomized 360 acute HF (AHF) patients with reduced or preserved ejection fraction to spironolactone 100 mg daily or usual care for 96 hours. The current analysis assessed effects of study therapy within tertiles of baseline estimated glomerular filtration rate (eGFR) and subgroups at heightened risk for diuretic resistance.
Results
Across eGFR tertiles, there was no incremental benefit of high-dose spironolactone on any efficacy endpoint, including changes in log N-terminal pro-B-type natriuretic peptide (NT-proBNP) and signs and symptoms of congestion (all p for interaction ≥0.06). High-dose spironolactone had no significant effect on NT-proBNP reduction regardless of blood pressure, DM status, and loop diuretic dose (all p for interaction ≥0.38). In-hospital changes in serum potassium and creatinine were similar between treatment groups for all GFR tertiles (all p for interaction ≥0.18). Rates of inpatient worsening HF, 30-day worsening HF, and 60-day all-cause mortality were numerically higher among patients with lower baseline eGFR, but relative effects of study treatment did not differ with renal function (all p for interaction ≥0.27).
Conclusions
High-dose spironolactone did not improve congestion over usual care among AHF patients, irrespective of renal function and risk factors for diuretic resistance. In-hospital initiation or continuation of spironolactone was safe during the inpatient stay, even when administered at high doses to patients with moderate renal dysfunction.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02235077
Keywords: spironolactone, clinical trial, heart failure, diuretic, safety
BRIEF SUMMARY
Among patients hospitalized for acute heart failure, the addition of high-dose spironolactone to usual care did not result in incremental improvements in congestion. This lack of treatment effect was consistent irrespective of renal function and patient risk factors for diuretic resistance. However, in-hospital initiation or continuation of spironolactone was safe during the inpatient stay, even when administered at high doses to patients with moderate renal dysfunction.
INTRODUCTION
Relief of signs and symptoms of congestion represents the cornerstone of inpatient care for patients hospitalized for heart failure (HF).1 However, effective decongestion is oftentimes difficult and a significant proportion of patients are discharged with persistent congestion and attendant heightened risks of death and HF rehospitalization.1, 2 Likewise, recent investigations have supported diuretic response, defined as the change in weight per 40 mg oral furosemide equivalent, as an objective measure of decongestive efficiency that predicts post-discharge outcomes.3–5 In these studies, poor diuretic response (i.e., diuretic resistance) consistently correlates with several patient characteristics, including poor renal function, lower systolic blood pressure, history of diabetes, and high doses of background loop diuretic therapy.3–6 Thus, these baseline characteristics may define patient populations where additive decongestive therapies offer greatest likelihood of benefit over standard in-hospital care.
Few studies have prospectively investigated decongestive strategies in the setting of acute HF (AHF) with renal insufficiency and diuretic resistance and there remain no definitively proven strategies.7–9 The recently completed ATHENA-HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) trial tested the hypothesis that addition of high-dose spironolactone would result in greater decongestion, as compared with standard care.10 Results from the overall trial population showed high-dose spironolactone to be well-tolerated but without laboratory or clinical benefits. However, although overall trial results were neutral, it is plausible that incremental decongestive benefit among patients with loop diuretic resistance was nullified by no benefit among patients with preserved renal function and robust response to background diuretic therapy. In this context, the purpose of this post-hoc analysis from the ATHENA-HF trial was to explore the incremental decongestive effects and safety of high-dose spironolactone over standard therapy in AHF patient subsets with renal dysfunction and high risk for diuretic resistance.
METHODS
Study Design
The design and primary results of the ATHENA-HF trial have been previously reported.10, 11 Briefly, ATHENA-HF was a prospective, multicenter, randomized trial investigating the efficacy and safety of high-dose spironolactone in addition to usual care versus usual care alone among patients hospitalized for AHF. Patients not taking spironolactone prior to enrollment were randomized to 100 mg spironolactone daily or placebo; patients already taking spironolactone were randomized to 100 mg spironolactone daily or 25 mg daily. The treatment period was 96 hours. Eligible patients were hospitalized with a clinical diagnosis of HF (≥1 sign and ≥1 symptom) irrespective of ejection fraction (EF) and an N-terminal pro-B-type natriuretic peptide (NT-proBNP) level ≥1000 pg/mL within 24 hours of randomization. Patients were required to have serum potassium level ≤5.0 mEq/L, an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73m2 determined by the Modification of Diet in Renal Disease (MDRD) equation, and systolic blood pressure >90 mmHg. Patients already receiving eplerenone or >25 mg daily of spironolactone were excluded. The trial was conducted in accordance with the Declaration of Helsinki and with institutional review board/ ethics committee approval at all sites. All patients provided written informed consent.
