Abstract
Children with chronic kidney disease suffer from excessive cardiovascular mortality and early alterations of the cardiovascular system. Tissue doppler imaging is a validated echocardiographic tool to assess early systolic and diastolic cardiac dysfunction. We hypothesized that tissue Doppler velocities would reveal reduced cardiac function in children with chronic kidney disease compared to healthy children. A standardized echocardiographic exam was performed in 128 patients of the Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) Study aged 6–17 years with an estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2. Tissue Doppler measurements included early (E’) and late (A’) diastolic and systolic (S’) velocity at the mitral and septal annulus of the left ventricle. Measured values were normalized to z-scores using published reference data. Predictors of E’/A’, E/E’, S’ and left ventricular mass index (LVMI) were assessed by multiple linear regression analyses. Tissue Doppler E’ was reduced and tissue Doppler A’ increased, resulting in a reduced tissue Doppler E’/A’ ratio (z-score −0.14, p < 0.0001) indicating reduced diastolic function compared to healthy children. Reduced tissue Doppler E’/A’ Z-Scores were independently associated with lower eGFR (p = 0.002) and increased systolic blood pressure (p = 0.02). While E/E’ Z-Scores were increased (Z-score 0.57, p < 0.0001), patients treated with pharmacological RAS blockade but not with other antihypertensive treatments had significantly lower E/E’ and higher E’/A’ Z-Scores. Systolic tissue Doppler velocities were significantly decreased (Z-score −0.24, p = 0.001) and inversely correlated with E/E’ Z-Scores (r = −0.41, p < 0.0001). LVMI was not associated with systolic or diastolic tissue Doppler velocities. Concentric left ventricular hypertrophy showed a tendency to lower S’ in multivariate analysis (p = 0.13) but no association to diastolic function. Concentric left ventricular geometry was significantly associated with lower midwall fractional shortening. In summary, systolic and diastolic function assessed by tissue Doppler is impaired. eGFR, systolic blood pressure and the type of antihypertensive medications are significant predictors of diastolic function in children with CKD. Left ventricular morphology is largely independent of tissue Doppler velocities. Tissue Doppler velocities provide sensitive information about early left ventricular dysfunction in this population.
Subject terms: Hypertension, Atherosclerosis, Carotid artery disease, Chronic kidney disease
Introduction
Chronic kidney disease (CKD) is associated with type 4 cardiorenal syndrome and an excessive mortality from cardiovascular disease (CVD). The rate of fatal cardiac events is highly increased in children on dialysis or after kidney transplantation compared to the general population and is the leading cause of death in this patient population1. Children with CKD are the population with the highest risk for cardiovascular disease. Alterations of the cardiovascular system can be identified at an early age in pediatric populations1,2.
Non-invasive exercise tests have shown impaired cardiac performance indices in asymptomatic adult pre-dialysis CKD patients3. Likewise in children with CKD, subclinical systolic dysfunction has been described using sophisticated echocardiographic measurements such as strain analysis and endocardial and midwall fractional shortening, while ejection fraction is usually normal4,5. These findings add to conventional echocardiographic studies with distinct changes in cardiac morphology and geometry in children with CKD4–7. Tissue Doppler measurements are a reasonable echocardiographic tool in pediatrics since they are non-invasive, hardly time-consuming, widely available and software independent compared to other measurements. The measurement of diastolic indices by Tissue doppler is of particular interest since diastolic dysfunction is regarded as the main abnormality in heart failure with preserved ejection fraction and in adults it is predictive of progress to overt heart failure8. Diastolic dysfunction has also been described as an integral part of cardiorenal syndrome type 4, which is increasingly diagnosed in adults with CKD and is associated with increased mortality rates up to an odds ratio of 4.4 for an eGFR of 40 ml/min/1.73 m2 9,10. Accordingly, the use of tissue Doppler imaging for evaluation of both diastolic and systolic function has been recommended by the American Society of Echocardiography and the European Society of Cardiology11,12.
Tissue Doppler measurements include both diastolic and systolic measurements (early diastolic mitral annulus velocity E’, late diastolic velocity A’, systolic velocity S’). Derived measures are the E’/A’ ratio describing diastolic function and conventional E to tissue Doppler E’ ratio (E/E’ ratio), a surrogate of left ventricular filling pressure.
Few tissue Doppler studies, in small cohorts, have been conducted to date to evaluate both myocardial diastolic or systolic function in pediatric kidney disease13–16. Since pediatric reference values have been provided, quantification of abnormal states across the pediatric age range is readily possible17.
The present study aimed to describe diastolic and systolic function by tissue Doppler parameters in a large cohort of children with CKD and to identify factors associated with impaired systolic or diastolic function.
Results
Subject characteristics and conventional echocardiographic parameters
The characteristics of the 128 patients are shown in Table 1. BP was elevated and increased with CKD stage. Serum parameters for albumin, hemoglobin and bicarbonate were stable across CKD stages, whereas serum inorganic phosphorus and iPTH levels increased with CKD stage. 61% of all patients were on antihypertensive medication; 44.5% received RAS inhibitors. 19.5% of patients were treated with combined antihypertensive medications, including a RAS inhibitor in 11.7%. For further description of antihypertensive medication, see Table 1.
Table 1.
