Abstract
Disrupting sleepiness and fatigue during the post-lunch dip by environmental factors may result in a decrease in human errors and accidents, and enhance job performance. Recent studies have shown that both red white light as well as blue white light can have a positive effect on human alertness and mental functioning. In the present study, the light intervention was evaluated for its effectiveness on alleviating the post-lunch dip. Twenty healthy volunteers experienced 117 min of four light conditions preceded by a 13-min initial dim light while performing a continuous performance test (CPT) and undergoing recording of the electroencephalogram (EEG): blue-enriched white light (12,000 K, 500 lx, BWL), red saturated white light (2,700 K, 500 lx, RWL), normal white light (4,000 K, 500 lx, NWL), and dim light (<5 lx, DL) conditions. Other outcome measures were subjective sleepiness, mood, and performance tests (working memory, divided attention, and inhibitory capacity). We found that exposure to both BWL and RWL conditions decreased the lower alpha-band power compared to the NWL and DL conditions. No significant differences were observed in subjective sleepiness and mental performance during sustained attention, working memory, and inhibitory capacity tasks between NWL, RWL, and BWL conditions. The present findings suggest that both RWL and BWL, compared to NWL condition, can improve the physiological correlates of alertness in EEG measurements. However, these changes did not translate to improvements in task performance and subjective alertness.
Keywords: Post-lunch dip, Alertness, Performance, Light, Electroencephalography
Introduction
Most people experience a significant reduction in alertness and concentration and an increase in sleepiness and fatigue during the midafternoon hours (between 14:00 and 16:00 pm) that is called a post-lunch dip1). The circadian rhythms for daytime alertness are not sufficiently strong to counteract the increased sleepiness during this specific time2, 3). A reduction in alertness during this period may lead to poor judgment and increased human error, resulting in an increase in the accident rate and reduced performance in the workplace4). Therefore, disrupting this decline in alertness by an environmental factor is expected to lead to improve safety and performance.
Light can have an acute effect on brain activity, as well as physiological, psychological and neurobehavioral responses and task performance5,6,7). Some studies have shown that exposure to short wavelength light (blue) has a potential for improving alertness and performance8,9,10). Recent studies indicated that long wavelength (red) or red saturated white light could improve subjective and objective measures of alertness during the night and daytime11,12,13). Furthermore, red lights provide stronger stimuli for alertness compared with short wavelength blue light during the midafternoon hours14). It is important to note that the use of monochromatic light in everyday life and work settings is not applicable5). Also, an increased proportion of blue wavelengths in polychromatic white light leads to an increase in the correlated color temperature (CCT), known as blue-enriched white light15). Also, an increased proportion of red wavelengths leads to a decrease in CCT called low CCT, warm light, or red saturated white light13).
Sahin et al. reported that red saturated white light enhanced alertness during the middle of the afternoon compared to dim lighting13). However, they did not compare blue-enriched white light with red saturated white light. Also, Baek and Min showed that exposure to blue-enriched white light significantly decreased alpha brainwave power and increased alertness and performance compared to white light during the post-lunch dip period5). However, the effect of red saturated white light was not investigated in their study.
The question is still open as to whether exposure to red saturated polychromatic white light and conforming to the commonly experienced illumination levels for the daytime work environment (500 lx on the desk) have a positive impact on alertness and performance during the midafternoon (post-lunch) hours. Focusing on this question, a simulated workplace environment was designed and by imposing activities such as perception, attention, and memory on participants, their mental state became similar to the time when they were in the actual environment5). Importantly, the measured effects of light may depend on the type of output measures such as self-report questionnaires (subjective measures) and objective measures (electroencephalogram (EEG) and performance tests)16). Thus a multi-measure approach was applied to investigate these potential effects of light in the current study. The main purpose of the present study was to investigate whether exposure to red saturated white light (RWL) compared to blue-enriched light (BWL), normal white light (NWL), and dim light (DL) conditions (control) could have any effect on alertness, mood, and performance during the post-lunch dip period. We hypothesized that exposure to RWL and BWL light conditions would enhance the alertness, mood, and mental performance and additionally decrease subjective sleepiness compared to NWL and DL conditions.
Materials and Methods
Participants
In this study, 20 healthy paid volunteers (male; mean ± SD age; 27.65 ± 3.65 yr) were recruited and followed the experimental protocol. All participants were nonsmokers and free from any health problems (physical, psychological or sleep disorders) and were not taking any medication. No participants had a history of eye disease and color blindness based on the Ishihara test (Kanehara Shupman Co., Tokyo, Japan). Volunteers with a good sleep quality (PSQI score <5) according to Pittsburgh sleep quality index17) without extreme early or extreme late chronotype18) who had a regular sleep-wake state (bedtimes 22:00 and 24:00 pm and wake up between 07:00 and 08:00 am) were included in the study. Besides, subjects filled out sleep/wake log, beginning one week before the onset of the investigation and were asked to keep a regular sleep/wake state during the study. The estimated marginal means (EMM) and standard errors (SE) of the average sleep duration (hour) were 7.061 ± 0.04 for BWL, 7.096 ± 0.025 for NWL, 7.047 ± 0.04 for RWL, and 7.079 ± 0.026 for the DL condition. No significant difference was observed in the average sleep duration in the week before the experiment between the light conditions (p>0.05). We excluded participants if they had traveled to a different time zone or experienced shift-work during the three months prior to the experiment session. The participants were asked to sleep between 22:00 and 23:00 pm at night before the experiment and not to wake up later than 07:30 am. Also, they were asked to avoid napping on the day of the experiment to ensure that measurements were made during post-lunch dip period that usually occurs 16 to 18 h after bedtime between 14:00 and 16:00 pm1). All participants had eaten 60 to 90 min before the experiment. Participants were asked to avoid drinking caffeine and alcohol 12 h before the experiment. The Ethics Committee at Hamadan University of Medical Sciences approved the current study and the participants signed an informed written consent.
