Abstract
Purpose of Review
Neuropsychiatric syndromes (NPS) are common in neurodegenerative disorders (NDD). This review describes the role of NPS in the diagnosis of NDD, criteria for the diagnosis of NPS, management of NPS, and agents in clinical trials for NPS.
Recent Findings
NPS play an increasingly important role in the diagnosis of NDD. Consensus diagnostic criteria have evolved for psychosis, depression, agitation, and apathy in NDD. With one exception—pimavanserin is approved for the treatment of hallucinations and delusions in Parkinson’s disease—there are no drugs approved by the FDA for treatment of NPS in NDD. Trials show that atypical antipsychotics reduce psychosis in AD and in Parkinson’s disease, although side effect concerns have constrained their use. Antidepressants show benefit in treatment of Parkinson’s disease with depression. Several agents are in clinical trials for treatment of NPS in NDD.
Summary
Neuropsychiatric syndromes play a major role in NDD diagnosis. Clinical criteria allow recognition of NPS in NDD. Psychotropic medications are often useful in the treatment of NPS in NDD; efficacious, safe, and approved agents are needed.
Keywords: Neurodegenerative disorders, Neuropsychiatric syndromes, Alzheimer’s disease, Parkinson’s disease, Depression, Psychosis, Apathy, Agitation
Introduction
Neuropsychiatric syndromes (NPS) are common in neurodegenerative disorders (NDD). They occur in nearly all patients with Alzheimer’s disease (AD) [1–3]. NPS have many adverse consequences including distress for the patient, reduction of patient and caregiver quality of life, increased risk of institutionalization, and increased cost [4–7].Depression and psychosis are associated with more rapid cognitive decline in AD [8••]. NPS are present in the prodromal phase of AD and other NDD and increase in frequency through the course of the illnesses [3, 9–11].
Despite their high prevalence and serious consequences, the only agent approved by the Food and Drug Administration (FDA) for any NPS of any NDD is pimavanserin for treatment of hallucinations and delusions of Parkinson’s disease (PD) psychosis. Past trials of antipsychotics suggest efficacy in psychosis and agitation; interest in advancing development of these agents for NPS in NDD may be limited by loss of patent protection and the generic status of many of these drugs. In the recent past, progress has been made toward developing effective therapies for NPS. Here we review advances in the pharmacologic treatment of the NPS of AD and related dementias. We discuss the role of NPS in diagnosing NDD, the definition of NPS, current approaches to treating NPS in NDD, and clinical trials and drug development for psychotropic agents used for NPS in NDD. We emphasize novel mechanisms and innovative approaches to trials and pharmacotherapy.
Role of Neuropsychiatric Syndromes in Diagnosing Neurodegenerative Disorders
Neuropsychiatric syndromes play an increasingly important role in the diagnosis of NDD. This reflects the growing recognition of the importance of NPS as expressions of neurological disease and the unique association between NPS and specific NDD. Diagnosis of probable behavioral variant frontotemporal dementia (bvFTD) requires the presence of NPS. Other diagnostic criteria allow NPS to fulfill profiles of diagnostic criteria but do not specifically require the presence of NPS. These include the National Institute of Aging-Alzheimer’s Association (NIA-AA) definition of dementia, the NIA-AA definition of AD-type dementia (AD), the criteria for Dementia with Lewy bodies (DLB), the definition of vascular cognitive impairment (VCI), and criteria for progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). NPS are supportive of the diagnosis of PD dementia.
In the criteria for bvFTD, if the neuropsychological profile for bvFTD is met, then the patient must have at least two of the following behavioral symptoms: early behavioral disinhibition; early apathy or inertia; early loss of sympathy or empathy; early perseverative, stereotyped or compulsive/ritualistic behavior; or hyperorality and dietary changes [12]. If the neuropsychological profile is not met, then the patient must have three of the categories of behavioral changes. For bvFTD, behavioral changes represent the core changes required for the diagnosis.
The NIA-AA approach to AD begins by identifying the syndrome of all-cause dementia. This includes cognitive or behavioral symptoms that interfere with work or activities, represent a decline from a previous level of function, are not better explained by delirium or a psychiatric disorder, are well-documented by neuropsychological assessments, and have at least two of the following features: memory impairment; impaired reasoning and complex thinking; impaired visual spatial abilities; impaired language; or changes in personality, behavior, or comportment [13]. This approach to dementia allows “changes in personality, behavior, or comportment” to be one of two clinical features leading to a diagnosis of dementia. In a second step, the NIA-AA criteria define AD dementia by the presence of a dementia syndrome as previously defined plus insidious onset, and gradual worsening. It may have either an amnestic or a non-amnestic presentation. Building on the criteria for dementia allows for behavior to be one of two necessary elements for a diagnosis of AD dementia [13•].
A similar strategy for diagnosis of dementia and AD is adopted by the Diagnostic and Statistical Manual, 5th ed. [14]. This approach defines major neurocognitive disorder as having evidence of significant decline from past cognitive ability, impairment of cognitive performance sufficient to interfere with activities of daily living (ADL), and not exclusively explained by a delirium or some other mental disorder. The clinical symptoms include memory impairment, language abnormalities, visuospatial disturbance, executive dysfunction, attention impairment, and social cognition decline [15]. The inclusion of social cognition as a key feature allows major cognitive disorder to be identified by decline in empathy, sympathy, social judgment, responsiveness to social cues, and appropriate social behavior. Major neurocognitive disorder due to AD requires the presence of memory impairment plus impairment of at least one of the other domains. In this setting, compromised social cognition in addition to memory impairment would allow fulfillment of the clinical criteria for major neurocognitive disorder due to AD.
There are four core clinical features of DLB, two of which must be present to allow a diagnosis of DLB in a patient with progressive cognitive decline [16••]. These core features include fluctuating cognition, recurrent visual hallucinations, rapid eye movement (REM) sleep behavior disorder, and parkinsonism. One can make a diagnosis with no behavioral features based on fluctuating cognition and parkinsonism, but behavioral features including hallucinations and REM sleep behavior disorder are core features that can fulfill the criteria for probable DLB and are invoked in a majority of DLB diagnoses [16••].
Diagnostic criteria for PD dementia [17] identify behavioral symptoms (apathy, depressed or anxious mood, hallucinations, delusions, excessive daytime sleepiness) as supportive of the diagnosis but note that the absence of behavioral symptoms does not exclude the diagnosis.
The diagnostic criteria for corticobasal degeneration include insidious onset and gradual progression in an individual 50 years of age or older. Symptoms must be present for a minimum of 1 year and include the corticobasal syndrome or a frontobehavioral syndrome plus at least one corticobasal feature, such as asymmetric limb rigidity or bradykinesia, asymmetric limb dystonia, asymmetric limb myoclonus, orobuccal or limb apraxia, cortical sensory deficit, or alien limb phenomenon [18•]. Thus, a behavioral type of frontobehavioral syndrome plus one corticobasal syndrome feature allows the diagnosis of corticobasal degeneration.
Diagnostic criteria for PSP do not allow behavioral abnormalities to meet criteria for level 1 certainty but do allow including behavioral abnormalities (frontal cognitive/behavioral presentation) to achieve a level 2 diagnostic certainty. There are three levels of certainty in this diagnostic approach [19•].
Other sets of diagnostic criteria for cognitive disorders also include reference to behavioral features. Vascular cognitive impairment (VCI) requires onset of the clinical syndrome to be related to a cerebrovascular event and evidence of decline in frontal executive functioning, plus one of the following: gait disturbance, urinary symptoms, or personality and mood changes. There must also be evidence on computerized tomography (CT) or magnetic resonance imaging (MRI) of cerebrovascular disease. In this set of criteria, personality and mood changes can contribute to meeting the criteria for diagnosis of VCI [20•].
Only a few diagnostic criteria sets do not include an overt inclusion of a behavioral alternative to meet diagnostic criteria. The NIA-AA criteria for mild cognitive impairment (MCI) due to AD do not include reference to behavioral changes [21•]. Similarly, primary progressive aphasia, including progressive non-fluent aphasia and progressive semantic dementia, does not include specific reference to behavioral features that would allow meeting the diagnostic criteria [22•].
