Abstract
Women report greater craving during certain phases of the menstrual cycle. As well, research indicates that pharmacotherapies for smoking may be less efficacious in women compared to men, which may be due to interactions with natural fluctuations in ovarian hormone levels. N-Acetylcysteine (NAC) is a glutamatergic compound that has shown some efficacy in treating substance use disorders and aids in the prevention of relapse. However, it is unclear whether NAC has sex-specific effectiveness for nicotine relapse treatment. Given that NAC has shown promise to reduce nicotine reinstatement in preclinical models using male rats, the exploration of potential sex differences in the efficacy of NAC is warranted. Using a rat model, we first investigated the ability of NAC treatment (100 mg/kg, i.p.) during nicotine withdrawal with extinction training to reduce cue-induced nicotine seeking in male and female rats. Next, we assessed whether NAC’s effects were estrous-cycle-dependent for female rats. Results show that following NAC treatment during extinction, reinstatement of nicotine seeking was significantly decreased in males but not females, indicating a sex-specific effect of NAC. Furthermore, for females, both vehicle- and NAC-treated groups significantly reinstated nicotine seeking behavior compared to extinction, regardless of estrous cycle phase. These results suggest that NAC is inefficacious in reducing nicotine relapse in females regardless of estrous cycle phase at the dose evaluated here. These collective findings could have important clinical implications for use and efficacy of NAC as a pharmacotherapy for freely-cycling women smokers.
Keywords: N-acetylcysteine, smoking, estrous cycle, nicotine, sex differences
Introduction
Tobacco use disorder remains a significant public health concern with over 480,000 deaths each year in the United States (U.S. Dept of Health and Human Services, 2014). Given the health impact of nicotine-containing products, there is a great need for novel treatments to reduce nicotine addiction and relapse. Indeed, the majority of smokers who attempt to quit will relapse (Babb et al., 2017), especially following an initial quit attempt when levels of craving and negative affect are highest (Brandon et al., 1990; Piper et al., 2017). Currently available pharmacotherapeutic treatments for tobacco use disorder nonspecifically target nicotinic acetylcholine receptors, and as such are often associated with aversive side effects and have checkered clinical success (Aubin et al., 2008; Cahill et al., 2014; Silagy et al., 2004; Stapleton et al., 2008). Thus, relapse rates remain high, and suitable alternative treatment options are lacking.
Specific to gender/sex differences in smoking cessation, clinical trials show that long-term smoking cessation is more difficult to achieve in women than men (Anker and Carroll, 2011; Becker et al., 2017; Perkins and Scott, 2008; Piper et al., 2007). Sex differences in response to smoking cessation pharmacotherapies such as the nicotine patch and varenicline have been reported with women demonstrating lower cessation rates than men (McKee et al., 2016; Perkins and Scott, 2008; Potter, 2014; Smith et al., 2016; Smith et al., 2017). As well, women experience greater withdrawal severity than men (Faulker et al., 2018). Women are also more sensitive to the subjective effects of intravenous nicotine such as self-reported “drug strength” and “bad effects”, commonly synonymous with “dislike” in nicotine studies, when using the Drug Effects Questionnaire (Sofuoglu et al, 2009). Using an intravenous nicotine self-administration paradigm in smokers, men demonstrate a choice preference for lower nicotine doses, while women do not display a preference (Jensen et al, 2016). These results indicate that women may be less sensitive than men to the reinforcing effects of nicotine. Similarly, women are less sensitive to pharmacological factors and more sensitive to non-pharmacological stimuli associated with cigarettes compared to men (Perkins et al, 2018). Clinical research studies using simultaneous menstrual tracking (via urine luteinizing hormone (LH) testing and serum hormone level monitoring) and nicotine withdrawal questionnaires (e.g., the Minnesota Nicotine Withdrawal Scale and the Shiffman-Jarvik withdrawal scale) suggest that one reason for this discrepancy is that hormonal fluctuations across the menstrual cycle can affect nicotine craving and the propensity to relapse to smoking following abstinence (Allen et al., 2008; Carpenter et al., 2006; Franklin et al., 2008). Of note, clinical studies often require women to use various forms of contraception in order to enroll (Gray et al., 2010; Prado et al., 2015), ultimately resulting in changes in hormonal milieu during the study.
Sex differences in preclinical animal models investigating nicotine seeking and relapse rates have been reported. Studies have found that female rats typically acquire and self-administer higher doses of nicotine than males in nicotine self-administration models (Donny et al., 2000; Pogun et al., 2017). One study found that males acquired nicotine self-administration in fewer days than females; however, there were no sex differences after both sexes had acquired nicotine self-administration (Swalve et al, 2016). In another study, no sex differences were found in nicotine intake with 0.03 and 0.05 mg/kg/infusion unit doses (Feltenstein et al., 2012). In a study examining the effects of nicotine dose reduction, female rats were less sensitive than males to dose reduction at lower unit prices, though no effects on sensitivity were observed across the whole range of doses tested (Grebenstein et al, 2013). Discrepancies in intravenous nicotine self-administration between males and females could be due in part to different reinforcement schedules (Pogun et al, 2017). As well, sex differences in nicotine intake may be due to cyclic reproductive hormone changes in females, as alterations in ovarian hormone levels impact nicotine-related behaviors. Circulating estrogen and progesterone levels likely play an important role in regulating the reinforcing effects of nicotine (Lynch et al., 2002). For example, exogenous 17β-estradiol (E2) treatment increases intake of nicotine in ovariectomized female rats during nicotine self-administration (Flores et al., 2016). Ovariectomized females also do not show nicotine conditioned place preference, whereas ovary-intact females do (Torres et al., 2009). Taken together, these studies suggest an interaction between nicotine and circulating ovarian hormone levels that merits further investigation.
The female rodent reproductive cycle, the estrous cycle, involves similar fluctuations in ovarian hormone levels to the human menstrual cycle; it is a 4-5 day cycle, and consists of the proestrus, estrus, metestrus, and diestrus cycle phases (Goldman et al., 2007; Koebele and Bimonte-Nelson, 2016). Regarding circulating levels, in proestrus there is a surge of E2 which leads to ovulation and there are high progesterone levels, in estrus E2 has a transient low-to-moderate increase and progesterone is at a low level, and in metestrus and diestrus E2 and progesterone are at moderately high levels (Koebele and Bimonte-Nelson, 2016). Preclinically, it is unclear if there are estrous cycle phase-dependent differences in motivated nicotine seeking, specifically with re-exposure to contingent nicotine-conditioned cues.
