ABSTRACT
Background
The Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) in Lombok, Indonesia showed that maternal multiple micronutrients (MMN), as compared with iron and folic acid (IFA), reduced fetal loss, early infant mortality, and low birth weight. Mitochondria play a key role during pregnancy by providing maternal metabolic energy for fetal development, but the effects of maternal supplementation during pregnancy on mitochondria are not fully understood.
Objective
The aim of this study was to assess the impact of MMN supplementation on maternal mitochondrial DNA copy number (mtDNA-CN).
Methods
We used archived venous blood specimens from pregnant women enrolled in the SUMMIT study. SUMMIT was a cluster-randomized double-blind controlled trial in which midwives were randomly assigned to distribute MMN or IFA to pregnant women. In this study, we selected 108 sets of paired baseline and postsupplementation samples (MMN = 54 and IFA = 54). Maternal mtDNA-CN was determined by real-time quantitative polymerase chain reaction in baseline and postsupplementation specimens. The association between supplementation type and change in mtDNA-CN was performed using rank-based estimation for linear models.
Results
In both groups, maternal mtDNA-CN at postsupplementation was significantly elevated compared with baseline (P < 0.001). The regression revealed that the MMN group had lower postsupplementation mtDNA-CN than the IFA group (β = −4.63, P = 0.003), especially for women with mtDNA-CN levels above the median at baseline (β = −7.49, P = 0.007). This effect was rapid, and observed within 33 d of initiation of supplementation (β = −7.39, P = 0.017).
Conclusion
Maternal MMN supplementation rapidly stabilized mtDNA-CN in pregnant women who participated in SUMMIT, indicating improved mitochondrial efficiency. The data provide a mechanistic basis for the beneficial effects of MMN on fetal growth and survival, and support the transition from routine IFA to MMN supplementation.
This trial was registered at www.isrctn.com as ISRCTN34151616.
Keywords: supplementation, multiple micronutrients, iron and folic acid, pregnancy, mitochondrial DNA copy number, mtDNA-CN, oxidative stress
Introduction
Deficiencies in macronutrients and micronutrients in low- and middle-income countries (LMICs) often occur because of poor diet, dietary taboos, or illness (1, 2). Micronutrient deficiencies can result in adverse pregnancy outcomes including fetal loss and intrauterine growth restriction (IUGR), indicated by being born small for gestational age (SGA), and greater risk of mortality (3). Supplementation with multiple micronutrients during pregnancy may reduce these risks (4–6). The Supplementation of Multiple Micronutrients Intervention Trial (SUMMIT) was a double-blind cluster-randomized trial of maternal supplementation with either multiple micronutrients (MMN) or iron and folic acid (IFA) conducted in Lombok, Indonesia from 2001 to 2004. The SUMMIT study showed the MMN group experienced a 10% reduced risk of fetal loss, an 18% reduced risk of early infant mortality, and a 14% reduction in low birth weight (LBW) and SGA, as compared with the IFA group (4, 7). Effects were stronger in women who were anemic at enrollment, with reductions of 29% for fetal loss and neonatal mortality, 38% for early infant mortality, and 25% for LBW. Short- and long-term effects on child cognition were also reported (8). A recent individual patient data meta-analysis of 112,953 pregnant women in 17 studies concluded that maternal MMN supplementation, compared with IFA, reduced stillbirth, LBW, preterm birth, SGA, and 6-mo mortality (6). Again, all effects were stronger in women who were anemic at enrollment. However, the biological mechanisms leading to these favorable outcomes remain unknown (9), and such knowledge may help improve supplement formulation or dosing regimens. Moreover, although the WHO 2016 guidelines for antenatal care suggest countries can decide to transition from IFA to MMN, a global recommendation is still pending and would be motivated by evidence of a pleiotropic biological mechanism for the effects of MMN supplementation (10).
In this study, we hypothesized that the modulation of mitochondrial factors by MMN components could optimize maternal mitochondrial function and thereby improve multiple fetal outcomes. The main mitochondrial function is to produce ATP via coupling with oxidative phosphorylation (OXPHOS) (11). Mitochondria have been reported to modulate the major stress–response pathways (12), and alterations in leukocyte mitochondrial DNA copy number (mtDNA-CN) in adulthood have been associated with early-life exposure to stress and psychopathology (13). Maternal mitochondrial ATP is essential for fetal growth and development (14), and an appropriate mtDNA-CN sensitive to energy demand and oxidative stress (15, 16) is needed to optimize mitochondrial function. MtDNA-CN has been reported to correlate inversely with pregnancy outcomes. Higher mtDNA-CN was found in IUGR pregnancy (17, 18), and we recently reported that higher maternal mtDNA-CN in peripheral blood was associated with reduced birth weight (19). MMN deficiencies observed in an ageing population showed a reduction of mitochondrial efficiency (20), leading to compensatory increases in mtDNA-CN. As pregnancy progresses, increased mtDNA-CN in cord blood has been observed (21), and high placental mtDNA-CN was reported in pregnant women with high oxidative stress (22). MMN supplementation during pregnancy could promote efficient mitochondrial function and protect against oxidative stress, both of which would reduce increases in mtDNA-CN.