Review of these criteria demonstrates that behavioral features are commonly recognized in NDD and contribute importantly to diagnostic approaches. Progress in understanding the biology of NPS will likely lead to an enhanced ability to use them for classification, course prediction, treatment, and therapeutic monitoring of NDD.
Diagnostic Criteria for Neuropsychiatric Syndromes
Neuropsychiatric syndromes are to be distinguished from symptoms. Symptoms are subjective phenomena, experienced by the individual. Syndromes are constellations of clinical features, signs, symptoms, or phenomena that are recognized by a clinician and comprise a medical condition. Most clinical phenotypes discussed here are syndromes.
Specific diagnostic criteria have been developed for psychosis in AD [23•], depression in AD [24•], apathy in AD and other NDD [25•], and agitation in cognitive disorders including AD [26••]. Criteria for specific neuropsychiatric syndromes in PD include psychosis in PD [27•] and depression in PD [28•].
Consensus criteria are typically derived by panels of experts. The sensitivity, specificity, and reliability of the criteria are shown following the introduction of the criteria and they are often refined based on clinical experience. For this reason, many criteria are labeled “provisional” or “draft” when they are first published. Criteria are critical to allow a common vocabulary for diagnosis. They facilitate many types of research including epidemiology, studies of pathophysiology, relationships to burden, and financial cost.
Criteria for neuropsychiatric syndromes are critical to facilitate clinical trials. In drug development programs, diagnostic criteria for neuropsychiatric syndromes allow the identification and quantification of the unmet need. They characterize the eventual “indication” that will comprise the reason for prescribing the drug. They are critical to planning clinical trials and anticipating the challenges of recruitment. They are essential when presenting the trial results to the FDA where they allow regulatory officials to understand who is included in the trial and what specific outcomes can be anticipated. Diagnostic criteria are essential to inform prescribers once a drug is approved and to explain the use of the medication to patients, caregivers, insurers, pharmacy benefit managers, regulatory oversight agencies, and advocacy groups.
Current Approaches to Treating Neuropsychiatric Syndromes in Neurodegenerative Disorders
The usual clinical approach to treating neuropsychiatric syndromes in NDD is to extrapolate from the use of psychotropic agents used in idiopathic psychiatric disorders for similar phenomena. Approved antidepressants are used for the treatment of depression in NDD; antipsychotics are used for the treatment of psychosis (hallucinations and delusions) and agitation in NDD; mood stabilizers are used in the management of agitation in NDD; anxiolytics are applied to anxiety in NDD; stimulants are prescribed for apathy in NDD; and hypnotics are used for sleep dysregulation in NDD. This approach is based on the approval of antidepressants for the indication of major depressive disorder, antipsychotics for schizophrenia or bipolar illness, mood stabilizers for bipolar disorder, stimulants for attention deficit disorder, anxiolytics for generalized anxiety disorder, and hypnotics for insomnia. This extrapolation is based on the clinical similarity of symptoms and behaviors of the neuropsychiatric syndromes occurring in NDD and non-NDD circumstances and the absence of alternatives. The implementation of treatment by analogy has been called the “the therapeutic metaphor” for the use of psychotropic agents [29]. However, patients with NDD are excluded from trials of patients used for testing of antidepressants, antipsychotics, anxiolytics, stimulants or hypnotics, and no data concerning the use of psychotropic medications in patients with NDD are available at the conclusion of trials involving patients with conventional psychiatric disorders. The extension of these therapies from non-NDD to NDD patients is based on many untested assumptions that may result in treating patients with agents that are ineffective or have safety and tolerability issues that differ from those observed in clinical trials of patients without NDD. The change of the clinical phenotype by the features of the NDD (e.g., apathy, cognitive impairment, sleep disturbances) and the brain changes induced by the NDD may alter the efficacy or side effect profile of the psychotropic agent. Likewise, brain changes may alter the necessary doses to impact behavior. Most patients with NDD are elderly compared to those with idiopathic disorders, and this may affect the pharmacokinetics and the pharmacodynamics of an agent. Doses of psychotropics used in the elderly are often lower than doses used in idiopathic disorders occurring in younger individuals; for example, the doses of antipsychotics that appear to be efficacious and tolerated in older AD patients with agitation are much lower than those used for psychosis in younger patients with schizophrenia. Data generated on use of psychotropic agents within patients with the NDD are vital for guiding use of psychotropics in these conditions.
Treatment of NPS in NDD
Only one agent—pimavanserin—is currently approved by the US FDA for the treatment of a neuropsychiatric syndrome of a NDD. This agent is approved for the treatment of hallucinations and delusions occurring in PD psychosis [30••]. All other use of psychotropics is “off label,” although it may be necessary for best practices, based on extensive experience, and endorsed by treatment guidelines [31, 32]. Table 1 summarizes current recommendations for treatment of NPS in NDD.
Table 1.
Recommended treatments for neuropsychiatric syndromes
| Neuropsychiatric syndrome | 1st-line therapies* | 2nd-line therapies | 3rd-line therapies |
|---|---|---|---|
| Agitation in AD |
Citalopram (10–30 mg/day)** Risperidone (0.5–1 mg/day) |
Aripiprazole (10 mg/day) Carbamazepine (300 mg/day) Dextromethorphan/quinidine (20/10 mg BID) Olanzapine (5–10 mg/day) Quetiapine (200 mg/day) Trazodone (50–100 mg/day) |
Lamotrigine (25–100 mg/day) THC (2.5–7 mg/day) |
| Apathy in AD | Methylphenidate (20 mg/day) | Modafinil (200 mg/day) | |
| Depression in AD |
Citalopram (10–40 mg/day)** Escitalopram (5–20 mg) Sertraline (50–150 mg) |
Aripiprazole as augmentation (2 mg–15 mg/day) Bupropion (100 mg–300 mg/day) Carbamazepine (augmentation) (300 mg/day) Duloxetine (20–60 mg/day) Fluoxetine (20–40 mg/day) Mirtazapine (7.5–30 mg/day) Paroxetine (10–40 mg/day) Quetiapine as augmentation (25–200 mg/day) Venlafaxine (37.5–225 mg/day) |
Electroconvulsive therapy Tricyclic antidepressants |
| Depression in PD |
Pramipexole (0.3–4.2 mg/day) Ropinirole (10 mg/day) |
Citalopram (10–20 mg/day) Desipramine (25–75 mg/day)*** Nortriptyline (25–75 mg/day)*** Sertraline(25–50 mg/day) |
Electroconvulsive therapy Buproprion (100–300 mg/day) Duloxetine (30–60 mg/day) Mirtazapine(30 mg/day) Paroxetine (10–40 mg/day) Venlafaxine (37.5–225 mg/day) |
| Psychosis in PD | Pimavanserin (40 mg/day) |
Clozapine (6.25–50 mg/day) Quetiapine (25–100 mg/day) |
Risperidone (0.5–2 mg/day) Olanzapine (5–7.5 mg/day) |
*Initiation of pharmacological interventions should occur after non-pharmacological approaches, cognitive enhancers, and comprehensive assessment of medical and environmental factors has been completed
**Maximum recommended dose for citalopram in patients over the age of 60 is 20 mg/day
***TCA should not be used in patients with cognitive impairment
Overview
Psychotropics are commonly used in AD and other NDD despite the absence of a specific FDA-approved indication in this setting. It is incumbent on the clinician in these circumstances to weigh potential benefit and harm to the patient, consider the urgency and the magnitude of the threat, review the consequences of not treating, and assess the appropriateness of non-pharmacological interventions prior to initiating use of a psychotropic agent [32, 33]. A shared decision-making paradigm to include family caregivers or others is an important aspect of the decision to treat [34].
Treatment of Agitation in AD
An unanswered question concerning agitation in NDD is whether there are different types of agitation and if these differing phenoptypes may respond differentially to treatments used for agitation. For example, among patients with agitation treated with citalopram, those with the mild-moderate symptoms appeared to respond best while those with more severe agitation demonstrated less efficacy and more side effects [35]. Different brain circuits may be involved in different types of behavioral dysregulation and may respond differentially to antipsychotics, antidepressants, or mood stabilizers [36]. This question represents an area where further study is warranted.