Study design and procedures
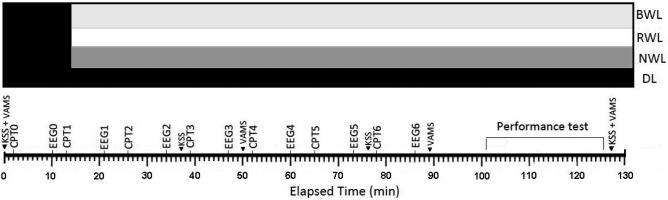
This study used a repeated-measure design and the participants were exposed to four lighting conditions in a counterbalanced order, separated by one week. Each participant was first exposed to a 13-min period of dim light (<5 lx at eye level from a 3,520 K compacted fluorescent lamp) followed by a different light condition each week: a dim light condition (DL, <5 lx at eye level from a 3520 K compact fluorescent lamp), or 500 lx illumination level on the desk level from a blue-enriched white light (BWL, CCT=12,000 K,), a red saturated white light (RWL, CCT=2,700 K), or a normal white light condition (NWL, CCT=4,000 K). In each lighting condition, after the initial dim period (baseline), the light source was turned on and the experiment started. Each experiment lasted for approximately 130 min. It should be noted that the light setting (500 lx) was selected in accordance with the norms for office environments19). Participants were asked to come to the laboratory about 13:00 pm to receive instructions and to be fitted with EEG electrodes. During this preparation period, they were kept seated in a dim room (<5 lx at eye level). The experiment started at 13:50 pm and the participants completed seven repeated sequences that included a continuous performance task (~8 min) followed by an EEG (3 min). After removing the EEG electrodes, the participants performed Go/No-Go, 2-back and divided attention tasks in a sequential order. The experimental set-up is presented in Fig. 1.
Fig. 1.
The experimental setup. The protocol consisted of collecting Continuous performance test (CPT), Karolinska Sleepiness Scale (KSS), Visual Analog Mood Scales (VAMS), electroencephalogram (EEG), and mental performance task. EEG0 that present the data recorded during the initial dim period were used as a baseline. BWL: blue-enriched white light; RWL: red saturated white light; NWL: normal white light; DL: dim light.
Routine daily activities usually need constant alertness and a minimal mental effort to remain alert. Therefore, in the current study, by imposing a sustained attention task (continuous performance test)20) on the participants, their mental state became similar to that in the actual workplace environment5). Sustained attention means the capability to focus on a task for an extended period of time21). Given that the alpha wave activity is related to the sustained attention task22), the focus of our analysis was on EEG alpha wave activity.
Lighting settings
The study was conducted in an air-conditioned simulated office environment room with an area of 19 m2. No daylight entered the experimental environment. The subjects sat behind a desk most of the time, making their position almost fixed in the experimental setting. They were also asked to keep their eyes open during the experiment.
The experiment room was lit with dimmable fluorescent light sources including seven ceiling tubes (Philips, MASTER TL-D Super 80 36W/840 and 36W/827, nominal CCT=4,000 K and 2,700 K), and 22 ceiling tubes (Philips, TL-D Snow White 18W, CCT=12,000 K). Each light group source had an independent circuit and control switch. Commercial lamps were intentionally applied to create a factual experimental lighting environment. Illuminance measures demonstrated illuminance uniformity (Emin/Eave) of at least 0.8 that reflected the homogeneity of illuminance in the experiment room.
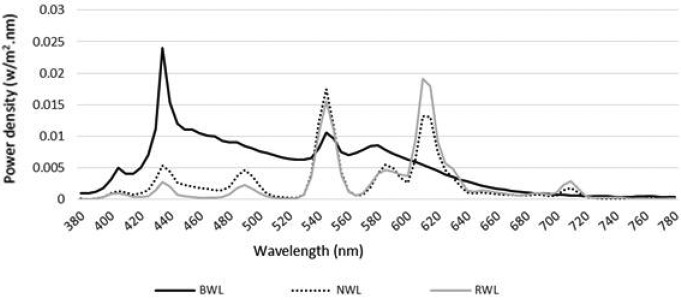
The vertical illuminance (1.2 m from the floor) and horizontal illuminance level (0.75 m above the floor at the work area) of each light condition was measured using an illuminance meter (Hagner, model E2, Solna, Sweden). Mean horizontal illuminance level at work area was 516.5 lx (SD=13 lx). The mean luminance in the participants’ field of view (1.2 m from the floor) was also measured by a luminance meter (Hagner Screen Master, Solna, Sweden). The mean of luminance was 41 ± 2.36 Cd/m2 and none of the light sources were in the direct view of the participants. Next, the actual CCT, Color Rendering Index, and chromaticity coordinates were measured at eye level using a spectrometer (C7000 SpectroMaster, Sekonic Corp., Tokyo, Japan). Technical details, photometric values, and spectral power distribution (at eye level) are shown in Table 1 and Fig. 2. The supplementary materials proposed by Lucas et al.23) were applied to calculate photometric values.
Table 1. Technical details and photometric values for BWL, RWL, and NWL light conditions.
| Variable | BWL | RWL | NWL |
|---|---|---|---|
| Nominal correlated color temperature (K) | 12,000 | 2,700 | 4,000 |
| Actual correlated color temperature (K) | 9,733 | 2,564 | 3,730 |
| Color Rendering Index (CRI Ra) | 83 | 81.9 | 82.9 |
| Chromaticity coordinates (x,y) 1931 CIE chromaticity coordinates | 0.281, 0.2926 | 0.4831, 0.4327 | 0.395, 0.3899 |
| Photopic illuminance (lx) | 317 | 333 | 332 |
| Cyanopic lx (α-opic lx) | 469 | 73 | 180 |
| Melanopic lx (α-opic lx) | 388 | 115 | 195 |
| Rhodopic lx (α-opic lx) | 371 | 169 | 236 |
| Chloropic lx (α-opic lx) | 345 | 259 | 291 |
| Erythropic lx (α-opic lx) | 314 | 326 | 319 |
| Photon flx (photons/cm2.s) | 3.18 × 1014 | 2.7 × 1014 | 2.7 × 1014 |
| Irradiance (µW/cm2) | 121 | 92 | 96 |
BWL: blue-enriched white light; RWL: red saturated white light; NWL: normal white light.
Fig. 2.