Study Endpoints
The pre-specified primary efficacy endpoint for the main ATHENA-HF trial and the present post-hoc analysis was the proportional change in log NT-proBNP level from baseline to 96 hours or hospital discharge (whichever occurred first). Pre-specified secondary congestion endpoints were measured from baseline to 96 hours/hospital discharge, and included (i) change in absolute NT-proBNP level, (ii) change in clinical congestion score, (iii) change in dyspnea (7-point Likert scale), (iv) change in dyspnea (100-point visual analogue scale), (v) net urine output, (vi) change in body weight, and (vii) change in furosemide equivalent diuretic dose. Secondary clinical endpoints included (i) inpatient worsening HF, defined as worsening signs and symptoms requiring additional therapy, (ii) 30-day worsening HF, defined as the composite of HF readmission, emergency department visit, or outpatient receipt of intravenous diuretic therapy, and (iii) 60-day all-cause mortality. Safety endpoints included (i) changes in serum potassium, creatinine, and eGFR from baseline to 96 hours/hospital discharge, (ii) serious adverse events at 30 days, and (iii) hyperkalemia ≥5.5 mEq/L at 30 days.
Statistical Analysis
Spironolactone Treatment Effect by Baseline eGFR
Patients were categorized by tertile of baseline eGFR and baseline characteristics were compared. Continuous variables were reported as median (25th percentile, 75th percentile) and compared using Wilcoxon rank-sum tests. Categorical variables were presented as frequencies and percentages, and compared using the proportion difference test or the Fisher’s exact test.
Within each eGFR tertile, patients were further stratified by study treatment arm and the effect of treatment was compared for all efficacy and safety endpoints. Interactions between tertiles and treatment arms were evaluated using general linear models for continuous outcomes and logistic models for categorical outcomes. For each endpoint, imputation for missing data was not performed and analyses were derived from patients with complete data for a given measure. To evaluate consistency of efficacy and safety results for high-dose spironolactone with alternate eGFR cutpoints, sensitivity analyses using clinical eGFR definitions aligned with the stages of chronic kidney disease were performed (i.e., eGFR 30-44, 45-59, and ≥60 mL/min/1.732). Further sensitivity analyses included separate evaluations among patients with EF<45% and ≥45% by baseline eGFR tertile.
Spironolactone Treatment Effect by Risk Factors for Diuretic Resistance
To further evaluate study treatment effect among patients with risk factors for diuretic resistance other than low eGFR, regression modelling with multiple imputation method for missing values of change in log NT-proBNP was used (rate of missing values, 12.5%). The effect of high-dose spironolactone on the primary endpoint was tested across multiple pre-specified subgroups of interest, including systolic blood pressure (≥/< median), presence versus absence of diabetes mellitus (DM), and baseline loop diuretic dose (≥/< median). Interaction p values, with adjustments for baseline log NTproBNP and stratification factor from randomization scheme, were computed to assess treatment effect for change in log NT-proBNP for specific subgroups.
Associations Between Baseline eGFR and Study Endpoints
Unadjusted and adjusted hazard ratios using Cox regression models were used to compare eGFR tertiles for time-to-event endpoints of 30-day worsening HF and 60-day all-cause mortality. Linearity and proportional hazards assumptions were tested for all models and no violations were found. Furthermore, unadjusted and adjusted general linear regression models were used to assess association between eGFR tertile and change in log NT-proBNP. All adjusted Cox regression and general linear regression models used 6 pre-specified covariates measured at baseline, including age, systolic blood pressure, history of DM, history of atrial fibrillation, ischemic HF etiology, and proportion of patients with HF with preserved EF (HFpEF). All statistical analyses were performed using SAS version 9.4 or later (SAS Institute, Cary, NC). Two-tailed p<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
Baseline characteristics by eGFR tertile (defined as eGFR≤50, eGFR 51-71, and eGFR≥72 mL/min/1.732) for all 360 patients enrolled in the ATHENA-HF trial are presented in Table S1 in Supplementary Materials. Patients with worse renal function tended to be older and were more likely to be white with preserved EF, ischemic HF etiology, and history of atrial fibrillation. Baseline NT-proBNP level increased markedly from highest to lowest eGFR tertile, but signs and symptoms of congestion were similar between groups with the exception of less orthopnea among those with worse renal function. Rates of baseline loop diuretic use were similar between eGFR tertiles, but dosing increased with progressively worse renal function. Patients in the lowest eGFR tertile were least likely to be receiving background angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker therapy, but rates of background mineralocorticoid receptor antagonist (MRA) therapy were similar across groups.
Effects of Spironolactone on Congestion and Clinical Events
Data on in-hospital changes in congestion and clinical events are displayed in Table 1. Regardless of treatment assignment, patients in all eGFR tertiles tended to have at least moderate reductions in NT-proBNP level from baseline to 96 hours. Similarly, all groups tended to have improvements in clinical congestion, including improvements in dyspnea and clinical congestion score and weight loss. Median (25th-75th) urine output from baseline to 96 hours ranged from 4,018 (1,586-7,416) to 7,060 (2,211-8,736) mL in all subgroups.