N-Acetylcysteine (NAC) has become a promising pharmacotherapy in the treatment of substance use disorders (Deepmala et al., 2015). NAC is an FDA-approved antioxidant that targets glutamatergic signaling within the nucleus accumbens core (NAcore). Specifically, NAC restores expression of the glial glutamate transporter (GLT-1) following cocaine self-administration, which is critical in its ability to reduce cocaine-seeking behavior (Reissner et al., 2015). NAC appears to be a safe, well-tolerated compound, and has shown some efficacy in the treatment of addiction at both the clinical and preclinical levels of analysis with different drugs of abuse, including nicotine (Roberts-Wolfe and Kalivas, 2015; Grant et al., 2014). NAC represents a novel pharmacotherapeutic approach of restoring neuroadaptations induced by drugs of abuse to attenuate craving and relapse (McClure et al., 2014). Furthermore, NAC is safe to combine with other pharmacotherapies such as varenicline to promote nicotine cessation (McClure et al, 2015). In addition to reducing cigarette smoking (Knackstedt et al., 2009; Grant et al., 2014; McClure et al., 2015), NAC has shown some efficacy in treating marijuana craving in adolescents (Gray et al., 2010). Finally, NAC may reduce cocaine craving in individuals maintaining abstinence (LaRowe et al., 2013).
NAC has shown promise preclinically in reducing nicotine self-administration and seeking. For example, two studies showed that acute NAC significantly decreased nicotine seeking (Ramirez-Nino et al., 2013; Moro et al., 2018). NAC administered chronically during nicotine self-administration decreased intake (Ramirez-Nino et al., 2013). To date, all preclinical NAC studies with nicotine self-administration and reinstatement have been conducted in males and thus it remains unclear if NAC is efficacious in reducing nicotine seeking in females. As well, it is unknown to what extent pharmacological targeting of glutamate via NAC could inhibit nicotine seeking in a reproductive cycle phase-dependent manner. A subchronic NAC treatment (e.g. more than one injection [acute], but less than several weeks [chronic]) was used in this study, which was based on similar regimens that have been used in the cocaine literature that have yielded positive results for reducing drug seeking and/or restoration of GLT-1 (Reissner et al., 2015; Knackstedt et al., 2010).
In conjunction with other pharmacotherapies, NAC has been used clinically as a treatment in female populations for indications relevant to reproductive health (e.g., polycystic ovary syndrome, hyperandrogenism, and cystic ovaries; Abu Hashim et al., 2010; Amin et al., 2008; Badawy et al., 2007; Salehpour et al., 2012). The utility of NAC in treating these conditions raises the possibility that NAC interacts with the hypothalamic-pituitary-ovarian axis, and therefore, sex-steroid hormone regulation is likely involved with NAC effects. While it is currently unknown whether and how ovarian hormones interact with NAC and glutamatergic signaling, estrogen receptors and glutamate receptor coupling could play an important role. For example, the estrogen receptor α has been shown to couple to metabotropic glutamate receptor 5 (mGluR5) in the striatum of female rats, and activation of these receptor complexes might be a mechanism through which E2 elicits rapid neurophysiological effects within striatal synapses (Grove-Strawser et al., 2010). Since reproductive cycle phase and ovarian hormones, as well as glutamate dysregulation, have been implicated in nicotine relapse, and as NAC has been shown to be a glutamatergic agent capable of restoring drug-induced glutamate alterations, NAC’s interaction with the female reproductive system may be an important consideration in the design of NAC treatment regimens for nicotine addiction in freely-cycling women.
In a series of studies in male and freely-cycling female rats, we examined if NAC administered sub-chronically during nicotine withdrawal with extinction training inhibited cue-induced nicotine seeking. Specifically, we examined: (1) the effects of NAC on reinstatement of nicotine seeking in males and females and (2) the effects of NAC on reinstatement according to estrous cycle phase in females. With regard to sex differences, we hypothesized that NAC treatment would decrease reinstated nicotine seeking in males, consistent with the literature. Given several clinical studies evaluating NAC with an overrepresentation of male study participants or sample sizes that were too small to assess sex differences, we hypothesized that NAC would be inefficacious at reducing reinstated nicotine seeking in female rats. Consistent with clinical literature indicating increasing progesterone levels as a protective factor against smoking (Saladin et al., 2015), we hypothesized that female rats in estrus (when E2 and progesterone levels are relatively lower; Koebele and Bimonte-Nelson, 2016) would have increased nicotine seeking behavior. As progesterone may be protective against nicotine reward and cigarette craving (Lynch and Sofuoglu, 2010; Saladin et al., 2015), we hypothesized that females in metestrus or diestrus, when progesterone levels are relatively higher (Koebele and Bimonte-Nelson, 2016) would not reinstate to nicotine-conditioned cues.
Methods
Experiment 1: Sex Differences in Nicotine Seeking Following NAC Treatment
Subjects
Two to three month old female (n=14) and male (n=17) Sprague Dawley rats (200-250 g and 250-300 g, respectively; Charles River, Riverside, CA) were housed on a 12-hour reverse-light cycle and had ad libitum access to food and water prior to surgical and self-administration procedures. All animals were handled daily, and all animal use practices were approved by the Institutional Animal Care and Use Committee (IACUC) of Arizona State University. Animals that lost catheter patency (n=2 males), did not meet self-administration (n=1 female) or extinction criteria (n=2 males) were excluded from the study. A total of 13 females and 13 males were included in the study. Of the animals that did not have patency concerns, 92% (13/14) of females and 100% of males (15/15) reached self-administration criteria. Among those that reached self-administration criteria, 100% (13/13) of females reached extinction criteria and 86% of males (13/15) reached extinction criteria.
Self-administration, Extinction, and Reinstatement Procedures
Prior to self-administration procedures, rats had to successfully reach 2:1 active to inactive ratio during food training (refer to Supplemental Materials S1). One week after catheterization surgery, rats were trained to self-administer nicotine (0.02 mg/kg/infusion; see Figure 1) self-administration on a fixed ratio-1 (FR-1) schedule of reinforcement. All animals were required to complete at least 10 qualifying sessions (>10 infusions across 10 non-consecutive sessions). Males and females that achieved acquisition criteria were moved into extinction training.
Figure 1. Nicotine self-administration timeline in male and female rats.
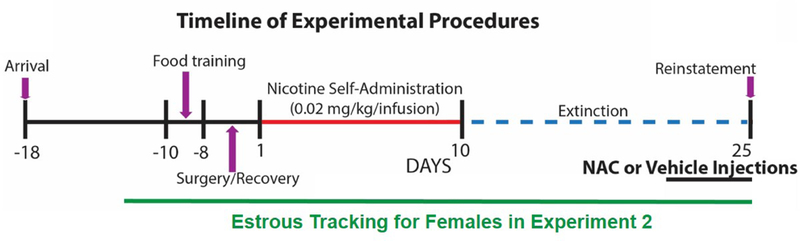
Nicotine self-administration (0.02 mg/kg/infusion), extinction, and reinstatement experimental procedures for male and female rats. Estrous cycle tracking in Experiment 2 began 4-5 days prior to surgical procedures, and continued throughout the duration of the experiment. NAC treatment was administered intraperitoneally 2-hr prior to each of the last four days of extinction and prior to reinstatement. Acquisition of self- administration was defined as ≥ 10 sessions of at least 10 infusions.