Methods
Sample selection and study design
We used archived venous blood specimens from pregnant women enrolled in SUMMIT, collected in 2001–2004 (ISRCTN34151616; approved by the National Institute of Health Research and Development of the Ministry of Health of Indonesia, the Provincial Planning Department of Nusa Tenggara Barat Province, and the Johns Hopkins Joint Committee on Clinical Investigation, Baltimore, MD; the current study was approved by the Eijkman Institute Research Ethics Commission). SUMMIT was a cluster-randomized double-blind controlled trial in which midwives were randomly assigned to distribute MMN or IFA to pregnant women when they sought prenatal care. Because SUMMIT was integrated with the government health system, pregnant women were enrolled at any gestational age, as assessed by reported last menstrual period or exam, at which time blood specimens were collected (baseline) (4). Participants were provided a daily supplement during pregnancy and until 3 mo postpartum. The SUMMIT study enrolled 31,290 pregnant women in the primary birth cohort, and 28,426 children were born alive. In a random subsample of 2369 participants, blood samples were drawn at 2 time points: 1) before supplementation for all participants in the subsample (baseline), and 2) at 1 of 4 postsupplementation time points: 1 mo after enrollment, 36 weeks of gestation, 1 wk postpartum, or 12 wk postpartum (23).
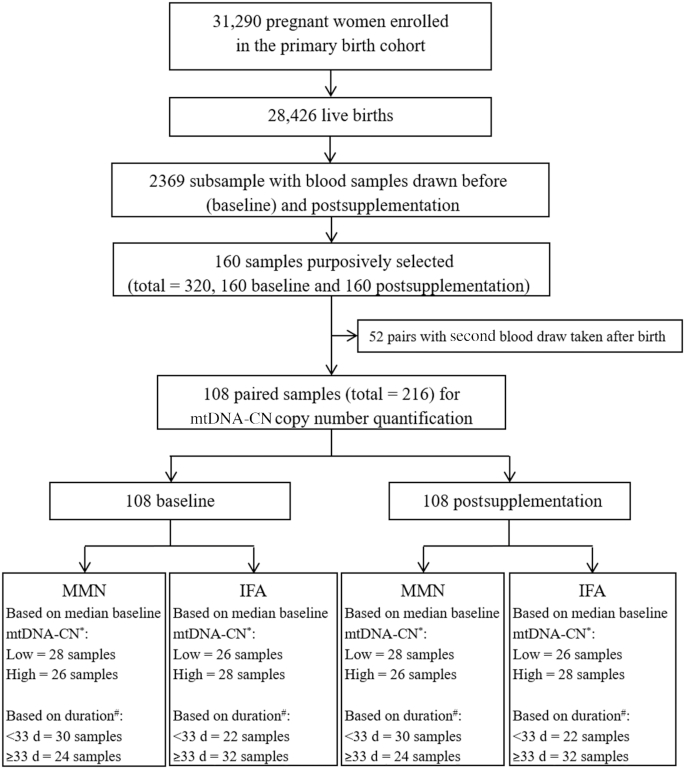
The plasma was separated from the blood cells, then the resulting packed cell pellets were washed 3 times with PBS and transferred to cryovials for archived storage at either −80 °C or −40 °C. We first purposively and blindly selected 160 sets of paired baseline and postsupplementation samples from the SUMMIT archives. We used all specimens which had postsupplementation time points of either 1 mo after initiation of supplementation or at ∼36 weeks of gestation, with no preference applied, excluding the 1 wk and 12 wk postpartum time points. Selected specimens would have had an equal chance of being eligible for the 1 mo postsupplementation or the 36 weeks of gestation time point. Therefore, the women would have enrolled earlier than 35 weeks of gestation, considering the average gestation period is 40 weeks. This selection resulted in 108 eligible paired baseline and postsupplementation prepartum samples ranging from 4 to 34 weeks of gestation at enrollment. The 52 postpartum sets were excluded. These 108 women were from 72 of the 262 clusters randomized for SUMMIT and the number of women per cluster ranged from 1 to 6, including 54 women from 39 clusters given MMN and 54 women from 33 clusters given IFA. Supplementation duration ranged from 20 to 197 d. We further categorized subjects as below (<33) or above (≥33) the median days of supplementation between baseline and postsupplementation blood draws, and below (<14.1) or above (≥14.1) the median baseline mtDNA-CN (Figure 1).
FIGURE 1.
Flowchart for mtDNA-CN assessment of 108 pregnant women enrolled in the SUMMIT study. The SUMMIT study enrolled 31,290 pregnant women in the primary birth cohort and 28,426 children were born alive. Blood samples were drawn before (baseline) and postsupplementation in a random subsample of 2369 participants. The postsupplementation blood collection was at 1 of 4 points: 1 mo after enrollment, 36 weeks of gestation, 1 wk postpartum, or 12 wk postpartum. We purposively and blindly elected 160 sets of paired baseline and postsupplementation samples. Of these samples, we selected those in which the postsupplementation blood draw was performed either 1 mo after initiation of supplementation or at ∼36 weeks of gestation (n = 108), and postpartum sets were excluded (n = 52). These 108 women were given MMN (n = 54) or IFA (n = 54) supplementation. Further, these samples were categorized as below (<33) or above (≥33) the median days of supplementation, and below (<14.1) or above (≥14.1) the median of baseline mtDNA-CN. *Low baseline < 14.1, and high baseline ≥ 14.1. #Short duration of supplementation <33 d, and long ≥33 d. IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number; SUMMIT, Supplementation with Multiple Micronutrients Intervention Trial.