Table 1.
Primary and Secondary Endpoints By Baseline Renal Function
| Outcomes |
High-dose Spironolactone GFR ≤50 (N=60) GFR 51-71 (N=55) GFR ≥72 (N=67) |
Usual Care GFR ≤50 (N=58) GFR 51-71 (N=65) GFR ≥72 (N=55) |
P value | Interaction P value |
|---|---|---|---|---|
| Primary Endpoint: Log NT-proBNP | ||||
| Baseline | ||||
| GFR ≤50 | 8.87 (8.26, 9.48) | 8.53 (7.80, 8.94) | 0.010 | |
| GFR 51-71 | 8.22 (7.94, 8.86) | 8.31 (7.59, 9.05) | 0.615 | |
| GFR ≥72 | 8.18 (7.39, 8.77) | 7.76 (7.27, 8.35) | 0.107 | |
| 96-h (or earlier discharge) | ||||
| GFR ≤50 | 8.52 (7.74, 9.14) | 7.93 (7.49, 8.53) | 0.013 | |
| GFR 51-71 | 7.80 (7.19, 8.63) | 7.66 (7.06, 8.50) | 0.499 | |
| GFR ≥72 | 7.40 (6.85, 7.97) | 6.93 (6.52, 7.51) | 0.030 | |
| Change | 0.797 | |||
| GFR ≤50 | −0.43 (−0.80, −0.13) | −0.42 (−0.72, −0.04) | 0.810 | |
| GFR 51-71 | −0.54 (−1.00, −0.14) | −0.38 (−0.99, −0.09) | 0.610 | |
| GFR ≥72 | −0.70 (−1.10, −0.47) | −0.76 (−1.16, −0.33) | 0.785 | |
| Secondary Endpoints: Measures of Congestion | ||||
| NT-proBNP (pg/mL) | ||||
| Baseline | ||||
| GFR ≤50 | 7097 (3860, 13140) | 5063 (2451, 7637) | 0.010 | |
| GFR 51-71 | 3720 (2811, 7031) | 4073 (1973, 8545) | 0.615 | |
| GFR ≥72 | 3563 (1627, 6452) | 2342 (1432, 4231) | 0.107 | |
| 96-h (or earlier discharge) | ||||
| GFR ≤50 | 4994 (2290, 9290) | 2781 (1794, 5065) | 0.013 | |
| GFR 51-71 | 2440 (1326, 5588) | 2121 (1161, 4914) | 0.499 | |
| GFR ≥72 | 1642 (939, 2889) | 1018 (680, 1830) | 0.030 | |
| Change | 0.073 | |||
| GFR ≤50 | −2189 (−6529, −610) | −833 (−2679, −204) | 0.045 | |
| GFR 51-71 | −1701 (−3004, −617) | −1089 (−2981, −162) | 0.503 | |
| GFR ≥72 | −1951 (−3676, −582) | −1059 (−2979, −482) | 0.195 | |
| 96-h change in clinical congestion score * | 0.063 | |||
| GFR ≤50 | −5 (−7, −3) | −7 (−9, −3) | 0.122 | |
| GFR 51-71 | −6 (−9, −4) | −5 (−7, −4) | 0.312 | |
| GFR ≥72 | −5 (−8, −4) | −7 (−8, −5) | 0.139 | |
| 96-h change in dyspnea – Likert † | 0.103 | |||
| GFR ≤50 | 2 (1, 3) | 1 (1, 3) | 0.025 | |
| GFR 51-71 | 2 (1, 2) | 2 (1, 2) | 0.702 | |
| GFR ≥72 | 2 (1, 3) | 2 (1, 3) | 0.409 | |
| 96-h change in dyspnea – VAS ‡ | 0.793 | |||
| GFR ≤50 | 12 (0, 27) | 15 (5, 25) | 0.333 | |
| GFR 51-71 | 18 (5, 35) | 20 (10, 37) | 0.812 | |
| GFR ≥72 | 15 (2, 28) | 12 (0, 30) | 0.752 | |
| 96-h net urine output (mL) | 0.687 | |||
| GFR ≤50 | 5702 (2780, 7455) | 4018 (1587, 7416) | 0.189 | |
| GFR 51-71 | 4631 (2825, 7770) | 5101 (3005, 7166) | 0.959 | |
| GFR ≥72 | 7060 (2211, 8737) | 6745 (3734, 8983) | 0.978 | |
| 96-h change in weight (kg) | 0.599 | |||
| GFR ≤50 | −3.9 (−7.3, −0.9) | −2.7 (−4.4, −0.4) | 0.084 | |
| GFR 51-71 | −2.9 (−5.5, −0.8) | −2.4 (−5.6, −0.8) | 0.878 | |
| GFR ≥72 | −3.7 (−5.7, −1.1) | −3.4 (−5.3, −0.9) | 0.629 | |
| 96-h change in furosemide equivalent diuretic dose (mg) | 0.058 | |||
| GFR ≤50 | −80 (−173, 95) | −80 (−160, 0) | 0.525 | |
| GFR 51-71 | −80 (−200, 0) | −80 (−160, 4) | 0.784 | |
| GFR ≥72 | −60 (−160, 0) | −40 (−120, 0) | 0.110 | |