Spectral power distribution measured at eye level in the BWL, NWL, and RWL conditions. BWL: blue-enriched white light; NWL: normal white light; RWL: red saturated white light.
Continuous performance test
Continuous performance test (CPT) is a standardized computer test that is used for the assessment of sustained attention over the course of time20). The present test (~8 min) reflected a demonstration of a randomly varying single number stimuli (0–9) shown on a monitor screen for 500 milliseconds (ms), with a 1,000 ms time interval between consecutive stimuli. The study subjects were trained to react with their dominant hand immediately when a target stimulus (‘4’) appeared by pressing the spacebar button on a keyboard in front of them. A randomized stimulus demonstration was applied for each experiment to prevent learning effects. The mean response time of correct responses and accuracy were used as dependent measures in all data analyses.
Electroencephalogram
A 3-minute sample of EEG data was recorded using an EEG analyzer, with Ag/Ag Cl electrodes (Encephalon 131–03, Medicom MTD, Russia). The EEG was derived from the Z-line on the participants’ scalps at positions Oz, Pz, Cz, and Fz, in line with the International 10–20 system24). Reference electrodes were located on the left and right earlobes (A1 and A2), and a ground electrode was placed on the forehead. Also, one electrode was located directly below the right eye for monitoring eye blinks. The participants were told to sit quietly, keep their eyes open, fix their gazes at an X printed on the opposite wall, and remain still without moving, blinking, or talking during EEG recording trials to minimize artifacts during EEG testing. EEG was recorded at 250 Hz (band-pass filter, 0.3–40 Hz) and impedance was kept below 5 KΩ. The values of the two reference channels were averaged together and subtracted from all other channels. Next, the obtained data were placed into 2-s epochs with one second overlapping from each epoch. Any epoch with an artifact of eye blinks, noise, and body movements was removed from the analysis. Subsequently, a 10% cosine window followed by a fast Fourier transform was applied to each epoch. This process yielded spectral power distributions from 0.3 to 40 Hz at 0.5 Hz intervals. Next, the power from each epoch was averaged together to gain average power for each trial. Power spectra from each of the four electrode sites were divided into two group frequency bins: lower alpha (8–10 Hz) and upper alpha (10–13 Hz). These frequency ranges were selected because both alpha band EEG activities are linked to cognitive function and attentional processing25). The MATLAB software package (ver. R2012a, Math-Works, USA) was applied to analyze EEG data.
Karolinska Sleepiness Scale (KSS)
The subjective sleepiness of participants was measured with Karolinska’s self-reporting Sleepiness Scale (KSS)26). The ranking response of this scale varied between 1 and 9, where 1=“extremely alert”, 3=“alert”, 5=“neither alert nor sleepy”, 7=“sleepy”, and 9=“very sleepy.
Visual Analog Mood Scales (VAMS)
The subjective mood was evaluated using an 11-point visual analog scale, varying from 0 for ‘very bad mood,’ to 100, for ‘very good mood.’ Both VAMS and KSS scores were normalized to the sample score obtained at initial adaptation time.
Mental performance tests
A three-minute visual Go/No-Go task was used for measuring inhibitory capacity27). During this test, a total of 120 pairs (yellow and white or white and blue) squares were presented randomly on a computer screen with a black background. Each character was presented for 200 milliseconds (ms) with an interval of 1,000 ms. The participants were asked to compare the pair squares appearing on the screen: if the white and blue squares and the blue square were presented on the right, the participants would immediately press the ‘?’ button and if the blue square was presented on the left, the participants pressed the ‘Z’ button on the keyboard. Also, if the yellow and white pair of squares appeared, the participants did not react. The previous study indicated that this test elicits reliable results in assessing motor activity28).
A four-minute two-Back task was used for measuring the working memory and executive functioning. This test is a popular test for assessing working memory performance29) and had acceptable reliability and validity30). In general, 120 numbers were presented with an interval of 1,500 ms on a black monitor screen after each other during the test. The participants were asked to compare the last number appearing on the screen with two numbers that were shown previously; if the numbers were the same (i.e., the same number as the previous one), the participants pressed the ‘?’ button immediately and if the numbers were different, the participants pressed the ‘Z’ button on the keyboard.
Also, in this study, a 4-min divided attention task was used31, 32). A total of 168 pairs of images were consecutively presented in random order on a black monitor screen. Each character was presented for 200 ms with an interval of 1,000 ms. If a candle image was presented on the right side of the screen, the participants pressed the ‘?’ button and if a circle appeared on the left side, the participants pressed the ‘Z’ button instantly on the keyboard. Also, if these two images appeared at the same time and in the correct order, the participants pressed the ‘?’ and ‘Z’ buttons together. To minimize unwanted impacts of individual differences on the results of the Go/No-Go, 2-Back, and, divided attention task, the ratio of the average of raw data of each participant to the average of all participants was used to transform the participant’s raw data12, 13). Therefore, the transformed value was applied to data analysis. The mean response time of correct responses and accuracy for all mental performance tasks were used as the dependent variables in the data analyses.
Statistical analysis
Linear mixed model (LMM) analyses were applied to assess the effect of the light conditions and trials on the EEG upper and lower-band alpha wave activities, the subjective measures of mood, sleepiness, and mental performance. Separate LMM analyses were run for each dependent variable. In the model, the light condition and trial were fixed and the participant was added as a random effect to the model. We controlled potential confounding variables such as general health status, sleep quality, sleep duration, chronotype, traveling time, and caffeine and alcohol consumption. The baseline dependent measurement for each participant (i.e., the first trial during the initial dim period) was added as covariate to the model for analyzing the CPT, KSS, mood, and EEG measures, except for the LMM models for the Go/No-Go, 2-Back and, divided attention task as these variables were only measured at the end of the experiment. The lower and upper alpha-band power, subjective sleepiness, and continuous performance task data were transformed by using Box-Cox transformation to correct non-normal data distribution and skewness33). The Bonferroni correction was applied to the pairwise comparisons. A p-value ≤0.05 was considered statistically as significant.