MtDNA-CN determination
DNA extraction was performed on packed cell samples using the Geneius Micro gDNA Extraction Kit (Geneaid). Determination of mtDNA-CN was done by real-time qPCR assay with SYBR Select MasterMix (Applied Biosystems), using primers for mitochondrial tRNAleu and nuclear β-2 microglobulin genes, based on the protocol from Venegas et al. (24), using the 7500 Real-Time PCR System (Applied Biosystems), as previously described (19). Primers, PCR mixture, and qPCR conditions for the mtDNA transfer RNA leucine gene (GenBank: KM102154.1) and the single-copy nDNA β-2 microglobulin gene (GenBank: NM_004048.2) amplifications are described in Supplemental Table 1. Raw fluorescence data were analyzed using the qPCR package in R version 3.4.0 (www.r-project.org) (25). The relative ratio of mtDNA-CN to nDNA was calculated according to the efficiency-corrected method by Pfaffl (26). The overall percentage change of mtDNA-CN (Δ) during pregnancy was calculated by subtracting mtDNA-CN at baseline from the mtDNA-CN postsupplementation and dividing by the mtDNA-CN at baseline, based on the following equation:
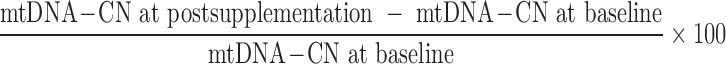 |
Statistical analysis
Statistical tests were performed using R version 3.4.0 with RStudio version 1.0.143 (www.rstudio.com), and SAS version 9.4. The normality of the continuous variables was tested with the Shapiro–Wilk test. Given the low number of women per cluster, we did not adjust for cluster group, and analysis of the null model revealed no association of cluster with mtDNA-CN. Continuous variables with nonnormally distributed data were analyzed using nonparametric tests and are presented with the median and IQR (Q1–Q3). To compare the mtDNA-CN at baseline and postsupplementation, Wilcoxon Signed Rank tests were used. The multivariate regression analysis of the association between supplementation type and the change in mtDNA-CN from baseline to postsupplementation was performed using rank-based estimation for linear models, adjusted for mtDNA-CN at baseline, supplementation duration, and maternal anemia (hemoglobin <11 g/dL). The interpretation of results was similar to those from linear regression models (27). P < 0.05 was considered statistically significant, and to test for interactions with duration or copy number at baseline a cutoff of P < 0.15 was used. Pearson's chi-square test was used to compare the Δ percentages of MMN and IFA groups. All tests were performed using either stats, ggplot2, or Rfit packages in R, or SAS PROC NPAR1WAY, PHREG, GENMOD, or GLIMMIX.
Results
Baseline characteristics of the participants
Overall, women in the MMN (n = 54) and the IFA (n = 54) groups did not differ at baseline (Table 1).
TABLE 1.
Characteristics of the 108 pregnant women enrolled in the Supplementation with Multiple Micronutrients Intervention Trial study by supplement group1
| Characteristics | MMN (n = 54) | IFA (n = 54) |
|---|---|---|
| Age of women at enrollment, y | 25 [22–27] | 25 [21–30] |
| Gestational age at enrollment, wk | 16.8 [12–22] | 16 [12–23] |
| Trimester at enrollment | ||
| First | 21 (39) | 21 (39) |
| Second | 30 (56) | 25 (46) |
| Third | 3 (6) | 8 (15) |
| Midupper arm circumference, mm | 250 [232–269] | 245 [235–260] |
| ≥235 | 40 (74) | 41 (76) |
| <235 | 14 (26) | 13 (24) |
| Hemoglobin at enrollment, g/dL | 10.8 [9–12] | 11.2 [11–12] |
| ≥11 | 25 (46) | 31 (57) |
| <11 | 29 (54) | 23 (43) |
| Maternal height, cm | 149.1 [147–153] | 149.8 [148–153] |
| ≥145 | 49 (91) | 51 (94) |
| <145 | 5 (9) | 3 (6) |
| Gestational age at birth, wk | 39.7 [37–41] | 39.8 [38–41] |
| ≥37 | 41 (76) | 45 (83) |
| <37 | 13 (24) | 9 (17) |
| Birth weight, g | 3,400 [3,000–3,500] | 3,050 [3,000–3,500] |
| AGA | 46 (85) | 42 (78) |
| SGA | 8 (15) | 12 (22) |
| Parity | ||
| 1 | 18 (33) | 17 (32) |
| >1 | 36 (67) | 37 (68) |
| Infants’ gender | ||
| Male | 31 (57) | 27 (50) |
| Female | 23 (43) | 27 (50) |
| Women's education, y | 6 [6–9] | 6 [6–9] |
| Men's education, y | 6 [6–11] | 6 [6–9] |
| Duration of supplementation, d | 32 [31–82] | 36 [31–119] |
Continuous data are shown as median [IQR]; categorical variables are shown as n (%). AGA, appropriate for gestational age; IFA, iron and folic acid; MMN, multiple micronutrient; SGA, small for gestational age.
MtDNA-CN at baseline and postsupplementation
The mtDNA-CN in the combined supplementation groups was elevated at postsupplementation [median (IQR) = 18.0 (14.4–23.0)], compared with baseline [14.1 (11.1–17.7)] (P < 0.001) (Figure 2).