Subject characteristics in 128 children with CKD. Findings are stratified by CKD stage.
| N | All | CKD 2-3b | CKD 4–5 | Dialysis |
|---|---|---|---|---|
| 128 | 46 | 69 | 13 | |
| Age (years) | 12.7 ± 3.5 | 12.8 ± 3.25 | 12.4 | 14.3 |
| % male | 72.7 | 69.6 | 73.9 | 76.9 |
| BMI z-score | 0.16 ± 1.10 | 0.13 ± 1.22 | 0.19 ± 1.08 | 0.07 ± 0.83 |
| Height z-score | −0.98 ± 1.08 | −0.83 ± 0.91 | −0.96 ± 1.08 | −1.66 ± 1.44 |
| Systolic BP z-score | 0.59 ± 1.14 | 0.46 ± 1.11 | 0.54 ± 1.10 | 1.33 ± 1.26 |
| Diastolic BP z-score | 0.31 ± 0.97 | 0.28 ± 0.83 | 0.3 ± 1.03 | 0.41 ± 1.16 |
| eGFR (ml/min/1.73 m2) | 25.8 ± 11.4 | 38.1 ± 6.83 | 21 ± 4.55 | n.a. |
| Serum albumin (g/L) | 39.9 ± 3.67 | 39.7 ± 3.71 | 40.3 ± 3.47 | 37.6 ± 4.12 |
| Serum hemoglobin (g/dl) | 11.9 ± 1.77 | 12.3 ± 2.3 | 11.8 ± 1.35 | 11.09 ± 1.37 |
| Serum LDL cholesterol (mg/dl) | 106 ± 36 | 105 ± 35 | 110 ± 37 | 85 ± 23 |
| Serum HDL cholesterol (mg/dl) | 53.6 ± 13.8 | 56.4 ± 15.0 | 52.0 ± 13.1 | 51.4 ± 11.6 |
| Serum bicarbonate (mM) | 22.9 ± 3.66 | 23.6 ± 2.78 | 21.9 ± 3.75 | 25.6 ± 4.5 |
| Serum calcium (mM) | 2.35 ± 0.18 | 2.33 ± 0.15 | 2.34 ± 0.20 | 2.46 ± 0.18 |
| Serum phosphate (mM) | 1.53 ± 0.29 | 1.40 ± 0.21 | 1.59 ± 0.31 | 1.69 ± 0.29 |
| Serum iPTH (pmol/l) | 13.7 (19.5) | 11.6 (32.7) | 15.3 (17.1) | 17.0 (18.4) |
| CRP (mg/l) | 0.39 (0.9) | 0.3 (0.91) | 0.37 (0.62) | 0.7 (1.57) |
| Albuminuria (mg/g creatinine) | 288 (849) | 177 (452) | 324 (967) | 618 (1197) |
| Antihypertensive medication (n, %) | 78 (60.9) | 25 (54.3) | 45 (65.2) | 8 (61.5) |
| RAS Inhibitor | 57 (44.5) | 21 (45.7) | 31 (44.9) | 5 (38.5) |
| Calcium blockers | 25 (19.5) | 6 (13) | 18 (26.1) | 1 (7.7) |
| Diuretics | 9 (7) | 1 (2.2) | 5 (7.3) | 3 (23.1) |
| Other BP medication | 18 (14.1) | 1 (2.2) | 13 (18.8) | 4 (30.8) |
| Mono- or combination therapy | ||||
| RAS Inhibitor monotherapy | 42 (32.8) | 18 (39.1) | 20 (29.0) | 4 (30.8) |
| RAS Inhibitor + other AHT | 15 (11.7) | 3 (6.5) | 11 (15.9) | 1 (7.7) |
| Non-RAS inhibitor monotherapy | 11 (8.6) | 3 (6.5) | 7 (10.1) | 1 (7.7) |
| Non-RAS combination therapy | 10 (7.8) | 1 (2.2) | 7 (10.1) | 2 (15.4) |
| Other medications | ||||
| Vitamin D supplement | 32 (25) | 9 (19.6) | 21 (30.4) | 2 (15.4) |
| Calcitriol | 74 (57.8) | 20 (43.5) | 46 (66.7) | 8 (61.5) |
| Phosphate binders | 54 (42.2) | 11 (23.9) | 30 (43.5) | 13 (100) |
| Erythropoiesis stimulating agent | 54 (42.2) | 12 (26.1) | 31 (44.9) | 11 (84.6) |
Values are Mean ± SD or Median (IQR) as appropriate; eGFR, estimated Glomerular Filtration Rate; BP, Blood Pressure; RAS, Renin Angiotensin System; AHT, Antihypertensive Therapy.
Diastolic function by conventional and tissue Doppler
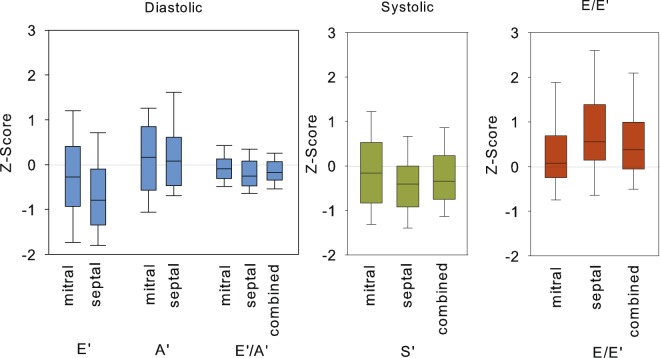
Diastolic function measured by conventional Doppler (E and E/A) ratio over the mitral valve was not different among CKD groups (Table 2). Tissue Doppler results are shown in Table 3 (TD Z-Scores), Table S1 (Unadjusted TD velocities) and Fig. 1 (TD Z-Scores). All correlation and regression analysis were performed with tissue Doppler Z-Scores. While diastolic TD velocities of the patients were mostly within normal ranges, Z-Scores of the early diastolic TD velocity (E’) were reduced significantly while the late diastolic TD velocity (A’) was significantly increased compared to a previously published healthy population17 (Table 3, Fig. 1). This resulted in a decrease of the TD E’/A’ ratio Z-Scores, suggesting reduced diastolic function compared to healthy controls. The reduction of TD E’/A’ Z-Scores were more pronounced at the septum. Diastolic TD velocities E’ and A’ Z-Scores were highly correlated to each other and to systolic TD velocity Z-Score (S’) and E/E’ Z-Scores (Table S2, Supplement). Univariate analysis revealed significantly decreased TD E’/A’ Z-Scores in dialysis patients and patients with CKD stage 4–5 compared to patients with milder chronic kidney disease (p < 0.05). In multivariate analysis, reduced TD E’/A’ Z-Scores were associated with lower eGFR and increased systolic blood pressure. Patients treated with RAS inhibitors had a higher TD E’/A’ Z-Score ratio compared to untreated patients (TD E’/A’ −0.07 ± 0.31 vs −0.20 ± 0.29, p = 0.02) in univariate and multivariate analysis.