Results
EEG
Lower alpha-band activity
Significant main effects of the light conditions (F3, 33.13=17.688, p<0.001), trials (F5, 107.688=16.003, p<0.001), and an interaction between the light conditions and trial (F15, 75.452=4.514, p<0.001) were found in the transformed lower alpha-band power. The estimated marginal means (EMM) and standard errors (SE) of the transformed lower alpha power were 2.33 ± 0.028 for BWL, 2.531 ± 0.031 for NWL, 2.368 ± 0.026 for RWL, and 2.583 ± 0.033 for the DL condition. The pairwise comparisons indicated a significantly lower power under BWL (p<0.001) and RWL (p<0.001) compared to the DL condition. In addition, a significant difference was observed between the BWL (p<0.001) and RWL (p=0.001) conditions compared to the NWL condition. The other comparisons did not indicate any significant differences. In general, the participants experienced reduced levels of alertness over the duration of the experiment.
Upper alpha-band activity
Significant main effects of the light conditions (F3, 29.998=23.979, p<0.001), trials (F5, 102.225=7.241, p<0.001) and an interaction between the light conditions × trial (F15, 80.875=3.148, p<0.001) were observed in the transformed upper alpha-band power. The transformed EMM ± SE of the upper alpha power were 2.41 ± 0.03 for BWL, 2.566 ± 0.046 for RWL, 2.608 ± 0.027 for NWL, and 2.753 ± 0.031 for the DL condition. The pairwise comparisons showed significant differences between BWL (p<0.001), NWL (p=0.003), and RWL (p=0.016) compared to the DL condition. Moreover, a significant difference was observed for the BWL condition compared to NWL (p<0.001) and RWL (p=0.023) conditions. The other comparisons did not reveal any significant differences. Figure 3 shows the transformed EMM ± SE of the lower and upper alpha-band powers in each trial. Furthermore, pairwise comparisons (post-hoc) for the interaction between light conditions and trial for lower and upper alpha-band are shown in Table 2.
Fig. 3.
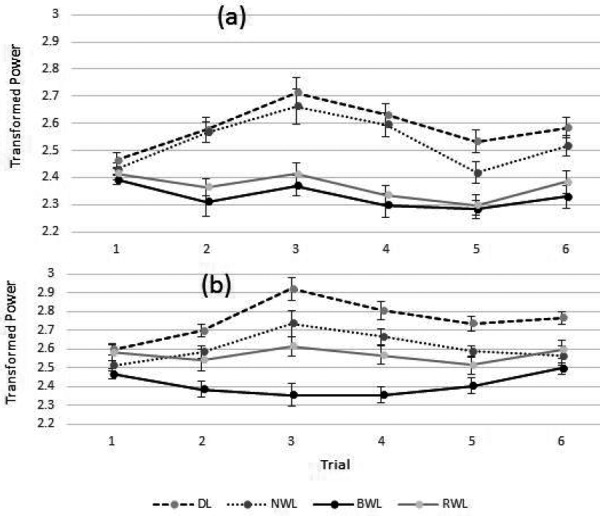
The estimated marginal means (EMM) and standard errors (SE) of the transformed EEG lower alpha (a) and upper alpha (b) powers during each trial for the BWL, NWL, RWL, and DL conditions. DL: dim light; NWL: normal white light; BWL: blue-enriched white light; RWL: red saturated white light.
Table 2. Pairwise comparisons (post-hoc) for the light conditions × trials interaction for lower and upper alpha-band powers.
| Variable | Trial | Pairs | Sig. | Variable | Trial | Pairs | Sig. |
|---|---|---|---|---|---|---|---|
| Lower alpha | 2 | BWL-DL | 0.004 | Upper alpha | 1 | BWL-DL | 0.007 |
| RWL-DL | 0.003 | 2 | BWL-DL | <0.001 | |||
| RWL-NWL | 0.001 | BWL-NWL | 0.003 | ||||
| BWL-NWL | 0.002 | 3 | BWL-DL | <0.001 | |||
| 3 | BWL-DL | <0.001 | RWL-DL | 0.003 | |||
| RWL-DL | <0.001 | BWL-NWL | 0.001 | ||||
| RWL-NWL | 0.013 | BWL-RWL | 0.013 | ||||
| BWL-NWL | 0.002 | 4 | BWL-DL | <0.001 | |||
| 4 | BWL-DL | <0.001 | RWL-DL | 0.007 | |||
| RWL-DL | <0.001 | BWL-NWL | <0.001 | ||||
| RWL-NWL | <0.001 | BWL-RWL | 0.008 | ||||
| BWL-NWL | <0.001 | 5 | NWL-DL | 0.019 | |||
| 5 | BWL-DL | <0.001 | BWL-DL | <0.001 | |||
| RWL-DL | 0.001 | RWL-DL | 0.014 | ||||
| 6 | BWL-DL | <0.001 | BWL-NWL | 0.004 | |||
| RWL-DL | 0.006 | 6 | NWL-DL | 0.008 | |||
| BWL-NWL | 0.007 | BWL-DL | <0.001 | ||||
| RWL-DL | 0.036 |
Only comparisons that were statistically significant are shown. p<0.05 was considered statistically significant.
Subjective Sleepiness (KSS)
Significant main effects of the light conditions (F3, 84.516=21.818, p<0.001), trials (F2, 154.023=30.547, p<0.001) and the light conditions × trial (F6, 187.78=6.144, p<0.001) were observed in the transformed subjective sleepiness scores. The EMM ± SE of transformed sleepiness scores were 1.911 ± 0.039 for BWL, 1.926 ± 0.039 for RWL, 2.027 ± 0.038 for NWL, and 2.254 ± 0.039 for the DL condition. The pairwise comparisons indicated that participants felt lower sleepiness under the BWL (p<0.001), RWL (p<0.001), and NWL (p<0.001) conditions as compared with the DL condition. The other comparisons did not indicate significant differences. The participants felt sleepier during trial 2 compared to trial 1 and in trial 3 compared to trials 1 and 2. This indicates that they generally experienced more sleepiness over the experiment. Figure 4 shows the transformed EMM ± SE of subjective sleepiness scores in each trial. The pairwise comparisons (post-hoc) for the interaction between the light conditions and trials showed that the BWL, RWL, and NWL conditions (p<0.001) decreased significantly subjective sleepiness compared to the DL condition in all trials except trial 1.