The potential benefit of antipsychotics must be weighed against the significant risks to patients, such as cerebrovascular adverse events and mortality. Antipsychotic use may have less risk of premature death or need for medical care in cases where careful control for cardiovascular risk factors was implemented [37]. Atypical antipsychotics are more beneficial than placebo and are associated with decreased caregiver burden, but the potential for adverse effects limits their overall usefulness [38, 39]. Not all atypical antipsychotics have been studied in the context of NPS of NDD, and the class effects are not established.
There is limited evidence to support the use of typical antipsychotics to manage aggression and agitation in clinically acute settings. Efficacy of the agents is modest [40]. Haloperidol is useful in treatment of aggression with agitation (but not general agitation behaviors, such as wandering or verbal agitation) [41]. Typical antipsychotics are not recommended in non-emergent treatment of agitation in dementia [42]. The use of typical antipsychotics in NDD even in acute situations has risk.
Atypical antipsychotics have a lower risk of certain side effect including parkinsonism, acute dystonic reactions, and tardive dyskinesia. Atypicals including risperidone, olanzapine, and aripiprazole are alternatives for use in management of severe agitation, aggression, and psychosis associated with AD where there is risk of harm to the patient and/or others [43–46]. Risperidone has the best evidence for short-term efficacy (6–12 weeks) in patients with agitation [38]. A meta-analysis of four large placebo-controlled clinical trials supported risperidone’s efficacy in the management of agitation and aggression even in severely impaired AD patients [47]. In AD patients with psychosis or agitation who had responded to risperidone therapy for 4–8 months, discontinuation of risperidone was associated with an increased risk of relapse [48•]. Risperidone may be considered as an option for short-term intervention in cases of acute, treatment-resistant agitation in AD and may be useful in long-term management of agitation where the agitation syndrome has become chronic.
Studies with quetiapine have provided mixed results. Ballard et al. [49] found no treatment benefit in reducing agitation compared to placebo. Zhong et al. [50] showed improvement of agitation in patients given 200 mg of quetiapine daily.
Olanzapine has been used for treatment of psychosis in AD [45]. It has anticholinergic effects and may decrease cognition while increasing the risk of anticholinergic delirium symptoms [51]. Olanzapine use in the treatment of delusions and hallucinations in PD has resulted in exacerbation of parkinsonism [52].
Brexpiprazole, a novel compound structurally similar to aripiprazole (the mechanism of action includes reduced partial agonism for D2, 5HT1A receptors, and enhanced antagonism for 5-HT2A and α1-adrenoreceptors), is being tested for agitation associated with AD [53–55].
Data supporting use of anticonvulsants/mood stabilizers are strongest for carbamazepine [56]. Its use is limited by the risk for side effects such as dizziness, sedation, ataxia, and confusion and the more rare but significant adverse effects of inappropriate antidiuretic hormone with hyponatremia, cardiac, and hepatotoxicity [57]. Patients should be informed of the black box warnings for aplastic anemia, agranulocytosis, and rare but sometimes fatal dermatologic adverse reactions.
The evidence for valproate as a treatment for agitation is mixed, with a meta-analysis of pooled results concluding that valproate is ineffective in reducing agitation in dementia and is associated with unacceptable rates of adverse events, notably sedation [43, 58]. There is a risk that valproate may worsen agitation compared to placebo as measured by both the Neuropsychiatric Inventory (NPI) and Cohen-Mansfield Agitation Inventory (CMAI) [59]. Among other treatments from this category, topiramate showed limited efficacy [60]. Gabapentin, lamotrigine, oxycarbamazepine, and levetiracetam have been the subject of observational or uncontrolled studies and are worthy of further investigation. One study suggested that lamotrigine may be effective and may make it possible to avoid increasing the dosage of antipsychotic medications prescribed to elderly patients with cognitive impairment [61].
Trazodone, a hypnotic and antidepressant (pharmacologically, a serotonin antagonist and reuptake inhibitor), is used for management of insomnia and night time behavioral disturbances, irritability, agitation, and aggression in AD. Trazodone has a favorable safety profile if administered in small doses and appears to produce a stabilization of the circadian rhythms in individuals with AD [62••]. Because of its hypnotic properties, trazodone may be particularly useful in patients with nocturnal agitation.
There is growing interest in selective serotonin reuptake inhibitors (SSRIs) to target agitation and aggression in dementia. A recent large randomized controlled trial found that citalopram significantly reduced agitation and caregiver distress compared to placebo. Worsening cognition and QT prolongation were significantly more common in the citalopram group (30 mg/day) [63]. Patients in the study were required to have treatment-requiring levels of agitation; those with major depression or psychosis requiring antipsychotics were excluded. Assessment of the 20 mg (the maximum dose recommended by the FDA in adults over 60) has not been conducted. Comparator studies indicate sertraline and citalopram are probably as effective as risperidone in treating agitation in dementia, especially among those with mild-moderate agitation severity [64].
Dextromethorphan/quinidine, a combination drug containing dextromethorphan, an n-methyl-d-aspartate receptor antagonist and high affinity sigma-1 receptor agonist, and the antiarrhythmic agent, quinidine, is the first FDA-approved drug for the treatment of pseudobulbar affect [65]. A recent study in patients with AD demonstrated that dextromethorphan/quinidine significantly improved AD-associated agitation, reduced caregiver burden, and was generally well tolerated [66••]. Two phase 3 studies are in progress.
Tetrahydrocannabinol (THC) studies have provided conflicting evidence regarding reduction of agitation. Low-dose THC (4.5 mg daily) did not significantly reduce NPS after 3 weeks, though it was well tolerated [67]. Previous studies with THC (2.5–7 mg daily) reported positive effects on NPS in dementia [68, 69].
Treatment of Depression in AD
Studies of depression in AD have usually used the Diagnostic and Statistical Manual definition of major depression and research definitions of AD based on clinical criteria. Clinically defined populations are heterogeneous with regard to the underlying biology with many patients having AD mixed with other pathology, some having pure AD, and some having non-AD phenocopy disorders [70]. How this biological heterogeneity may affect treatment response is unknown.
Despite the widespread use of antidepressants (30–50% of patient with AD/dementia are on antidepressants), there is mixed evidence regarding the benefits from their use in depression of AD patients. Individual studies have shown a trend toward tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRIs), and serotonin and norepinephrine reuptake inhibitors (SNRI), being effective in management of depressive syndromes in patients with AD but meta-analyses do not support a reliable benefit [71]. Many trials have been carried out on small numbers of patient and were underpowered to detect drug-placebo differences. Variable trial methods, comorbid conditions and differences in the administered antidepressants further confound findings [72]. One SSRI trial with sertraline was positive, but three others with sertraline, fluoxetine, citalopram, and escitalopram were not [73]. While several smaller trials have found SSRIs to be effective, the Depression of Alzheimer’s Disease-2 (DIADS-2 trial) a large, multicenter trial found no difference between sertraline (100 mg) and placebo in patients with depression of AD [74–76•]. The study showed that the sertraline group experienced more side effects (diarrhea, dizziness, and dry mouth) and pulmonary serious adverse events. Treatment with sertraline was not associated with significantly greater reductions in caregiver distress than placebo [77]. Escitalopram (citalopram’s metabolite), although safe, failed to show significant antidepressant benefit for AD patients in one study [73].
In cases of severe depression when the patient is suicidal or when other NPS cluster together (e.g., agitation or/and aggression) or in situations of high risk of self- harm or self-neglect, general guidelines for treating depression in the geriatric population should be considered. The American Psychiatric Association (APA) recommends a trial of an antidepressant to treat clinically significant, persistent depressed mood in patients with dementia, and SSRIs are preferred because of their favorable safety profile [78]. Sertraline, citalopram, or escitalopram in low doses are the most appropriate first-line agents. Other SSRIs like fluoxetine and paroxetine are not recommended as a first-line SSRI due to uncertain efficacy or unfavorable (mostly anticholinergic) side effects [79]. The dose might be incrementally increased, if tolerated, to a maximum of 150 mg of sertraline or 40 mg of citalopram per day with close monitoring of side effects. Although improvement should occur within 4 to 6 weeks at the target dose, a longer period may be required to reach full effect [80].