Table 2.
Conventional echocardiographic findings in 128 children with CKD, stratified by CKD stage.
| N | All | CKD 2-3b | CKD 4-5 | Dialysis |
|---|---|---|---|---|
| 128 | 46 | 69 | 13 | |
| LVMI (g/m2.16 + 0.09) | 45.2 ± 10.4 | 41.2 ± 8.15 | 46.5 ± 10.5 | 50.6 ± 13.5 |
| Relative Wall Thickness | 0.43 ± 0.25 | 0.42 ± 0.11 | 0.43 ± 0.13 | 0.49 ± 0.20 |
| LV hypertrophy | 60 (46.9%) | 16 (34.8%) | 35 (50.7%) | 9 (69.2%) |
| Eccentric | 20 (15.6%) | 5 (10.9%) | 22 (20.3%) | 1 (7.7%) |
| Concentric | 40 (31.2%) | 22 (23.9%) | 14 (30.4%) | 8 (61.5%) |
| Concentric LV remodeling | 38 (29.7%) | 16 (34.8%) | 22 (31.9%) | — |
| Endocardial fractional shortening (EFS) | 37.9 ± 7.72 | 39.5 ± 8.19 | 36.3 ± 7.49 | 40.6 ± 5.60 |
| Midwall fractional shortening (MFS) | 16.4 ± 3.58 | 17.3 ± 3.48 | 15.8 ± 3.58 | 15.9 ± 3.54 |
| E | 99.3 ± 16.8 | 98.2 ± 14.6 | 99.4 ± 1.85 | 103.1 ± 17.15 |
| E/A Ratio | 1.89 ± 0.57 | 1.94 ± 0.55 | 1.86 ± 0.61 | 1.87 ± 0.46 |
| IVS diastolic (cm) | 0.90 ± 0.19 | 0.86 ± 0.16 | 0.91 ± 0.18 | 0.96 ± 0.26 |
| LVID diastolic (cm) | 3.95 ± 0.56 | 3.95 ± 0.54 | 3.95 ± 0.57 | 3.99 ± 0.63 |
| PW diastolic (cm) | 0.86 ± 0.19 | 0.84 ± 0.16 | 0.86 ± 0.19 | 0.98 ± 0.25 |
| IVS systolic (cm) | 1.4 ± 1.27 | 1.59 ± 2.06 | 1.26 ± 0.27 | 1.45 ± 0.24 |
LVMI, Left ventricular mass index; E, Early diastolic velocity (cm/s); A, Atrial; E/A ratio Early to atrial diastolic Doppler flow velocity; IVS Interventricular septum (cm); LVID Left ventricular internal diameter (cm); PW Posterior wall (cm).
Table 3.
Tissue Doppler Results in 128 children with CKD, stratified by CKD stage. Tissue Doppler Velocities (Z-Scores, Mean ± SD).
| All | CKD 2-3b | CKD 4-5 | Dialysis | |
|---|---|---|---|---|
| E’ | ||||
| Mitral anular | −0.24 ± 1.07* | −0.14 ± 1.20 | −0.24 ± 0.99 | −0.62 ± 0.94 |
| Septal | −0.69 ± 1.02*** | −0.60 ± 1.10 | −0.77 ± 1.00 | −0.58 ± 0.88 |
| Combined | −0.45 ± 0.94*** | −0.37 ± 1.05 | −0.48 ± 0.90 | −0.60 ± 0.77 |
| A’ | ||||
| Mitral anular | 0.21 ± 1.11* | −0.02 ± 0.97B;C | 0.21 ± 1.11A | 1.01 ± 1.35A |
| Septal | 0.22 ± 0.96*** | 0.04 ± 1.02 | 0.33 ± 0.95 | 0.29 ± 0.74 |
| Combined | 0.23 ± 0.95* | 0.01 ± 0.90B | 0.30 ± 0.96A | 0.65 ± 0.89 |
| S’ | ||||
| Mitral anular | −0.11 ± 1.03 | −0.17 ± 1.05 | −0.10 ± 1.01 | 0.09 ± 1.10 |
| Septal | −0.38 ± 0.93*** | −0.39 ± 0.81 | −0.43 ± 1.03 | −0.09 ± 0.81 |
| Combined | −0.24 ± 0.85* | −0.28 ± 0.84 | −0.26 ± 0.86 | 0.002 ± 0.90 |
| E’/A’ (diastolic function) | ||||
| Mitral anular | −0.08 ± 0.34* | 0.03 ± 0.35C | −0.09 ± 0.31C | −0.40 ± 0.18B |
| Septal | −0.20 ± 0.40*** | −0.10 ± 0.43 | −0.26 ± 0.36 | −0.19 ± 0.40 |
| Combined | −0.14 ± 0.3*** | −0.03 ± 0.34B,C | −0.18 ± 0.28A | −0.29 ± 0.18 A |
| E/E’ (LV compliance) | ||||
| Mitral anular | 0.34 ± 0.98** | 0.18 ± 0.83 | 0.35 ± 1.01 | 0.82 ± 1.18 |
| Septal | 0.81 ± 1.21*** | 0.63 ± 1.10 | 0.95 ± 1.22 | 0.85 ± 1.54 |
| Combined | 0.57 ± 1.00*** | 0.40 ± 0.89 | 0.64 ± 1.01 | 0.83 ± 1.31 |
*p < 0.05;**p < 0.001;***p < 0.0001 compared to reference population.