Fig. 4.
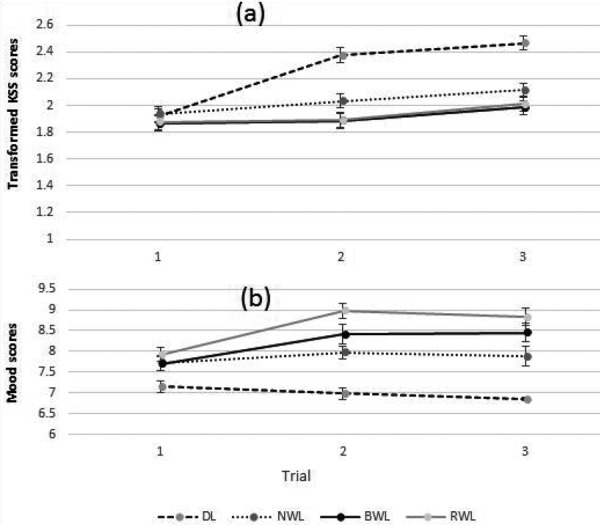
The estimated marginal means (EMM) and standard errors (SE) of transformed subjective sleepiness (a) and mood scores (b) from each trial for the BWL, NWL, RWL, and DL conditions. DL: dim light; NWL: normal white light; BWL: blue-enriched white light; RWL: red saturated white light.
Subjective mood
Significant main effects of the light conditions (F3, 42.453=46.525, p<0.001), trials (F2, 70.351=8.461, p=0.001) and light conditions × trial (F6, 41.875=5.094, p=0.001) were observed in the subjective mood scores. The pairwise comparisons showed a significantly better mood under the BWL (8.185 ± 0.175; p<0.001), NWL (7.858 ± 0.125; p<0.001), and RWL (8.576 ± 0.125; p<0.001) conditions compared to the DL (6.998 ± 0.077) condition. In addition, the mood was significantly improved under the RWL compared to the NWL (p=0.001) condition. The other comparisons did not show any significant differences. Figure 4 indicates the EMM ± SE of subjective mood scores in each trial. The pairwise comparisons for the interaction between light conditions and trials revealed better emotional status (mood) under the BWL, RWL, and NWL conditions compared to the DL condition in trials 2 and 3. Furthermore, there were significant differences between the RWL compared to the NWL condition in trials 2 (p=0.001) and 3 (p=0.025).
Continuous performance test
Significant main effects of the light conditions (F3, 38.694=17.414, p<0.001), trials (F5, 112.341=2.721, p=0.023) and interactions between the light conditions and trial (F15, 82.773=1.926, p=0.032) were observed in the transformed mean response time of CPT task. The pairwise comparisons revealed significant lower transformed response times under BWL (5.979 ± 0.011; p<0.001), RWL (5.984 ± 0.014; p<0.001) and NWL (6.032 ± 0.016; p=0.006) compared to the DL (6.115 ± 0.017) condition. The other comparisons did not indicate any significant differences. No significant main effect of light and the light conditions × trial was observed in the accuracy of the CPT test. Generally, the accuracy was reduced over the experiment. Figure 5 indicates the EMM ± SE of mean response times and accuracies of CPT task in each trial. Furthermore, pairwise comparisons (post-hoc) for interaction between the light conditions and trials for the mean response time of continuous performance test are shown in Table 3.
Fig. 5.
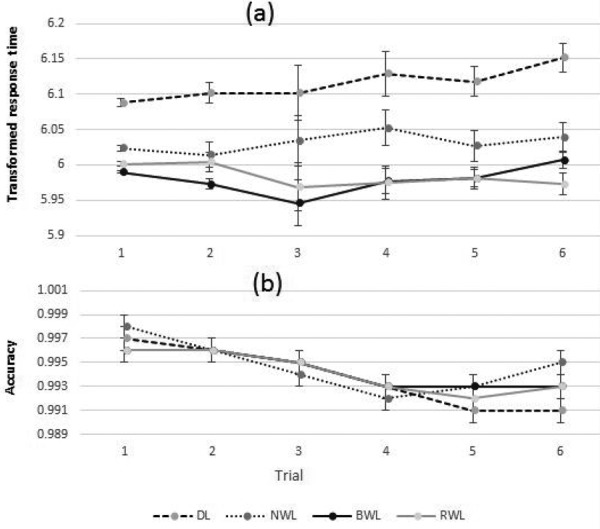
The estimated marginal means (EMM) and standard errors (SE) of the transformed mean response time (a) and the accuracy (b) of the continuous performance test during each trial for the BWL, NWL, RWL, and DL conditions. DL: dim light; NWL: normal white light; BWL: blue-enriched white light; RWL: red saturated white light.
Table 3. Pairwise comparisons (post-hoc) for the light conditions × trials interaction for the transformed mean response time of continuous performance test.
| Trial | Pairs | Sig. |
|---|---|---|
| 1 | NWL-DL | <0.001 |
| RWL-DL | <0.001 | |
| BWL-DL | <0.001 | |
| BWL-NWL | <0.001 | |
| RWL-NWL | <0.001 | |
| BWL-RWL | 0.011 | |
| 2 | NWL-DL | 0.03 |
| RWL-DL | <0.001 | |
| BWL-DL | <0.001 | |
| 3 | BWL-DL | 0.021 |
| 4 | RWL-DL | 0.002 |
| BWL-DL | 0.001 | |
| 5 | NWL-DL | 0.032 |
| RWL-DL | <0.001 | |
| BWL-DL | <0.001 | |
| 6 | NWL-DL | 0.002 |
| RWL-DL | <0.001 | |
| BWL-DL | <0.001 | |
Only comparisons that were statistically significant are shown. p<0.05 was considered statistically significant.
