Abstract
Background:
The prevention of surgical site infection is one of the most concerning issues in operating rooms. Surgical gowns are worn as one of the intraoperative strategies for infection prevention. The present study investigated whether the gowns remained sterile during the surgical procedure. Furthermore, this study examined which parts of the surgical gown were more prone to contamination.
Methods:
The sterility of the gowns was investigated during eight total joint arthroplasties all of which were performed by four surgeons. The samples were taken from the arms and frontal part of the sterile gowns pre- and postoperatively. In the anterior surface of the gown, the sampling was initiated at a strip with 50 cm height from the ground followed by the strips with 15 cm distances from caudal to cephalad. Furthermore, the frontal part of the gown was divided into three parts in relation to the operating room table. Finally, the contamination rate was evaluated in each part. A semiquantitative method was used for the analysis of bacterial culture.
Results:
Before the operation, there were four samples tested positive for bacterial culture (1.06%). All of these samples were taken from the most proximal strip near the neckline. After the surgery, the rate of contamination in the strips on the frontal part of the gown was reported as 3.1% to 53%. Based on the operating table, the contamination rate was 35.9%, 8.9%, and 47.3% in the distal, middle, and proximal parts of the gown, respectively. The contamination rate at the elbow crease was 23%, and at 5 and 10 cm above the creases were 24% and 36%, respectively.
Conclusion:
The high rate of gown contamination during the operation is concerning. However, part of the gown that was in contact with the operating room table remained clean most of the time. More safe strategies should be used for infection prevention in operating rooms.
Key Words: Contamination, Infection, Operating room, Sterility, Surgical gown
Introduction
Total joint arthroplasty (TJA), especially in the hip and knee, are among the most successful surgeries associated with compromising outcomes and improved quality of life (1, 2). However, there are some complications affecting the outcomes of TJA. Periprosthetic joint infection (PJI) is one of the most concerning complications associated with morbidity and mortality after TJA and a leading cause for revision surgery (3-5). The rate of PJI has been reported within 1% to 2% after arthroplasty that is expected to substantially increase with the increased number of arthroplasties (6-10).
Regarding the terrible clinical and socioeconomic consequences of infection in patients after TJA, it is critical for surgeons and medical researchers to identify the risk factors of infection and sources of contamination. Previously, some conditions or surgical factors increasing the risk of PJI have been suggested, such as smoking, obesity, immunosuppressive drugs, diabetes mellitus, lasting operation, power equipment, and higher number of staff in the operating room (11-14).
Among these factors, the clothing of the surgeon and other staff of the operating room is of considerable importance for the intraoperative prevention of infection. Most of the guidelines recommended utilizing surgical gowns, gloves, and masks, which have been shown to be effective in the prevention of surgeon-related contamination (15-19). Merollini et al. observed that the use of surgical gown is the most important strategy to reduce the risk of surgical site infection identified by the experts (16).
However, there are some drawbacks with the use of surgical gowns. For example, it has been demonstrated that gown-glove interface can be a source of contamination (20). Furthermore, the type of gown can significantly affect the count of airborne microorganisms or increase the risk of intraoperative contamination without being airborne (5, 21-23).
Accurate knowledge about the sterile parts of the surgical gown can be helpful to minimize the risk of surgeon-related contamination. To the best of our knowledge, this was exclusively investigated in an original study (24) that necessitates the precise conduction of further studies to determine the sterile parts of a surgical gown. In the present study, the boundaries of the most sterile parts of the surgical gowns used during arthroplasty procedures were determined pre- and postoperatively.
Materials and Methods
During August 2017, eight TJAs (five total hip arthroplasties and three total knee arthroplasties) were performed by the same surgeon from whom the required data were collected. Before the study, the institutional review board approved the proposal. In each surgery, the disposable gowns of the same four staff, including the senior surgeon and three of his co-workers and residents were investigated. None of these participants were involved in the dressing of the patients.