Extinction training consisted of a 2-hour session with active and inactive levers present, but neither lever produced conditioned stimuli or infusions of nicotine. Animals received 10 extinction sessions prior to beginning daily injections of NAC (100 mg/kg, i.p.) or saline vehicle. Injections occurred 2 hours prior to operant sessions as previously published (Zhou and Kalivas, 2008), and were administered on the last four days of extinction and on the day of reinstatement (see Figure 1A). Rats that did not extinguish lever pressing (i.e., >75 lever presses prior to reinstatement) were excluded. During reinstatement sessions, nicotine-conditioned cues were presented contingent upon an active lever press; however, no nicotine was delivered during reinstatement.
Data Analysis
Behavioral data were analyzed using repeated measures and mixed model analyses of variance (ANOVAs) with post-hoc Bonferroni multiple comparison tests where appropriate. Greenhouse-Geiser corrections of degrees of freedom were utilized for violations of sphericity, where necessary. Two-way repeated measures ANOVA was used to analyze self-administration and extinction data. For self-administration and extinction data, independent variables included sex, time and treatment. Dependent variables included lever presses and infusions. Linear trend analyses (also referred to as linear regression; see Gipson et al., 2011; Montgomery et al., 2012) were conducted on self-administration data to determine nicotine acquisition. Reinstatement data were analyzed by mean of each treatment group compared to the average of the previous two extinction sessions prior to NAC injections. Data were then analyzed using appropriate ANOVAs with post-hoc Bonferroni-corrected pairwise comparisons. A two-way repeated measures ANOVA was used to analyze time course data during reinstatement. Two-tailed t-tests were used to analyze mean cue presentations from time course data. A four way, mixed-design model ANOVA was used to analyze reinstatement data for male and females with lever, sex, session, and treatment as independent variables. For male and female reinstatement data, independent variables included session (extinction versus reinstatement), NAC treatment (vehicle versus 100 mg/kg), and lever (active versus inactive). The dependent variable was number of lever presses. Statistical tests were performed in Graphpad Prism software package or SPSS, and p values <0.05 were considered statistically significant.
Experiment 2: Cycle-Phase Dependent Differences in Nicotine-Seeking Following NAC Treatment.
Subjects
Two to three month old female Sprague Dawley rats (N=60, 200-250 g; Charles River, Riverside, CA) were housed in identical conditions and underwent identical surgical and behavioral sessions as in Experiment 1 (see Figure 1A for timeline). Rats were monitored for estrous cycle phase for 4-5 days prior to experimental manipulations and then daily following their behavioral sessions (see detailed estrous tracking methods in S1, as well as Figure 2 for representative images of vaginal cytology). Data from estrous cycle-tracked females were then analyzed with cycle phase as a factor. Animals that lost catheter patency (n=5), had post-operative complications (n=10), did not meet self-administration (n=5) or extinction criteria (n=2) were excluded from the study. A total of 38 females were included in the study, with 88% (40/45) meeting self-administration criteria, and 95% (38/40) meeting extinction criteria.
Figure 2. Representative samples of vaginal cytology across cycle.
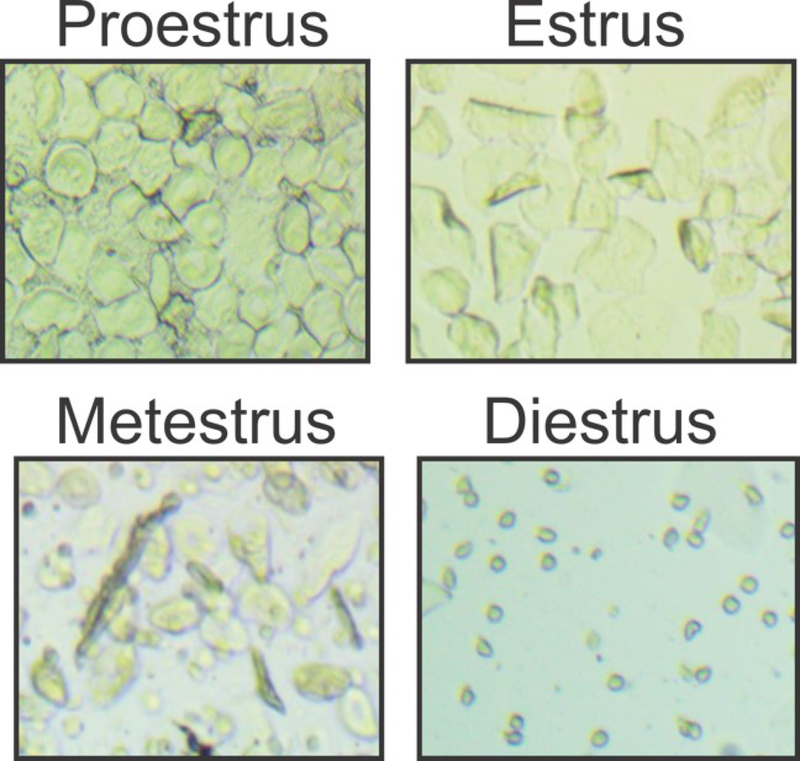
Vaginal cytology was qualified for female rats during nicotine self-administration, extinction, and reinstatement phases. Proestrus (predominantly round epithelial cells), estrus (predominantly cornified cells), metestrus (mixture of leukocytes, round epithelial cells and cornified cells), and diestrus (predominantly leukocytes) were identified after daily behavioral sessions. No differences in self-administration training were observed due to cycle phase (see Table 1).
Food Training, Self-administration, Extinction, and Reinstatement Procedures
In Experiment 2, all behavioral procedures were identical as in Experiment 1, except females were monitored for estrous cycle phase.
Data Analysis
To determine if NAC- or vehicle-treated females would differentially reinstate to nicotine-conditioned cues due to estrous cycle phase, reinstatement data were analyzed via a three-way ANOVA with session (extinction vs. reinstatement) considered a within-subjects factor, and treatment group and cycle phase considered between-subjects factors. Proestrus was not included as none of the rats were in this cycle phase during reinstatement. However, proestrus was observed during self-administration and extinction.
