Abstract
Background:
As the frequency of nonalcoholic fatty liver disease (NAFLD) continues to rise in the United States (US) community, more patients are hospitalized with NAFLD. However, data on the prevalence and outcomes of hospitalizations with NAFLD are lacking. We investigated the prevalence, trends and outcomes of NAFLD hospitalizations in the US.
Methods:
Hospitalizations with NAFLD were identified in the National Inpatient Sample (2007-2014) by their ICD-9-CM codes, and the prevalence and trends over an 8-year period were calculated among different demographic groups. After excluding other causes of liver disease among the NAFLD cohorts (n=210,660), the impact of sex, race and region on outcomes (mortality, discharge disposition, length of stay [LOS], and cost) were computed using generalized estimating equations (SAS 9.4).
Results:
Admissions with NAFLD tripled from 2007-2014 at an average rate of 79/100,000 hospitalizations/year (P<0.0001), with a larger rate of increase among males vs. females (83/100,000 vs. 75/100,000), Hispanics vs. Whites vs. Blacks (107/100,000 vs. 80/100,000 vs. 48/100,000), and government-insured or uninsured patients vs. privately-insured (94/100,000 vs. 74/100,000). Males had higher mortality, LOS, and cost than females. Blacks had longer LOS and poorer discharge destination than Whites; while Hispanics and Asians incurred higher cost than Whites. Uninsured patients had higher mortality, longer LOS, and poorer discharge disposition than the privately-insured.
Conclusions:
Hospitalizations with NAFLD are rapidly increasing in the US, with a disproportionately higher burden among certain demographic groups. Measures are required to arrest this ominous trend and to eliminate the disparities in outcome among patients hospitalized with NAFLD.
Keywords: Ethnicity, charge, length of stay, cost, discharge disposition
Introduction
With the increasing adoption of the Western diet and sedentary lifestyle, the prevalence of obesity, insulin resistance, type II diabetes, lipid disorders, and metabolic syndrome has been increasing [1-3]. Individuals with these disorders have a propensity to accumulate abnormal fat deposits in their liver; this is called nonalcoholic fatty liver disease (NAFLD). NAFLD is currently the most common liver disease and is estimated to affect 33% of adults worldwide (approx. 1 billion) [1]. NAFLD may progress to hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [2-4]. NAFLD represents a spectrum of liver diseases ranging from simple steatosis, steatohepatitis, to fibrosis and cirrhosis, with an increased risk for hepatocellular carcinoma. Clinical outcomes are poorer as patients’ progress from the benign end of the spectrum (steatosis) to the severe phenotypes [5-7].
Although studies have reported the rising prevalence of NAFLD in the community [8-10], the occurrence and burden among hospitalized patients have not been studied. Furthermore, gender, racial, socioeconomic, and regional disparities have been revealed in both prevalence and management outcomes for NAFLD-associated conditions [11,12]. However, no study has evaluated such disparities among patients hospitalized with NAFLD.
Considering the alarming increase in NAFLD and that end-stage liver disease from NAFLD is projected to be the number one reason for liver transplantation by 2020 [13], it is essential to evaluate the burden of and disparities among NAFLD-associated hospitalizations in the United States (US). This will allow early formulation of public health measures to quickly arrest any ominous trend. Therefore, we carried out this population-based study to investigate the prevalence and trends in the NAFLD in different demographic categories and the disparities among hospitalization outcomes of subjects admitted with NAFLD. We hypothesized that the prevalence and burden of NAFLD among hospitalized patients would be increasing, mirroring the pattern in the community.
Patients and methods
Data source
A retrospective cross-sectional analysis of the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) database was performed. The NIS is administered by the Agency for Healthcare Research and Quality, through a multi-stage clustered sampling by states, strata, and hospitals within the US for every year. Data from each year represent 20% of all the discharges across over 4500 non-federal community hospitals (public and academic centers) from about 40 states. Currently, 40 of 50 states in the US participate in the NIS. Each year of the NIS has about 7 million hospitalization records (weighted to 35 million hospitalizations) [14]. The NIS provides a fairly accurate representation of hospitalizations, because all the large and diverse states in the US participate in the program, including California, Florida, Texas, and New York. In this study, we used data from the years 2007-2014 (n=61,324,882) that contained per discharge 15 procedures and about 25-30 diagnoses all coded with the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes. Since the NIS is a completely de-identified publicly available data, no Institutional Review Board approval was required.
