Recent data suggest that up to 11 percent of the United States population uses antidepressants such as Serotonin Reuptake Inhibitors (SRIs; Pratt, Brody, & Gu, 2011). This statistic includes pregnant women, 5 to 10% of whom report antidepressant use during pregnancy (Huybrechts et al., 2013; Austin, 2006; Cooper, Willy, Pont, & Ray, 2007), and this number may be increasing over time (Bérard, Boukhris, & Sheehy, 2016). SRIs are a mainstay treatment for depression and have historically been preferred by depressed pregnant women due to their relative safety profile, a view which emerged following initial reports of low risk for poor health or for congenital malformations (Harrington, Lee, Crum, Zimmerman, & Hertz-Picciotto, 2013; Lattimore et al., 2005). However, more recent research calls this view into more question, with studies suggesting potential increased risk for cardiac malformations among children exposed (Huybrechts, et al., 2014; Bérard, Zhao & Sheehy, 2017). In addition, a large cohort study exploring the association between prenatal SRIs and later child outcomes linked prenatal SRI exposure with slightly greater risk for Autism Spectrum Disorders (ASD; Croen, Grether, Yoshida, Odouli, & Hendrick, 2011), generating new concerns regarding increased risk for neurodevelopmental disorders. This initial report was followed by a plethora of studies and reviews on prenatal SRI exposures and ASD (Andrade, 2017; Freire, de Oliveira, & Pereira Pondé, 2016; Gentile, 2015; Healy, Le Noury, & Mangin, 2016; Kobayashi, Matsuyama, Takeuchi, & Ito, 2016; Mezzacappa et al., 2017; Rai et al., 2017). Similarly, recent studies have found significant associations between prenatal SRI exposure and atypical language development among toddlers and adolescents (Brown et al., 2016; Handal, Skurtveit, Roth, Hernandez-Diaz, & Selmer, 2016; Skurtveit, Selmer, Roth, Hernandez-Diaz, & Handal, 2014).
Serotonin is a critical regulator of fetal neural development, influencing many processes such as neural cell division, neuronal migration, and synaptogenesis (Harrington et al., 2013 for a review; Whitaker-Azmitia, 2001). SRIs can cross the placenta to influence the serotonin system (Rampono et al., 2009) and may also be able to influence fetal neural circuitry. While not fully understood, these and other potential mechanisms suggest that medication exposures that influence the serotonin system may also influence fetal neural development, and, in turn, postnatal behaviors.
Although meta-analytic and narrative reviews of the prenatal SRI exposure-ASD relationship tend to support a significant association (Boukhris & Bérard, 2015; Gentile, 2015; Man et al., 2015), a number of published studies fail to replicate this effect, especially when including important covariates (Castro et al., 2016; Clements et al., 2015; Harrington, Lee, Crum, Zimmerman, & Hertz-Picciotto, 2014; Hviid, Melbye, & Pasternak, 2013; Malm et al., 2016; Rai et al., 2013; Sørensen et al., 2013; Sujan et al., 2017). In addition, while the literature now contains a number of studies with substantial sample sizes, primarily due to the availability of medical record and registry databases, methodological weaknesses remain. For example, levels of prenatal SRI exposure have typically been determined on the basis of medical and pharmacy record searches (Bérard et al., 2016; Boukhris, Sheehy, & Mottron, 2015; Brown et al., 2017; Castro et al., 2016; Gidaya et al., 2014; Liu et al., 2017; Malm et al., 2016; Rai et al., 2013; Sørensen et al., 2013; Viktorin, Uher, Reichenberg, Levine, & Sandin, 2017) without independent verification of maternal medication use. Such proxies for fetal SRI exposure may not always be an accurate indicator of use, with one report suggesting that only 73% of women who filled a prescription for SRIs in pregnancy reported actually taking them (Skurtveit et al., 2014).
Similarly, many studies of fetal SRI exposure have relied solely on health or school registry data to determine ASD and language delay outcome status (Brown et al., 2016; Croen et al., 2011; Hviid et al., 2013; Malm et al., 2016; Rai et al., 2013; Sørensen et al., 2013; Viktorin et al., 2017). A large Swedish study tracking the prevalence of autism symptom phenotypes and registered ASD diagnoses over ten years found a significant linear increase in registered ASD diagnoses over time, despite a relatively stable prevalence of ASD symptoms as measured by validated parental interviews (Lundström, Reichenberg, Anckarsäter, Lichtenstein, & Gillberg, 2015). These findings suggest that registry diagnoses of ASD, which are commonly used in studies on this topic, may not always accurately reflect child symptomatology or DSM-5 criteria. Only a few prenatal SRI exposure studies in the extant literature have directly assessed children for ASD outcomes using parental reports or clinical observation (Harrington et al., 2014; Johnson, Smith, Stowe, Newport, & Brennan, 2016). The current study employs both of these assessment methods.
A major concern in the SRI exposure literature is how to best control for the potentially confounding influence of maternal depression severity during pregnancy. While some studies on maternal depression and child ASD outcomes have had mixed findings depending on which maternal assessments and comparison groups were used, psychiatric difficulties seem to be more common among parents of children with ASD (for review see Yirmiya & Shaked, 2005). More broadly, a number of studies have found associations between maternal depression and a range of poor child outcomes (Brand & Brennan, 2009; Davalos, Yadon, & Tregellas, 2012; Nulman et al., 2015). Despite this, not all SRI exposure studies have incorporated measures of maternal prenatal depression (e.g., Rai et al., 2013), while others have inferred prenatal depression status based on diagnoses from medical records (e.g., historical diagnoses of Major Depressive Disorder), which may not reflect the actual symptomatology that a mother experienced during pregnancy (Brown et al., 2017; Castro et al., 2016; Viktorin et al., 2017). Others have also used maternal retrospective reports of prenatal depression (Harrington et al., 2014), which may underestimate true exposure (Newport et al., 2008). Review of this literature suggests a need for studies that prospectively measure maternal depression severity as well as medication use in pregnancy and directly assess child social behaviors later in childhood to better understand the stability of behavioral effects. This is the primary goal of the current study.
Our research group previously found significant associations between prospectively measured prenatal SRI use and parent-reported Pervasive Developmental Disorder (PDD) symptoms (which are closely tied to ASD symptomology) during preschool (Johnson et al., 2016). We also found that prenatal SRI exposure was associated with children’s expressive language delays. Notably, few other studies on prenatal SRI use and child outcomes have focused on both social and language deficits. Most studies only assess one of these outcomes (though at least one study has assessed both outcomes during infancy; Pedersen, Henriksen & Olsen, 2010). Interestingly, a recent preclinical study found that prenatal SRI administration resulted in reduced rodent pup ultrasonic vocalizations, which are thought to reflect affective and communicative behaviors intended to elicit a maternal response (Maloney et al., 2017). However, the influence of prenatal SRI exposure on social behavior outcomes in this study was mixed, with no significant effects noted for juvenile play behavior, but rodents prenatally exposed to SRIs showed a significant decrease in preference for social stimuli in adulthood.