A: different from CKD 2-3b; B: different from CKD 4–5; C: different from Dialysis (all p < 0.05).
Figure 1.
Distribution of left ventricular tissue Doppler measures in 128 children with CKD. Data are expressed as Z-scores. (LVFP = Left ventricular filling pressure, boxes and whiskers: Median, 10th, 25th, 75th and 90th percentiles).
E/E’ Z-Score, a surrogate for left ventricular filling pressure was significantly elevated both at the mitral and septal annulus (Table 3, Fig. 1). E/E’ Z-Score was highly negatively correlated to systolic TD velocity Z-Score (Fig. 2). While E/E’ Z-Score was not associated to either eGFR or systolic BP SDS, patients treated with RAS inhibitors had significantly lower E/E’ Z-Score than patients treated with other antihypertensive drug classes or receiving no treatment in univariate and multivariate analysis (Table 4). The Z-Score for E/E’ in patients with RAS inhibition was 0.32 ± 0.64 vs 0.76 ± 1.18 without RAS inhibition (p = 0.02) in univariate analysis.
Figure 2.
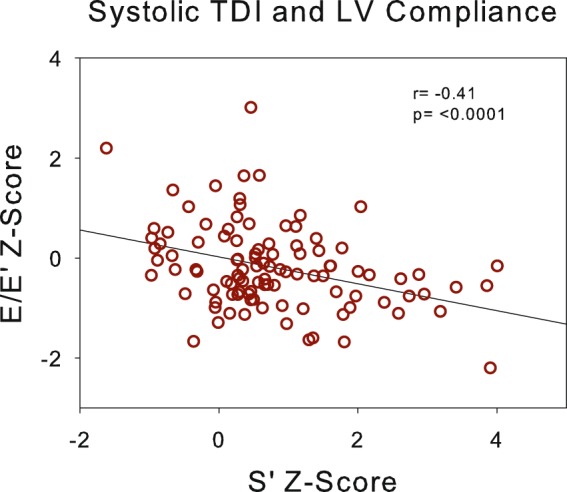
Association of left ventricular filling pressure (E/E’) with systolic tissue Doppler Velocity (S’).
Table 4.
Multivariable models of Tissue Doppler velocities, including E’/A’, E/E’ and S’.
| E’/A' | E/E’ | S' | ||||
|---|---|---|---|---|---|---|
| Estimate | P | Estimate | P | Estimate | P | |
| Intercept | −0.28 ± 0.14 | 0.05 | 0.09 ± 0.50 | 0.86 | −0.59 ± 0.39 | 0.14 |
| Age (years) | −0.008 ± 0.008 | 0.27 | 0.04 ± 0.03 | 0.2 | 0.05 ± 0.02 | 0.03 |
| Height Z-score | −0.03 ± 0.03 | 0.35 | −0.26 ± 0.10 | 0.008 | −0.001 ± 0.08 | 0.98 |
| Male sex | 0.57 ± 0.16 | 0.0009 | ||||
| eGFR | 0.008 ± 0.002 | 0.002 | −0.003 ± 0.008 | 0.71 | −0.01 ± 0.006 | 0.07 |
| Systolic BP Z-score | −0.07 ± 0.02 | 0.02 | ||||
| ACEi/ARB therapy | 0.13 ± 0.05 | 0.02 | −0.37 ± 0.19 | 0.05 | ||
| E/E' | −0.05 ± 0.03 | 0.08 | −0.40 ± 0.08 | <0.0001 | ||
| Concentric LV hypertrophy | 0.08 ± 0.06 | 0.18 | −0.25 ± 0.16 | 0.13 | ||
eGFR, estimated Glomerular Filtration rate (ml/min/1.73 m2); ACE, Angiotensine converting enzyme inhibitor; ARB, Angiotensine receptor blockade, E/E’ Early conventional to tissue Doppler diastolic velocity (cm/s); LV, left ventricular.
Systolic function
Systolic tissue Doppler velocity Z-Scores were again mostly within normal ranges, however significantly reduced at the septal but not at the mitral annulus (Table 3, Fig. 1) compared to healthy controls. As described above, systolic function declined with increasing E/E’ Z-Scores. By multivariate analysis renal function and blood pressure were not associated with systolic tissue Doppler velocity Z-Scores, nor was any other recorded clinical or biochemical parameter predictive of reduced systolic function. Systolic function assessed by tissue Doppler Z-Scores was slightly lower in patients with concentric LVH in multivariate analysis. Systolic function assessed by midwall fractional shortening (MFS) and endocardial fractional shortening (EFS) were highly inter-correlated (r = 0.66, p < 0.0001, Table S2), MFS was positively correlated to E’ (r = 0.27, p < 0.05) and E’/A’ Z-Scores (r = 0.21, p < 0.05), no other tissue Doppler measurement was correlated to MFS or EFS.
Left ventricular hypertrophy (LVH) and left ventricular geometry
Conventional echocardiographic analysis showed elevated LVMI and a high prevalence of LVH, which increased with declining renal function and was highest in dialysis patients (Table 2). Relative wall thickness was also increased, resulting in classification as concentric LV remodeling or concentric LV hypertrophy depending on LVMI in a substantial percentage of patients (Table 2).
LVMI increased with age, was negatively associated with height and higher in male patients. It was associated with systolic function expressed by midwall fractional shortening in univariate and multivariate analysis but not with Doppler velocity Z-Scores or systolic BP SDS (Tables S3 and S4). Concentric left ventricular remodeling and concentric LVH were associated with increased systolic BP SDS and reduced midwall fractional shortening but not with tissue Doppler velocities in multivariable logistic regression (Table 5). Left ventricular systolic tissue Doppler velocity Z-Scores showed a borderline negative association with concentric LV hypertrophy in multivariable analysis (Table 4).
Table 5.