If patients do not respond to the SSRI, switching to a different agent or augmenting treatment with a second agent should be considered. Following the “therapeutic metaphor” mentioned above, an atypical antipsychotic in a small dose is appropriate for patients who have psychotic symptoms or agitation along with depression [45, 81]. An anticonvulsant in smaller doses (the best evidence is for carbamazepine) might be considered as additional therapy to an antidepressant if there is moderate or severe agitation [56].
Switching antidepressants to a different class (as opposed to augmentation) is recommended in cases with severe side effects induced by the initial medication. Preferred second-line agents are SNRIs such as venlafaxine or duloxetine or antidepressants with a mixed pharmacology (mirtazapine, bupropion). Evidence for benefit from use of non-SSRI antidepressants specifically for depression in AD is lacking. Tricyclic antidepressants are generally not recommended due to anticholinergic side effects [82].
Psychiatric hospitalization should be considered an option in cases where self-harm is a threat. For patients with severe, refractory depression, electroconvulsive therapy (ECT) might be considered, especially if there is risk of self-harm or harm to others [80, 83].
Vortioxetine has a unique pharmacologic profile inhibiting the serotonin (5-HT) transporter and acting as a serotonin receptor agonist (5-HT1A), a partial agonist (5-HT1B), and an antagonist (5-HT3, 5-HT7, and 5-HT1D) at different receptors [84••]. This agent is of interest in NDD because in major depression, it has shown benefit on the digit symbol substitution test independent of its effect on mood and suggesting a beneficial effect on executive function. The effects have not been adequately studied in AD or other NDD.
Treatment of Apathy in AD
Dopaminergic reward circuitry dysfunction is strongly implicated in the symptoms of apathy in AD and drugs enhancing dopaminergic transmission have been proposed as treatment. Methylphenidate acts by blocking the dopamine and norepinephrine transporters, leading to increased concentrations of synaptic dopamine and norepinephrine. This effect in turn leads to increased dopamine and norepinephrine at receptor sites. Methylphenidate is also a weak 5-hydroxytryptamine-1A (5HT1A) receptor agonist.
Methylphenidate, but not modafinil, was shown to be effective in reducing apathy in AD in a small cross-over trial [85] and was further assessed in the larger, multicenter, double blind trial—Alzheimer’s Disease Methylphenidate Trial (ADMET) [86]. In ADMET, methylphenidate (20 mg daily for 6 weeks) was associated with a significant reduction in apathy symptoms. Two study outcomes measures—the Clinical Global Impression of Change (CGI-C) and NPI apathy score—showed diminished apathy with methylphenidate treatment. A trend toward increased Mini-Mental State Examination (MMSE) scores suggested that methylphenidate treatment was associated with improved global cognition. Adverse events and side effects were modest. The results suggest that methylphenidate treatment may have clinical utility in treating apathy of AD.
Treatment of Psychosis in PD
Pimavanserin is a selective-serotonin 5-HT2A inverse agonist. It is approved by the FDA for the treatment of hallucinations and delusions of PD psychosis. Approval of pimavanserin was based on the results of a trial in which adults with PD psychosis were randomly assigned to take 40 mg of pimavanserin or placebo daily for 6 weeks [30]. Patients taking pimavanserin experienced fewer and less severe hallucinations and delusions without worsening of the primary motor symptoms of PD. The most common adverse effects reported by patients taking pimavanserin included peripheral edema, nausea, and confusion.
Clozapine is efficacious in the treatment of PD psychosis as shown by randomized controlled trials of even very small doses (6.25 to 50 mg daily) [87, 88]. The risk of agranulocytosis and the necessity of blood monitoring with clozapine have led many experts to recommend a trial of other antipsychotics, mainly quetiapine (12.5–150 mg) before implementing use of clozapine [28, 88].
Efforts to treat psychosis of PD with antipsychotics commonly used for schizophrenia have shown limited efficacy and were associated with significant deterioration of the motor symptoms [89, 90]. Typical antipsychotics, especially potent blockers of dopaminergic receptors, are contraindicated in PD because of motor worsening.
Quetiapine is the most frequently prescribed agent targeting psychotic symptoms in PD. Trial findings are, however, inconsistent, and firm conclusions about its efficacy cannot be drawn. One open-label trial found a significant improvement in psychotic symptoms (assessed by the Brief Psychiatric Rating Scale [BPRS]) in PD patients treated with quetiapine for 12 weeks [91]. In this study, the benefit of quetiapine (mean dose 91.5 mg/daily) was comparable with a benefit of clozapine (mean dose 26 mg/daily). Several studies report significant improvement in the level of global clinical functioning of the PD patients with psychosis treated with quetiapine (Clinical Global Impression-Improvement [CGI-I] and Clinical Global Impression-Severity [CGI-S]) [91–93]. A meta-analysis of data from different trials including a total of 241 participants randomized to either quetiapine or a comparator (placebo or clozapine) [93] failed to support efficacy. All the studies show that patients taking quetiapine experienced fewer side effects than occur with other antipsychotics, but the efficacy superior to placebo was not demonstrated.
Risperidone was beneficial in managing psychotic symptoms of PDD as assessed by the BPRS and CMAI scores. In the same group, treatment with risperidone improved levels of social, occupational, and psychological functioning [94]. An unfavorable safety profile limits risperidone use.
Olanzapine was assessed for treatment of PD psychosis, but worsening of motor function and overall psychiatric symptoms were reported in up to 80% of individuals [28].
Treatment of Depression in PD
Several studies demonstrate efficacy of antidepressants for PD depression. Ideally, pharmacological and non-pharmacological intervention should be initiated concomitantly to promote optimal response in the management of PD depression.
Dopamine agonists are commonly used as a first step in the management of depression of PD if the patient is not already receiving this treatment. Studies show pramipexole (range of doses 0.3–4.2 mg/day) and ropinirole (10 mg/day) have antidepressant properties in patients with PD [95, 96]. In a 12-week randomized double-blind, placebo-controlled trial of 323 patients, pramipexole was found to improve mood independently of changes in motor function [95]. Similarly in a longer (8-month) prospective randomized study of 41 patients, pramipexole but not pergolide was effective in reducing depression [96]. As an adjunct medication, ropinirole (daily dose of 10 mg) improved both anxiety and depressive symptoms in PD patients with motor fluctuations [97]. Despite benefits, dopamine agonist use may be limited since this group of medications is linked to risk for developing impulse control disorders. Regular monitoring to capture symptoms such as pathologic gambling, hypersexuality, and overspending in the course of dopamine agonist therapy is crucial [98].
If an antidepressant agent is warranted and there is no cognitive impairment, SSRIs or TCAs can be considered as first-line therapy. The TCAs inhibit reuptake of dopamine, norepinephrine, and, to a lesser extent, serotonin. The TCAs also possess alpha-adrenergic antagonist, antimuscarinic, and antihistaminic properties. Almost all TCAs—amitriptyline, desipramine, imipramine, and nortriptyline (with the exception of doxepine)—have been demonstrated to be potent antidepressants in randomized controlled trials involving patients with PD. The secondary amine TCAs (e.g., desipramine and nortriptyline) are preferred due to better tolerability over the tertiary TCAs. Desipramine and imipramine improved motor symptoms of PD as well as mood [28, 99]. In a subset of patients, such as those with hypersalivation or overactive bladder, the antimuscarinic activity of TCAs may be of additional clinical benefit. Sedating TCAs may be helpful for the treatment of depression with insomnia. Anticholinergic properties of TCAs limit their use in PD patients with existing or emerging cognitive dysfunction. Although infrequent, the TCAs have the potential to induce cardiac conduction disturbances, and obtaining a baseline electrocardiogram prior to treatment initiation is recommended for best practice.