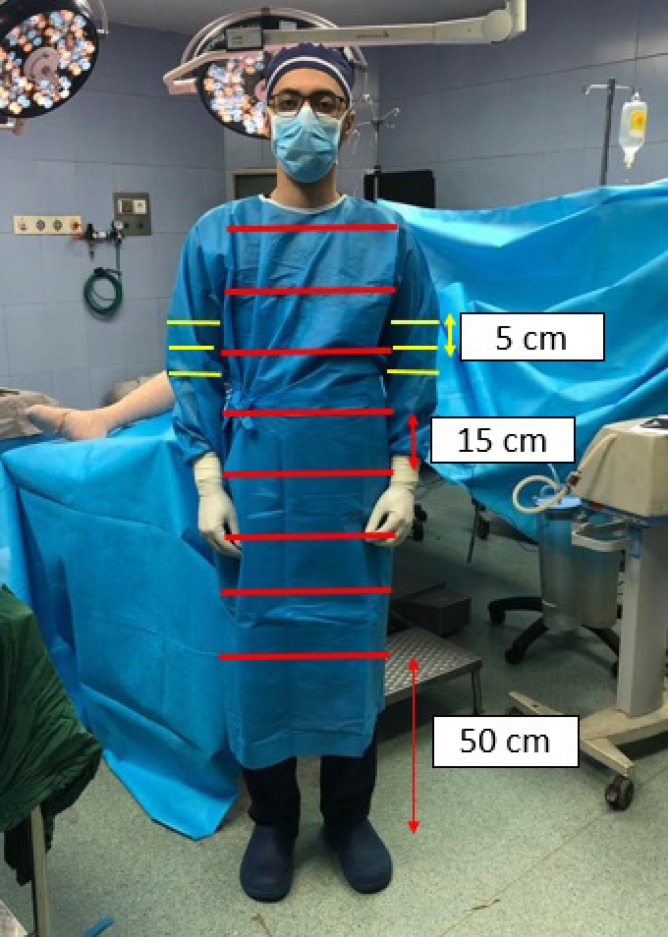
For sampling, the protocol explained by Bible et al. was utilized with some modifications (24). The samples were taken from the frontal part of the gowns and sleeves using sterile swabs. The first sampling strip was located at 50 cm height from the ground. Since then, sampling was performed in the strips of 15 cm distances.
With this method and considering the height of the enrolled staff (176-187 cm), it was possible to take 7 samples of the frontal parts of the gown from each of the participants. It is important that the sampling was conducted on the entire length of each strip. In addition, sampling was performed on the gown sleeves at the elbow creases, as well as 5 and 10 cm, proximal to the creases [Figure 1]. Therefore, 52 samples were taken at each sampling stage for each surgery.
Figure 1.
The location of the sampling at the anterior surface and sleeves of the gown
The sampling of the gowns was performed in 2 stages at each surgical procedure as follows: 1) after wearing the gowns and just before the beginning of the procedure and 2) at the end of the procedure and just before the gown removal. In addition, just before wearing the gowns, the sampling was performed from the same points or strips of the unsterile staff’s clothes or bodies to control the reliability of the culture results presented by the laboratory. The laboratory was blind about the characteristics of the samples (the stage in which the sample was taken and part of sampling).
Culture analysis was performed based on a semiquantitative technique using a sheep blood agar plate, which was sufficient for the growth of Gram-negative and Gram-positive bacteria (25). Each plate was divided into four quadrants and graded by the number of quadrants with positive growth (0-4+). The contamination was defined as the presence of one or more quadrants with positive growth (24).
For more evaluation, the frontal parts of the gowns were divided to three proximal (with the most proximal two strips), distal (two strips below the level of operating room table), and middle (three strips between the proximal and distal areas) parts. The height of the operating table was within 90-125 cm from the ground. Finally, the prevalence of contamination was distinctly investigated in these separate parts.
Results
In total, out of 1248 samples, 416 samples were taken from the unsterile clothes or skin. All of these samples were tested positive after culture analysis confirming the efficacy of laboratory studies. Among the samples taken from the gowns immediately after wearing them (n=376), 4 samples were contaminated (one sample 1+ and the others 2+; 1.06%). All of these samples were taken from the higher strip of the gowns. The rest of the samples obtained from the newly worn gowns (n=372) were not contaminated.
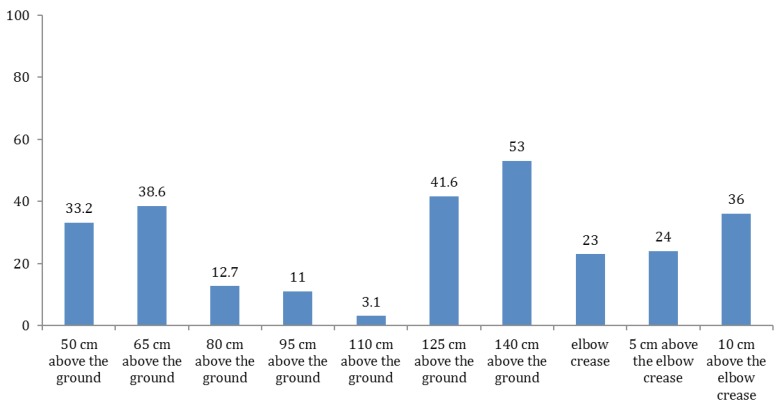
In the samples taken at the second stage, the growth was observed in all of the strips with a considerably different prevalence [3.1-53%; Figure 2]. Based on the operating table, the average rates of contamination were reported as 35.9%, 8.9%, and 47.3% below the table, in the middle part of the gown and higher part, respectively. In total, 23% of the samples taken from the elbow crease were contaminated. Furthermore, 24% and 36% of the samples obtained from 5 and 10 cm above the elbow crease were tested positive after culture analysis, respectively.