Results
Experiment 1
Nicotine Self-Administration in Male and Female Rats
In Experiment 1, a two-way mixed model repeated measures ANOVA revealed no significant differences in active lever presses between male and female rats during nicotine self-administration (F(1,24)= 0.01, p>0.05). A main effect of time was found for both females and males (F(9,120.87)= 4.24, p<0.05; Figure 3A), indicating that responding had increased over time in both sexes. No main effects of sex or time, nor interactions between the two factors, were found for inactive lever presses (Figure 3B). When comparing nicotine infusions using a similar statistical model, no main effect of sex or interaction between sex and time was found for infusions (p>0.05); however, there was a significant main effect of time (F(9,114.34)=8.17, p<0.0001; Figure 3C). To determine discrimination of levers, active and inactive lever pressing was compared within sex using a two-way mixed model repeated measures ANOVA with lever and time as main factors. In males, active lever pressing was significantly higher than inactive lever pressing (F(1,12)=81.15, p<0.0001), with an additional significant effect of time (F(9,41.27)=2.74, p<0.05), but no interaction between lever presses and time. For female rats, active lever pressing was significantly higher than inactive lever pressing (F(1,12)= 13.23, p<0.005). There was no significant effect of time nor any interaction between lever presses and time. Linear trend analyses were conducted on the results shown in Figure 3C to determine if nicotine self-administration acquisition was obtained in males and females. There was significant acquisition of nicotine self-administration in males (F(1,12)= 7.29, p<0.05) and females (F(1,12)= 23.21, p<0.0005). Slopes of acquisition curves were not significantly different between males and females (p>0.05), indicating that the rates at which males and females acquired nicotine self-administration were not different. Extinction curves were then compared between male and female rats and no significant differences were found in rate of extinction between males and females (Figure 3D). A significant main effect of time was found (F(13,102.2)= 8.79, p<0.0001) indicating that rats significantly decreased responding across sessions. Females required 12.62±1.16 (mean±SEM) sessions and males required 12.54±0.95 sessions to reach self-administration acquisition criteria.
Figure 3. Nicotine self-administration in male and female rats.
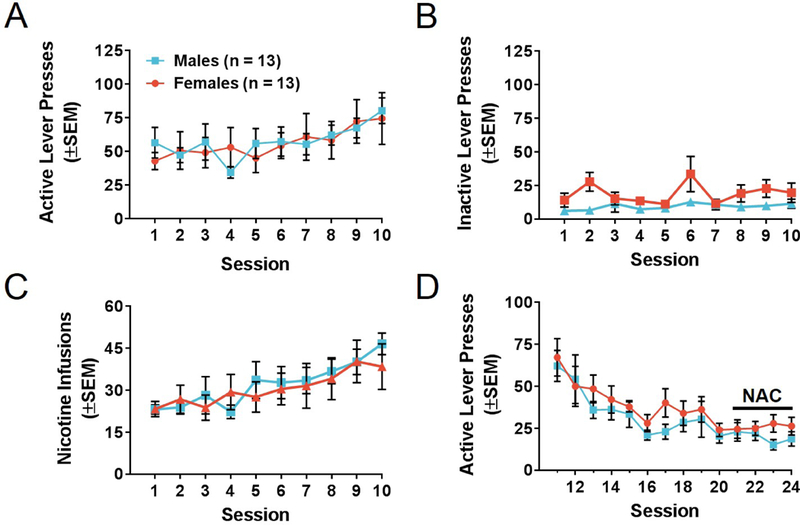
(A) Active lever presses, (B) inactive lever presses and (C) infusions during nicotine self-administration for male and female rats. Using individual two-way ANOVAs with sex as a between-subjects factor and session as a within-subjects factor, no significant differences due to sex were observed. (D) Active lever presses during withdrawal with extinction training for male and female rats. NAC administration occurred 2-hr prior to the last four extinction sessions and prior to the reinstatement session, as marked by the line in (D). Male and female rats extinguished nicotine-seeking behavior at similar rates according to linear regression modeling. Number of animals is noted in (A). Error bars represent standard error of the mean (SEM).
NAC Decreases Reinstated Nicotine Seeking in a Sex-Specific Manner
The effects of NAC treatment on nicotine-seeking behaviors in male and freely-cycling, unmonitored female rats was compared using the withdrawal with extinction and reinstatement models. Using a two-way ANOVA with extinction session as a repeated measures factor and treatment as a within-subjects factor, a significant effect of extinction session was observed (F(13,143)=5.18, p<0.0001) indicating active lever pressing decreased over time. No main effect of treatment or session x treatment interaction was found (p’s>0.05). NAC treatment did not significantly alter extinction lever pressing in males during sessions 21-24 compared to vehicle-treated males (p>0.05; Figure 4A). Additionally, there was no significant difference in male active lever presses during the four NAC treatment sessions compared to the four sessions prior to NAC treatment (p>0.05). NAC treatment also did not significantly alter extinction lever pressing compared to vehicle-treated females during sessions 21-24, or in the comparison of NAC extinction sessions with pre-NAC extinction sessions (p>0.05; Figure 4D). A four-way mixed-design ANOVA (2 × 2 × 2 × 2 with sex, session, treatment and lever as factors) was utilized to compare extinction and reinstatement lever pressing between males and females. Significant main effects of session (F(1,22)=10.21, p<0.005) as well as lever (F(1,22)=27.96, p<0.0001) were found, indicating significant reinstatement and discrimination between active and inactive levers. Additionally, no significant main effects of sex or treatment were found (p’s>0.05). Only the treatment x lever interaction (F(1,22)= 4.41, p<0.05) and the session x lever interaction (F(1,22)=13.58, p<0.005) were significant, with no significant sex x treatment interaction (F(1,22)= 2.11, p=0.161).
Figure 4. NAC attenuates nicotine-seeking behavior in male but not female rats.