Multivariable logistic regression model for Concentric LVH and Concentric Remodeling
| Concentric LVH | Concentric Geometry | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.22 (1.05, 1.42) | 0.011 | 0.991 (0.85, 1.15) | 0.903 |
| Height Z-score | 0.65 (0.40, 1.07) | 0.090 | 1.441 (0.84, 2.48) | 0.185 |
| Male sex | 4.78 (1.36, 16.79) | 0.015 | 1.37 (0.42, 4.44) | 0.602 |
| eGFR | 1.00 (0.95, 1.04) | 0.826 | 1.035 (0.99, 1.08) | 0.149 |
| Systolic BP Z-score | 1.83 (1.17, 2.86) | 0.008 | 1.61 (1.03, 2.52) | 0.036 |
| MFS | 0.79 (0.68, 0.92) | 0.002 | 0.59 (0.48, 0.73) | <0.0001 |
eGFR, estimated Glomerular Filtration rate (ml/min/1.73 m2; MFS, midwall fractional shortening; LVH, left ventricular hypertrophy.
Discussion
This study in a large cohort of children and adolescents with CKD provides novel information about systolic and diastolic left ventricular function in this condition. Our study encompassed a comprehensive analysis of tissue Doppler derived indicators of diastolic function (early E’, late A’ wave, E’/A’, E/E’) and systolic function (S’). Measurements were normalized to age-independent z-scores17. We demonstrate a significant reduction of systolic and diastolic function in children with CKD compared to healthy children. A critical impact of renal function and blood pressure on diastolic function and a significant association of antihypertensive treatment with RAS blockade with improved left ventricular filling pressure (E/E’ Z-Score) and diastolic function (E’/A’ Z-Score) was evident. In turn, systolic function (S’ Z-Score) was positively influenced by reduced left ventricular filling pressure.
Smaller studies have obtained tissue Doppler measurements in children with CKD, but their interpretation of the results was usually based on small control cohorts of healthy children. Z-Scores based on published reference data have not been calculated. We therefore demonstrated that available normal data can and should be used to calculate valid Z-scores to interpret tissue Doppler measurements in children.
While there was a high prevalence of LVH typical of many CKD and dialysis cohorts1,18,19, there was no significant association of diastolic function assessed by tissue Doppler imaging to left ventricular mass or geometry and only a borderline association of systolic TD velocity (S’ Z-Score) to LV hypertrophy. Midwall fractional shortening was the only functional measure associated to concentric left ventricular morphology. Previous studies have linked E/E’ to left ventricular hypertrophy, but only if LVH was considered very pronounced (>97.5th percentile)18 and in a smaller analysis unadjusted for age20. However, in the presence of LVH, diastolic or systolic dysfunction may play an additional predictive role for future adverse events21 or outcomes, as longitudinal analysis may reveal. More importantly, these results suggest that LV morphology alone does not sufficiently identify risk factors for cardiac involvement in children with CKD nor does it precisely enough describe cardiac alterations.
In previous small-sample studies in children with CKD variable Doppler indices such as tissue Doppler E’16, E’/A’13,14 or conventional pulse wave Doppler E/A22 were found to be reduced. While an association of diastolic function to serum Cystatin C levels has been shown23, no relationship to eGFR has been demonstrated. Our study, investigating a much larger patient cohort, is the first to show independent associations of both lower GFR and higher blood pressure with reduced diastolic function measured by TDI E’/A’ Z-Score. Additionally, diastolic function measured by E’/A’ Z-Score tended to be better preserved in patients treated with RAS blockade independently of renal function and blood pressure.
The Heart Failure and Echocardiography Associations of the European Society of Cardiology have recommended the evaluation of E/E’ in the workup of diastolic heart failure in adults24, however its role in childhood is not clear. Here, we observed a globally increased E/E’ Z-Scores, indicative of increased left ventricular filling pressure. In hypertensive adults and children with hypertrophic cardiomyopathy, E/E’ ratio emerged as a powerful predictor of cardiac events21,25. Also, the finding of the inverse correlation of E/E’ Z-Score with systolic TD velocitiy Z-Score may be of importance since our group previously described abnormal systolic mechanics in children with CKD similar to alterations in hypertrophic cardiomyopathy5.
We describe for the first time an independent association of RAS inhibition with better preservation of LV diastolic function in children with CKD. Notably, the association appeared to be specific for RAS blockers whereas the use of other antihypertensive agents, including diuretics, did not show any relationship with E/E’ Z-Score. Previous studies in pediatric CKD failed to show associations of LV filling pressure (E/E’) with antihypertensive treatment protocols, possibly due to insufficient sample size or lacking adjustment of Doppler measurements for patient age14,16,22,26. We therefore hypothesize that RAS blockade should be the preferred treatment for hypertension in children with CKD independent of the level of proteinuria.
Our study is also the first to demonstrate a close inverse relationship of E/E’ Z-Score with systolic function in children with CKD. This pathophysiological relationship emphasizes the importance of optimizing diastolic function, preferably by RAS inhibition although other aspects such as the fluid status of patients should not be neglected in order to improve diastolic function.
Furthermore, our results show a reduction not only of diastolic but also of systolic tissue Doppler velocities in children with CKD compared to healthy individuals, suggesting reduced systolic LV function. This finding confirms earlier findings obtained in pediatric CKD cohorts with various methodologies including analysis of fractional shortening4, TD26, and strain imaging5. Our analysis thus endorses the use of tissue Doppler imaging as a simple method to screen for subtle systolic dysfunction in the setting of normal left ventricular ejection.
While tissue Doppler imaging of the left ventricle is supposed to be more independent of preload than flow Doppler27–29, acute preload changes can impact also on tissue Doppler measurements30. In this study fluid status was not assessed directly. However, none of our patients was in a critically ill condition with acute volume changes, and patients on hemodialysis were examined between dialysis sessions in order to minimize the effects of acute volume changes. Future studies should however look more closely into the effect of fluid status on cardiac function in children with CKD.