Figure 2.
The rate of growth in the sampling strips at the second stage
Discussion
The results of the present study revealed that in spite of preoperative gown sterilization, cautions about the prevention of contamination during surgery, and adherence to the current guidelines, gown contamination can occur during the operation. There are several strategies and techniques introduced to prevent or minimize the incidence of postoperative infections in major orthopedic surgeries. In many of these guidelines, wearing sterile gowns was introduced as one of the most important intraoperative infection prevention strategy (15, 16).
It has been reported that more than 75% of the surgeons believe that using sterile surgical gowns is significant or critical for the intraoperative prevention of infection prevention (15). Knowing the fact that most of the surgical wound infections are developed by intraoperative surgical site contamination, the importance of using sterile surgical gowns becomes more prominent (26).
However, there are several concerns using surgical gowns. One of the most important features of a surgical gown is the material of which the gown is made. It has been shown that the infection rate and bacterial strike-through are higher when reusable woven gowns are used (27-31). Surgical drapes and gowns can release thousands of airborne particles in space carrying microorganisms, such as methicillin-resistant Staphylococcus aureus.
Although surgical gowns and drapes are believed to release more airborne particles, Noguchi et al. showed that even when nonwoven gowns and drapes are used, the space of operating room may be filled up by scattered airborne particles produced during unfolding the gowns and drapes, as well as donning the gowns (23). Furthermore, one of the problems with surgical gowns is increased perspiration, especially when nonwoven paper gowns are used. The sweat may aggregate in several parts of the body surface, such as axillae, arms, and waist which are loaded by several microorganisms (21, 24, 32). The sweat may affect the permeability of the gown (33). Then, it may be possible for microorganisms to cross the gown to the surgical wound without being airborne (22).
It has been demonstrated that the staff of operating room produce thousands of skin fragments prone to carrying microorganisms, which can contaminate other parts of the body or clothes in uncovered skin (28, 34-39). In the present study, contamination was preoperatively observed in four samples and many other samples after the operation completion. The high rate of postoperative contamination, when the worldwide accepted strategies were followed, was greatly concerning. The authors think that although the main source of contamination was not clearly detected, all or some of the above-mentioned causes may lead to positive culture in the present study.
Preoperatively, the contamination was observed in the most proximal frontal part of surgical gown near the neckline. It may be possible that the skin fragments released from the uncovered neck skin were responsible for the contamination of these newly worn gowns. In addition, the potential contact of the oral mask or the surgeon’s hands with this part when securing the mask may be the reason for the contamination.
The third potential reason for the preoperative positive culture is the airborne particles produced during unfolding the gowns and uncovering the gloves. The authors presumed that these particles may be suspended in higher parts of the space due to the lightweight. Fourthly, in a study carried out by Fraser et al., it was shown that particle contamination usually occurs in the gown-glove interface (20). It seems that when the surgeon flexes the elbows, the arms are tucked to the frontal part of the gown, and the contamination can transfer from gown-glove interface to this part of the gown.
The considerable contamination rate at the elbows and arms may be caused by accumulated sweat in these regions as previously explained. Furthermore, Bible et al. explained that the potential contact of the elbows and arms with the lateral sides and back of the gown may contribute to the contamination of these parts (24). In the present study, the middle part of the gown front was contaminated less than the upper and lower parts.
The potential reasons for the higher rate of contamination in upper parts may include the skin fragments and more perspiration in the upper part of the body; accordingly, the gown encloses the upper part of the body more strictly. Furthermore, it is possible that the contaminated arms of the gown in contact with the frontal part lead to the contamination of this part. Regarding the higher contamination rate of the lower part of the gown in comparison to the middle part, the authors assumed that some airborne particles arising from the ground due to the stepping of the staff may be the reason for contamination.
Additionally, the lower parts of the body, such as the perineal region and groins, are accumulated by microorganisms, which can pass from the body surface through the gown material and contaminate the frontal part of the gown. Although there are several studies that addressed the sterility of the surgical settings, including the surgical gowns, it seems that a limited number of studies investigated the contamination of the gowns during operation (24). The results of the present study are in line with the findings of a study conducted by Bible et al. (24).