(A) Extinction curve of male rats with NAC administered 2-hr prior to sessions 21-24 (highlighted by the line). During extinction, no lights or cues were presented following a press of the active lever. NAC treatment (blue) did not significantly alter extinction behavior on sessions 21-24 in males relative to control (black). (B) Active and inactive lever press responses during reinstatement of contingent cues for male rats. A compound stimulus (light + tone) was contingently presented upon an active lever press (FR-1). Vehicle (0 mg/kg)-treated males significantly reinstated to nicotine-conditioned cues, whereas NAC (100 mg/kg)-treated males did not. Additionally, NAC treatment significantly reduced active lever presses compared to vehicle. No differences were found in inactive lever pressing. (C) Time course of cumulative cue presentations during reinstatement session of male rats. NAC (blue) significantly reduced the mean number of contingent cue presentations (inset) and produced significantly different linear regressions across the reinstatement session in males, compared to control (black). (D) Extinction curve of female rats with NAC administered 2-hr prior to sessions 21-24 (highlighted by the line). NAC (red) did not significantly alter extinction behavior on sessions 21-24 in females compared to vehicle treatment (black). (E) Active and inactive lever press responses during reinstatement of contingent cues for female rats. Identical to reinstatement sessions in males, a compound stimulus (light + tone) was contingently presented upon an active lever press (FR-1). Both vehicle and NAC-treated females significantly reinstated to nicotine-conditioned cues compared to extinction lever pressing. (F) Time course of cumulative cue presentations during reinstatement session of female rats. There was no effect of NAC treatment (red) found in either mean (inset) or cumulative cue presentations in females during cue-induced nicotine reinstatement compared to vehicle treatment (black). Error bars represent SEM. *p<0.05, extinction versus vehicle reinstatement and vehicle reinstatement versus NAC treatment active lever pressing in (B); vehicle versus NAC at various time points within the reinstatement session (C) or in mean cue presentations (C, inset).
To further determine if NAC has sex-specific effects on nicotine-seeking behavior, a one-way ANOVA was used to examine the effect of treatment on extinction and reinstatement active lever pressing within each sex. In males, this analysis revealed significant differences between groups (F(2,23)= 8.25, p<0.005; Figure 4B). Post hoc analysis with Bonferroni corrections revealed that vehicle-treated male rats significantly reinstated to nicotine-conditioned cues compared to extinction (p<0.05), whereas NAC-treated males did not significantly reinstate above extinction lever pressing (p>0.05). As well, reinstatement of nicotine seeking was significantly decreased in NAC- versus vehicle-treated males (p<0.05). These results indicate that NAC significantly decreased cue-induced nicotine seeking in male rats. Contingent cue presentations across the reinstatement session were then analyzed in 15-minute bins. A two-way repeated measures ANOVA on reinstatement time course data revealed significant main effects of time (F(7,12.35)= 24.89, p<0.0001) and treatment (F(1,11)= 9.67, p<0.05), as well as a significant time x treatment interaction (F(7,77)= 4.44, p<0.001; Figure 4C). Finally, a significant difference in NAC treatment was found in mean cue presentations during reinstatement (t(11)=2.39, p<0.05; Figure 4C inset).
Using the same one-way ANOVA structure for freely-cycling, unmonitored females following vehicle or NAC treatment, no significant group differences in reinstatement were detected (F(2,23)= 1.37, p>0.05, Figure 4E). These results indicate a lack of efficacy of NAC in freely-cycling females. Time course data were then analyzed using a repeated measures two-way ANOVA. No main effect of treatment in cumulative cue presentations or time x treatment interaction was found (p’s>0.05, Figure 4F); there was a significant main effect of time (F(7,77)=7.84, p<0.0001, Figure 4F), which reflects an increase in the number of cue presentations across the session. No significant differences in NAC treatment were found in mean cue presentations (Figure 4F, inset). Taken together, these results indicate that NAC treatment during withdrawal with extinction training reduces nicotine-seeking behavior in males, but does not decrease nicotine seeking in freely-cycling females, at least at the given dose of 100 mg/kg.
Experiment 2
Nicotine Intake Does Not Vary Across the Estrous Cycle
In female rats monitored for estrous cycle phase during self-administration training, a one-way ANOVA revealed no significant differences in active lever presses across the estrous cycle. As well, no significant differences were found in the number of inactive lever presses or infusions across estrous cycle phases (see Table 1).
Table 1. Estrous cycle phase did not alter nicotine consumption or operant responding during self-administration training.
Following each training session of 0.02 mg/kg/infusion nicotine self-administration, female rats were vaginally smeared as described in the Supplemental S1 section. Self-administration criteria were set at least 10 non-consecutive sessions with a minimum of 10 infusions. No significant differences were found in active lever presses, inactive lever presses, or infusions using a two-way, mixed model ANOVA for each variable, with session as a within-subjects factor and estrous cycle phase as a between-subjects factor.
| Self-Administration Measure | Significance (p<0.05) |
|---|---|
| Active Lever | n.s. F(2,524)=0.1373 p=0.8717 |
| Inactive lever | n.s. F(2,535)=1.276, p=0.2799 |
| Infusions | n.s. F(2,534)=0.03926, p=0.9615 |
NAC Does Not Alter Reinstatement of Nicotine Seeking in an Estrous Cycle Phase-Dependent Manner
To determine if estrous cycle phase impacted reinstatement of nicotine-seeking, the estrous cycle was monitored in Experiment 2. Reinstatement data from vehicle and NAC-treated females were analyzed according to estrous cycle phase (combined metestrus/diestrus phase versus estrus phase). Due to the short duration of proestrus (<24 hours; Koebele and Bimonte-Nelson, 2016), no rats were observed in this phase when monitored after reinstatement. A three-way ANOVA was used to analyze NAC treatment, cycle phase, and session effects on active lever pressing. The three-way ANOVA revealed a significant effect of session on reinstatement lever pressing (F(1,34)=29.68, p<0.0001: Figure 5A), indicating that females significantly reinstated to nicotine-conditioned cues; there was no main effect of treatment (F(1,34)=0.01, p>0.05) or cycle phase (F(1,34)=0.64, p>0.05), and no significant interactions. Time course data were analyzed according to cycle phase using a three-way mixed model ANOVA. A significant main effect of time (F(7,238)=68.17, p<0.0001) was observed, but no effect of treatment, cycle phase, or any interactions reached statistical significance (Figure 5B). As well, no difference in mean cue presentations was observed (Figure 5C).
Figure 5. NAC does not alter nicotine seeking in an estrous cycle phase-dependent manner.