FIGURE 2.
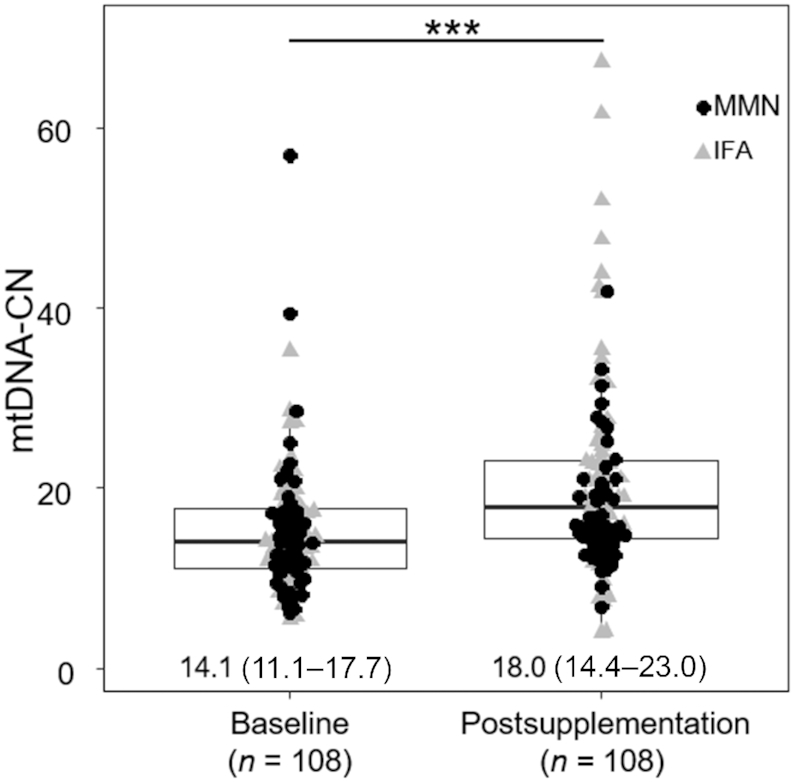
Paired plot between baseline and postsupplementation mtDNA-CN of 108 pregnant women enrolled in the Supplementation with Multiple Micronutrients Intervention Trial study. MtDNA-CN significantly increased in the postsupplementation sample (P < 0.001). MtDNA-CN data are shown as median (IQR). Comparison between baseline and postsupplementation was performed using Wilcoxon's Signed Rank test. Shown below the bars are medians (IQRs). ***Statistically significant difference between mtDNA-CN at baseline and postsupplementation, P < 0.001. IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number.
MMN supplementation stabilized mtDNA-CN during pregnancy
In women given MMN supplementation, the postsupplementation mtDNA-CN was lower by 4.63 units than in the IFA group (P = 0.003). This effect was greater in women whose baseline mtDNA-CN was above the median (β = −7.49, P = 0.007), although the low baseline mtDNA-CN group showed a similar trend (β = −3.09, P = 0.09). However, the interaction between MMN and baseline mtDNA-CN was nonsignificant (P-interaction = 0.16) (Table 2).
TABLE 2.
Multivariate regression results of mtDNA-CN at baseline, MMN, duration of supplementation, and maternal anemia for predicting postsupplementation mtDNA-CN in total samples, low baseline, high baseline, supplementation duration <33 d, and supplementation duration ≥33 d groups1
| MtDNA-CN at postsupplementation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All samples2 (n = 108: MMN = 54, IFA = 54) | Low baseline2 (n = 54: MMN = 28, IFA = 26) | High baseline2 (n = 54: MMN = 26, IFA = 28) | Duration < 33 d2 (n = 52: MMN = 30, IFA = 22) | Duration ≥ 33 d2 (n = 56: MMN = 24, IFA = 32) | ||||||
| β | P | β | P | β | P | β | P | β | P | |
| Baseline mtDNA-CN | 0.26 | 0.017 | −0.27 | 0.48 | 0.35 | 0.06 | 0.88 | <0.001 | 0.03 | 0.75 |
| MMN | −4.63 | 0.003 | −3.09 | 0.09 | −7.49 | 0.007 | −7.39 | 0.017 | −2.91 | 0.026 |
| Duration of supplementation, d | −0.02 | 0.09 | −0.02 | 0.40 | −0.04 | 0.07 | 0.04 | 0.96 | −0.01 | 0.28 |
| Maternal anemia (Hb <11 g/dL) | 0.51 | 0.74 | −0.13 | 0.94 | 0.87 | 0.75 | −3.67 | 0.22 | 1.47 | 0.27 |
| Interaction model | ||||||||||
| MMN:high baseline3 | −4.45 | 0.13 | ||||||||
| MMN:duration <33 d4 | −6.16 | 0.035 | ||||||||
High baseline mtDNA-CN ≥ 14.1; low baseline mtDNA-CN < 14.1. P values < 0.05 are significant, and for interactions P values < 0.15 are significant. Hb, hemoglobin; IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number; β, β coefficient.
The model used for this regression was postsupplementation mtDNA-CN ∼ baseline mtDNA-CN + supplementation type + duration of supplementation + anemic mother.
The interaction model used for this regression was postsupplementation mtDNA-CN – baseline mtDNA-CN + supplementation type + duration + anemic mother + supplementation type: high baseline.