It seems that using the sampling protocol introduced by Bible et al. in the present study was the main reason for similar findings (24). In the aforementioned study, the rate of contamination ranged from 6% to 48% that was observed in all of the sampling parts, including the front, elbows, and arms of the gowns (24). Similar to the results of the study carried out by Bible et al., the authors demonstrated that the contamination rate was the lowest in the middle part of the gown front, compared to the higher and lower parts (24).
It should be noted that the middle part of the gown was in contact with the operating room table that is a promising finding. Noguchi et al. demonstrated that although thousands of airborne particles were detected in the operating room, a small number of these particles were observed on the surface of the operating table (23).
Similar to the findings of other studies, there were some limitations in the present study. The study was limited by small sample size and fixed participants. It seems that obtaining more samples and enrolling more participants may result in more reliable findings. Furthermore, the type of contaminating microorganism was not investigated in the present study.
The obtained results of the present study demonstrated that despite following the guidelines regarding the prevention of surgical wound infection, intraoperative contamination of surgical gown can occur many times. These findings are concerning and necessitate the knowledge about the sources of contamination. Fortunately, the middle part of the gown front was contaminated less frequently, compared to the higher and lower parts. In addition, the rate of contamination in arms of the gowns was considerable that may demonstrate the importance of changing some of the surgeons’ intraoperative behaviors, including the position in which the upper extremities are held. It is suggested to carry out further studies in the future.
Conflicts of Interest:
The authors declare that there is no conflict of interest.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–19. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Skou ST, Roos EM, Laursen MB. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(7):692. doi: 10.1056/NEJMoa1505467. [DOI] [PubMed] [Google Scholar]
- 3.Rowan FE, Donaldson MJ, Pietrzak JP, Haddad FS. The role of one-stage exchange for prosthetic joint infection. Curr Rev Musculoskelet Med. 2018;11(3):370–9. doi: 10.1007/s12178-018-9499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozic KJ, Ong K, Lau E, Berry DJ, Vail TP, Kurtz SM, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574–83. doi: 10.1007/s11999-012-2605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulihar A, Taub NA, Taylor GJ. A randomised prospective comparison of Rotecno versus new Gore occlusive surgical gowns using bacterial air counts in ultraclean air. J Hosp Infect. 2009;73(1):54–7. doi: 10.1016/j.jhin.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Del Pozo JL, Patel R. Infection associated with prosthetic joints. N Engl J Med. 2009;361(8):787–94. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowsey MM, Choong PF. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577–81. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90(7):915–9. doi: 10.1302/0301-620X.90B7.20498. [DOI] [PubMed] [Google Scholar]
- 9.Glenny AM, Song F. Antimicrobial prophylaxis in total hip replacement: a systematic review. Health Technol Assess. 1999;3(21):1–57. [PubMed] [Google Scholar]
- 10.Darouiche R. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 11.Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT. Short-term complication risk following primary and revision total joint arthroplasty. 55th Annual Meeting of the Orthopaedic Research Society; February 22-25, 2009; Las Vegas, NV. [Google Scholar]
- 12.Hansen E, Belden K, Silibovsky R, Vogt M, Arnold W, Bicanic G, et al. Perioperative antibiotics. J Orthop Res. 2014;32(Suppl 1):S31–59. doi: 10.1002/jor.22549. [DOI] [PubMed] [Google Scholar]
- 13.Ritter MA. Surgical wound environment. Clin Orthop Relat Res. 1984;190(1):11–3. [PubMed] [Google Scholar]