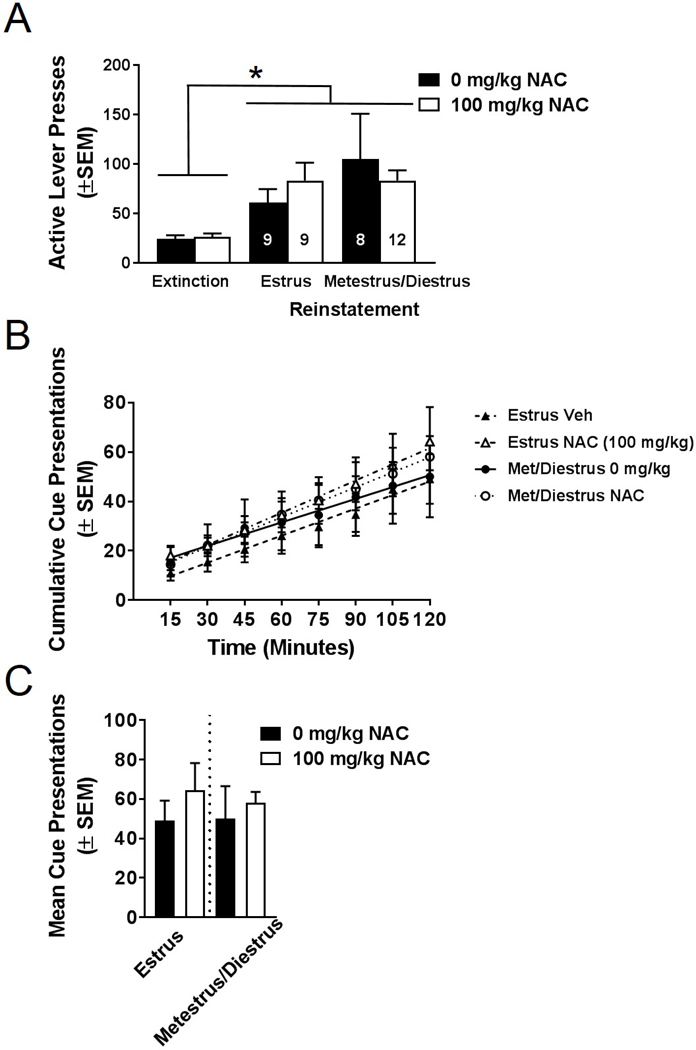
(A) Active lever presses of female rats during reinstatement based on estrous phase determined immediately following the reinstatement session. Female rats in estrus and metestrus/diestrus cycle phases significantly reinstated compared to extinction, but no effect of NAC treatment was observed. (B) Time course of cumulative cue presentation during reinstatement based on estrous cycle phase and NAC treatment. Neither mean (C) nor cumulative time course contingent cue presentations during reinstatement differed based on NAC treatment in estrus or metestrus/diestrus phases cycle phases. *p<0.05, extinction versus reinstatement active lever pressing. Error bars represent SEM.
Discussion
In the current report, no sex differences in acquisition of nicotine self-administration or extinction were found between males and females. However, while NAC reduced nicotine seeking in males, it did not reduce this behavior in females. Additionally, we showed that NAC did not reduce nicotine seeking in females, regardless of estrous cycle phase.
Sex Differences in Nicotine Self-Administration and Extinction.
In the present study, although we did not find sex differences in nicotine self-administration acquisition or in rate of extinction, our methods share similarity with the study by Donny et al. as both 2-hr access and a FR-1 schedule of reinforcement were utilized. Similar to Swalve et al. (2016), we observed no differences in rate of extinction between males and females at similar lower unit doses. Additionally, our study used a discrete compound conditioned stimulus (CS+) paired with nicotine infusions, similar to Chaudri and colleagues (Chaudri et al., 2005). Several studies have reported sex differences in nicotine self-administration in rodents, and specifically, enhanced nicotine reward in females. For example, one study found that female rats acquire nicotine self-administration at a faster rate than males at lower unit doses (0.02 mg/kg/infusion; Donny et al., 2000), but few other sex differences were found in active lever presses, infusions, or total intake under limited access conditions, using FR schedules of reinforcement. Progressive ratio schedules, however, elicited large sex differences with females reaching higher breakpoints. Chaudri and colleagues found that combining nicotine infusions with a CS+ significantly increased active lever responding compared to intake without the CS+, and this increase was greater in females than males. Furthermore, Chaudri et al. and others (e.g., Flores et al., 2016) found that females have higher nicotine intake than males at higher unit doses than that utilized in the present study. In another study, however, no sex differences were found in nicotine intake (Feltenstein et al., 2012). Neither dose tested by Feltenstein and colleagues (0.03 or 0.05 mg/kg/infusion) were as high as doses used in the previous studies discussed (i.e., 0.06 mg/kg/0.1 mL infusion or 0.15 mg/kg/infusion). Feltenstein et al. (2012) speculated that females had relatively higher intake in Chaudri et al. (2005) within the 1-hr session compared to the 2-hr session utilized in their study, and this effect could have driven the significant sex difference. One other study found no differences in nicotine maintenance on a FR-1 schedule at 0.03 mg/kg/infusion (Swalve et al., 2016), lending support to a hypothesis of dose-dependent sex differences in nicotine intake. Taken together, it is possible that sex differences in nicotine self-administration emerge more consistently at higher unit doses, and thus no significant differences in self-administration training were observed here for males and females. Of note, male but not female smokers display choice preference for low doses of nicotine over high doses (Jensen et al., 2016), illustrating the translational value of modeling nicotine-related sex differences in rodents. Consistent with a dose-dependent sex difference hypothesis, Feltenstein et al. (2012) found that females exhibit increased lever presses compared to males during the first few sessions of extinction after self-administration of the higher dose (Feltenstein et al., 2012). It is therefore possible that similar to intake, sex differences in extinction rate could emerge after self-administration of a higher unit dose of nicotine.
Sex Differences in Nicotine Reinstatement.
Here we report no significant difference in reinstated nicotine seeking between vehicle-treated males and females, and significant reinstatement in females in Experiment 2 (Figure 5). Sex differences in reinstated nicotine seeking have been reported in one other study to date. In Swalve et al. (2016), males but not females reinstated to nicotine-conditioned cues, counter to studies reporting higher vulnerability to nicotine-related behaviors in females (e.g., Donny et al., 2000; Chaudri et al., 2005; Flores et al., 2016) as well as no sex differences in reinstated nicotine seeking (Feltenstein et al., 2012). The discrepant results between our study and Swalve et al. (2016) could be due to higher levels of nicotine intake with the 2-hr access model utilized here, versus a 1-hr session and lower reported levels of nicotine intake in the previous study.
NAC Reduces Nicotine Seeking in a Sex-Specific Manner.
Here we show that NAC, a clinically relevant glutamatergic agent currently under investigation to treat substance use disorders (including tobacco use disorder), has sex-specific efficacy in reducing cue-induced nicotine seeking. Freely-cycling females in Experiment 1 did not reinstate robustly to nicotine-conditioned cues regardless of NAC treatment (Figure 4E), whereas males administered vehicle-treatment demonstrated significant reinstatement compared to extinction and significant reduction of reinstatement pressing due to NAC treatment (Figure 4B). In the present study, subchronic NAC treatment administered during extinction training reduced conditioned cue-induced nicotine seeking in male rats, consistent with previous findings (Ramirez-Niño et al., 2013; Moro et al., 2018). Conversely, NAC did not significantly reduce nicotine seeking in freely-cycling female rats. Preclinical studies examining the effects of NAC on nicotine seeking behavior show dose-dependent efficacy of NAC in male rats (Ramirez-Niño et al., 2013) with higher doses reducing nicotine seeking more effectively. In our study, male and female rats were administered a subchronic high dose of NAC (100 mg/kg) that has previously been used in preclinical NAC studies showing efficacy in reducing cocaine (Ducret et al., 2016; Reissner et al., 2015) and nicotine seeking in male rats (Ramirez-Niño et al., 2013; Moro et al., 2018). Only two studies thus far have examined sex differences in cue-induced nicotine seeking (Feltenstein et al., 2012; Swalve et al., 2016), and this study represents the first evaluation of the effects of NAC in a sex-specific manner. The specific hormonal actions of the rodent estrous cycle on nicotine-seeking behavior and a potential explanation of why NAC was ineffective at reducing nicotine cued-reinstatement warrant further investigation, as they could provide vital information for development of new smoking cessation agents.