The interaction model used for this regression was postsupplementation mtDNA-CN – baseline mtDNA-CN + supplementation type + anemic mother + supplementation type: duration <33 d.
We further assessed the impact of short or long duration based on the median days of supplementation, in this case 33 d. The result showed that baseline mtDNA-CN strongly predicted short-duration postsupplementation mtDNA-CN levels (β = 0.88, P < 0.001). In addition, women given MMN had lower postsupplementation mtDNA-CN in both the short- and long-duration groups compared with IFA, and the effect was greater in the short-duration group (P-interaction = 0.03). Compared with the IFA treatment, MMN supplementation lowered the mtDNA-CN by 7.39 units in the shorter studies (<33 d, P = 0.017) and by 2.91 units in the longer studies (P = 0.026) (Table 2).
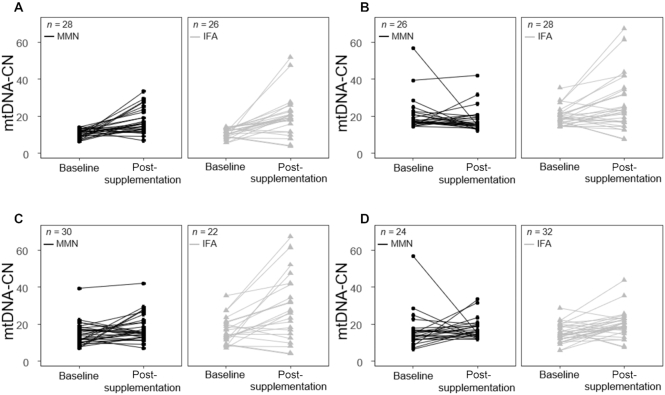
The difference in baseline and postsupplementation mtDNA-CN of the MMN and IFA groups was visualized with a paired plot that illustrated the effect of MMN on stabilizing mtDNA-CN, particularly for women with baseline mtDNA-CN above the median. The paired plot also illustrated the MMN stabilizing effect on mtDNA-CN in women with short duration of supplementation, i.e., <33 d. The plot revealed the greater dispersion of mtDNA-CN in the postsupplementation IFA group than in the MMN group, again indicating the stabilizing effect of MMN supplementation (Figure 3).
FIGURE 3.
Paired plot comparison of the MMN group with the IFA group for low baseline mtDNA-CN (A), high baseline mtDNA-CN (B), supplementation duration <33 d (C), and supplementation duration ≥33 d (D) among 108 pregnant women enrolled in the Supplementation with Multiple Micronutrients Intervention Trial study. High baseline mtDNA-CN ≥ 14.1; low baseline mtDNA-CN < 14.1. IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number.
To further affirm the stabilizing effect of MMN on baseline to postsupplementation mtDNA-CN, we divided the samples into 3 groups: >10% decrease, >10% increase, and no change (i.e., <10% decrease or increase), and compared the proportions in the MMN and IFA groups with Pearson's chi-square test. The results showed that the MMN group had significantly fewer numbers of >10% increase and greater numbers of no change than the IFA group (Table 3).
TABLE 3.
The Δ mtDNA-CN proportions of 108 pregnant women enrolled in the Supplementation with Multiple Micronutrients Intervention Trial study by supplementation group
| Supplement | ||
|---|---|---|
| MtDNA-CN change1 | MMN (n = 54) | IFA (n = 54) |
| >10% decrease | 14 (25.9) | 13 (24.1) |
| No change | 12 (22.2) | 3 (5.6) |
| >10% increase | 27 (51.9) | 38 (70.4) |
| P 2 | 0.021 | |
Values are n (%). IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number.
Pearson's chi-square test was used to compare the Δ percentages of the MMN and IFA groups. P < 0.05 was considered statistically significant.
Discussion
Our study showed that maternal MMN supplementation stabilized maternal peripheral blood mtDNA-CN as compared with IFA. We also observed that mtDNA-CN significantly increased from baseline onwards, indicating an increase with gestational age, regardless of the type of supplementation (MMN or IFA) given. Nevertheless, MMN was able to allow only a relatively small change in mtDNA-CN from early (baseline) to later pregnancy. Of note, this study is the first, to our knowledge, to compare the mtDNA-CN at baseline and at follow-up from the same pregnant woman, to be carried out in an LMIC where the majority of global pregnancies occur (28), and to present evidence of a biomedical mechanism for the effect of MMN on pregnancy outcomes, although it was conducted in a modest sample size, which is a limitation of this study.
Previous studies have reported that oxidative stress increased with gestational age during normal pregnancy, especially in the third trimester, marked by the rise of plasma lipid hydroperoxides, urinary 8-hydroxydeoxyguanosine, and plasma 8-isoprostane (29–31), and that peripheral blood mtDNA-CN was higher when exposed to oxidative stress (32, 33). Oxidative stress in pregnant women can lead to reduced birth weight (34), and although we did not measure oxidative stress directly, which is another limitation of the study, our previous study in this population, along with other studies, reported that elevated levels of mtDNA-CN were associated with LBW (17–19).
Our study provides evidence for how the mtDNA-CN may change during pregnancy and supports the idea that elevation of mtDNA-CN is a compensatory mechanism to meet energy requirements and overcome increased oxidative stress during pregnancy. Given that each person has different mitochondrial efficiency and heterogeneity when exposed to oxidative stress and its consequences, MMN may provide pleiotropic effects across populations and persons depending on their micronutrient status.