- 14.Ritter MA, French ML, Hart JB. Microbiological studies in a horizontal wall-less laminar air-flow operating room during actual surgery. Clin Orthop Relat Res. 1973;97(1):16–8. doi: 10.1097/00003086-197311000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ricciardi BF, Bostrom MP, Lidgren L, Ranstam J, Merollini KM, W-Dahl A. Prevention of surgical site infection in total joint arthroplasty: an international tertiary care center survey. HSS J. 2014;10(1):45–51. doi: 10.1007/s11420-013-9369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221–6. doi: 10.1016/j.ajic.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Baldini A, Blevins K, Del Gaizo D, Enke O, Goswami K, Griffin W, et al. General assembly, prevention, operating room - personnel: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2018;34(2S):S97–104. doi: 10.1016/j.arth.2018.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seto WH, Tsang D, Yung RW, Ching TY, Ng TK, Ho M, et al. Advisors of Expert SARS group of Hospital Authority Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361(9368):1519–20. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100–14. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser JF, Young SW, Valentine KA, Probst NE, Spangehl MJ. The gown-glove interface is a source of contamination: a comparative study. Clin Orthop Relat Res. 2015;473(7):2291–7. doi: 10.1007/s11999-014-4094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward WG Sr, Cooper JM, Lippert D, Kablawi RO, Neiberg RH, Sherertz RJ. Glove and gown effects on intraoperative bacterial contamination. Ann Surg. 2014;259(3):591–7. doi: 10.1097/SLA.0b013e3182a6f2d9. [DOI] [PubMed] [Google Scholar]
- 22.Lankester BJ, Bartlett GE, Garneti N, Blom AW, Bowker KE, Bannister GC. Direct measurement of bacterial penetration through surgical gowns: a new method. J Hosp Infect. 2002;50(4):281–5. doi: 10.1053/jhin.2001.1154. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi C, Koseki H, Horiuchi H, Yonekura A, Tomita M, Higuchi T, et al. Factors contributing to airborne particle dispersal in the operating room. BMC Surg. 2017;17(1):78. doi: 10.1186/s12893-017-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bible JE, Biswas D, Whang PG, Simpson AK, Grauer JN. Which regions of the operating gown should be considered most sterile? Clin Orthop Relat Res. 2009;467(3):825–30. doi: 10.1007/s11999-008-0341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151–3. doi: 10.1302/0301-620X.68B1.3510215. [DOI] [PubMed] [Google Scholar]
- 26.Maki D. Lister revisited: surgical antisepsis and asepsis. N Engl J Med. 1976;294(23):1286–7. doi: 10.1056/NEJM197606032942311. [DOI] [PubMed] [Google Scholar]
- 27.Moylan JA, Kennedy BV. The importance of gown and drape barriers in the prevention of wound infection. Surg Gynecol Obstet. 1980;151(4):465–70. [PubMed] [Google Scholar]
- 28.Moylan JA, Fitzpatrick KT, Davenport KE. Reducing wound infections: improved gown and drape barrier performance. Arch Surg. 1987;122(22):152–7. doi: 10.1001/archsurg.1987.01400140034003. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin BC, Fox IL, Russ C. Affect of disposable draping on wound infection rate. Va Med. 1981;108(7):477. [PubMed] [Google Scholar]
- 30.Smith JW, Nichols RL. Barrier efficiency of surgical gowns: are we really protected from our patients’ pathogens? Arch Surg. 1991;126(6):756–63. doi: 10.1001/archsurg.1991.01410300102016. [DOI] [PubMed] [Google Scholar]
- 31.Granzow JW, Smith JW, Nichols RL, Waterman RS, Muzik AC. Evaluation of the protective value of hospital gowns against blood strike-through and methicillin-resistant Staphylococcus aureus penetration. Am J Infect Control. 1998;26(2):85–93. doi: 10.1016/s0196-6553(98)80027-8. [DOI] [PubMed] [Google Scholar]
- 32.Hill J, Howell A, Blowers R. Effect of clothing on dispersal of Staphylococcus aureus by males and females. Lancet. 1974;2(7889):1131–3. doi: 10.1016/s0140-6736(74)90885-x. [DOI] [PubMed] [Google Scholar]
- 33.Mills SJ, Holland DJ, Hardy AE. Operative field contamination by the sweating surgeon. Aust N Z J Surg. 2000;70(12):837–9. doi: 10.1046/j.1440-1622.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- 34.Davies RR, Noble WC. Dispersal of bacteria in desquamated skin. Lancet. 1962;2(7269):1295–7. doi: 10.1016/s0140-6736(62)90849-8. [DOI] [PubMed] [Google Scholar]
- 35.Whyte W, Vesley D, Hodgson R. Bacterial dispersion in relation to operating room clothing. J Hyg. 1976;76(3):367–8. doi: 10.1017/s0022172400055297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dineen P, Drusin L. Epidemics of postoperative wound infections associated with hair carriers. Lancet. 1973;302(7839):1157–9. doi: 10.1016/s0140-6736(73)92933-4. [DOI] [PubMed] [Google Scholar]
- 37.Whyte W. The role of clothing and drapes in the operating room. J Hosp Infection. 1988;11(Suppl C):2–17. doi: 10.1016/0195-6701(88)90019-9. [DOI] [PubMed] [Google Scholar]
- 38.Andersson AE, Bergh I, Karlsson J, Eriksson BI, Nilsson K. Traffic flow in the operating room: an explorative and descriptive study on air quality during orthopedic trauma implant surgery. Am J Infect Control. 2012;40(8):750–5. doi: 10.1016/j.ajic.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79–84. doi: 10.1053/jhin.2002.1217. [DOI] [PubMed] [Google Scholar]