The current ASD literature has also largely focused on the DSM-IV conceptualization of ASD. However, in the DSM-5, ASD is separated from Social (Pragmatic) Communication Disorder, providing a classification for children who present with social communication deficits without other aspects of ASD (e.g., without restricted interests or repetitive behaviors). This revised diagnostic classification suggests that studies should examine the association between prenatal SRI exposures and both social communication problems and ASD symptoms. A secondary goal in the current study was to employ clinician-administered assessments specifically designed to measure child ASD symptoms and child social communication (pragmatic language) abilities among a subgroup of our sample.
Overall, the current study aims to address limitations in the current literature by drawing on a prospective, longitudinal sample of mother-child dyads who have been followed throughout pregnancy into early childhood. This study reports on two follow-ups of this established cohort, (1) an online questionnaire follow-up evaluating maternal and alternative caregiver report of child social behavior outcomes (N=137), and (2) an in-depth laboratory follow-up utilizing maternal report and clinician administered assessments of autism spectrum disorder behaviors as well as pragmatic language outcomes (N=44). We specifically assessed whether these outcomes associate with prospectively measured prenatal SRI exposure, while controlling for prenatal depression.
Method
Participants
The present study followed mother-child dyads from an existing longitudinal cohort of women recruited from the Emory Women’s Mental Health Program (WMHP), an outpatient treatment facility for women suffering from mental health conditions during the prenatal period. A total of 229 women with prospectively collected prenatal medication use and self-reported depression data were invited to participate in an initial follow-up study when their children were 2–5 years of age; 178 (77%) agreed to participate, thereby establishing the longitudinal cohort (Johnson et al. 2016).
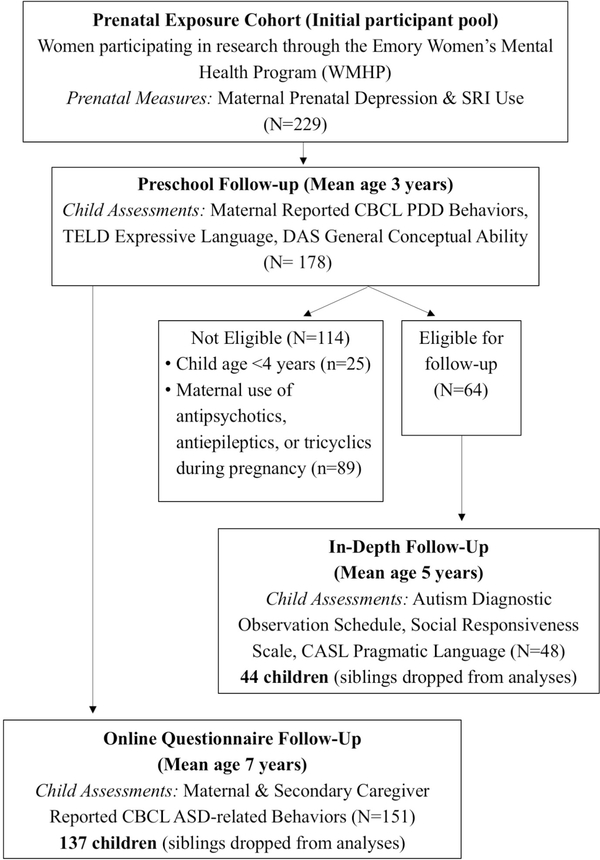
The present study reports on findings from two distinct follow-ups of this cohort (see Figure 1). One follow-up was an online questionnaire study initiated when the children were in elementary school (ages 6 and older). Mother-child dyads were eligible if they had participated in both the prenatal and preschool phases of the overarching study. A total of 151 children participated (85%). In order to maintain statistical assumptions of independence, children were excluded from the final database if their mother completed questionnaires on an older sibling in the cohort (n=14), resulting in a total of 137 children whose mother consented to the follow-up and who had online questionnaires completed by a mother and/or alternate caregiver. The majority of this sample had information from both reporters: 136 children (99%) had questionnaires filled out by the mother and 100 children (73%) had questionnaires filled out by an alternative-caregiver.
Figure 1.
Initial and follow-up visits of mother-child dyads in the current longitudinal cohort. CASL = Comprehensive Assessment of Spoken Language; CBCL PDD = Child Behavior Checklist Pervasive Developmental Disorder; CBCL ASD = Child Behavior Checklist Autism Spectrum Disorder; DAS = Differential Ability Scales; SRI = Serotonin Reuptake Inhibitor; TELD = Test of Early Language Development.
The second follow-up was an in-depth laboratory study focused on a subsample from the longitudinal cohort. This in-depth study included two comparison groups with similar levels of maternal prenatal depression severity—a group whose mothers used SRIs during pregnancy and a group whose mothers remained medication-free throughout pregnancy. To be included in this in-depth laboratory follow-up, mothers needed prospective data available from the prenatal phase of our longitudinal study that indicated either maternal SRI use or no use of any psychotropic medications during pregnancy, and subsequent participation in the preschool phase. Additional exclusion criteria included: (a) child age younger than 4 years, given that they may not yet evidence verbal fluency (n=25) and (b) maternal use of antipsychotics, antiepileptics, or tricyclic antidepressants during pregnancy (n=89). A total of the 64 children were eligible; 48 (75%) participated (age range 4–7 years). In order to maintain assumptions regarding statistical independence of observations, participants with prior participation of an older sibling were excluded from the final database (n=4), resulting in a final total of 44 mother-child dyads.
Procedure
In the online questionnaire follow-up, data were collected via a secure database called REDCap. Permission to re-contact, along with contact information, was obtained during the preschool phase of the study. Mothers received a direct hyperlink to the online measures, and were instructed to click on the link, read consent information thoroughly, and complete the online questionnaires if they agreed to study details. Participants were not required to complete every questionnaire in one sitting, and were re-contacted if measures were left incomplete for longer than two weeks. Behavioral questionnaires were also completed by an alternate caregiver (nominated by the child’s mother) using the same REDCap procedures. During the in-depth laboratory follow-up study, mothers and their children were assessed during a two-hour lab visit. Children were evaluated for ASD symptoms and pragmatic language abilities, and mothers completed standardized questionnaires about their child’s behavior and ASD-related symptomology. Test administrators and coders were blind to prenatal exposure status. Mothers and alternate caregivers were financially compensated and children received a toy for participation. The Emory University Institutional Review Board approved these studies.
Measures
Prenatal Measures
Maternal Prenatal Depression Symptoms and SRI Use.
During pregnancy, mothers were evaluated prospectively at 4–6 week intervals. Depressive symptoms were assessed using the Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996), a 21-item measure that addresses the presence/absence and severity of physical symptoms, behaviors, thoughts, and feelings that are associated with depression and were experienced in the last two weeks. BDI scores were used to calculate an area under the curve (AUC) measure of symptom levels across pregnancy, normalized to 40 weeks to account for differences in timing of delivery. Using the same methodology as in Johnson et al. (2016), information on prenatal SRI exposure (as well as any other psychotropic and/or substance-related exposures) was gathered on weekly tracking sheets verified by study physicians. The tracking sheets asked about medication use over the week, with room to specify use of one (or more) types of SRIs taken that week. Of the women who took SRIs, 75% did so throughout the entirety of their pregnancy. Overall SRI exposure was next calculated by multiplying each SRI used by the number of weeks taken, standardized to a 40-week pregnancy. The standardized drug weeks for each SRI were then added to obtain a final overall calculation of prenatal SRI exposure. The mean number of drug weeks of SRI exposure was 34.7 (SD=10.8) among the SRI group in the online follow up study and 35.4 (SD=9.2) among the SRI group in the in-depth laboratory study.