If the use of a TCA is limited or contra-indicated due to cognitive impairment, comorbid medical conditions, or treatment-emergent adverse effects, SSRIs can be considered as first-line treatment. In a 30-day randomized, double-blind, placebo-controlled trial, desipramine and citalopram both improved depressive symptoms compared with placebo [100]. In practice, SSRIs are well tolerated and with the exception of tremor, which is occasionally induced by SSRIs. They rarely exacerbate PD motor symptoms. In uncontrolled studies, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline were similarly effective for the treatment of depression in PD [101•]. Among the SSRIs, citalopram and sertraline are preferred due to efficacy results, tolerability, and low drug-drug interaction potential. Paroxetine is a less favorable choice due to its profile of side effects and the observations that it failed to demonstrate superiority over placebo in some randomized trials [99].
In addition to SSRIs and TCAs, several other antidepressants including bupropion, duloxetine, mirtazapine, moclobemide, nefazodone, and venlafaxine have been evaluated in controlled trials for the management of depression in PD. Both open-label and controlled data demonstrate that duloxetine and venlafaxine are well tolerated and improve depressive symptoms in patients with PD. In a 12-week open-label study of 151 patients, duloxetine was found to be well tolerated and improved depressive symptoms in patients with PD [102]. In another 12-week randomized, double-blind, placebo controlled study of 115 patients, venlafaxine-extended release was found to be as effective as paroxetine for improving depression in PD; both agents were superior to placebo [101•]. SNRIs however were found to have lower acceptability and tolerability than SSRIs in PD [102].
Non-selective monoamine oxidase (MAO) inhibitors (isocarboxazid, phenelzine, and tranylcypromine) should be avoided in levodopa-treated patients because of the risk of hypertensive crisis. The selective monoamine oxidase (MAO)-B inhibitors may be beneficial in the treatment of PD depression. An open-label, randomized, prospective, parallel group study of augmentation of levodopa with selegiline, a MAO-B inhibitor, showed that it prevented progression of minor depression in PD over a 1-year interval. MAO-B inhibitors represent an alternative treatment for patients not responsive to more widely used first choice antidepressants.
Mirtazapine, a multiple-mechanism antidepressant (presynaptic alpha-2 antagonist blocker of 5-HT2a, 5-HT2c, 5-HT3, and H-1 receptors), is a promising treatment option for depression of PD. In a randomized double-blind, placebo-controlled study involving 20 depressed PD patients, mirtazapine (30 mg/day) in combination with brief psychotherapy was superior to placebo in reducing depression [103]. Preliminary evidence suggests that mirtazapine may improve tremor and may ameliorate levodopa-induced dyskinesias [104].
ECT should be considered for patients with severe and treatment resistant PD-related depression especially if complicated by psychosis or when the patient is at high risk for self-harm. Safety of ECT is acceptable and does not produce additional cognitive impairment [105]. Motor symptoms—as well as the mood disorder—of PD often improve in the course of ECT [105].
Agents in Clinical Trials for Treatment of Neuropsychiatric Syndromes in Neurodegenerative Disorders
Review of the available treatments for NPS in NDD demonstrates the need for better understanding of the neurobiology of NPS, more information on behavioral subtypes and their treatment responsiveness, improved interventions for patients with NPS, novel trial designs to more rapidly assess efficacy and safety, and innovative approaches to regulatory review.
Table 2 summarizes the agents currently in clinical trials for neuropsychiatric syndromes in patients with neurodegenerative diseases. Agitation in AD is a particularly active area with 11 trials currently in progress. There is substantial innovation in the mechanisms of actions of these agents and there is extensive use of repurposing as a strategy for developing new therapies. For example, gabapentin, escitalopram, carbamazepine, mirtazapine, and lithium are all repurposed agents that are approved for specific indications and are being assessed in clinical trials for agitation in patients with AD. Novel agents include multi-receptor molecules such as ITI-007 and AVP-786.
Table 2.
Drugs in currently active double-blind placebo-controlled clinical trials for neuropsychiatric aspects of neurodegenerative disorders (from clinicaltrials.org; accessed May 21, 2018)
| Disorder | Neuropsychiatric syndrome | Agent | Phase | Sponsor |
|---|---|---|---|---|
| Alzheimer’s disease | Agitation | Gabapentin | 4 | University of Texas, Austin |
| Agitation | Pimavanserin | 2 | ACADIA Pharmaceuticals | |
| Agitation | Dronabinol | 2 | Johns Hopkins University | |
| Agitation | AVP-786 | 3 | Avanir Pharmaceuticals | |
| Agitation | Escitalopram | 3 | Johns Hopkins Bloomberg School of Public Health | |
| Agitation | Nabilone | 3 | Sunnybrook Health Sciences Center | |
| Agitation | Carbamazepine and mirtazapine | 3 | University of Success | |
| Agitation or psychosis | MP-101 | 2 | Mediti Pharmaceuticals Inc | |
| Agitation | Lithium | 2 | New York State Psychiatric Institute | |
| Agitation (mild) | Piromelatine | 2 | Neurim Pharmaceuticals Ltd | |
| Apathy | Methylphenidate | 3 | Johns Hopkins Bloomberg School of Public Health | |
| Sleep | Zolpidem; zoplicone | 3 | Brasilia University Hospital | |
| Lemborexant | 2 | Eisai Inc. | ||
| Suvorexant | 3 | Merck Sharp & Dohme Corp. | ||
| Dementia with Lewy bodies | REM Sleep Behavior Disorder | Nelotanserin | 2 | Axovant Sciences Ltd. |
| Parkinson’s disease | Psychosis | SEP-363856 | 2 | Sunovion |
| REM Sleep Behavior Disorder | Nelotanserin | 2 | Axovant Sciences Ltd. | |
| REM Sleep Behavior Disorder | Melatonin and clonazepam | 2 | Seoul National University Hospital | |
| Excessive sleepiness | JZP-110 | 2 | Jazz Pharmaceuticals | |
| Sleep disturbances | Melatonin | 4 | KIMJisun | |
| Depression (inadequately controlled) | Pimavanserin | 2 | ACADIA Pharmaceuticals | |
| Impulse control disorder | N-acetylcysteine | 3 | Center Hospitaliere Universitaire, Amiens | |
| Huntington’s disease | Irritable mood | SRX246 | 1/2 | Azevan Pharmaceuticals |
| Dementia* | Psychosis | Pimavanserin | 3 | ACADIA Pharmaceuticals |
*Dementia-related psychosis includes psychosis occurring in Alzheimer’s disease, vascular dementia, frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration
Sleep dysregulation in NDD is another area in which there is substantial interest. There are three sleep studies in AD. Some trials use traditional hypnotics such as zolpidem and zoplicone; others utilizing novel pharmacologic approaches are evaluating orexin antagonists including suvorexant and lemborexant. Sleep is addressed in a nelotanserin trial of REM sleep behavior disorder in patients with DLB. In addition, there is a nelotanserin study of REM sleep behavior disorder in PD; a study of melatonin and clonazepam in REM sleep behavior disorder in PD; an investigation of JZP-110 for excessive sleepiness in PD; and a study of melatonin for sleep disturbances in PD. There is a trial of SEP-363856 for the treatment of PD psychosis. Additional studies in PD include a study of pimavanserin for inadequately controlled depression and N-acetylcysteine for impulse control disorder in PD. There is a study of irritable mood using SRS246 in patients with Huntington’s disease.
Innovation in clinical trial design is also apparent in drug development programs. A novel approach in drug development for NPS of NDD is the trial of pimavanserin for treatment of dementia-related psychosis (DRP). DRP includes psychosis occurring in patients with AD, vascular dementia, FTD, PSP, and CBD. The structure of this study assumes that there is a final common pathway for the emergence of psychosis in patients with NDD and that modulation of this pathway with a 5-HT2A inverse agonist will result in amelioration of psychotic symptoms across the different NDD types. This trial is also innovative in having a withdrawal design with all patients placed on pimavanserin at the beginning of the trial; those who improve are eventually withdrawn in a double-blind phase of the trial. Withdrawal trials have the advantage of putting all patients onto medications when symptoms are present, responding immediately to the presence of symptoms without the threat of being placed on a placebo, and limiting the withdrawal population to those patients who responded in the treatment period. The withdrawal design limits the placebo effects that affect many trials of NPS in NDD.