Two-Back
Significant main effects of the light conditions (F3, 12.851=7.67, p=0.003) were observed in the mean response time of the two-Back test. The EMM ± SE of mean response time were 388.77 ± 27.747 for BWL, 389.49 ± 27.797 for RWL, 396.79 ± 28.214 for NWL, and 408.22 ± 28.162 for DL condition. The pairwise comparisons revealed significant differences under BWL (p=0.003), RWL (p=0.001) compared to the DL condition. The remaining comparisons did not show any significant differences.
Go/No-Go
Significant main effects of the light conditions (F3, 21.304=6.896, p=0.002) were observed in the mean response time of the Go/No-Go test. The EMM ± SE of mean response times were 311.12 ± 12.724 for NWL, 303.94 ± 12.313 for BWL, 333.66 ± 14.467 for DL, and 304.58 ± 12.279 for RWL condition. The pairwise comparisons revealed a significantly lower response time under BWL (p=0.006) and RWL (p=0.008) than the DL condition. The other comparisons revealed no significant differences.
Divided attention
No significant main effect of the light was found on the mean response time of divided attention test (F3, 45.168=2.337, p=0.086). The EMM ± SE of mean reaction times were 326.46 ± 11.024 for RWL, 330.9 ± 11.024 for DL, 325.18 ± 11.024 for BWL, and 327.58 ± 11.024 for the NWL condition. No significant effect of the light conditions was observed in the accuracy of all performance tasks.
Discussion
In the present study, both BWL and RWL conditions significantly decreased lower alpha-band power compared to the NWL and DL conditions. Notably, the alpha power was negatively correlated with light-induced alertness. On the other hand, reductions in alpha power indicate an enhancement in objective alertness5, 25, 34, 35). These findings are consistent with previous studies that showed the alerting effect of both blue and red lights (40 lx at eye level) compared to a DL condition11, 36). In this regard, Baek and Min reported that exposure to BWL condition (40 lx at eye level) significantly decreased lower alpha-band power compared to NWL condition during the post-lunch dip period5). However, unlike our study, they did not compare the EEG alpha activity between BWL and RWL conditions. Furthermore, Sahin et al. indicated that RWL condition (2568 K, 361 lx at eye level) decreased EEG alpha power compared to a DL condition during the middle of the afternoon13). But the effects of BWL and NWL conditions were not assessed in their study.
The results showed that the BWL condition reduced upper alpha-band power more than the NWL, RWL, and DL conditions. In line with these results, Baek and Min reported that BWL condition (40 lx at eye level) significantly decreased upper alpha-band power compared to NWL condition during the post-lunch dip period5). However, they did not examine the resultant objective alertness between the RWL and BWL conditions. Importantly, we evaluated the effect of light at a higher illumination level (i.e., 500 lx on the desk) to provide realistic levels for daytime office environments.
Neurophysiological evidence supports our findings of the relative effectiveness of RWL and BWL conditions in improving objective alertness compared to the NWL and DL condition. Besides cones and rods, intrinsically photosensitive retinal ganglion cells (ipRGCs) are a new type of retinal photoreceptors that transmit light into neural signals for the circadian system37, 38). These cells are particularly sensitive to blue light, expressing the non-imaging effect of light such as melatonin suppression, regulation of circadian system, as well as neuroendocrine and neurobehavioral functions such as improvement in alertness and performance39,40,41). It is well accepted that melatonin suppression is required to influence the light on alertness at night10, 42). Recent studies have shown that melatonin level is low during the daytime and that light-induced alertness is not always mediated by suppression of melatonin14, 43, 44).
Vandewalle et al. reported that blue light mediated the enhancement of brain activity and may cause an improvement in alertness and performance45). Also, another study has shown that light enhances the activity of the brain that is related to executive control and working memory46). Rautkylä et al. suggested that light can use the amygdala in the limbic system to send signals to the cerebral cortex, and that light can modulate emotions that induce alerting responses47). In addition, some studies suggested that the red light-induced alerting response may be expressed from other types of photoreceptors (i.e., ipRGCs, cones and rods)13, 14). Finally, it can be concluded that there may be different mechanisms beyond physiological and neuropsychological effects of light.
In the present study, the participants had significantly better moods under the RWL condition compared to the NWL and DL conditions. These findings are consistent with those by Smolders and de Kort, who reported the beneficial effects of the RWL condition on the vitality and emotional status during the afternoon hours16). Some studies have suggested that warm colors such as red, yellow, and orange increase feelings of arousal and excitation that affect emotional brain activity and motor responses. These factors can explain the better mood of the participants during the RWL condition48, 49).
As shown in Fig. 4, the participants felt sleepier during the DL condition compared to the BWL, RWL and, NWL conditions. These are consistent with earlier studies that showed RWL and BWL light conditions reduced subjective sleepiness compared to the DL condition36). In the present study, the participants felt sleepier during trial 2 compared to trial 1, and in trial 3 compared to trials 1 and 2. These findings are consistent with our expectations and with earlier studies that participants would get sleepier in the afternoon and that participants would get sleepier as time passed13, 14).
The present study showed that the BWL and RWL conditions were effective in reducing the response times during inhibitory capacity, working memory and sustained attention tasks compared to the DL condition. Consistent with this finding, Baek and Min reported that exposure to BWL condition (40 lx at eye level) during the post-lunch dip period significantly decreased the response time in a sustained attention task compared to a DL condition5). Despite the effect of BWL and RWL on enhancing performance compared to the DL condition, these light conditions did not improve performance on any of the tasks examined in this study compared to the NWL condition. These findings are in line with those of Smolders and de Kort16) who indicated no significant difference in performances under 2,700 compared to 6,000 K light conditions (500 lx on the desk) during the daytime. Furthermore, another study showed that blue-enriched white light (40 lx at eye level) did not lead to an improvement in performance compared to a white light condition that was defined according to the 1931 CIE chromaticity coordinates (x: 0.3303, y: 0.331, 40.2 lx at eye level) during afternoon hours5).
Contrary to the present study, Keis et al. reported that long-term exposure to blue-enriched classroom lighting (e.g., several weeks) during the daytime leads to improvements in the students’ performance, processing speed, and the ability to concentrate50). However, that study used a higher level of blue-enriched light (17,000 K vs. 4,000 K) than the current study. Furthermore, field studies have shown that exposure to blue-enriched light could improve performance during the daytime15, 51, 52). However, in these studies, a higher level of blue-enriched light (17000 K vs. 4000 K) was used than the present study and only subjective measures were employed.