| Secondary Endpoints: Clinical Events | ||||
| Inpatient worsening HF events § | 0.270 | |||
| GFR ≤50 | 17 (29.3%) | 10 (17.5%) | 0.137 | |
| GFR 51-71 | 9 (17.0%) | 13 (20.3%) | 0.646 | |
| GFR ≥72 | 7 (11.1%) | 8 (14.8%) | 0.550 | |
| 30-day HF hospitalization, ED visit, or death | 0.612 | |||
| GFR ≤50 | 7 (13.2%) | 7 (12.5%) | 0.912 | |
| GFR 51-71 | 2 (3.8%) | 4 (7.0%) | 0.680 | |
| GFR ≥72 | 10 (16.7%) | 6 (12.0%) | 0.489 | |
| 60-day all-cause mortality | 0.635 | |||
| GFR ≤50 | 6 (10.0%) | 6 (10.3%) | 0.951 | |
| GFR 51-71 | 1 (1.8%) | 4 (6.2%) | 0.373 | |
| GFR ≥72 | 1 (1.5%) | 0 (0.0%) | 1.000 |
Data expressed as n (%) or median (25th, 75th). Data derived from patients with complete data for each endpoint (i.e., no imputation). Change refers to change in measure from baseline to 96-hours or hospital discharge, whichever occurred first.
Clinical congestion score was calculated by finding the sum of the individual scores of orthopnea, jugular venous distention, and pedal edema on a standardized 4-point scale ranging from 0 to 3.
Measured by Likert scale ranging from 1 =markedly improved to 7 =markedly worse.
Measured by VAS ranging from 0 to 100 with higher values indicating better status.
Defined as worsening HF with signs and symptoms requiring additional therapy.
GFR, estimated glomerular filtration rate; HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; VAS, visual analogue scale
There was no significant difference in the primary endpoint of change in log NT-proBNP between high-dose spironolactone and usual care, regardless of baseline eGFR tertile (p for interaction =0.80). Likewise, there was no differential effect of high-dose spironolactone by baseline renal function for any of the secondary congestion endpoints (all p for interaction ≥0.058). Rates of inpatient worsening HF, 30-day worsening HF, and 60-day all-cause mortality were numerically higher among patients with lower eGFR, but there was no interaction with study treatment (all p for interaction ≥0.27). Sensitivity analyses for all primary and secondary endpoints using clinical eGFR cutpoints of 30-44 (N=71), 45-59 (N=109), and ≥60 mL/min/1.732 (N=180) are presented in Table S2 in Supplementary Materials. Further sensitivity analyses for efficacy endpoints limited to patients with EF<45% and ≥45% are displayed in Tables S3 and S4 in Supplementary Materials, respectively. Results of all sensitivity analyses were consistent with the primary analysis, with no suggestion of an advantage for high-dose spironolactone for any endpoint, irrespective of eGFR group.
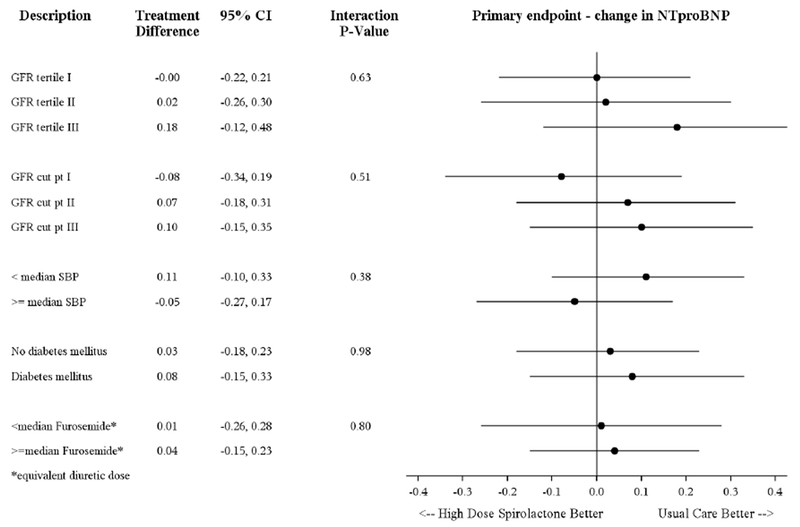
Figure 1 displays results for the primary efficacy endpoint among subgroups at heightened risk of diuretic resistance. In addition to a neutral effect among patients with lower eGFR, there was no benefit of high-dose spironolactone regardless of stratification by median systolic blood pressure, DM status, or median loop diuretic dose.
Figure 1. Forest plot of pre-specified subgroup analyses.