MMN supplementation can counteract elevated oxidative stress during pregnancy (35, 36). Previously, SUMMIT showed that MMN supplementation reduced fetal loss and early infant mortality (4) and improved birth weight (37) in particular in anemic women (4). In this study we could not see a difference in supplement effect in anemic women, which might be due to the modest sample size. Nevertheless, as causes of anemia in LMICs include nutrition, infectious disease, and genetics, which influence several hemoglobin-independent pathways (38), MMN components most probably have a positive effect on overall maternal health status, including reductions in maternal and fetal inflammation and improvements in oxidative metabolism and placental function (6). We propose that MMN supplementation could mitigate the need to increase mtDNA-CN due to higher energy demand by supporting more efficient mitochondrial function, and thereby stabilizing the copy number. The MMN supplement contained vitamins and minerals such as zinc, copper, iodine, and selenium which could reduce oxidative stress and improve mitochondrial functions (4). For example, vitamin C protects mtDNA from oxidative damage by reactive oxygen species (ROS) (39), whereas riboflavin is needed to increase the activity of mitochondrial complex I and II (40). A study in pregnant Nigerian women concluded that vitamin C treatment could reduce oxidative stress during pregnancy (41). Moreover, a study in diabetic pregnant rats showed that a combination of vitamins C and E supplementation decreased oxidative stress and improved fetal outcomes (42).
Zinc is the cofactor for antioxidant enzymes and induces synthesis of metallothionein, which is involved in the reduction of hydroxyl radicals (43, 44). Zinc can also reduce DNA strand breaks and modify circulating plasma protein concentrations involved in DNA repair, oxidative stress, and inflammation (45). Copper is associated with the reduction of oxidative stress (46). Cu,Zn-superoxide dismutase, an antioxidant enzyme that provides cells with a defense against oxygen toxicity, requires copper and zinc for its catalytic function (47). Copper is also needed for mitochondrial OXPHOS, in particular for the activity of the mitochondrial cytochrome c oxidase enzyme (48). Iodine is associated with oxidative stress (49) and mild iodine deficiency was correlated with decreased total antioxidant status and superoxide dismutase activity in pregnant women in Mexico (50). Selenium is the cofactor for the glutathione peroxidase antioxidant enzyme. Low selenium serum concentrations were associated with pre-eclampsia (51), and selenium supplementation has been reported to significantly enhance mitochondrial respiration and increase mitochondrial content in trophoblasts (52). Thus, efficient antioxidant enzymes will indirectly protect mtDNA damage from ROS. These data suggest that the presence of additional components in the MMN, as compared with IFA, may maintain mitochondrial efficiency and protect against oxidative damage, most likely by the preservation of mitochondria and mtDNA quality. This abates a potential harmful cycle by sustaining efficient mitochondrial function that, in turn, reduces the production of ROS as an OXPHOS by-product, while enabling better fetal growth and protection from IUGR.
We also observed that the response to MMN supplementation was rapid, appearing within <33 d, whereas effects of IFA supplementation alone might require a longer period. This suggests that MMN is likely to be beneficial when started at any time during pregnancy. We note that other studies report duration-specific effects, as opposed to dose-specific effects, of nutrients. For example, the impact of preconceptional folate consumption in reducing preterm birth (53) or the impact of folate in reducing the risk of stroke (54). The timing of initiation of supplementation might also be important, but the limited sample size of our study precluded such analysis. It was reported in 2 meta-analyses that if MMN is given before the third trimester the birth weight will be improved (55), and if given before 20 weeks of gestation the incidence of preterm birth will be decreased (6). However, later supplementation would still give positive effects, as SUMMIT reported that initiation of supplementation during the third trimester could still improve birth weight and reduce infant mortality (4). The effect of supplementation in the third trimester could be partly explained by the antioxidative mechanism of MMN in reducing the high oxidative stress that is commonly observed in the third trimester (29–31, 56). In this study, we did not measure oxidative stress biomarkers; therefore, future study on mitochondrial function and MMN that also includes measurement of oxidative stress biomarkers is needed to elucidate the mechanisms of oxidative stress reduction and mitochondrial improvement due to micronutrient supplementation.
In conclusion, as pregnancy progresses, the energy demands and oxidative stress both increase. This leads to an increase in mtDNA-CN with gestational age, possibly as a compensatory mechanism. MMN supplementation may improve mitochondrial function, reduce oxidative stress, stabilize mtDNA-CN, and improve fetal growth, birth weight, and survival. Our results provide a valuable scientific basis in support of policy change toward MMN supplementation for pregnant women to fulfill their micronutrient needs in pregnancy, particularly in LMICs.
Supplementary Material
Acknowledgments
We gratefully acknowledge Husni Muadz, PhD, and Mandri Apriatni, MA, from the SUMMIT Institute of Development for their contribution during the initial data collection, and Sukma Oktavianthi, MBiomed, from the Eijkman Institute for Molecular Biology for her expertise in assisting with the statistical analyses. The authors’ responsibilities were as follows—SGM and AHS: designed the study; LP: performed the analysis of blood specimens and associated data processing; LP, ELP, RR, AHS, and SGM: analyzed the data and performed the statistical analysis; AHS: is the principal investigator and guarantor of SUMMIT, and its data used herein; and all authors: wrote and revised the manuscript and read and approved the final manuscript.