In the present study, no significant effect of the light conditions on the mean response time of the divided attention task was observed. These unexpected findings might originate from the limitations of the current study, i.e., we did not measure the mean response time during the initial dim period as a baseline measurement. In addition, no significant main effect of the light conditions was found on the accuracy of all mental performance tasks. These findings are consistent with previous studies that showed the accuracy of mental performance is less affected by light conditions compared to the response time5, 6, 42, 53, 54). Generally, the results of other studies that tested the effect of light on performance have yielded mixed results. The following parameters may explain these heterogeneous results: circadian phase and homeostatic conditions45, 55), the difficulty of the task, the time of day, illuminance level56), and previous emotional level of the participants57). Thus, further studies are needed to investigate the relationship between the alertness level and task performance.
It should be noted that post-lunch dip might depend on the composition of lunch58). The fatigue and sleepiness at this period could be worsened by eating a high-carbohydrate lunch; however, this effect still exists even with a light meal59). Also, the post-lunch dip can occur even when people have had no lunch60). Monk reported that the post-lunch dip is rooted in human biology and may be linked to the harmony of human circadian rhythms1). In the current study, we did not measure participants’ lunch composition, so, this factor may have affected the results.
Another limitation of the present study was that the participants were relatively young. Therefore, due to potential changes in the retina and neural structure of the eye due to aging, our observations cannot directly be generalized to older groups of people. Given that the majority of activities carried out in the daytime office environment is of a kind of visual task, we employed only a limited set of visual performance tasks during the current study. Because in addition to non-image forming effects of light, subjective evaluation (visual comfort) of participants about the light conditions had a potential impact on their performance during visual mental performance task16); hence, it is not possible to specify the mechanisms that induced improvement in the participants’ performance in the present study. Additionally, the application of the dim adaptation period before the actual light manipulation might decrease the generalizability of the present results to a real-life setting. Future studies are recommended to use different types of task performance, other age groups, and actual workplace setting.
Conclusion
The findings of the present study indicated that both blue-enriched and red-saturated white lights (500 lx on the desk) had a more efficient stimulating effect on modulating the brain activities associated with daytime alertness than the dim and normal light conditions during the post-lunch dip hours. However, no significant improvements were observed in subjective sleepiness and mental performance during sustained attention, working memory, and inhibitory capacity tasks compared to the normal light condition. Thus, it can be concluded that both blue-enriched (BWL) and red-saturated white (RWL) lights can improve physiological correlates of alertness compared to the normal light condition (NWL) that is generally used in workplaces such as office environments. But these changes did not translate to improvements in task performance and subjective alertness. Further studies are required to decide on the optimal light intervention characteristics such as light spectrum and illumination level to improve alertness and mental performance during the daytime hours.
Conflicts of Interest
The authors declare that they have no competing interest.
Acknowledgments
The authors would like to thank the vice-chancellor for Research and Technology, Hamadan University of Medical Sciences for their financial support.
References
- 1.Monk TH. (2005) The post-lunch dip in performance. Clin Sports Med 24, e15–23, xi–xii. [DOI] [PubMed] [Google Scholar]
- 2.Cajochen C, Blatter K, Wallach D. (2004) Circadian and sleep-wake dependent impact on neurobehavioral function. Psychol Belg 44, 59–80. [Google Scholar]
- 3.Edgar DM, Dement WC, Fuller CA. (1993) Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci 13, 1065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson A, Lombardi DA, Folkard S, Stutts J, Courtney TK, Connor JL. (2011) The link between fatigue and safety. Accid Anal Prev 43, 498–515. [DOI] [PubMed] [Google Scholar]
- 5.Baek H, Min BK. (2015) Blue light aids in coping with the post-lunch dip: an EEG study. Ergonomics 58, 803–10. [DOI] [PubMed] [Google Scholar]
- 6.Smolders KC, de Kort YA, Cluitmans PJ. (2012) A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol Behav 107, 7–16. [DOI] [PubMed] [Google Scholar]
- 7.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. (2000) Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res 115, 75–83. [DOI] [PubMed] [Google Scholar]
- 8.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. (2006) Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 29, 161–8. [PubMed] [Google Scholar]
- 9.Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, Maquet P. (2007) Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex 17, 2788–95. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A. (2005) High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab 90, 1311–6. [DOI] [PubMed] [Google Scholar]
- 11.Figueiro MG, Bierman A, Plitnick B, Rea MS. (2009) Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci 10, 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiro MG, Sahin L, Wood B, Plitnick B. (2016) Light at night and measures of alertness and performance: implications for shift workers. Biol Res Nurs 18, 90–100. [DOI] [PubMed] [Google Scholar]
- 13.Sahin L, Wood BM, Plitnick B, Figueiro MG. (2014) Daytime light exposure: effects on biomarkers, measures of alertness, and performance. Behav Brain Res 274, 176–85. [DOI] [PubMed] [Google Scholar]
- 14.Sahin L, Figueiro MG. (2013) Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav 116-117, 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Viola AU, James LM, Schlangen LJ, Dijk DJ. (2008) Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health 34, 297–306. [DOI] [PubMed] [Google Scholar]
- 16.Smolders KC, de Kort YA. (2017) Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J Environ Psychol 50, 80–93. [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- 18.Roenneberg T, Wirz-Justice A, Merrow M. (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18, 80–90. [DOI] [PubMed] [Google Scholar]
- 19.EN U (2011) Light and lighting. Lighting of work places, Part 1: Indoor work places.
- 20.Riccio CA, Reynolds CR, Lowe P, Moore JJ. (2002) The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol 17, 235–72. [PubMed] [Google Scholar]
- 21.Glisky EL. (2007) Changes in cognitive function in human aging. Brain aging: models, methods, and mechanisms. 3–20.