Median SBP was 122 mmHg and median daily furosemide equivalent dose was 80 mg. X-axis represents treatment difference for change in log NT-proBNP. GFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure
Renal Function, Changes in Natriuretic Peptide Level, and Clinical Events
Compared to patients in the highest eGFR tertile, lower eGFR tertiles were associated with less reduction in log NT-proBNP from baseline to 96 hours/discharge (Table 2). This relationship persisted after adjustment for clinical factors. Regarding clinical endpoints, eGFR≤50 was independently associated with greater risk of 60-day all-cause mortality. Baseline renal function was not associated with risk of 30-day worsening HF events.
Table 2.
Associations Between Baseline Renal Function and Primary and Clinical Endpoints (Combined High-dose Spironolactone and Usual Care Groups)
| Primary Endpoint | Unadjusted Change (95% CI); P value | Adjusted Change (95% CI); P value* |
|---|---|---|
| Absolute change in log NT-proBNP | ||
| GFR ≤50 | 0.37 (0.20, 0.55); <0.001 | 0.22 (0.01, 0.43); 0.041 |
| GFR 51-71 | 0.30 (0.10, 0.50); 0.004 | 0.23 (0.01, 0.45); 0.041 |
| GFR ≥72 | Referent | Referent |
| Clinical Endpoints | Unadjusted Hazard Ratio (95% CI); P value | Adjusted Hazard Ratio (95% CI); P value* |
| 30-day HF hospitalization, ED visit, or death | ||
| GFR ≤50 | 1.01 (0.52-1.97); 0.968 | 0.98 (0.43-2.21); 0.960 |
| GFR 51-71 | 0.66 (0.31-1.41); 0.287 | 0.51 (0.22-1.18); 0.117 |
| GFR ≥72 | Referent | Referent |
| 60-day all-cause mortality | ||
| GFR ≤50 | 12.75 (1.66-98.07); 0.014 | 12.11 (1.37-107.22); 0.025 |
| GFR 51-71 | 5.14 (0.60-43.95); 0.135 | 5.54 (0.57-53.88); 0.140 |
| GFR ≥72 | Referent | Referent |
Adjusted for age, systolic blood pressure, history of DM, history of atrial fibrillation, ischemic HF etiology, and proportion of patients with HF with preserved ejection fraction.
ED, emergency department; GFR, estimated glomerular filtration rate; HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Safety of Spironolactone
Changes in serum potassium and serum creatinine from baseline to 96 hours/discharge were similar between high-dose spironolactone and usual care for all eGFR tertiles (all p for interaction ≥0.18) (Table 3). Patients in the highest eGFR tertile tended to have less reduction in GFR with high-dose spironolactone, while change in eGFR within lower tertiles was similar between treatment arms (p for interaction =0.033). Only 1 patient randomized to usual care and 0 patients randomized to high-dose spironolactone developed a serum potassium level between 5.5-5.9 mEq/L during the 96-hour treatment period; no patient developed a serum potassium level ≥6.0 mEq/L. Serious adverse events through 30 days were similar between study treatment groups for all eGFR tertiles (all p for interaction =0.68). Rates of hyperkalemia through 30 days were similarly low (≤2%) for high-dose spironolactone and usual care, irrespective of eGFR tertile. Sensitivity analyses for safety endpoints using clinical eGFR cutpoints (Table S5 in Supplementary Materials) and stratified by EF<45% (Table S6 in Supplementary Materials) and ≥45% (Table S7 in Supplementary Materials) did not demonstrate any statistically significant treatment interactions (all p for interaction ≥0.14).
Table 3.
Safety Endpoints By Baseline Renal Function
|
High-dose Spironolactone GFR ≤50 (N=60) GFR 51-71 (N=55) GFR ≥72 (N=67) |
Usual Care GFR ≤50 (N=58) GFR 51-71 (N=65) GFR ≥72 (N=55) |
P value | Interaction P value | |
|---|---|---|---|---|
| Inpatient (i.e., study treatment phase) | ||||
| 96-h change in serum potassium (mEq/L) | 0.948 | |||
| GFR ≤50 | 0.10 (−0.10, 0.70) | 0.10 (−0.30, 0.60) | 0.582 | |
| GFR 51-71 | 0.40 (0.00, 0.70) | 0.20 (−0.30, 0.50) | 0.157 | |
| GFR ≥72 | 0.40 (0.00, 0.60) | 0.10 (−0.40, 0.80) | 0.316 | |
| 96-h change in serum creatinine (mg/dL) | 0.182 | |||
| GFR ≤50 | 0.20 (−0.10, 0.40) | 0.13 (−0.04, 0.41) | 0.777 | |
| GFR 51-71 | 0.20 (0.04, 0.46) | 0.04 (0.00, 0.30) | 0.133 | |
| GFR ≥72 | 0.00 (−0.09, 0.11) | 0.12 (−0.01,0.20) | 0.026 | |
| 96-h change in GFR (mL/min/1.732) | 0.033 | |||
| GFR ≤50 | −4.09 (−9.58, 3.96) | −4.74 (−10.10, 1.33) | 0.765 | |
| GFR 51-71 | −9.35 (−17.43, −1.88) | −1.58 (−13.55, 0.00) | 0.118 | |
| GFR ≥72 | 0.00 (−11.70, 13.01) | −10.05 (−19.16, 0.84) | 0.053 | |
| 30-day Adverse Event Rates | ||||
| Serious Adverse Event | 0.682 | |||
| GFR ≤50 | 11 (18.3) | 5 (8.6) | 0.123 | |
| GFR 51-71 | 4 (7.3) | 4 (6.2) | 1.000 | |
| GFR ≥72 | 6 (9.0) | 4 (7.3) | 1.000 | |
| Hyperkalemia ≥5.5 mEq/L | -- | |||
| GFR ≤50 | 1 (1.7) | 0 (0.0) | -- | |
| GFR 51-71 | 0 (0.0) | 1 (1.5) | -- | |
| GFR ≥72 | 0 (0.0) | 1 (1.8) | -- |
Data expressed as median (25th,75th). Data derived from patients with complete data for each endpoint (i.e., no imputation). Change refers to change in measure from baseline to 96-hours or hospital discharge, whichever occurred first.