Notes
Supported in part by a block grant from the Government of the Republic of Indonesia through the Ministry of Research, Technology and Higher Education, in part by the Women Reach Initiative grant from the Harvard TH Chan School of Public Health, and in part by the Indonesian Danone Institute Foundation.
Author disclosures: LP, ELP, RR, DEW, AHS, and SGM, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
AHS current affiliation is the Eijkman-Oxford Clinical Research Unit, Eijkman Institute for Molecular Biology, Jakarta, Indonesia and the Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Abbreviations used: IFA, iron and folic acid; IUGR, intrauterine growth restriction; LBW, low birth weight; LMIC, low- and middle-income country; MMN, multiple micronutrient; mtDNA, mitochondrial DNA; mtDNA-CN, mitochondrial DNA copy number; OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; SGA, small for gestational age; SUMMIT, Supplementation with Multiple Micronutrients Intervention Trial.
References
- 1. Gittelsohn J, Thapa M, Landman LT. Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Soc Sci Med. 1997;44:1739–49. [DOI] [PubMed] [Google Scholar]
- 2. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 3. Katz J, Wu LA, Mullany LC, Coles CL, Lee ACC, Kozuki N, Tielsch JM. Prevalence of small-for-gestational-age and its mortality risk varies by choice of birth-weight-for-gestation reference population. PLoS One. 2014;9:e92074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, Muadz H, Soesbandoro SDA, Tjiong R et al.. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371:215–27. [DOI] [PubMed] [Google Scholar]
- 5. Roberfroid D, Huybregts L, Lanou H, Habicht J-P, Henry M-C, Meda N, Kolsteren P. Prenatal micronutrient supplements cumulatively increase fetal growth. J Nutr. 2012;142:548–54. [DOI] [PubMed] [Google Scholar]
- 6. Smith ER, Shankar AH, Wu LS-F, Aboud S, Adu-Afarwuah S, Ali H, Agustina R, Arifeen S, Ashorn P, Bhutta ZA et al.. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health. 2017;5:e1090–100. [DOI] [PubMed] [Google Scholar]
- 7. Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH; SUMMIT Study Group. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Trop Med Int Health. 2012;17:938–50. [DOI] [PubMed] [Google Scholar]
- 8. Prado EL, Sebayang SK, Apriatni M, Adawiyah SR, Hidayati N, Islamiyah A, Siddiq S, Harefa B, Lum J, Alcock KJ et al.. Maternal multiple micronutrient supplementation and other biomedical and socioenvironmental influences on children's cognition at age 9–12 years in Indonesia: follow-up of the SUMMIT randomised trial. Lancet Glob Health. 2017;5:e217–28. [DOI] [PubMed] [Google Scholar]
- 9. Ladipo OA. Nutrition in pregnancy: mineral and vitamin supplements. Am J Clin Nutr. 2000;72:280S–90S. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization, editor. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 11. Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. [DOI] [PubMed] [Google Scholar]
- 12. Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci USA. 2015;112:E6614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Kleist-Retzow J-C, Cormier-Daire V, Viot G, Goldenberg A, Mardach B, Amiel J, Saada P, Dumez Y, Brunelle F, Saudubray J-M et al.. Antenatal manifestations of mitochondrial respiratory chain deficiency. J Pediatr. 2003;143:208–12. [DOI] [PubMed] [Google Scholar]
- 15. Shay JW, Pierce DJ, Werbin H. Mitochondrial DNA copy number is proportional to total cell DNA under a variety of growth conditions. J Biol Chem. 1990;265:14802–7. [PubMed] [Google Scholar]
- 16. Liu C-S, Tsai C-S, Kuo C-L, Chen H-W, Lii C-K, Ma Y-S, Wei Y-H. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37:1307–17. [DOI] [PubMed] [Google Scholar]
- 17. Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, Cetin I. Higher mitochondrial DNA content in human IUGR placenta. Placenta. 2008;29:1029–33. [DOI] [PubMed] [Google Scholar]
- 18. Colleoni F, Lattuada D, Garretto A, Massari M, Mandò C, Somigliana E, Cetin I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol. 2010;203:365.e1–6. [DOI] [PubMed] [Google Scholar]
- 19. Priliani L, Febinia CA, Kamal B, Shankar AH, Malik SG. Increased mitochondrial DNA copy number in maternal peripheral blood is associated with low birth weight in Lombok, Indonesia. Placenta. 2018;70:1–3. [DOI] [PubMed] [Google Scholar]
- 20. Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA. 2006;103:17589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pejznochová M, Tesarová M, Honzík T, Hansíková H, Magner M, Zeman J. The developmental changes in mitochondrial DNA content per cell in human cord blood leukocytes during gestation. Physiol Res. 2008;57:947–55. [DOI] [PubMed] [Google Scholar]
- 22. Qiu C, Hevner K, Abetew D, Sedensky M, Morgan P, Enquobahrie DA, Williams MA. Mitochondrial DNA copy number and oxidative DNA damage in placental tissues from gestational diabetes and control pregnancies: a pilot study. Clin Lab. 2013;59:655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prado EL, Ullman MT, Muadz H, Alcock KJ, Shankar AH, SUMMIT Study Group. The effect of maternal multiple micronutrient supplementation on cognition and mood during pregnancy and postpartum in Indonesia: a randomized trial. PLoS One. 2012;7:e32519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venegas V, Wang J, Dimmock D, Wong L-J. Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet. 2011;68:19.7.1–12. [DOI] [PubMed] [Google Scholar]