Preschool-Age Measures
Child Cognitive Ability.
During the original preschool follow-up, children were administered the Differential Ability Scales, Second Edition (DAS-II; Elliott, 2007). An overall reasoning and conceptual ability composite score, the General Conceptual Ability (GCA), was calculated as a proxy for IQ. Higher scores represent higher ability.
Maternal and Alternate Caregiver Reported PDD Behaviors.
Mothers and alternate caregivers completed the Preschool-Age Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000). The Pervasive Developmental Disorder (PDD) subscale of the CBCL assesses the occurrence of 13 child behaviors over the past two months that are considered to be risk factors for ASD, such as avoiding eye contact and not getting along with others. Higher scores indicate more PDD-related behaviors.
Child Expressive Language.
Children were administered the Expressive Language subtest from the Test of Early Language Development, Third Edition (TELD-3), a standardized measure of expressive language with high test-retest reliability and criterion validity (Hresko, Reid, & Hammill, 1999). The measure evaluates a child’s ability to answer open-ended questions and provide age-appropriate responses. Higher scores reflect greater expressive language abilities.
Online Questionnaire Follow-up Measures
Maternal and Alternate Caregiver Reported Child ASD-Related Behaviors.
ASD behaviors were measured using the School-Age Child Behavior Checklist (CBCL), which was completed by the child’s mother and an alternate caregiver (Achenbach, 2001). In our sample, alternate caregivers included fathers (74%), grandparents (15%), teachers and day-care providers (7%), as well as other relatives (4%). T-scores on the Withdrawn, Social Problems and Thought Problems subscales were summed to create a CBCL ASD-related behavior score for each child. This score has been shown to discriminate between children with and without ASD, particularly among high functioning youth (Biederman et al., 2010). Mother and alternate caregiver scores on child ASD-related behaviors were moderately correlated in this sample (r=0.43).
In-depth Laboratory Follow-up Measures
Clinician Assessed ASD-Related Behaviors.
Children were administered the Autism Diagnostic Observation Schedule (ADOS), a semi-structured assessment of communication, social interaction, and play with demonstrated interrater and test-retest reliability as well as criterion validity (Lord et al., 2000). Children in the sample met basic verbal fluency (i.e., produced a range of sentence types, were able to provide information about events), and were administered Module 3 of the ADOS. The ADOS was administered by one of three trained clinical psychology graduate students, and was simultaneously observed and co-coded by another trained student. Following administration, both coders scored independently, and then met to determine a consensus rating. Each task loaded onto one of three domains: Communication (e.g., conversation, reporting of events), Reciprocal Social Interaction (e.g., quality of social response, overall quality of rapport), and Restricted and Repetitive Behaviors (e.g., compulsions or rituals). The goal of the current study was to assess the full range of ASDrelated symptoms. Therefore, the total ADOS score was calculated by summing the three domain scores. Higher scores represent more ASD symptomatology.
Quantitative Measure of Autistic Traits.
Mothers completed the Social Responsiveness Scale (SRS; Constantino & Gruber, 2007) about their child. The SRS is a 65-item scale that evaluates the severity of ASD symptoms occurring in day-to-day scenarios. Previous studies note high interrater and test-retest reliability, discriminant validity, and a single factor structure (Bölte, Poustka, & Constantino, 2008). The overall standard score was used. Higher scores reflect more ASD symptoms.
Child Pragmatic Language.
Child pragmatic language was assessed using the Pragmatic Judgment test from the Comprehensive Assessment of Spoken Language (CASL; Carrow-Woolfolk, 1999). This test has been shown to have high test-retest reliability and appropriate criterion-related and construct validity (Reichow, Salamack, Paul, Volkmar, & Klin, 2008). The test is comprised of 60 open-ended questions where an examiner asks the child to describe what s/he would do in social scenarios (e.g., “What can a child say to her mother after spilling milk at the table?”). The examiner scores each response on a scale of 0 to 2 based on norm-referenced criteria. The Pragmatic Judgment test standard score was used, with higher scores reflecting more advanced language ability.
Statistical Analyses and Identification of Covariates
Characteristics of dyads who did and did not participate in the current follow-up studies are presented in Table 1. There were no statistically significant differences in depression between those who did and did not participate in the follow-up studies (see Table 1). In the online questionnaire sample, reported prenatal depression symptoms were higher for mothers who took SRIs in comparison to those who did not take any psychotropic medications, though both groups reported a range of maternal depression levels (see Table 2). However, in the in-depth study, depression was not significantly different among those who did or did not take SRIs (Table 2). Of note, there were no differences between the original pregnancy cohort and those who participated in the preschool follow-up; (t=−0.99, p=0.32). Because a major concern in the literature is controlling the confounding effects of maternal prenatal depression, we included this variable as a covariate in all analyses. In addition, child IQ was included as a covariate in all analyses in order to assess the impact of prenatal SRI use over and above any impact of global intellectual functioning. Additional variables that were evaluated as potential covariates included maternal, paternal, and child age at the time of follow-up, child sex and race, mother’s highest level of education and marital status, number of children living in the home, child daycare attendance, and birth record data on gestational age, birthweight, delivery complications, and Apgar scores. Prenatal exposures to alcohol, tobacco, hypnotics, anxiolytics (and in the questionnaire follow-up, antipsychotics and antiepileptics) were also tested as potential covariates, as were postnatal exposures to maternal psychiatric illness, psychotropic medication, and breastfeeding. The relationship between potential covariates and the outcomes of interest (ASD-related behaviors and pragmatic language) were examined via t-tests or Pearson correlations. Any variables that were associated with study outcomes were included as covariates in the relevant analyses. Child age was positively associated with maternal report of CBCL ASD-related behaviors in the online questionnaire follow-up (r(136)=0.21, p=0.02). Child race and Apgar scores were associated with ADOS scores, such that Caucasian children, on average, had higher ADOS scores than children of other races (t(42)=2.28, p=0.03), and lower Apgar scores were associated with higher ADOS scores (r(43)=−0.34, p=0.03). Therefore, all analyses with CBCL mother-reported ASD-related behavior scores as the dependent variable included child age as a covariate while all analyses with ADOS score as the dependent variable included child race and Apgar scores as covariates. These potential covariates were not significantly associated with CBCL alternate caregiver-reported ASD-related behaviors, CASL pragmatic language scores, or SRS scores.
Table 1.