GFR, estimated glomerular filtration rate
DISCUSSION
In this cohort of patients hospitalized for AHF, 50% had an eGFR <60 mL/min/1.732 and approximately 20% of patients had an eGFR <45 mL/min/1.732. Patient profile and clinical outcomes differed by baseline renal function, with worse renal function associated with older age, higher likelihood of preserved EF, and higher all-cause mortality at 60 days. Worse baseline renal function correlated with greater elevation in baseline NT-proBNP level and was independently associated with less in-hospital NT-proBNP reduction as compared with patients with better renal function. Regarding study treatment, addition of high-dose spironolactone did not offer decongestive or clinical advantages over usual care alone among AHF patients with impaired renal function, nor was it effective in subsets at heightened risk for poor response to standard loop diuretic therapy. However, the safety profile of in-hospital use of spironolactone was reassuring, with no signal of excess hyperkalemia, worsening renal function, or adverse clinical events during the inpatient stay, even in patients with moderate renal dysfunction.
Potential issues specific to spironolactone metabolism notwithstanding,12 it was posited that robust diuretic response to standard therapy among patients with preserved renal function prevented detection of incremental decongestion with high-dose spironolactone in the overall ATHENA population. The current post-hoc analysis does not support this hypothesis. Reflecting on the present results, patient characteristics of the lowest eGFR tertile deserve attention. Despite an attempt to identify a subset who would demonstrate diuretic resistance, this was not accomplished. Notably, patients in the lowest eGFR tertile had reasonable urine output with 96 hours of standard care (i.e., median >4.0L, 25% with urine output >7.4L). Likewise, limited by trial selection criteria mandating eGFR ≥30 mL/min/1.732, the severity of renal dysfunction in the lowest eGFR tertile was modest with a median eGFR of 44, median serum creatinine of 1.6 mg/dL, and median blood urea nitrogen level of 32 mg/dL. Stratification by other factors previously associated with poor diuretic response (including lower systolic blood pressure, history of DM, and high background dosing of loop diuretic therapy) also failed to detect an efficacy signal, potentially due to small numbers of patients in the overall cohort with true diuretic resistance. A low prevalence of diuretic resistance has been seen in prior HF trials of decongestive therapies and may have similarly contributed to neutral results in the ROSE (Renal Optimization Strategies Evaluation) study of low-dose dopamine and nesiritide.9 Despite the ROSE program requiring renal dysfunction for enrollment, median eGFR was roughly 45 mL/min/1.732 and patients receiving placebo produced a median 8.3L of urine with 72 hours of standard therapy.9 Together with the ROSE findings, the current data from ATHENA-HF suggest isolated moderate renal insufficiency may be an inadequate selection criterion for future trials of additive decongestive therapies in AHF. Rather, enrollment of patients with confirmed oliguria despite usual care may maximize chances of demonstrating incremental benefit on congestive endpoints and may more closely align with the unmet therapeutic need in clinical practice. Likewise, given the reassuring in-hospital safety profile of high-dose spironolactone seen here, future evaluation of efficacy and safety of spironolactone among AHF patients with severe renal dysfunction (i.e., eGFR <30 mL/min/1.732) may be considered.