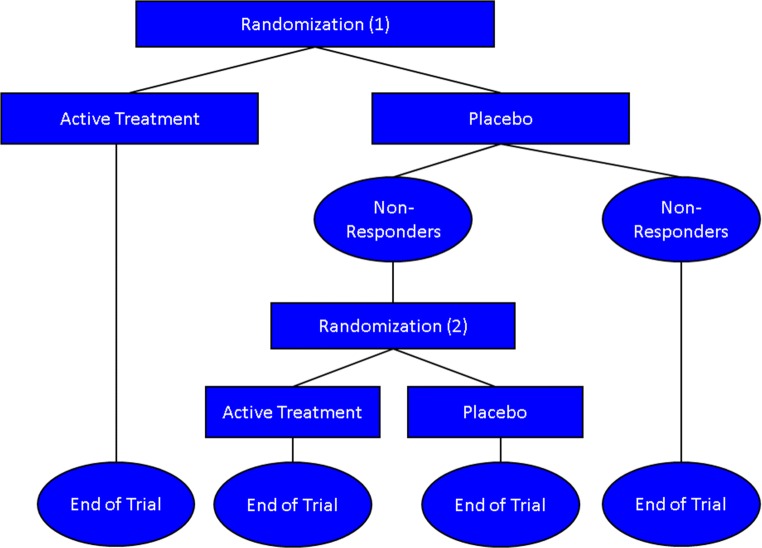
Another means of exploring and limiting placebo effects is the sequential parallel comparison design (SPCD) in which a 2-stage approach is used with the first consisting of a standard randomization to drug or placebo and the second consisting of a re-randomization of placebo non-responders to drug or placebo [106] (Fig. 1). The placebo response in the second stage is typically lower than in the first and allows drug activity to be determined. The first study using this design in NDD was in a trial of dextromethorphan/quinidine for agitation in AD [26]. The trial demonstrated a robust effect of dextromethorphan/quinidine on agitation in AD.
Fig. 1.
Serial parallel comparative design (SPCD). Placebo non-responders are re-randomized to drug or placebo
Substantial innovation is apparent in drug development for neuropsychiatric symptoms in patients with AD, and the emergence of new therapies based on a robust pipeline of new treatments in combination with novel trial designs is promising.
Conclusions
It is increasingly recognized that NPS play a major role in NDD diagnosis. The presence of NPS is required for the diagnosis of bvFTD, and NPS have a role in syndromic diagnosis of dementia, AD, PD, VCI, PSP, and CBD. Consensus clinical criteria have emerged for psychosis, apathy, agitation, and depression in AD and for psychosis and depression in PD. Treatment of NPS in NDD is based primarily on the “therapeutic metaphor” analogy [29] with idiopathic psychiatric disorders; increasing understanding of the neurobiology of NPS in NDD may provide new insights into how best to approach therapies of these complex syndromes. A review of clinical trials shows that antipsychotics can benefit psychosis and agitation in AD and methylphenidate can reduce apathy in AD. For psychosis of PD, pimavanserin is approved by the FDA and clozapine is often effective. Quetiapine is widely used but the data supporting its efficacy are variable. Antidepressant trials in AD have shown variable efficacy in improving mood; antidepressants consistently show benefit in improving mood in depression with PD. Clinical trials of emerging agents show that drugs in the pipeline have diversified mechanisms of action. There are many repurposed agents among pipeline treatments for NPS in NDD. Innovation in clinical trial designs for NPS is apparent. The pipeline promises to deliver new and more effective therapies for NPS in NDD.
Funding Information
JLC received funding from the National Institute of General Medical Sciences (Grant: P20GM109025) and support from Keep Memory Alive.
Compliance with Ethical Standards
Conflict of Interest
Aaron Ritter and Kasia Rothenberg each declare no potential conflict of interest.
Jeffrey Cummings has provided consultation to Acadia, Accera, Actinogen, ADAMAS, Alkahest, Allergan, Alzheon, Avanir, Axovant, Axsome, BiOasis Technologies, Biogen, Bracket, Eisai, Genentech, Global Alzheimer Platform, Grifols, Kyowa, Lilly, Lundbeck, MedAvante, Merck, Neurocog, Nutricia, Otsuka, QR Pharma, Resverlogix, Roche, Samus, Servier, Suven, Takeda, Toyoma, and United Neuroscience companies. JLC owns stock in ADAMAS, Alzheon, BiOasis, EIP Pharma, Prana, Sonexa, MedAvante, Neurotrax, and QR Pharma. JLC receives research support from Avid and Teva. JLC owns the copyright of the Neuropsychiatric Inventory (NPI).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Complex Medical-Psychiatric Issues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Aalten P, de Vugt ME, Lousberg R, Korten E, Jaspers N, Senden B, Jolles J, Verhey FRJ. Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2003;15(2):99–105. doi: 10.1159/000067972. [DOI] [PubMed] [Google Scholar]
- 2.Boccardi V, Conestabile Della Staffa M, Baroni M, Ercolani S, Croce MF, Ruggiero C, Mecocci P, ReGAL study group Prevalence and correlates of behavioral disorders in old age subjects with cognitive impairment: results from the ReGAl Project. J Alzheimers Dis. 2017;60(4):1275–1283. doi: 10.3233/JAD-170494. [DOI] [PubMed] [Google Scholar]
- 3.Poletti M, Nuti A, Cipriani G, Bonuccelli U. Behavioral and psychological symptoms of dementia: factor analysis and relationship with cognitive impairment. Eur Neurol. 2013;69(2):76–82. doi: 10.1159/000341956. [DOI] [PubMed] [Google Scholar]
- 4.Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17(5):403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann N, Lanctot KL, Sambrook R, Lesnikova N, Hebert R, McCracken P, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21(10):972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 6.Hurt C, Bhattacharyya S, Burns A, Camus V, Liperoti R, Marriott A, Nobili F, Robert P, Tsolaki M, Vellas B, Verhey F, Byrne EJ. Patient and caregiver perspectives of quality of life in dementia. An investigation of the relationship to behavioural and psychological symptoms in dementia. Dement Geriatr Cogn Disord. 2008;26(2):138–146. doi: 10.1159/000149584. [DOI] [PubMed] [Google Scholar]
- 7.Richardson TJ, Lee SJ, Berg-Weger M, Grossberg GT. Caregiver health: health of caregivers of Alzheimer’s and other dementia patients. Curr Psychiatry Rep. 2013;15(7):367. doi: 10.1007/s11920-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 8.Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23(2):130–140. doi: 10.1016/j.jagp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler CA, Magalhaes TF, Oliveira JM, Alves GS, Knochel C, Oertel-Knochel V, et al. Neuropsychiatric disturbances in mild cognitive impairment (MCI): a systematic review of population-based studies. Curr Alzheimer Res. 2016;13(10):1066–1082. doi: 10.2174/1567205013666160502123129. [DOI] [PubMed] [Google Scholar]
- 10.Thompson C, Brodaty H, Trollor J, Sachdev P. Behavioral and psychological symptoms associated with dementia subtype and severity. Int Psychogeriatr. 2010;22(2):300–305. doi: 10.1017/S1041610209991220. [DOI] [PubMed] [Google Scholar]
- 11.Van der Mussele S, Fransen E, Struyfs H, Luyckx J, Marien P, Saerens J, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239–1250. doi: 10.3233/JAD-140405. [DOI] [PubMed] [Google Scholar]
- 12.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.• McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. 10.1016/j.jalz.2011.03.005. These are updated criteria for the diagnosis of Alzheimer’s disease dementia based on the recommendations of an expert work group. [DOI] [PMC free article] [PubMed]
- 14.American Psychiatric Association. Diagnostic and statistical manual, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
- 15.Simpson JR. DSM-5 and neurocognitive disorders. J Am Acad Psychiatry Law. 2014;42(2):159–164. [PubMed] [Google Scholar]
- 16.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias: diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8(1):29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, Breitner JC, Bruce ML, Caine ED, Cummings JL, Devanand DP, Jeste DV, Krishnan KRR, Lyketsos CG, Lyness JM, Rabins PV, III CFR, Rovner BW, Steffens DC, Unützer J, Lebowitz BD. Provisional diagnostic criteria for depression of Alzheimer’s disease: description and review. Expert Rev Neurother. 2003;3(1):99–106. doi: 10.1586/14737175.3.1.99. [DOI] [PubMed] [Google Scholar]
- 25.Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27(1):7–17. doi: 10.1017/S1041610214001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061–1068. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- 28.Marsh L, Williams JR, Rocco M, Grill S, Munro C, Dawson TM. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology. 2004;63(2):293–300. doi: 10.1212/01.WNL.0000129843.15756.A3. [DOI] [PubMed] [Google Scholar]
- 29.Tariot PN. Treatment strategies for agitation and psychosis in dementia. J Clin Psychiatry. 1996;57:21–29. [PubMed] [Google Scholar]
- 30.Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgrad Med 2005;Spec No:6–22. [PubMed]