Differences between dyads who did or did not participate in the current follow-up studies.
| Online Questionnaire Study | Participated (n=137) | Eligible but did not Participate (n=23) | Not Eligible (n=18) |
|---|---|---|---|
| Child gender, n (% out of 100) | 76 (55.5) Male | 10 (43.5) | 8 (44.4) |
| Child race, n (%) | 108 (78.8) Caucasian | 19 (82.6) | 16 (88.9) |
| Child IQ, Mean (SD) | 105.4 (14.3) | 104.5 (10.3) | 104.7 (12.6) |
| Children in residence, Mean (SD) | 2.1 (0.9) | 2.4 (1.2) | 2.5 (0.9) |
| EGA at delivery, Mean (SD) | 38.8 (1.5) Weeks | 39.0 (1.6) | 38.5 (0.9) |
| Marital status, n (%) | 115 (85.2) Married | 18 (78.3) | 17 (94.4) |
| Maternal age in years, Mean (SD) | 37.3 (4.6) | 36.0 (6.3) | 38.3 (2.8) |
| Prenatal depression AUC, Mean (SD) | 411.6 (311.2) | 509.3 (387.0) | 359.5 (363.8) |
| Maternal PDD t-score, Mean (SD) | 54.4 (6.7) | 54.6 (6.5) | 52.3 (5.4) |
| Alternate PDD t-score, Mean (SD) | 53.4 (6.1) | 55.7 (7.6) | 53.7 (6.4) |
| Child expressive language, Mean (SD) | 106.5 (13.9) | 103.4 (14.7) | 106.9(10.7) |
| In-Depth Laboratory Study | Participated (n=44) | Eligible but did not Participate (n=14) | Not Eligible (n=120) |
| Child gender, n (% out of 100) | 19 (43.2) Male | 10 (71.4) | 63 (52.5) |
| Child race, n (%) | 35 (79.5) Caucasian | 12 (85.7) | 101 (84.2) |
| Child IQ, Mean (SD) | 108.3 (13.3) | 104.6 (12.9) | 104.2 (13.8) |
| Children in residence, Mean (SD) | 2.1 (0.9) | 2.4 (1.1) | 2.1 (.9) |
| EGA at delivery, Mean (SD) | 39.1 (1.4) Weeks | 38.6 (1.6) | 38.8 (1.4) |
| Marital status, n (%) | 38 (86.4) Married | 14 (100) | 98 (83.1) |
| Maternal age in years, Mean (SD) | 38.4 (4.7) | 38.2 (4.1) | 36.7 (4.8) |
| Prenatal depression AUC, Mean (SD) | 394.6 (274.2) | 404.4 (283.2) | 429.7 (352.4) |
| Maternal PDD t-score, Mean (SD) | 53.7 (5.6) | 55.3 (6.0) | 54.2 (7.0) |
| Alternate PDD t-score, Mean (SD) | 52.3 (4.8) | 51.9 (2.6) | 54.4 (6.9) |
| Child expressive language, Mean (SD) | 111.2 (13.5)* | 96.9 (10.6) | 105.4(13.5) |
Note:
p<0.05 difference between referent and comparison groups.
EGA = estimated gestational age. Maternal age represents the age at the indicated follow-up. Prenatal depression AUC = depression scores across pregnancy calculated as the area under the curve from the Beck Depression Inventory assessed multiple times throughout pregnancy. Maternal PDD, Alternate Caregiver PDD and child expressive language were assessed at the pre-school aged follow-up. PDD = maternal or caregiver-reported child pervasive developmental disorder as measured by the Child Behavior Checklist (CBCL).
Table 2.
Differences between dyads who were and were not prenatally exposed to SRIs in the current follow-up studies.
| Online Questionnaire Study | SRI Exposed (n=75) | Unexposed (n=62) | Between-Group Differences |
|---|---|---|---|
| Child age in years, Mean (SD) | 7.4 (1.2) | 7.2 (1.3) | t(135) = −1.0 |
| Child gender, n (% out of 100) | 37 (49.3) Male | 39 (62.9) | Χ2(1,137) = 2.5 |
| Child race, n (%) | 57 (76.0) Caucasian | 51 (82.3) | Χ2(1,127) = 0.8 |
| Child IQ, Mean (SD) | 105.9 (15.1) | 104.9 (13.3) | t(135) = −0.4 |
| Child Apgar scores Mean (SD) | 8.8(0.5) | 8.9(0.5) | t(135)=0.7 |
| Children in residence, Mean (SD) | 1.9 (0.3) | 2.0 (0.2) | t(135) = 0.7 |
| No. of delivery complications, Mean (SD) | 0.9 (0.9) | 0.6 (1.0) | t(135) = −1.7 |
| EGA at delivery, Mean (SD) | 38.8 weeks (1.4) | 38.9 (1.6) | t(135) = 0.4 |
| Maternal Marital status, n (%) | 59 (78.7) Married | 53 (85.5) | Χ2(1, 137) = 0.8 |
| Maternal age in years, Mean (SD) | 41.8 (4.7) | 40.2 (4.7) | t(135) = −1.9 |
| Prenatal depression AUC, Mean (SD) | 465.1(342.6) | 340.9(254.9) | t(135)=−2.3* |
| In-Depth Laboratory Study | SRI Exposed (n=27) | Unexposed (n=17) | Between-Group Differences |
| Child age in years, Mean (SD) | 5.6 (1.0) | 5.0 (0.9) | t(42) = −1.8 |
| Child gender, n (% out of 100) | 10 (37.0) Male | 9 (52.9) | Χ2(1,44) = 1.1 |
| Child race, n (%) | 22 (81.5) Caucasian | 12 (70.6) | Χ2(1,44) = 0.4 |
| Child IQ, Mean (SD) | 108.5 (14.5) | 107.8 (11.6) | t(42) = −0.2 |
| Child Apgar scores Mean (SD) | 8.9 (0.3) | 8.94 (0.3) | t(42)=0.5 |
| Children in residence, Mean (SD) | 2.2 (1.0) | 2.0 (0.8) | t(42) = −0.7 |
| No. of delivery complications, Mean (SD) | 0.9 (0.8) | 1.1 (1.1) | t(42) = 0.7 |
| EGA at delivery, Mean (SD) | 38.9 weeks (1.1) | 39.4 (1.7) | t(42) = 1.0 |
| Maternal Marital status, n (%) | 24 (88.9) Married | 14 (82.4) | Χ2(1,44) = 0.4 |
| Maternal age in years, Mean (SD) | 40.1 (4.2) | 38.7 (5.4) | t(42) = −1.5 |
| Paternal age in years, Mean (SD) | 41.5 (5.7) | 40.2 (6.1) | t(42) = −0.8 |
| Prenatal depression AUC, Mean (SD) | 432.4 (290.3) | 330.7(239.8) | t(42)=−1.2 |
Note:
p<0.05 difference between referent and comparison groups.
EGA = estimated gestational age. Child, maternal, and paternal age represents the age at the indicated follow-up. Prenatal depression AUC = depression scores across pregnancy calculated as the area under the curve from the Beck Depression Inventory assessed multiple times throughout pregnancy.