Another obvious limitation of our study is the cross-sectional design of our analysis, which precludes any firm conclusions about causalities.
Chinappa et al. emphasized that the finding of subclinically decreased cardiac function in adults with CKD requires more research into functional mechanisms of cardiac disease in CKD3. Similarly, the Investigator Network Initiative Cardiovascular and Renal Clinical Trialists (INI-CRCT) has recently called for more trials evaluating the benefits and risks of cardiovascular medications in patients with CKD31. The observations made in this study underscore the presence of functional myocardial alterations and both the need and feasibility of using non-invasive imaging in interventional clinical trials in the pediatric subset of the CKD population.
In conclusion, we demonstrated that TD velocities are independent of LV mass and morphology and provide sensitive additional information about early alterations of the left ventricular function in children with CKD. These changes are independently related to the degree of renal functional impairment and systolic blood pressure. Treatment with RAS blockade may be beneficial not only for the prevention of CKD progression but also for cardiac sequelae.
Methods
Subjects and Study Design
The patients were participants of the 4C Study (The Cardiovascular Comorbidity in Children with Chronic Kidney Disease Study, registered at ClinicalTrials.gov NCT0104644832. The study prospectively observes 688 patients who were enrolled 2010–2012 at age 6 to 17 years with CKD stage III-V (eGFR < 60 ml/min/1.73 m2). The patients are followed prospectively with annual comprehensive cardiovascular assessments (including oscillometric BP measurements as previously described2 and echocardiographies) and 6-monthly clinical assessments, blood and urine collection. The measurement of Tissue doppler velocities was not part of the standard echocardiography protocol of the 4C study but was carried out as an ancillary measurement and therefore only available in a subset of patients who were seen by one investigator (A.D.). The patients were not selected specifically for this analysis but were included randomly due to their assignment to the performing investigator. The first available echocardiography per patient during the course of the study was included in the analysis.
The following Ethics committees (EC) and Institutional Review Boards (IRB) approved the 4C Study: Austria: EC of the University of Vienna; EC of the University of Innsbruck. Czech Republic: EC of the University of Prague. France: Comité de protection des personnes “Est IV”, Strasbourg. Germany: EC Charité— Universitätsmedizin Berlin; EC of the Medical Faculty of the University of Cologne; Ethikkommission der sächsischen Länderkammer; EC of Friedrich-Alexander-University Erlangen; EC of the University of Duisburg-Essen; EC of Albert-Ludwig University Freiburg; Ethikkommission der Ärztekammer Hamburg; EC and IRB of Hannover Medical School; EC and IRB of the University of Heidelberg; EC of the Medical Faculty of the University of Jena; EC of the Medical Faculty of the University of Leipzig; EC of Philipps University Marburg; Ethikkommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms Universität Münster; EC of the Medical Faculty of the University of Rostock. Italy: Comitato Etico Indipendente dell’Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi; Comitato di Etica dell’IRCCS Istituto Giannina Gaslini di Genova; Comitato Etico dell’IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena di Milano; Ethical Committee for clinical practice of the General Hospital-University of Padova; Comitato Etico per la Sperimentazione Clinica dell’IRCCS Ospedale Pediatrico Bambino Gesu’ di Roma. Lith- uania: EC and IRB of Vilnius University. Poland: EC at Children’s Memorial Health Institute, Warsaw. Portugal: EC of the University of Porto. Serbia: EC of the University of Belgrade. Switzerland: EC of the Canton Bern; EC of the Canton Zurich. Turkey: EC of the University of Cukurova, Adana; Non-interventional Clinical Researches Ethics Boards of Hacettepe Univer- sity, Ankara; EC of the University of Cerrahpasa, Istanbul; EC and IRB of the University of Ege, Izmir. United Kingdom: Great Ormond Street Hospital and UCL Institute of Child Health research EC. The study was performed in accordance with the ethical standards of the Declaration of Helsinki of 1964 and its later amendments. Written informed consent was given by the parents and adolescents, and oral assent by younger children.
For the present analysis 128 patients at 12 study sites underwent a standardized echocardiographic exam including tissue Doppler measurements at the time of the study visit. Clinical information and biospecimens collected at the same study visit were analyzed for the present study. GFR was calculated by the updated Schwartz formula33.
Echocardiographic measurements
The transthoracic echocardiographies were performed according to the pediatric echocardiography standards issued by the American Society of Cardiology34. Patients on hemodialysis were examined between hemodialysis session in order to avoid acute volume changes. None of the patients was critically ill at the time of the investigation. The echocardiographic exam included standard M-mode images, pulsed Doppler and tissue Doppler echocardiography. The left ventricular mass index (LVMI) was calculated according to a recently published allometric formula providing age independent normalization of left ventricular mass (LVM/(height2.16 + 0.09)). An LVM index 45.0 m2 16 was considered to identify LVH, representing the 95th percentile35. Endocardial and midwall fractional shortening was calculated as suggested by Lang et al.36. Relative wall thickness was normalized to 10 years of age, using a cut-off of 0.38 as equivalent of the 95th percentile37.
Tissue Doppler measurements included early (E’) and late (A’) diastolic and systolic (S’) velocity at the mitral and septal annulus of the left ventricle. Diastolic function was described by E’/A’ ratio and left ventricular filling pressure by E/E’ ratio.
All exams were performed by a single trained paediatrician (A.D.) and reviewed by an expert paediatric cardiologist (M.C.). All exams were performed on an Acuson P50, Siemens Healthcare, Erlangen. Image analysis was carried out using Syngo (Syngo US Workplace, Siemens Medical Solutions, USA Inc) as digital image evaluation software.