It is possible that a higher dose, longer duration of treatment, or different route of administration of NAC would decrease motivated nicotine seeking, as NAC has been shown to have poor bioavailability (Borgström et al. 1986; Olsson et al. 1988). While previous investigations with NAC have demonstrated success at reducing nicotine-seeking behavior in male rats, successful dosing ranges have not been uniform. For example, one study reported successful reduction of nicotine-seeking behavior at 60 and 90 mg/kg NAC, i.p., but not 30 mg/kg (Ramirez-Niño et al., 2013), whereas others only report success at 100 mg/kg NAC and not 30 or 60 (Moro et al., 2018). Furthermore, NAC is presumed to work in part by upregulating expression of the glial glutamate transporter GLT-1 (Reissner et al., 2015). However, studies have found that E2 regulates glutamate transporter expression and increases glutamate uptake in primary astrocytes (Pawlak et al., 2005; Lee et al., 2013), possibly limiting the restorative properties of NAC in female rats. This is a potentially important limitation of our study, as clinical studies evaluating substance use disorders have shown efficacy of NAC following longer, chronic NAC treatment, but with limited inclusion of women. For example, following 8 weeks of NAC treatment, adolescents of either sex were twice as likely to show negative urine cannabinoid tests (Gray et al., 2012). A small pilot study with smokers of both sexes indicated a modest reduction in weekly cigarette use after 4 weeks of NAC treatment, although this was not significant (Knackstedt et al., 2009). Thus, it is possible that a longer duration of NAC treatment (at least 8 weeks) may have greater efficacy in reducing nicotine use, including reduced nicotine seeking in females as well as further reductions in nicotine seeking in males.
Estrous Cycle and Nicotine-Motivated Behavior.
Ovarian hormone deprivation, and estrogen and progesterone supplementation, have been shown to have marked effects on nicotine-related behaviors. For example, ovariectomized female rats do not readily acquire nicotine self-administration (Flores et al., 2016), but dose-dependent increases in nicotine intake occur with E2 supplementation. This is important because previous research has shown that dopaminergic transmission is reduced in ovariectomized female rats (Ohtani et al., 2001), which is then restored by E2 supplementation. Heavy smoking in women is associated increases of estrogen and progesterone metabolites during the early follicular phase, as well as a decrease in post-ovulatory progesterone metabolite levels (Windham et al., 2005). Clinically, either micronized progesterone (e.g., Prometrium®) or synthetic progestins are typically prescribed to women for hormone therapy due to poor oral bioavailability and short half-life of the endogenous hormone (Kuhl, 2005). Interestingly, in women, progesterone administration during the follicular menstrual cycle phase attenuates the subjective effects of d-amphetamine (Justice and de Wit, 1999) and cocaine (Sofuoglu et al., 2002). Exogenous progesterone administration attenuates smoking urges in human smokers (Sofuoglu et al., 2009), and exogenous micronized progesterone treatment significantly attenuates nicotine craving in women (Sofuoglu et al., 2001).
In our current study, female rats during estrous cycle phases associated with higher progesterone levels (i.e., metestrus and diestrus) did not reinstate less than females in phases associated with lower progesterone (i.e., estrus), and there were no differences in nicotine consumption rates due to estrous cycle phase (see Table 1). Thus, our results reported here, while not directly measuring or manipulating circulating hormonal levels, provide preliminary indication that there may not be a role of endogenous cycling ovarian hormones in estrogen or progesterone-associated protection against nicotine seeking behavior. However, it is possible that exogenous progestogen administration could reduce nicotine craving via direct actions of the synthetic progestin on nicotine reward neural circuits, or via a disruption of normal cyclicity due to feedback mechanisms (Kuhl, 2005; Rosen and Cedars, 2007). Further investigation of nicotine self-administration and seeking behaviors coupled with exogenous estrogen and progesterone administration, as well as direct measurement of hormone levels, is warranted. Of additional note, we did not observe any female rats in proestrus during reinstatement, when circulating E2 and progesterone levels are high (Koebele and Bimonte-Nelson, 2016). Thus, we cannot exclude the possibility that differences in nicotine-motivated behaviors occur during proestrus in rats, even though this temporal period is shorter than all other phases, lasting less than a day (Koebele and Bimonte-Nelson, 2016).
Estrous Cycle, Glutamate, and NAC.
Our study was the first to examine NAC treatment and nicotine-motivated behavior in females. Despite the results seen here where no significant effects of estrus cycle phase on nicotine self-administration, extinction, or reinstatement, and no significant effects of NAC on reinstatement of nicotine-seeking behaviors in female rats were observed, understanding the interactions between glutamatergic signaling and hormone levels could play a vital role in addressing nicotine cessation in women smokers. Mechanistically, it is unknown if interactions between glutamatergic signaling and regulation of cellular activity within the reward circuitry by shifting ovarian hormone levels impacts nicotine relapse vulnerability. Importantly, inhibition of mGluR5 is known to attenuate cue-induced reinstatement of nicotine seeking (Bespalov et al., 2005), and it has been hypothesized that glutamate overflow induced by drug-paired cues activates mGluR5 to potentiate postsynaptic excitability and subsequent drug-seeking behavior (Kalivas, 2009). Moreover, both estrogen receptor α and mGluR5 are expressed on astrocytes and mGluR5 plays a critical role in regulating both basal and activity-dependent synaptic transmission (Panatier and Robitaille, 2016). As well, activation of mGluR5 is imperative for E2 to increase cocaine self-administration in female rats (Martinez et al., 2016). NAC’s ability to reduce drug seeking is dependent on restoration of GLT-1, an effect blocked by antagonism of mGluR5 (Reissner et al., 2015). However, no studies to date have examined the ability of NAC to restore GLT-1 in nicotine-withdrawn male or female rats. Taken together, it is possible that attenuating effects of NAC on reinstated nicotine seeking could be occluded when estrogen levels are elevated, an effect that warrants further investigation. Specifically, when E2 levels rise, more estrogen receptor α receptors coupled to mGluR5s are bound, which could facilitate the ability of mGluR5 activation to drive nicotine seeking. This in turn could occlude the ability of NAC to decrease nicotine-motivated behavior in females during phases of the estrous cycle when E2 levels rise. However, additional work is needed to determine the impact of E2 on glutamate systems during drug seeking, as we did not observe females in proestrus during reinstatement and did not measure hormone levels.