- 32.Kales HC, Gitlin LN, Lyketsos CG, Detroit Expert Panel on A, Management of Neuropsychiatric Symptoms of D Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Tariot P, Yaffe K. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider LS, Frangakis C, Drye LT, Devanand DP, Marano CM, Mintzer J, Mulsant BH, Munro CA, Newell JA, Pawluczyk S, Pelton G, Pollock BG, Porsteinsson AP, Rabins PV, Rein L, Rosenberg PB, Shade D, Weintraub D, Yesavage J, Lyketsos CG, for the CitAD Research Group Heterogeneity of treatment response to citalopram for patients with Alzheimer’s disease with aggression or agitation: the CitAD randomized clinical trial. Am J Psychiatry. 2016;173(5):465–472. doi: 10.1176/appi.ajp.2015.15050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez OL, Becker JT, Chang YF, Sweet RA, Aizenstein H, Snitz B, Saxton J, McDade E, Kamboh MI, DeKosky ST, Reynolds CF, III, Klunk WE. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051–1058. doi: 10.1176/appi.ajp.2013.12081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA, CATIE-AD Study Group Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed S, Rosenheck R, Lyketsos CG, Kaczynski R, Sultzer DL, Schneider LS. Effect of second-generation antipsychotics on caregiver burden in Alzheimer’s disease. J Clin Psychiatry. 2012;73(1):121–128. doi: 10.4088/JCP.10m06574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553–563. doi: 10.1111/j.1532-5415.1990.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 41.Lonergan E, Luxenberg J, Colford J. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002(2):CD002852. 10.1002/14651858.CD002852. [DOI] [PubMed]
- 42.American Psychiatric Association. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Washington, DC. 2016. [DOI] [PubMed]
- 43.Herrmann N, Lanctot KL, Hogan DB. Pharmacological recommendations for the symptomatic treatment of dementia: the Canadian Consensus Conference on the diagnosis and treatment of dementia 2012. Alzheimers Res Ther. 2013;5(Suppl 1):S5. doi: 10.1186/alzrt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Dement. 2003;18(4):205–214. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry. 2000;57(10):968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- 46.Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918–931. doi: 10.1097/JGP.0b013e3181557b47. [DOI] [PubMed] [Google Scholar]
- 47.Katz I, de Deyn PP, Mintzer J, Greenspan A, Zhu Y, Brodaty H. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer’s disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry. 2007;22(5):475–484. doi: 10.1002/gps.1792. [DOI] [PubMed] [Google Scholar]
- 48.Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, Gupta S, Colon S, Schimming C, Pelton GH, Levin B. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497–1507. doi: 10.1056/NEJMoa1114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, O’Brien J, Everratt A, Sadler S, Maddison C, Lee L, Bannister C, Elvish R, Jacoby R. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ. 2005;330(7496):874. doi: 10.1136/bmj.38369.459988.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res. 2007;4(1):81–93. doi: 10.2174/156720507779939805. [DOI] [PubMed] [Google Scholar]
- 51.Mulsant BH, Gharabawi GM, Bossie CA, Mao L, Martinez RA, Tune LE, Greenspan AJ, Bastean JN, Pollock BG. Correlates of anticholinergic activity in patients with dementia and psychosis treated with risperidone or olanzapine. J Clin Psychiatry. 2004;65(12):1708–1714. doi: 10.4088/JCP.v65n1217. [DOI] [PubMed] [Google Scholar]
- 52.Ondo WG, Levy JK, Vuong KD, Hunter C, Jankovic J. Olanzapine treatment for dopaminergic-induced hallucinations. Mov Disord. 2002;17(5):1031–1035. doi: 10.1002/mds.10217. [DOI] [PubMed] [Google Scholar]
- 53.Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Santamato A, Lozupone M, Prete C, Greco A, Pilotto A, Logroscino G. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16(17):2581–2588. doi: 10.1517/14656566.2015.1092520. [DOI] [PubMed] [Google Scholar]
- 54.Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, Yamashita H, Ito N, McQuade RD, Mork A, Pehrson AL, Hentzer M, Nielsen V, Bundgaard C, Arnt J, Stensbol TB, Kikuchi T. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 55.Maeda K, Lerdrup L, Sugino H, Akazawa H, Amada N, McQuade RD, et al. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605–614. doi: 10.1124/jpet.114.213819. [DOI] [PubMed] [Google Scholar]
- 56.Tariot PN, Erb R, Podgorski CA, Cox C, Patel S, Jakimovich L, Irvine C. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry. 1998;155(1):54–61. doi: 10.1176/ajp.155.1.54. [DOI] [PubMed] [Google Scholar]
- 57.Rothenberg KG, Wiechers IR. Antipsychotics for neuropsychiatric symptoms of dementia - safety and efficacy in the context of informed consent. Psychiatr Ann. 2015;45(7):348–353. doi: 10.3928/00485713-20150626-06. [DOI] [Google Scholar]
- 58.Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, Mintzer J, Brenner R, Schafer K, Thal L, Alzheimer’s Disease Cooperative Study. Valproate Nursing Home Study Group Divalproex sodium in nursing home residents with possible or probable Alzheimer disease complicated by agitation: a randomized, controlled trial. Am J Geriatr Psychiatry. 2005;13(11):942–949. doi: 10.1176/appi.ajgp.13.11.942. [DOI] [PubMed] [Google Scholar]
- 59.Herrmann N, Lanctot KL, Rothenburg LS, Eryavec G. A placebo-controlled trial of valproate for agitation and aggression in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(2):116–119. doi: 10.1159/000097757. [DOI] [PubMed] [Google Scholar]
- 60.Mowla A, Pani A. Comparison of topiramate and risperidone for the treatment of behavioral disturbances of patients with Alzheimer disease: a double-blind, randomized clinical trial. J Clin Psychopharmacol. 2010;30(1):40–43. doi: 10.1097/JCP.0b013e3181ca0c59. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki H, Gen K. Clinical efficacy of lamotrigine and changes in the dosages of concomitantly used psychotropic drugs in Alzheimer’s disease with behavioural and psychological symptoms of dementia: a preliminary open-label trial. Psychogeriatrics. 2015;15(1):32–37. doi: 10.1111/psyg.12085. [DOI] [PubMed] [Google Scholar]
- 62.Grippe TC, Goncalves BS, Louzada LL, Quintas JL, Naves JO, Camargos EF, et al. Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol Int. 2015;32(9):1311–1314. doi: 10.3109/07420528.2015.1077855. [DOI] [PubMed] [Google Scholar]
- 63.Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. doi: 10.1001/jama.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viscogliosi G, Chiriac IM, Ettorre E. Efficacy and safety of citalopram compared to atypical antipsychotics on agitation in nursing home residents with Alzheimer dementia. J Am Med Dir Assoc. 2017;18(9):799–802. doi: 10.1016/j.jamda.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Pioro EP, Brooks BR, Cummings J, Schiffer R, Thisted RA, Wynn D, Hepner A, Kaye R, Safety, Tolerability, and Efficacy Results Trial of AVP-923 in PBA Investigators Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693–702. doi: 10.1002/ana.22093. [DOI] [PubMed] [Google Scholar]
- 66.Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 67.van den Elsen GA, Ahmed AI, Verkes RJ, Kramers C, Feuth T, Rosenberg PB, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338–2346. doi: 10.1212/WNL.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walther S, Schupbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. 2011;31(2):256–258. doi: 10.1097/JCP.0b013e31820e861c. [DOI] [PubMed] [Google Scholar]
- 69.Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–419. doi: 10.1016/j.jagp.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 71.Sepehry AA, Lee PE, Hsiung GY, Beattie BL, Jacova C. Effect of selective serotonin reuptake inhibitors in Alzheimer’s disease with comorbid depression: a meta-analysis of depression and cognitive outcomes. Drugs Aging. 2012;29(10):793–806. doi: 10.1007/s40266-012-0012-5. [DOI] [PubMed] [Google Scholar]