- 25. Ritz C, Spiess A-N. qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics. 2008;24:1549–51. [DOI] [PubMed] [Google Scholar]
- 26. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kloke JD, McKean JW. Rfit: rank-based estimation for linear models. R J. 2012;4:57–64. [Google Scholar]
- 28. Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45:301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf). 2002;57:609–13. [DOI] [PubMed] [Google Scholar]
- 30. Hung T-H, Lo L-M, Chiu T-H, Li M-J, Yeh Y-L, Chen S-F, Hsieh T-T. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci. 2010;17:401–9. [DOI] [PubMed] [Google Scholar]
- 31. Matsuzaki M, Haruna M, Ota E, Murayama R, Yamaguchi T, Shioji I, Sasaki S, Yamaguchi T, Murashima S. Effects of lifestyle factors on urinary oxidative stress and serum antioxidant markers in pregnant Japanese women: a cohort study. Biosci Trends. 2014;8:176–84. [DOI] [PubMed] [Google Scholar]
- 32. Lee H-C, Wei Y-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y-C, Lee W-C, Liao S-C, Lee L-C, Su Y-J, Lee C-T, Chen J-B. Mitochondrial DNA copy number correlates with oxidative stress and predicts mortality in nondiabetic hemodialysis patients. J Nephrol. 2011;24:351–8. [DOI] [PubMed] [Google Scholar]
- 34. Kim Y-J, Hong Y-C, Lee K-H, Park HJ, Park EA, Moon H-S, Ha E-H. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–92. [DOI] [PubMed] [Google Scholar]
- 35. Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011:e841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS One. 2014;9:e111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sebayang SK, Dibley MJ, Kelly P, Shankar AV, Shankar AH. Modifying effect of maternal nutritional status on the impact of maternal multiple micronutrient supplementation on birthweight in Indonesia. Eur J Clin Nutr. 2011;65:1110–17. [DOI] [PubMed] [Google Scholar]
- 38. Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–35. [DOI] [PubMed] [Google Scholar]
- 39. Sagun KC, Cárcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19:1657–67. [DOI] [PubMed] [Google Scholar]
- 40. Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R. A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol. 2009;11:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olayaki AO, Ajao M, Jimoh AAG, Soladoye A. Effect of vitamin C on malondialdehyde (MDA) in pregnant Nigerian women. J Basic Appl Sci. 2008;4:105–8. [Google Scholar]
- 42. Cederberg J, Simán CM, Eriksson UJ. Combined treatment with vitamin E and vitamin C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatr Res. 2001;49:755–62. [DOI] [PubMed] [Google Scholar]
- 43. Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–63S. [DOI] [PubMed] [Google Scholar]
- 44. Do Nascimento Marreiro D, Cruz KJC, Morais JBS, Beserra JB, Severo JS, De Oliveira ARS. Zinc and oxidative stress: current mechanisms. Antioxidants (Basel). 2017;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zyba SJ, Shenvi SV, Killilea DW, Holland TC, Kim E, Moy A, Sutherland B, Gildengorin V, Shigenaga MK, King JC. A moderate increase in dietary zinc reduces DNA strand breaks in leukocytes and alters plasma proteins without changing plasma zinc concentrations. Am J Clin Nutr. 2017;105:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson WT. Oxidative stress in cardiac mitochondria caused by copper deficiency may be insufficient to damage mitochondrial proteins. FASEB J. 2009;23(1_Supplement):727.1. [Google Scholar]
- 47. Harris ED. Copper as a cofactor and regulator of copper,zinc superoxide dismutase. J Nutr. 1992;122:636–40. [DOI] [PubMed] [Google Scholar]
- 48. Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cuellar-Rufino S, Navarro-Meza M, García-Solís P, Xochihua-Rosas I, Arroyo-Helguera O. Iodine levels are associated with oxidative stress and antioxidant status in pregnant women with hypertensive disease. Nutr Hosp. 2017;34:661–6. [DOI] [PubMed] [Google Scholar]
- 50. Vidal ZEO, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, Rodríguez RC, Arroyo-Helguera O. Oxidative stress increased in pregnant women with iodine deficiency. Biol Trace Elem Res. 2014;157:211–17. [DOI] [PubMed] [Google Scholar]
- 51. Maleki A, Fard MK, Zadeh DH, Mamegani MA, Abasaizadeh S, Mazloomzadeh S. The relationship between plasma level of Se and preeclampsia. Hypertens Pregnancy. 2011;30:180–7. [DOI] [PubMed] [Google Scholar]
- 52. Khera A, Dong L-F, Holland O, Vanderlelie J, Pasdar EA, Neuzil J, Perkins AV. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta. 2015;36:863–9. [DOI] [PubMed] [Google Scholar]
- 53. Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GDV, Eddleman K, Gross SJ, Dugoff L, Craigo SD et al.. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–82. [DOI] [PubMed] [Google Scholar]
- 55. Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;11:CD004905. [DOI] [PubMed] [Google Scholar]
- 56. Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24(Suppl A):S21–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.