- 22.Orekhova EV, Stroganova TA, Posikera IN. (2001) Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin Neurophysiol 112, 740–9. [DOI] [PubMed] [Google Scholar]
- 23.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. (2014) Measuring and using light in the melanopsin age. Trends Neurosci 37, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomenclature SEP. (1991) American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol 8, 200–2. [PubMed] [Google Scholar]
- 25.Klimesch W. (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29, 169–95. [DOI] [PubMed] [Google Scholar]
- 26.Åkerstedt T, Gillberg M. (1990) Subjective and objective sleepiness in the active individual. Int J Neurosci 52, 29–37. [DOI] [PubMed] [Google Scholar]
- 27.Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. (2007) Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Arch Clin Neuropsychol 22, 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessel JR. (2018) Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology 55, 12871. [DOI] [PubMed] [Google Scholar]
- 29.Cook MJ.(2000) Working memory, age, crew downsizing, system design and training. Univ of Abertay Dundee Scotland (United Kingdom) Centre for Usability Test and Evaluation. [Google Scholar]
- 30.Chen YN, Mitra S, Schlaghecken F. (2008) Sub-processes of working memory in the N-back task: an investigation using ERPs. Clin Neurophysiol 119, 1546–59. [DOI] [PubMed] [Google Scholar]
- 31.Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M. (2005) On the neural basis of focused and divided attention. Brain Res Cogn Brain Res 25, 760–76. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. (1991) Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11, 2383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Box G, Cox D .(1981) An Analysis of Transformations Revisited, Rebutted. Wisconsin Univ-Madison Mathematics Research Center. [Google Scholar]
- 34.Klimesch W. (2012) α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16, 606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min BK, Jung YC, Kim E, Park JY. (2013) Bright illumination reduces parietal EEG alpha activity during a sustained attention task. Brain Res 1538, 83–92. [DOI] [PubMed] [Google Scholar]
- 36.Plitnick B, Figueiro M, Wood B, Rea M. (2010) The effects of red and blue light on alertness and mood at night. Light Res Technol 42, 449–58. [Google Scholar]
- 37.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. (2000) A novel human opsin in the inner retina. J Neurosci 20, 600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berson DM, Dunn FA, Takao M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–3. [DOI] [PubMed] [Google Scholar]
- 39.Hatori M, Panda S. (2010) The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med 16, 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holzman DC. (2010) What’s in a color? The unique human health effects of blue light. Environ Health Perspect 118, 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeGates TA, Fernandez DC, Hattar S. (2014) Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 15, 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motamedzadeh M, Golmohammadi R, Kazemi R, Heidarimoghadam R. (2017) The effect of blue-enriched white light on cognitive performances and sleepiness of night-shift workers: a field study. Physiol Behav 177, 208–14. [DOI] [PubMed] [Google Scholar]
- 43.Leichtfried V, Mair-Raggautz M, Schaeffer V, Hammerer-Lercher A, Mair G, Bartenbach C, Canazei M, Schobersberger W. (2015) Intense illumination in the morning hours improved mood and alertness but not mental performance. Appl Ergon 46 Pt A, 54–9. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto Y, Nakagawa S. (2015) Effects of daytime light exposure on cognitive brain activity as measured by the ERP P300. Physiol Behav 138, 313–8. [DOI] [PubMed] [Google Scholar]
- 45.Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, Dijk DJ, Maquet P. (2011) Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms 26, 249–59. [DOI] [PubMed] [Google Scholar]
- 46.Cabeza R, Nyberg L. (2000) Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12, 1–47. [DOI] [PubMed] [Google Scholar]
- 47.Rautkylä E, Puolakka M, Halonen L. (2012) Alerting effects of daytime light exposure—a proposed link between light exposure and brain mechanisms. Light Res Technol 44, 238–52. [Google Scholar]
- 48.Hanford N, Figueiro M. (2013) Light therapy and Alzheimer’s disease and related dementia: past, present, and future. J Alzheimers Dis 33, 913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danilenko KV, Cajochen C, Wirz-Justice A. (2003) Is sleep per se a zeitgeber in humans? J Biol Rhythms 18, 170–8. [DOI] [PubMed] [Google Scholar]
- 50.Keis O, Helbig H, Streb J, Hille K. (2014) Influence of blue-enriched classroom lighting on students’ cognitive performance. Trends Neurosci Educ 3, 86–92. [Google Scholar]
- 51.Iskra-Golec I, Wazna A, Smith L. (2012) Effects of blue-enriched light on the daily course of mood, sleepiness and light perception: a field experiment. Light Res Technol 44, 506–13. [Google Scholar]
- 52.Mills PR, Tomkins SC, Schlangen LJ. (2007) The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smolders KC, de Kort YA. (2014) Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J Environ Psychol 39, 77–91. [Google Scholar]
- 54.Huang RH, Lee L, Chiu YA, Sun Y. (2015) Effects of correlated color temperature on focused and sustained attention under white LED desk lighting. Color Res Appl 40, 281–6. [Google Scholar]
- 55.Gaggioni G, Maquet P, Schmidt C, Dijk DJ, Vandewalle G. (2014) Neuroimaging, cognition, light and circadian rhythms. Front Syst Neurosci 8, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huiberts LM, Smolders KC, de Kort YA. (2015) Shining light on memory: effects of bright light on working memory performance. Behav Brain Res 294, 234–45. [DOI] [PubMed] [Google Scholar]
- 57.Correa Á, Barba A, Padilla F. (2016) Light effects on behavioural performance depend on the individual state of vigilance. PLoS One 11, e0164945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoyland A, Lawton CL, Dye L. (2008) Acute effects of macronutrient manipulations on cognitive test performance in healthy young adults: a systematic research review. Neurosci Biobehav Rev 32, 72–85. [DOI] [PubMed] [Google Scholar]
- 59.Craig A, Baer K, Diekmann A. (1981) The effects of lunch on sensory-perceptual functioning in man. Int Arch Occup Environ Health 49, 105–14. [Google Scholar]
- 60.Colquhoun WP .(1971) Circadian variations in mental efficiency (Circadian rhythms in human mental performance from waking day, round of clock and simulated shiftwork studies). Biological rhythms and human performance (A 73–33154 16–04). 39–107, Academic Press, London and New York. [Google Scholar]