In the online questionnaire sample, linear regression analyses were used to assess whether prenatal SRI exposure predicted child CBCL ASD-related behaviors per maternal and caregiver report, while controlling for covariates. The maternal and alternative caregiver ASDrelated behavior scores were log transformed prior to analyses to reduce skewness and kurtosis. In the smaller in-depth follow-up, linear regressions were used to explore whether SRI exposure predicted to the ADOS and SRS assessments of ASD symptomology as well as to pragmatic language, while controlling for covariates. Further, linear regressions were used to evaluate whether prenatal SRI exposure predicted to ADOS and SRS while controlling for pragmatic language, in order to examine whether any relationship between prenatal SRI use and ASD symptomology held after discounting variance explained by child pragmatic language. In all analyses, missing data were handled through listwise deletion. With the exception of alternative caregiver report of CBCL ASD-related behaviors (27% missing), missing data were minimal for all other variables.
Results
Preliminary analyses
For the online questionnaire follow-up, no differences emerged between mothers and children who (a) were not eligible for the follow-up, (b) were eligible but did not participate, and (c) were eligible and did participate. For the in-depth laboratory follow-up, children who were eligible and participated had higher expressive language scores than children in the other groups; no other group differences were statistically significant (see Table 1).
Among children who were or were not exposed to SRIs, there were no differences on any of the participant demographics examined (see Table 2). As noted above, SRI exposure was significantly associated with higher prenatal maternal depression scores in the questionnaire follow-up, but not in the in-depth laboratory study (Table 2). In both follow-ups, Zoloft (Sertraline) and Prozac (Fluoxetine) were the most common SRIs used during pregnancy.
Prenatal SRI Exposure and Child ASD-Related Social Behaviors
Results of linear regression analyses assessing the separate associations between SRI use in pregnancy and ASD-related outcomes are presented in Table 3. In the online questionnaire follow-up, prenatal SRI exposure was associated with child CBCL ASD-related behaviors per maternal report, but not per alternate caregiver report. Prior work suggests that sex may moderate associations between prenatal exposures and child outcomes (Swales et al., 2018), and that SRI-ASD associations may be stronger among males (Harrington et al., 2014). Therefore, post hoc analyses exploring sex as a moderator of associations were conducted. These analyses revealed that SRI exposure predicted alternate caregiver report of ASD-related behaviors among male children, but not among females (overall SRI-sex multiplicative interaction term: R2 change=0.07 β=0.26, 95% CI [0.09, 0.44], p=0.01; SRI exposure effect for males only: R2 change=0.09, β=0.28, 95% CI [0.02, 0.55], p=0.04; SRI exposure effect for females only: R2 change=0.03, β=−0.21, 95% CI [−0.63, 0.08], p=0.15). SRI exposure did not interact with child sex to predict maternal report of ASD-related behaviors (overall SRI-sex multiplicative interaction term: R2 change=0.001, β=0.04, 95% CI [−0.10, 0.21], p=0.66).
Table 3.
Prenatal SRI exposure and child ASD and language outcomes in the current follow-up studies.
| Dependent Variable | Mean, SD, Range | R2 Change | β | 95% C.I. β | p- Value |
|---|---|---|---|---|---|
| Mother-reported Child ASD-related behaviors | 166.10, 17.62, 150–240 | 0.06 | 0.24 | 0.07 to 0.48 | 0.01 |
| Alternate Caregiverreported Child ASD-related behaviors | 166.02, 17.18, 150–227 | <0.01 | 0.00 | −0.15 to 0.26 | 0.64 |
| Mother-reported SRS | 49.18, 9.56, 35–90 | <0.01 | 0.08 | −0.25 to 0.40 | 0.61 |
| The Autism Diagnostic Observation Schedule (ADOS) | 5.64, 4.50, 0–17 | 0.02 | 0.14 | −0.15 to 0.41 | 0.31 |
| CASL Pragmatic Language Score | 114.36, 14.12, 81–145 | 0.07 | −0.27 | −0.53 to −0.01 | 0.04 |
Note: All analyses controlled for maternal prenatal depression and child IQ. Mother-reported ASD-related behavior analyses also controlled for child age, and ADOS analyses controlled for child race and Apgar scores. ASD = autism spectrum disorder. SRS = social responsiveness scale. CASL = Comprehensive Assessment of Spoken Language. Child ASD-related behaviors were measured by the CBCL. Missing data were minimal except for the alternative caregiverreported ASD-related behaviors (27% missing), and were handled by listwise deletion. In analyses, CASL was a standardized score, ADOS was the sum total of symptoms, SRS was the sum total of symptoms, and ASD-related behaviors were the sum total of t scores of 3 subscales from the CBCL. Both the maternal and alternative caregiver-reported ASD-related behavior scores were log transformed prior to analyses to reduce skewness and kurtosis.
In the smaller in-depth laboratory study, prenatal SRI exposure did not predict clinician-evaluated child ASD symptoms on the ADOS or maternal-reported symptoms on the SRS (see Table 3). Given the smaller sample in the in-depth laboratory study, child sex was not examined as a moderator of outcomes on the ADOS or SRS.
Prenatal SRI Exposure and Child Pragmatic Language
Prenatal SRI exposure was negatively associated with child pragmatic language during the in-depth follow-up (see Table 3). Moreover, prenatal exposure to SRIs accounted for 7.1 percent of the variance associated with language scores, even after controlling for child cognitive ability and maternal prenatal depression.
Disentangling Child Pragmatic Communication and ASD-Related Behaviors
Pragmatic language and total ADOS scores were significantly correlated in this sample (r=−0.55). Because problems with language may negatively impact social interactions, lower pragmatic language scores may contribute to elevated ADOS scores. While the initial SRI exposure and ADOS model was not statistically significant in our sample, we re-tested the model controlling for pragmatic language to assess for any change in variance. In this model, the amount of variance that SRI exposure accounted for in child ADOS scores dropped from 1.8 percent to 0.001 percent. Similarly, the amount of variance in SRS scores explained by SRI exposure dropped from 0.5 to 0.1 percent when pragmatic language was included as a covariate in the model. In this sample, prenatal SRI exposure explains little to no variance in ADOS and SRS scores once pragmatic language deficits are controlled.
Discussion
Recent studies suggest an association between prenatal SRI exposure and increased risk for ASD (Gentile, 2015; Man et al., 2015), though findings remain mixed. Previously, our group noted an association between prenatal SRI exposure and higher scores on caregiver-reported child ASD-related behaviors during preschool age (Johnson et al., 2016). These results, combined with those in our current school-age questionnaire follow-up, suggest that associations between prenatal SRI exposure and caregiver ratings of children’s ASD-related behaviors are relatively persistent across early childhood and across raters, particularly for boys.