Data analysis
Results of Tissue Doppler Imaging (TDI) measurements were normalized to patient age by calculation of Z scores based on a published reference cohort of 325 healthy children17. Standardized height and BMI were calculated from the WHO growth charts (http://www.who.int/growthref/en). Blood pressure values were standardized according to the Fourth Report on Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents38.
Data was tested for normal distribution by the Shapiro-Wilk test. Correlations of all variables of Table 1 to tissue Doppler measurements were tested with Spearman rank order correlation and group comparisons were carried out using chi-squared, t, or Mann–Whitney tests in case of two groups and chi- squared test, ANOVA, or Kruskal–Wallis test in case of more than two groups as appropriate. The influence of anthropometric, biochemical and disease–associated covariates as well as medication on tissue Doppler measurements and LV morphology was analyzed using multivariable linear, mixed and logistic regression modeling. Backward variable selection was performed with a threshold of P = 0.15. All multivariable models were controlled for the influence of age, height and eGFR.
Supplementary information
Impaired Systolic and Diastolic Left Ventricular Function in Children with Chronic Kidney Disease - Results from the 4C Study, Supplemental Material
Acknowledgements
We thank all children and adolescents for their participation in the study. We gratefully acknowledge the support of our colleagues and friends. This study has been performed within the framework of the 4C Study Consortium. The study has been made possible by grants of the KfH Foundation for Preventive Medicine,the European Renal Association – European Dialysis and Transplant Association (www.era-edta.org) and the German Federal Ministry of Education and Research (reference number: 01EO0802). Several authors of this publication are members of the European Reference Network for Rare Kidney Diseases (ERKNet).
Author Contributions
A.D. and F.S. conceived and planned the investigation. A.D. performed and M.C. reviewed the echocardiographies, P.H. performed the offline analysis of echocardiographic images. A.D. and P.H. performed the statistical analysis. S.E., B.R., B.K., F.M., F.L., J.H., M.C., S.H., A.Z., S.T., E.V., C.G. and K.A. performed study visits and contributed to the final version of the manuscript. A.K. and U.Q. contributed to the interpretation of the results and the final version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
On behalf of all authors, the corresponding author states that there is no conflict of interest. The results presented in this manuscript have not been published (neither in English nor in any other language) previously in whole or part, except in abstract format and the work is not under consideration for publication elsewhere.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46653-3.
References
- 1.Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23:578–585. doi: 10.1681/ASN.2011111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer F, et al. Querfeld, U.4C Study Consortium. Cardiovascular Phenotypes in Children with CKD: The 4C Study. Clin J Am Soc Nephrol. 2017;12:19–28. doi: 10.2215/CJN.01090216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnappa S, et al. Early and asymptomatic cardiac dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2017;33:450–458. doi: 10.1093/ndt/gfx064. [DOI] [PubMed] [Google Scholar]
- 4.Chinali Marcello, de Simone Giovanni, Matteucci Maria Chiara, Picca Stefano, Mastrostefano Antonio, Anarat Ali, Çaliskan Salim, Jeck Nikola, Neuhaus Thomas J., Peco-Antic Amira, Peruzzi Licia, Testa Sara, Mehls Otto, Wühl Elke, Schaefer Franz. Reduced Systolic Myocardial Function in Children with Chronic Renal Insufficiency. Journal of the American Society of Nephrology. 2007;18(2):593–598. doi: 10.1681/ASN.2006070691. [DOI] [PubMed] [Google Scholar]
- 5.Chinali M, et al. Advanced Parameters of Cardiac Mechanics in Children with CKD: The 4C Study. Clin J Am Soc Nephrol. 2015;10:1357–1363. doi: 10.2215/CJN.10921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matteucci MC, et al. & ESCAPE Trial Group. Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol. 2006;17:218–226. doi: 10.1681/ASN.2005030276. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone LM, et al. Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int. 1996;50:998–1006. doi: 10.1038/ki.1996.401. [DOI] [PubMed] [Google Scholar]
- 8.Kane GC, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C, Di Lullo L. Cardiorenal Syndrome in Western Countries: Epidemiology, Diagnosis and Management Approaches. Kidney Dis. 2017;2:151–163. doi: 10.1159/000448749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonelli M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. Journal of the American Society of Echocardiography. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 12.McMurray, J. J. V. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. European Journal of Heart Failure14, 803–869 (2014). [DOI] [PubMed]
- 13.Mencarelli Francesca, Fabi Marianna, Corazzi Valentina, Doyon Anke, Masetti Riccardo, Bonetti Simone, Castiglioni Laura, Pession Andrea, Montini Giovanni. Left ventricular mass and cardiac function in a population of children with chronic kidney disease. Pediatric Nephrology. 2013;29(5):893–900. doi: 10.1007/s00467-013-2710-6. [DOI] [PubMed] [Google Scholar]
- 14.Lindblad YT, et al. Left ventricular diastolic dysfunction by tissue Doppler echocardiography in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28:2003–2013. doi: 10.1007/s00467-013-2504-x. [DOI] [PubMed] [Google Scholar]
- 15.Tranæus Lindblad Ylva, Vavilis Georgios, Axelsson Jonas, Herthelius Maria, Bárány Peter. Assessing longitudinal trends in cardiac function among pediatric patients with chronic kidney disease. Pediatric Nephrology. 2016;31(9):1485–1497. doi: 10.1007/s00467-016-3371-z. [DOI] [PubMed] [Google Scholar]