- 72.Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011;59(4):577–585. doi: 10.1111/j.1532-5415.2011.03355.x. [DOI] [PubMed] [Google Scholar]
- 73.An H, Choi B, Park KW, Kim DH, Yang DW, Hong CH, Kim SY, Han SH. The effect of escitalopram on mood and cognition in depressive Alzheimer’s disease subjects. J Alzheimers Dis. 2017;55(2):727–735. doi: 10.3233/JAD-160225. [DOI] [PubMed] [Google Scholar]
- 74.Martin BK, Frangakis CE, Rosenberg PB, Mintzer JE, Katz IR, Porsteinsson AP, Schneider LS, Rabins PV, Munro CA, Meinert CL, Niederehe G, Lyketsos CG. Design of depression in Alzheimer’s disease study-2. Am J Geriatr Psychiatry. 2006;14(11):920–930. doi: 10.1097/01.JGP.0000240977.71305.ee. [DOI] [PubMed] [Google Scholar]
- 75.Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Weintraub D, Porsteinsson AP, Schneider LS, Rabins PV, Munro CA, Meinert CL, Lyketsos CG, DIADS-2 Research Group Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):136–145. doi: 10.1097/JGP.0b013e3181c796eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weintraub D, Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, et al. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. Am J Geriatr Psychiatry. 2010;18(4):332–340. doi: 10.1097/JGP.0b013e3181cc0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flynn Longmire CV, Drye LT, Frangakis CE, Martin BK, Meinert CL, Mintzer JE, Munro CA, Porsteinsson AP, Rabins PV, Rosenberg PB, Schneider LS, Weintraub D, Lyketsos CG, DIADS-2 Research Group Is sertraline treatment or depression remission in depressed Alzheimer patients associated with improved caregiver well being? Depression in Alzheimer’s disease study 2. Am J Geriatr Psychiatry. 2014;22(1):14–24. doi: 10.1016/j.jagp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Disease APAWGoAs, other D. Rabins PV, Blacker D, Rovner BW, Rummans T, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(12 Suppl):5–56. [PubMed] [Google Scholar]
- 79.Petracca GM, Chemerinski E, Starkstein SE. A double-blind, placebo-controlled study of fluoxetine in depressed patients with Alzheimer’s disease. Int Psychogeriatr. 2001;13(2):233–240. doi: 10.1017/S104161020100761X. [DOI] [PubMed] [Google Scholar]
- 80.Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243–252. doi: 10.1016/S0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- 81.Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999;60(2):107–115. doi: 10.4088/JCP.v60n0207. [DOI] [PubMed] [Google Scholar]
- 82.Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, Lyketsos C. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22(3):346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 83.Hausner L, Damian M, Sartorius A, Frolich L. Efficacy and cognitive side effects of electroconvulsive therapy (ECT) in depressed elderly inpatients with coexisting mild cognitive impairment or dementia. J Clin Psychiatry. 2011;72(1):91–97. doi: 10.4088/JCP.10m05973gry. [DOI] [PubMed] [Google Scholar]
- 84.McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 2016;19:pyw055. doi: 10.1093/ijnp/pyw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, Lanctôt KL. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg PB, Lanctot KL, Drye LT, Herrmann N, Scherer RW, Bachman DL, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810–816. doi: 10.4088/JCP.12m08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollak P, Tison F, Rascol O, Destee A, Pere JJ, Senard JM, et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689–695. doi: 10.1136/jnnp.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldman JG, Holden S. Treatment of psychosis and dementia in Parkinson’s disease. Curr Treat Options Neurol. 2014;16(3):281. doi: 10.1007/s11940-013-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedman JH. Parkinson’s disease psychosis 2010: a review article. Parkinsonism Relat Disord. 2010;16(9):553–560. doi: 10.1016/j.parkreldis.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Ballard C, Isaacson S, Mills R, Williams H, Corbett A, Coate B, Pahwa R, Rascol O, Burn DJ. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16(10):898 e1–898 e7. doi: 10.1016/j.jamda.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 91.Morgante L, Epifanio A, Spina E, Zappia M, Di Rosa AE, Marconi R, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27(4):153–156. doi: 10.1097/01.wnf.0000136891.17006.ec. [DOI] [PubMed] [Google Scholar]
- 92.Fernandez HH, Trieschmann ME, Burke MA, Jacques C, Friedman JH. Long-term outcome of quetiapine use for psychosis among parkinsonian patients. Mov Disord. 2003;18(5):510–514. doi: 10.1002/mds.10374. [DOI] [PubMed] [Google Scholar]
- 93.Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol. 2016;29(4):227–236. doi: 10.1177/0891988716640378. [DOI] [PubMed] [Google Scholar]
- 94.Workman RH, Jr, Orengo CA, Bakey AA, Molinari VA, Kunik ME. The use of risperidone for psychosis and agitation in demented patients with Parkinson’s disease. J Neuropsychiatr Clin Neurosci. 1997;9(4):594–597. doi: 10.1176/jnp.9.4.594. [DOI] [PubMed] [Google Scholar]
- 95.Barone P, Scarzella L, Marconi R, Antonini A, Morgante L, Bracco F, Zappia M, Musch B, and the Depression/Parkinson Italian Study Group Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601–607. doi: 10.1007/s00415-006-0067-5. [DOI] [PubMed] [Google Scholar]
- 96.Rektorova I, Rektor I, Bares M, Dostal V, Ehler E, Fanfrdlova Z, Fiedler J, Klajblova H, Kulist’ak P, Ressner P, Svatova J, Urbanek K, Veliskova J. Pramipexole and pergolide in the treatment of depression in Parkinson’s disease: a national multicentre prospective randomized study. Eur J Neurol. 2003;10(4):399–406. doi: 10.1046/j.1468-1331.2003.00612.x. [DOI] [PubMed] [Google Scholar]
- 97.Rektorova I, Balaz M, Svatova J, Zarubova K, Honig I, Dostal V, Sedlackova S, Nestrasil I, Mastik J, Bares M, Veliskova J, Dusek L. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol. 2008;31(5):261–266. doi: 10.1097/WNF.0b013e31815d25ce. [DOI] [PubMed] [Google Scholar]
- 98.Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67(7):1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- 99.Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Buyske S, Bienfait K, Dicke A. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009;72(10):886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devos D, Dujardin K, Poirot I, Moreau C, Cottencin O, Thomas P, Destée A, Bordet R, Defebvre L. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord. 2008;23(6):850–857. doi: 10.1002/mds.21966. [DOI] [PubMed] [Google Scholar]
- 101.Richard IH, McDermott MP, Kurlan R, Lyness JM, Como PG, Pearson N, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology. 2012;78(16):1229–1236. doi: 10.1212/WNL.0b013e3182516244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pae CU. Use of antidepressants for depression in patients with Parkinson’s disease. Expert Opin Pharmacother. 2013;14(3):255–257. doi: 10.1517/14656566.2013.759213. [DOI] [PubMed] [Google Scholar]
- 103.Chen JJ, Marsh L. Depression in Parkinson’s disease: identification and management. Pharmacotherapy. 2013;33(9):972–983. doi: 10.1002/phar.1314. [DOI] [PubMed] [Google Scholar]
- 104.Pact V, Giduz T. Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology. 1999;53(5):1154. doi: 10.1212/WNL.53.5.1154-a. [DOI] [PubMed] [Google Scholar]
- 105.Borisovskaya A, Bryson WC, Buchholz J, Samii A, Borson S. Electroconvulsive therapy for depression in Parkinson’s disease: systematic review of evidence and recommendations. Neurodegener Dis Manag. 2016;6(2):161–176. doi: 10.2217/nmt-2016-0002. [DOI] [PubMed] [Google Scholar]
- 106.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72(3):115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]