Conclusions and Future Directions
Given the potential impact of ovarian hormones on smoking relapse vulnerability, conditions such as exogenous hormone treatment, which can impact reproductive cycles, are an important aspect of treatment strategies that should be taken into account. In addition, many clinical studies are not sufficiently powered to examine sex differences. Clinical literature has demonstrated a role of progesterone in improving odds of abstinence from smoking (Saladin et al., 2015), thus measuring endogenous ovarian hormone levels in animal models may provide important insights into nicotine relapse vulnerability in females. In the present set of studies, male and female rats did not significantly differ in self-administration or extinction. However, nicotine-seeking behavior was successfully attenuated with subchronic NAC administration in male but not female rats. Additionally, NAC’s ineffectiveness was not due to differences in nicotine seeking based on estrous cycle phase, as similar levels of reinstatement during estrus and metestrus/diestrus cycle phases were found. The impact of exogenous estrogen treatment in NAC’s clinical efficacy as an addiction therapeutic has not been systematically evaluated, but is a critical factor that should be further explored. Given the literature showing strong evidence of nicotine-related behavioral differences in males and females, conditions that impact reproductive cycles are an important aspect of treatment strategies that should be taken into account.
Supplementary Material
Acknowledgements
The authors thank Vincent Carfagno, Paula Overby, Hanaa Ulangkaya, Andrea Sekito, Laura Vesala, Amanda Ariola, Jason Hguyen, Jackie Moreno, Armani Del Franco and Joseph McCallum for their technical assistance with self-administration. This work was supported by the National Institutes of Health Grant DA036569, DA044479, and DA045881 (to CDG) and the Arizona State University Institute for Social Science Research Seed Grant (to CDG and HBN).
Footnotes
The authors report no conflicts of interest.
References (see Supplemental S2 for additional references)
- Abu Hashim H, Anwar K, El-Fatah RA (2010) N-Acetyl Cysteine Plus Clomiphene Citrate Versus Metformin and Clomiphene Citrate in Treatment of Clomiphene-Resistant Polycystic Ovary Syndrome: A Randomized Controlled Trial. Journal of Women’s Health 19:2043–2048. [DOI] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D (2008) Menstrual phase effects on smoking relapse. Addiction (Abingdon, England) 103:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AF, Shaaban OM, Bediawy MA (2008) N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reproductive BioMedicine Online 17:722–726. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96. [DOI] [PubMed] [Google Scholar]
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, Williams KE, Reeves KR (2008) Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 63:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, Jamal A (2017) Quitting Smoking Among Adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep 65:1457–1464. [DOI] [PubMed] [Google Scholar]
- Badawy A, State O, Abdelgawad S (2007) N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: a cross-over trial. Acta obstetricia et gynecologica Scandinavica 86:218–222. [DOI] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A (2005) Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology 49 Suppl 1:167–178. [DOI] [PubMed] [Google Scholar]
- Borgstrom L, Kagedal B, Paulsen O (1986) Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol 31:217–222. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB (1990) Postcessation cigarette use: The process of relapse. Addictive Behaviors 15:105–114. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Lancaster T (2014) Pharmacological Treatments for Smoking Cessation. JAMA 311:193. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Upadhyaya H, LaRowe S, Saladin M, Brady K (2006) Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine & Tobacco Research 8:627–638. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ (2010) Sex differences and ovarian hormones in animal models of drug dependence. Hormones and Behavior 58:44–56. [DOI] [PubMed] [Google Scholar]
- Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R (2015) Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neuroscience and Biobehavioral Reviews 55:294–321. [DOI] [PubMed] [Google Scholar]
- Dhouib IE, Jallouli M, Annabi A, Gharbi N, Elfazaa S, Lasram MM (2016) A minireview on N-acetylcysteine: An old drug with new approaches. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S (2000) Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology 151:392–405. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE (2012) Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend 121:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE (2016) Estradiol promotes the rewarding effects of nicotine in female rats. Behavioural Brain Research 307:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR (2008) Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. Journal of women’s health (2002) 17:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT (2011) Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 214:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America 110:9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J, Murr A, Cooper R (2007) Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res Part B 80:84–97/ [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR, Potenza MN, Schreiber LR, Donahue CB, & Kim SW (2014). A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. The Journal of clinical psychiatry, 75(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, Larowe SD (2010) N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict 19:187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG (2013) Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav 114-115:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG (2010) Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, London ED (2018) Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology (Berl) 235:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Stamp JA (2011) Ovarian hormones and propensity to drug relapse: A review. Neuroscience & Biobehavioral Reviews 35:427–436. [DOI] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016) Intravenous Nicotine Self-Administration in Smokers: Dose-Response Function and Sex Differences. Neuropsychopharmacology 41:2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, de Wit H (1999) Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 145:67–75. [DOI] [PubMed] [Google Scholar]
- Kuhl H (2005) Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 8 Suppl 1:3–63. [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience 10:561–572. [DOI] [PubMed] [Google Scholar]
- Kash TL, Winder DG (2007) NMDAR LTP and LTD induction: 2B or Not 2B...is that the question? Debates in Neuroscience 1:79–84. [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA (2016) Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 87:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi Bolin B, Alcorn JL 3rd, Lile JA, Rush CR, Rayapati AO, Hays LR, Stoops WW (2017) N-Acetylcysteine reduces cocaine-cue attentional bias and differentially alters cocaine self-administration based on dosing order. Drug Alcohol Depend 178:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148:196–200. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164:121–137. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M (2010) Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol 18:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG (2016) Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM (2014) Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs 28:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gipson CD, Carpenter MJ, Roper AP, Froeliger BE, Kalivas PW, Gray KM (2015) An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse 41:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH (2016) Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis. Nicotine Tob Res 18:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG (2000) Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. The Journal of physiology 524 Pt 1:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery DC, Peck EA, Vining GG (2012) Introduction to linear regression analysis.645. [Google Scholar]
- Moro F, Orru A, Marzo CM, Di Clemente A, Cervo L (2018) mGluR2/3 mediates short-term control of nicotine-seeking by acute systemic N-acetylcysteine. Addict Biol 23:28–40. [DOI] [PubMed] [Google Scholar]
- Olsson B, Johansson M, Gabrielsson J, Bolme P (1988) Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. European Journal of Clinical Pharmacology 34:77–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