- 16.Mitsnefes MM, et al. Impaired left ventricular diastolic function in children with chronic renal failure. Kidney Int. 2004;65:1461–1466. doi: 10.1111/j.1523-1755.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 17.Eidem BW, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. Journal of the American Society of Echocardiography. 2004;17:212–221. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Shamszad P, Slesnick TC, Smith EO, Taylor MD, Feig DI. Association between left ventricular mass index and cardiac function in pediatric dialysis patients. Pediatr Nephrol. 2011;27:835–841. doi: 10.1007/s00467-011-2060-1. [DOI] [PubMed] [Google Scholar]
- 19.Matteucci Maria Chiara, Chinali Marcello, Rinelli Gabriele, Wühl Elke, Zurowska Aleksandra, Charbit Marina, Pongiglione Giacomo, Schaefer Franz. Change in Cardiac Geometry and Function in CKD Children During Strict BP Control: A Randomized Study. Clinical Journal of the American Society of Nephrology. 2012;8(2):203–210. doi: 10.2215/CJN.08420811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogan CS, et al. Assessment of left ventricular function by tissue Doppler echocardiography in pediatric chronic kidney disease. Ren Fail. 2015;37:1094–1099. doi: 10.3109/0886022X.2015.1061301. [DOI] [PubMed] [Google Scholar]
- 21.Sharp ASP, et al. ASCOT Investigators. Tissue Doppler E/E’ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31:747–752. doi: 10.1093/eurheartj/ehp498. [DOI] [PubMed] [Google Scholar]
- 22.Schoenmaker NJ, et al. Diastolic dysfunction measured by tissue Doppler imaging in children with end-stage renal disease: a report of the RICH-Q study. Cardiol Young. 2014;24:236–244. doi: 10.1017/S1047951113000188. [DOI] [PubMed] [Google Scholar]
- 23.Mitsnefes M, et al. Serum cystatin C and left ventricular diastolic dysfunction in children with chronic kidney disease. Pediatr Nephrol. 2006;21:1293–1298. doi: 10.1007/s00467-006-0132-4. [DOI] [PubMed] [Google Scholar]
- 24.Paulus WJ, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 25.McMahon CJ, et al. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation. 2004;109:1756–1762. doi: 10.1161/01.CIR.0000124723.16433.31. [DOI] [PubMed] [Google Scholar]
- 26.Simpson JM, Rawlins D, Mathur S, Chubb H, Sinha MD. Systolic and Diastolic Ventricular Function Assessed by Tissue Doppler Imaging in Children with Chronic Kidney Disease. Echocardiography. 2012;30:331–337. doi: 10.1111/echo.12015. [DOI] [PubMed] [Google Scholar]
- 27.Gorcsan J. Tissue Doppler echocardiography. Current Opinion in Cardiology. 2000;15:323–329. doi: 10.1097/00001573-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ogunyankin KO. Assessment of Left Ventricular Diastolic Function: The Power, Possibilities, and Pitfalls of Echocardiographic Imaging Techniques. Can J Cardiol. 2011;27:311–318. doi: 10.1016/j.cjca.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 29.Connelly KA, Royse C, Royse AG. Tissue Doppler Em and Instantaneous End-diastolic Stiffness: Validation Against Pressure-Volume Loops in Patients Undergoing Coronary Artery Bypass Surgery. ‘. Heart, Lung and Circulation’. 2011;20:223–230. doi: 10.1016/j.hlc.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Quintard H, Muller L, Philip I, Lena P, Ichai C. Influence of acute preload changes on mitral annulus velocity measured by tissue Doppler echocardiography in critically ill patients. J Clin Ultrasound. 2012;40:419–423. doi: 10.1002/jcu.21882. [DOI] [PubMed] [Google Scholar]
- 31.Rossignol Patrick, Agarwal Rajiv, Canaud Bernard, Charney Alan, Chatellier Gilles, Craig Jonathan C, Cushman William C, Gansevoort Ronald T, Fellström Bengt, Garza Dahlia, Guzman Nicolas, Holtkamp Frank A, London Gerard M, Massy Ziad A, Mebazaa Alexandre, Mol Peter G M, Pfeffer Marc A, Rosenberg Yves, Ruilope Luis M, Seltzer Jonathan, Shah Amil M, Shah Salim, Singh Bhupinder, Stefánsson Bergur V, Stockbridge Norman, Stough Wendy Gattis, Thygesen Kristian, Walsh Michael, Wanner Christoph, Warnock David G, Wilcox Christopher S, Wittes Janet, Pitt Bertram, Thompson Aliza, Zannad Faiez. Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. European Heart Journal. 2017;40(11):880–886. doi: 10.1093/eurheartj/ehx209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Querfeld Uwe, Anarat Ali, Bayazit Aysun K., Bakkaloglu Aysin S., Bilginer Yelda, Caliskan Salim, Civilibal Mahmut, Doyon Anke, Duzova Ali, Kracht Daniela, Litwin Mieczyslaw, Melk Anette, Mir Sevgi, Sözeri Betül, Shroff Rukshana, Zeller René, Wühl Elke, Schaefer Franz. The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) Study: Objectives, Design, and Methodology. Clinical Journal of the American Society of Nephrology. 2010;5(9):1642–1648. doi: 10.2215/CJN.08791209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz GJG, Furth SLS. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 34.Leo Lopez MD, et al. Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report From the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. Journal of the American Society of Echocardiography. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Chinali M, et al. Left Ventricular Mass Indexing in Infants, Children, and Adolescents: A?Simplified Approach for the Identification of Left Ventricular Hypertrophy in Clinical Practice. J Pediatr. 2016;170:193–198. doi: 10.1016/j.jpeds.2015.10.085. [DOI] [PubMed] [Google Scholar]
- 36.Lang RM, et al. Chamber Quantification Writing Group, American Society of Echocardiography’s Guidelines and Standards CommitteeEuropean Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.de Simone G, et al. Evaluation of Concentric Left Ventricular Geometry in Humans. Hypertension. 2005;45:64–68. doi: 10.1161/01.HYP.0000150108.37527.57. [DOI] [PubMed] [Google Scholar]
- 38.Falkner B. Summary of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Hypertension. 2004;44:387–388. doi: 10.1161/01.HYP.0000143545.54637.af. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impaired Systolic and Diastolic Left Ventricular Function in Children with Chronic Kidney Disease - Results from the 4C Study, Supplemental Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.