In our in-depth study, however, there was not a significant association between prenatal SRI exposure and ASD symptoms according to gold-standard clinician assessments or parent report as measured by the ADOS and SRS, respectively. Sample size in this study was small, and inherently limited, as the sample was drawn from those eligible in an established longitudinal cohort of mother-child dyads. It is possible that a larger sample may have detected an association, especially since the effect sizes in large population-based studies to date have been relatively small (for reviews see Man et al., 2015; Gentile, 2015). However, the association between prenatal SRI exposure and maternal report of CBCL ASD-related behaviors from the online questionnaire follow-up remained significant when tested among the smaller in-depth study subsample (R2 change=0.13, β=0.42, 95% CI [0.04, 0.60], p<0.01), suggesting that power may not be the only factor at play in the null findings from the in-depth assessment. Scores from three subscales on the CBCL (Social Problems, Thought Problems, and Withdrawn/Depressed) were combined to create the measure of maternal-reported ASD-related behaviors for the online questionnaire study. These subscales capture behaviors that are often associated with ASD, but are not necessarily unique to the disorder (for instance, sleep problems can be present in children with ASD, anxiety, depression, or attention deficit/hyperactivity disorder). Other items from these subscales include the presence of obsessions, compulsions, general difficulty getting along with peers (e.g., being bullied), and self-injurious behaviors. Again, such symptoms can be present among children with ASD, but are not part of the diagnostic criteria or exclusively present in ASD. This is of importance as there is also a growing literature on prenatal SRIs and other behavioral outcomes, such as internalizing behaviors (Hanley, Brain, & Oberlander, 2015) and anxious/depressive behaviors (Lupattelli et al., 2018). In our in-depth study, the SRS, a measure of parent-reported ASD symptoms, more closely mirrors diagnostic criteria for ASD, including symptoms such as restricted interests, poor eye contact, and sensory sensitivity. These symptoms are not included on the school-age version of the CBCL. Therefore, an alternative explanation could be that ASD-related behavior scores for children in the questionnaire followup (which are based on the CBCL measure) better reflect atypical social behaviors and not ASD per se.
If this alternative explanation is correct, it highlights the need for gold-standard assessments of both ASD and language, as well as other socially-related behaviors, in order to avoid misdiagnosis. Research conducted at the Marcus Autism Center has found that 35% of children previously diagnosed with ASD by a community care provider did not meet criteria for this diagnosis when they were re-assessed by clinicians using gold-standard measures including the ADOS and a developmental/IQ assessment. When re-assessed, these children received a different diagnosis such as a behavior disorder or language delay (Hall & Hamel, 2012). Further, other studies have suggested that autism symptoms may not explicitly track with registered ASD diagnoses. Specifically, the number of registered ASD diagnoses appears to be increasing over time, even though the prevalence of ASD symptoms as measured by validated parental interviews was found to be stable (Lundström et al., 2015). These findings, in addition to the findings from the present study, hold important implications for research, and particularly for research that relies solely upon registry-based diagnoses. An integral step in this field will be the use of direct, clinical assessments to provide more thorough, standardized evaluations and diagnoses.
Our lab previously found an association between SRI exposure and reduced expressive language at preschool age (Johnson et al., 2016). The current follow-up of this cohort at early school-age also suggests an association between SRI exposure and language problems, with lower overall pragmatic language scores among those with SRI exposure. No prior studies have examined pragmatic language as a specific outcome following prenatal SRI exposure. Given a growing literature on prenatal SRIs and language concerns (Brown et al., 2016; Johnson et al., 2016; Skurtveit et al., 2014), the overlap between pragmatic language deficits and ASD, and the current study’s findings of an association between prenatal SRI use and pragmatic language ability, it would be interesting to explore whether prior ASD findings in the literature have been partly due to specific effects of SRI exposure on child pragmatic language. Although preliminary, the current findings could further support the utility of separate classifications of ASD and Social (Pragmatic) Communication Disorder in the DSM-5, if they replicate in independent samples. Studies that conduct assessments of ASD and language in larger cohorts are necessary to further tease apart the potential risks of prenatal SRI exposure on these related domains of functioning.
Although SRIs explained a moderate amount of variance in pragmatic language scores, it is important to note that language scores among those with SRI exposure generally fell within the low-normal range in our sample, and those in the current sample had a verbal fluency level that allowed them to complete module 3 of the ADOS. It is possible that this study’s cohort, which was of fairly high socioeconomic status and primarily from intact families, may have relatively high language exposure or social support, both of which may offer protective benefit against prenatal SRI exposure. Children with SRI exposure who are raised in more stressful or less supportive contexts may be at greater risk for below average pragmatic language ability. Further work is needed to explore this possibility.
Few studies to date have explored sex differences in SRI-ASD outcomes, though the potential for such differences has been suggested. Specifically, Harrington et al. (2014) found that prenatal SRI exposure was more strongly tied to ASD among male children, rather than all children combined, and our study also suggests the potential for more robust findings among males. Sex differences have been reported for other studies on prenatal SRI exposure and child outcomes (Pedersen et al., 2010) and serotonin itself is influenced by sex hormones (Bethea, Pecins-Thompson, Schutzer, Gundlah & Lu, 1998; Bethea, Lu, Gundlah & Streicher, 2002). Further, Harrington et al. (2014) is one of the few prior studies to also utilize parent and child assessments to confirm child ASD diagnoses, compared to registry databases. It may be useful for future studies to further explore whether sex may moderate associations between prenatal SRI exposure and child outcomes.
Nearly 75 percent of the women who took SRIs in our sample did so consistently throughout their pregnancy, limiting our ability to examine whether exposure timing is differentially related to offspring outcomes. Future research on timing may further inform clinical decision-making or point to potential risk mechanisms. For example, some studies suggest a stronger ASD association when SRI exposure occurs during the first trimester (Croen et al., 2011; Harrington et al., 2014), while others suggest that later exposure may associate with increased risk of other child behavioral outcomes such as anxious/depressive behaviors (Lupattelli et al., 2018). In the first weeks of development, fetal serotonin is thought to be of maternal origin and can be transferred across the placenta. Further, serotonin transporters, the site of action for SRIs, are not thought to appear in the fetal brain until after the first trimester (Anderson, 2002; Harrington et al., 2013). Therefore, early trimester effects may point to a potential role of maternal or placental changes in serotonin levels, while later effects may be due to more direct effects of SRIs on fetal neural circuitry. It is also possible that differences in the expression of serotonin transporters or serotonin receptors moderate the impact of SRI exposure.
Strengths of this study include its prospective, longitudinal design and use of standardized, thorough child assessments, filling an important gap in a field that is largely dominated by studies with substantial sample sizes, but are limited to medical and registry data (e.g., El Marroun et al., 2014; Liu et al., 2017). Additionally, our maternal prenatal depression measures were collected prospectively, allowing for a more accurate assessment of SRI exposure above and beyond maternal depressive symptomatology. However, our sample size limited our ability to assess potential interactions between SRI exposure and maternal depression exposure; it may be that children at the greatest risk are those who are exposed to both SRIs and continued high levels of maternal depression. Parsing this out will be important for improved clinical decision making. Further, there is concern that those with severe depression may be less likely to participate or continue in research. Although the difference was not statistically significant, prenatal depression scores appear to be slightly higher among those who were eligible, but did not participate in the online questionnaire follow-up study. Concern and attention to the representativeness of women and children followed in longitudinal studies will continue to be important in research exploring the impact of depression and medication use on later outcomes.