Although efficacy findings were neutral, the present data add significant strength to previously reported ATHENA-HF results regarding relative safety of in-hospital use of spironolactone.10 In the current analysis, there were no heightened risks of hyperkalemia or worsening renal function i) despite administration of spironolactone doses above those generally used in clinical practice and ii) even among patients with reduced baseline eGFR where safety concerns are greatest. Despite proven survival benefits and strong guideline recommendations, utilization of MRA therapy among eligible HF with reduced EF (HFrEF) patients in routine practice has remained consistently low, with concerns over hyperkalemia and worsening renal function as significant factors.13–15 Following publication of the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, guidelines now also endorse consideration of spironolactone among HFpEF patients.16, 17 To improve long-term adherence to guideline-directed medical therapy, the HF hospitalization has been championed as a key opportunity to optimize chronic HF medical therapy.2, 18, 19 Nonetheless, only one-third of eligible hospitalized HF patients may be prescribed an MRA at discharge.20, 21 Prior observational data demonstrate strong associations between MRA prescription at discharge and longitudinal post-discharge adherence, but randomized data regarding safety of inpatient MRA use are scarce.22, 23 In this context, the current analysis from ATHENA-HF provides strong evidence for the relative safety of in-hospital initiation or continuation of MRA therapy during a hospitalization for AHF. Specifically, these findings inform in-hospital care for the substantial proportion of HF patients in routine practice with concomitant renal dysfunction. In combination with appropriate post-discharge laboratory and clinical surveillance, the present data support current guidelines regarding in-hospital initiation of MRA therapy in this high-risk subset as a generally safe means of improving quality of care.18, 24
Aside from study treatment effects, associations between baseline renal function and study endpoints warrant mention. The present findings are consistent with prior HF literature linking poor baseline renal function with increased risk of subsequent clinical events.25 However, unique to this analysis is the independent association between worse baseline renal function and less in-hospital reduction in NT-proBNP. While previous work has shown correlation between worse baseline renal function and higher baseline natriuretic peptide levels, our data have more direct application to future HF clinical trials using reduction in NT-proBNP as an endpoint.26 Similar to a previous analysis suggesting prevalent atrial fibrillation/ flutter may impact ability of a HF clinical trial to meet an NT-proBNP defined endpoint, the current study highlights baseline renal dysfunction as an additional independent factor potentially limiting sensitivity of a trial to detect significant reduction in NT-proBNP, irrespective of any cardiac effects of study therapy.27
Limitations
Limitations of this analysis should be recognized. First, these results should be viewed in the context of the ATHENA-HF inclusion criteria for eGFR ≥30 mL/min/1.73m2. The efficacy and safety findings seen here may not generalize to patients with more severe renal impairment. Nonetheless, this eGFR cutpoint is consistent with clinical guidelines for spironolactone and facilitates applicability to routine practice. Second, the trial protocol did not require post-discharge use of spironolactone. Thus, post-discharge clinical and safety data must be interpreted in the setting of most patients no longer actively receiving study drug. Third, despite multivariable modeling with pre-specified covariates, associations between renal function, clinical outcomes, and NT-proBNP change may be subject to residual confounding and this retrospective observational work cannot definitively determine cause-effect relationships. Fourth, given the moderate size of the overall trial cohort, subgroup analyses were subject to modest numbers of patients and limited statistical power to detect treatment effects. This issue also increased vulnerability to imbalances in baseline NT-proBNP levels (as was seen among patients in the lowest eGFR tertile receiving high-dose spironolactone) which may favor regression to the mean during follow-up and limit utility of change in NT-proBNP as an endpoint. Fifth, eGFR estimated at time of hospital admission for HF may differ from renal function measured under chronic stable conditions and the MDRD equation may be less accurate in the setting of rapidly changing renal function. Thus, the degree to which acute cardio-renal instability contributed to categorization of patients in this analysis and the results is unclear. Lastly, these data do not reflect treatment effect of spironolactone among patients with confirmed diuretic resistance during hospitalization. However, the decision to forego such analysis was pre-specified, as it was noted that stratification of patients by a feature measured after study randomization would be an improper subgroup analysis. Thus, the present analysis was limited to characteristics measured at study baseline that are risk factors for subsequent diuretic resistance.
CONCLUSIONS
In this AHF clinical trial population, renal dysfunction was associated with a distinct patient profile, less in-hospital reduction of NT-proBNP levels, and worse clinical outcomes. High-dose spironolactone did not offer incremental improvement in congestion over usual care, irrespective of renal function and risk factors for diuretic resistance. In-hospital initiation or continuation of spironolactone was safe during the inpatient stay, even when administered at high doses to patients with moderate renal dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SOURCES OF FUNDING
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers U10 HL084904, U10 HL110297, U10 HL110342, U10 HL110309, U10 HL110262, U10 HL110338, U10 HL110312, U10 HL110302, U10 HL110336, and U10 HL110337.