When left untreated, maternal prenatal depression has been associated with a number of adverse child outcomes including behavioral problems and altered stress regulation (Brand & Brennan, 2009; Brennan et al., 2000; Davalos, Yadon, & Tregellas, 2012; O’Donnell et al., 2013; Grigoriadis et al., 2013; Nulman et al., 2015). Therefore, treatment decisions are multifaceted and require weighing a number of differential risks. Recent literature suggests an association between prenatal SRIs and slight increased risk for ASD and language delays, and the current study preliminarily suggests a specific risk for child pragmatic language deficits. Studies that can incorporate and simultaneously assess language and ASD symptoms directly in larger cohorts are an important next step to allow for a thorough assessment of the potential risks, and benefits, of prenatal SRI use on maternal health and child outcomes.
Acknowledgements:
This research was supported by NIH (P50ES026071, R01MD009746 and RC1MH088609). Author E.L.S has received support from grant R01 MD009746 and T32 GM008169. Author C.L.H is supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1444932.
References
- Achenbach T (2001). Child Behavior Checklist for Ages 6–18. Burlington, VT: ASEBA, University of Vermont. [Google Scholar]
- Achenbach T, & Rescorla L (2000). Child Behavior Checklist for Ages 1 1/2–5. Burlington, V.T.: ASEBA, University of Vermont. [Google Scholar]
- Anderson GM (2002). Genetics of childhood disorders: XLV. Autism, part 4: serotonin in autism. Journal of the American Academy of Child {&} Adolescent Psychiatry, 41(12), 1513–1516. [DOI] [PubMed] [Google Scholar]
- Andrade C (2017). Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: Do the new studies add anything new? The Journal of Clinical Psychiatry, 78(8), e1052–e1056. 10.4088/JCP.17f11916 [DOI] [PubMed] [Google Scholar]
- Austin MP (2006). To treat or not to treat: maternal depression, SSRI use in pregnancy and adverse neonatal effects. Psychological Medicine, 36(12), 1663–1670. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, & Brown G (1996). Beck Depression Inventory-II. San Antonio. [Google Scholar]
- Bérard A, Boukhris T, & Sheehy O (2016). Selective serotonin reuptake inhibitors and autism: additional data on the Quebec Pregnancy/Birth Cohort. American Journal of Obstetrics and Gynecology, 215(6), 803–805. 10.1016/j.ajog.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Bérard A, Zhao JP, & Sheehy O (2017). Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec Pregnancy Cohort. BMJ open, 7(1), e013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, & Streicher JM (2002). Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in neuroendocrinology, 23(1), 41–100. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah CLZN, & Lu ZN (1998). Ovarian steroids and serotonin neural function. Molecular neurobiology, 18(2), 87–123. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Wozniak J, Micco JA, Henin A, … Faraone SV (2010). Child behavior checklist clinical scales discriminate referred youth with autism spectrum disorder: A preliminary study. Journal of Developmental & Behavioral Pediatrics, 31(6), 485–490. 10.1097/DBP.0b013e3181e56ddd [DOI] [PubMed] [Google Scholar]
- Bölte S, Poustka F, & Constantino JN (2008). Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Research, 1(6), 354–363. 10.1002/aur.49 [DOI] [PubMed] [Google Scholar]
- Boukhris T, & Bérard A (2015). Selective serotonin reuptake inhibitor use during pregnancy and the risk of autism spectrum disorders: a review. Journal of Pediatric Genetics, 4(2), 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, & Mottron L (2015). Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA pediatrics, 170(2), 117–124. [DOI] [PubMed] [Google Scholar]
- Brand SR, & Brennan PA (2009). Impact of antenatal and postpartum maternal mental illness: How are the children? Clinical Obstetrics and Gynecology, 52(3), 441–455. 10.1097/GRF.0b013e3181b52930 [DOI] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, & Williams GM (2000). Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Developmental Psychology, 36(6), 759–766. [DOI] [PubMed] [Google Scholar]
- Brown AS, Gyllenberg D, Malm H, McKeague IW, Hinkka-Yli-Salomäki S, Artama M, … Sourander A (2016). Association of Selective Serotonin Reuptake Inhibitor Exposure During Pregnancy With Speech, Scholastic, and Motor Disorders in Offspring. JAMA Psychiatry, 73(11), 1163–1170. 10.1001/jamapsychiatry.2016.2594 [DOI] [PubMed] [Google Scholar]
- Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, & Vigod SN (2017). Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA, 317(15), 1544–1552. 10.1001/jama.2017.3415 [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E (1999). CASL: Comprehensive assessment of spoken language. American Guidance Services. [Google Scholar]
- Castro VM, Kong SW, Clements CC, Brady R, Kaimal a J., Doyle a E., … Perlis RH (2016). Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Translational Psychiatry, 6(1), e708 10.1038/tp.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, … Perlis RH (2015). Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Molecular Psychiatry, 20(6), 727–734. 10.1038/mp.2014.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2007). Social responsiveness scale (SRS).
- Cooper WO, Willy ME, Pont SJ, & Ray WA (2007). Increasing use of antidepressants in pregnancy. American Journal of Obstetrics and Gynecology, 196(6), 544.e1–5. 10.1016/j.ajog.2007.01.033 [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, & Hendrick V (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Archives of General Psychiatry, 68(11), 1104–1112. 10.1001/archgenpsychiatry.2011.73 [DOI] [PubMed] [Google Scholar]
- Davalos DB, Yadon CA, & Tregellas HC (2012). Untreated prenatal maternal depression and the potential risks to offspring: a review. Archives of Women’s Mental Health, 15(1), 1–14. 10.1007/s00737-011-0251-1 [DOI] [PubMed] [Google Scholar]
- El Marroun H, White TJH, van der Knaap NJF, Homberg JR, Fernández G, Schoemaker NK, … Tiemeier H (2014). Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. The British Journal of Psychiatry : The Journal of Mental Science, 205(2), 95–102. 10.1192/bjp.bp.113.127746 [DOI] [PubMed] [Google Scholar]
- Freire C, de Oliveira CCM, & Pereira Pondé M (2016). Antidepressants in pregnancy and autism spectrum disorder: A systematic review. Journal of Psychiatry, 19, 392 10.4172/2378-5756.1000392 [DOI] [Google Scholar]
- Gentile S (2015). Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods? Journal of Affective Disorders, 182, 132–137. 10.1016/j.jad.2015.04.048 [DOI] [PubMed] [Google Scholar]
- Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, & Newschaffer CJ (2014). In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2558–2567. 10.1007/s10803-014-2128-4 [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, ... & Martinovic J (2013). The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. The Journal of clinical psychiatry, 74(4), e321–41. 10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- Hall C, & Hamel J (2012). The role of comprehensive evaluation in the differential diagnosis of autism in a clinic setting. In International Meeting for Autism Research Toronto, Ontario. [Google Scholar]
- Handal M, Skurtveit S, Roth C, Hernandez-Diaz S, & Selmer R (2016). Prenatal exposure to folic acid and antidepressants and language development: A population-based cohort study. Journal of Clinical Psychopharmacology, 36(4), 333–339. 10.1097/JCP.0000000000000519 [DOI] [PubMed] [Google Scholar]
- Hanley GE, Brain U, & Oberlander TF (2015) Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatric research, 78(2):174–80. doi: 10.1038/pr.2015.77 [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee L-C, Crum RM, Zimmerman AW, & Hertz-Picciotto I (2013). Serotonin hypothesis of autism: Implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Research, 6(3), 149–168. 10.1002/aur.1288 [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee L-C, Crum RM, Zimmerman AW, & Hertz-Picciotto I (2014). Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics, 133(5), e1241–e1248. 10.1542/peds.2013-3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D, Le Noury J, & Mangin D (2016). Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: A systematic review of epidemiological and physiological evidence. International Journal of Risk & Safety in Medicine, 28(3), 125–141. 10.3233/JRS-160726 [DOI] [PubMed] [Google Scholar]
- Hresko WP, Reid DK, & Hammill DD (1999, April). Test of Early Language Development.