DISCLOSURES
Dr. Greene is supported by National Institutes of Health grant 5T32HL069749-14 and a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis, and has received research support from Amgen and Novartis. Dr. Felker reports research support from Otsuka, Novartis, Roche Diagnostics, Amgen, Merck, American Heart Association, and the National Heart, Lung, and Blood Institute; and has served as a consultant for Novartis, Roche Diagnostics, Amgen, Trevena, Cytokinetics, Madeleine, Myokardia, Bristol-Myers Squibb, Stealth Biotherapeutics, and GlaxoSmithKline. Dr. Ambrosy is supported by National Institutes of Health grant 5T32HL069749. Dr. DeVore reports research support from the American Heart Association, Amgen, the National Institutes of Health, and Novartis; and has served as a consultant for Novartis. Dr. Fudim is supported by an American Heart Association Grant 17MCPRP33460225 and National Institutes of Health T32 grant 5T32HL007101, reports consulting for Coridea and AxonTherapies. Dr. Mentz reports research support from the NIH, Amgen, Novartis, Merck, Luitpold and has served as a consultant for Novartis, Amgen and Bayer. Dr. Vaduganathan is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). Dr. Hernandez reports consulting fees from AstraZeneca, Bayer, Boston Scientific, Merck, Novartis, Sanofi, and research support from AstraZeneca, GlaxoSmithKline, Luitpold, Merck, Novartis. Dr. Butler has received research support from the National Institutes of Health, PCORI and the European Union; and serves as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmacautical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. All other authors report no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L Udelson JE, Zannad F, Gheorghiade M and Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. [DOI] [PubMed] [Google Scholar]
- 2.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J and Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–9. [DOI] [PubMed] [Google Scholar]
- 3.Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC and Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–93. [DOI] [PubMed] [Google Scholar]
- 4.ter Maaten JM, Dunning AM, Valente MA, Damman K, Ezekowitz JA, Califf RM, Starling RC, van der Meer P, O’Connor CM, Schulte PJ, Testani JM, Hernandez AF, Tang WH and Voors AA. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am Heart J. 2015;170:313–21. [DOI] [PubMed] [Google Scholar]
- 5.Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G, Greenberg BH, Pang PS, Levin B, Hua TA, Severin T, Ponikowski P, Metra M and Investigators R-A. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palazzuoli A, Testani JM, Ruocco G, Pellegrini M, Ronco C and Nuti R. Different diuretic dose and response in acute decompensated heart failure: Clinical characteristics and prognostic significance. Int J Cardiol 2016;224:213–219. [DOI] [PubMed] [Google Scholar]
- 7.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28. [DOI] [PubMed] [Google Scholar]
- 9.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne-Nickens P, Hernandez AF, Braunwald E. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol 2017;2:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, Tang WH, Felker GM, Shah MR, Braunwald E. Rationale and Design of the ATHENAHF Trial: Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure. JACC Heart Fail 2016;4:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira JP, Girerd N, Zannad F. Interpretation of the ATHENA Trial-Caveats and Future Directions. JAMA Cardiol 2018;3:89–90. [DOI] [PubMed] [Google Scholar]
- 13.Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from Get With the Guidelines-Heart Failure). Am J Cardiol 2011;107:1818–23. [DOI] [PubMed] [Google Scholar]
- 14.Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018. [DOI] [PubMed] [Google Scholar]
- 15.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 17.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 19.Fonarow GC, Gheorghiade M, Abraham WT. Importance of in-hospital initiation of evidence-based medical therapies for heart failure-a review. Am J Cardiol 2004;94:1155–60. [DOI] [PubMed] [Google Scholar]
- 20.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004;351:543–51. [DOI] [PubMed] [Google Scholar]
- 21.Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–65. [DOI] [PubMed] [Google Scholar]
- 22.Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Setoguchi S, Hernandez AF, Fonarow GC. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986 e1. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med 2014;25:67–72. [DOI] [PubMed] [Google Scholar]
- 24.Cooper LB, Hammill BG, Peterson ED, Pitt B, Maciejewski ML, Curtis LH and Hernandez AF. Consistency of Laboratory Monitoring During Initiation of Mineralocorticoid Receptor Antagonist Therapy in Patients With Heart Failure. JAMA. 2015;314:1973–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandimarte F, Vaduganathan M, Mureddu GF, Cacciatore G, Sabbah HN, Fonarow GC, Goldsmith SR, Butler J, Fedele F, Gheorghiade M. Prognostic implications of renal dysfunction in patients hospitalized with heart failure: data from the last decade of clinical investigations. Heart Fail Rev 2013;18:167–76. [DOI] [PubMed] [Google Scholar]
- 26.Schaub JA, Coca SG, Moledina DG, Gentry M, Testani JM, Parikh CR. Amino-Terminal Pro-B-Type Natriuretic Peptide for Diagnosis and Prognosis in Patients With Renal Dysfunction: A Systematic Review and Meta-Analysis. JACC Heart Fail 2015;3:977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene SJ, Fonarow GC, Solomon SD, Subacius HP, Ambrosy AP, Vaduganathan M, Maggioni AP, Bohm M, Lewis EF, Zannad F, Butler J, Gheorghiade M. Influence of atrial fibrillation on post-discharge natriuretic peptide trajectory and clinical outcomes among patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur J Heart Fail 2017;19:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.