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, ... & Hernández-Díaz S (2014). Antidepressant use in pregnancy and the risk of cardiac defects. New England Journal of Medicine, 370(25), 2397–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Palmsten K, Mogun H, Kowal M, Avorn J, Setoguchi-Iwata S, & Hernández-Díaz S (2013). National trends in antidepressant medication treatment among publicly insured pregnant women. General hospital psychiatry, 35(3), 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid A, Melbye M, & Pasternak B (2013). Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. New England Journal of Medicine, 369(25), 2406–2415. 10.1056/NEJMoa1301449 [DOI] [PubMed] [Google Scholar]
- Johnson KC, Smith AK, Stowe ZN, Newport DJ, & Brennan PA (2016). Preschool outcomes following prenatal serotonin reuptake inhibitor exposure: differences in language and behavior, but not cognitive function. The Journal of Clinical Psychiatry, 77(2), e176–82. 10.4088/JCP.14m09348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsuyama T, Takeuchi M, & Ito S (2016). Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: A systematic review and metaanalysis. Reproductive Toxicology, 65, 170–178. 10.1016/j.reprotox.2016.07.016 [DOI] [PubMed] [Google Scholar]
- Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, & Vazquez DM (2005). Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: A meta-analysis. Journal of Perinatology, 25(9), 595–604. 10.1038/sj.jp.7211352 [DOI] [PubMed] [Google Scholar]
- Liu X, Agerbo E, Ingstrup KG, Musliner K, Meltzer-Brody S, Bergink V, & Munk-Olsen T (2017). Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ, 358, j3668 10.1136/BMJ.J3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, & Gillberg C (2015). Autism phenotype versus registered diagnosis in Swedish children: Prevalence trends over 10 years in general population samples. BMJ, 350, h1961 10.1136/BMJ.H1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, & Nordeng H (2018). Effect of Time-Dependent Selective Serotonin Reuptake Inhibitor Antidepressants During Pregnancy on Behavioral, Emotional, and Social Development in Preschool-Aged Children. Journal of the American Academy of Child & Adolescent Psychiatry, 57(3), 200–208. doi: 10.1016/j.jaac.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, ... & Sourander A (2016). Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. Journal of the American Academy of Child & Adolescent Psychiatry, 55(5), 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney SE, Rahn R, Akula S, Rieger MA, McCullough KB, Jakes C, … Dougherty JD (2017). Maternal SSRI treatment during offspring development results in long-term behavioral, cellular, and neuroimaging disruptions. bioRxiv, 205708 10.1101/205708 [DOI] [Google Scholar]
- Man KKC, Tong HHY, Wong LYL, Chan EW, Simonoff E, & Wong ICK (2015). Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neuroscience and Biobehavioral Reviews, 49, 82–89. 10.1016/j.neubiorev.2014.11.020 [DOI] [PubMed] [Google Scholar]
- Mezzacappa A, Lasica P-A, Gianfagna F, Cazas O, Hardy P, Falissard B, … Gressier F (2017). Risk for autism spectrum disorders according to period of of prenatal antidepressant exposure: A systematic review and meta-analysis. JAMA Pediatrics, 171(6), 555–563. 10.1001/jamapediatrics.2017.0124 [DOI] [PubMed] [Google Scholar]
- Newport D, Brennan P, Green P, Ilardi D, Whitfield T, Morris N, … Stowe Z (2008). Maternal depression and medication exposure during pregnancy: comparison of maternal retrospective recall to prospective documentation. BJOG: An International Journal of Obstetrics and Gynaecology, 115(6), 681–688. 10.1111/j.1471-0528.2008.01701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Koren G, Rovet J, Barrera M, Streiner DL, & Feldman BM (2015). Neurodevelopment of children prenatally exposed to selective reuptake inhibitor antidepressants: Toronto sibling study. The Journal of clinical psychiatry, 76(7), e842–7. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, & O’Connor TG (2013). Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology, 38(9), 1630–1638. 10.1016/j.psyneuen.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LH, Henriksen TB, & Olsen J (2010). Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics, 125(3), e600–e608. doi: 10.1542/peds.2008-3655 [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, & Gu Q (2011). Antidepressant use in persons aged 12 and over: United States, 2005–2008. NCHS Data Brief, (76), 1–8. [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, & Magnusson C (2013). Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: Population based case-control study. BMJ (Clinical Research Ed.), 346, f2059 10.1136/BMJ.F2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lee BK, Dalman C, Newschaffer C, Lewis G, & Magnusson C (2017). Antidepressants during pregnancy and autism in offspring: population based cohort study. bmj, 358, j2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, … Forman T (2009). Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry, 42(3), 95–100. 10.1055/s-0028-1103296 [DOI] [PubMed] [Google Scholar]
- Reichow B, Salamack S, Paul R, Volkmar FR, & Klin A (2008). Pragmatic assessment in autism spectrum disorders: A comparison of a standard measure with parent report. Communication Disorders Quarterly, 29(3), 169–176. 10.1177/1525740108318697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurtveit S, Selmer R, Roth C, Hernandez-Diaz S, & Handal M (2014). Prenatal exposure to antidepressants and language competence at age three: results from a large populationbased pregnancy cohort in Norway. BJOG: An International Journal of Obstetrics & Gynaecology, 121(13), 1621–1631. 10.1111/1471-0528.12821 [DOI] [PubMed] [Google Scholar]
- Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, & Pedersen LH (2013). Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clinical Epidemiology, 5, 449–459. 10.2147/CLEP.S53009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, … D’Onofrio BM (2017). Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA, 317(15), 1553–1562. 10.1001/jama.2017.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales DA, Winiarski DA, Smith AK, Stowe ZN, Newport DJ, Brennan PA (2018). Maternal depression and cortisol in pregnancy predict offspring emotional reactivity in the preschool period. Developmental Psychobiology, 60(5), 557–566. 10.1002/dev.21631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorin A, Uher R, Reichenberg A, Levine SZ, & Sandin S (2017). Autism risk following antidepressant medication during pregnancy. Psychological Medicine, 47(16), 2787–2796. 10.1017/S0033291717001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM (2001). Serotonin and brain development: role in human developmental diseases. Brain Research Bulletin, 56(5), 479–485. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, & Shaked M (2005). Psychiatric disorders in parents of children with autism: a meta‐analysis. Journal of child psychology and psychiatry, 46(1), 69–83. [DOI] [PubMed] [Google Scholar]