Study population and variables
After using the ICD-9-CM code of 571.8 to abstract records for patients aged 18 years and above with a discharge diagnosis of NAFLD (n=307,651, 0.009%), we eliminated records with other chronic liver disease—alcoholic liver disease, hemochromatosis, hepatitis C and B virus, primary biliary cirrhosis, autoimmune hepatitis, toxic liver disease, and other poorly defined liver diseases—and those with an organ transplant (Fig. 1 and Supplementary Table 1 (230.4KB, tif) ). The ICD-9-CM code for NAFLD has been used by many recent studies [15-20]. We also eliminated records with missing inputs, and abstracted demographic, patient, and hospital-related information. All the information used in this study either consisted of variables already available within the dataset or was created by us. We had 4 primary outcome variables: total hospital charge (THC; in US dollars), duration of hospitalization (length of stay in days: LOS), in-hospital mortality, and unfavorable disposition on discharge. For the THC we inflated the values before 2014 to the 2014 levels, using the Consumer Price Index from the US Bureau of Labor. Unfavorable disposition on discharge was derived from a multinomial variable in NIS to generate a binary variable: routine to home/home healthcare (as favorable) vs. transfer to another health facility (short-term acute hospital, skilled nursing, intermediate care, psychiatric, or rehabilitation centers) as unfavorable.
Figure 1.
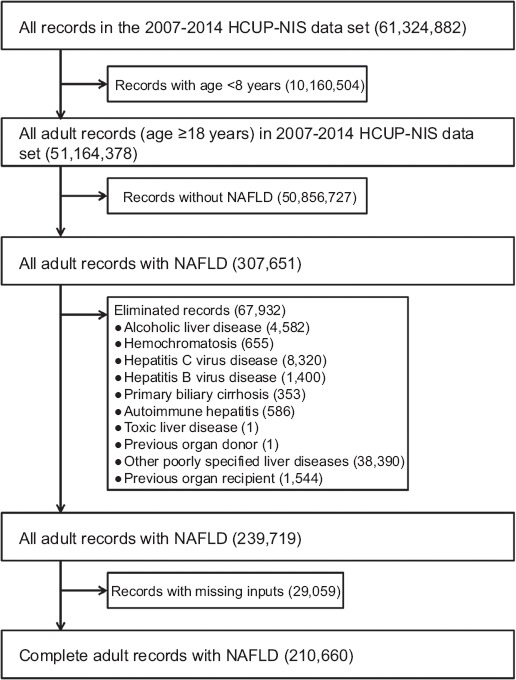
Selection flowchart for patients hospitalized for nonalcoholic fatty liver disease (NAFLD) in the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) data 2007-2014
We collected demographic information on sex (male and female), race (Whites, Blacks, Hispanics, and others [Asian, Pacific Islanders, Native Americans, and others]), health insurance (government [Medicare, Medicaid], private, and others [self-pay, uninsured and other charges]), and median household income of residence zip-code (first to fourth quartile). We also extracted comorbid clinical conditions using the ICD-9-CM codes. Over 50 comorbid conditions were selected and combined to produce the Deyo-Charlson index, an extensively studied and widely used guide [21]. These comorbidities captured chronic diseases across all the systems in the body and have been used in many studies [22-26]. Furthermore, since the outcomes of liver diseases vary with the severity of liver injury, we stratified the NAFLD subjects into three, based on the Baveno IV consensus criteria: no cirrhosis, compensated cirrhosis, and decompensated cirrhosis [27]. Cirrhosis and its decompensation (hepatorenal syndrome, jaundice, hepatic encephalopathy, ascites, variceal bleeding, and portal hypertension) were identified through ICD-9-CM codes (Supplementary Table 1 (230.4KB, tif) ). The Baveno IV classification has been extensively used and validated in the HCUP-NIS for the reliable identification of liver cirrhosis and assessment of its severity [28]. Finally, hospital characteristics that could impact outcomes were determined from the dataset and included hospital region (Northeast, South, Midwest and West), and hospital teaching status (rural, urban-nonteaching and urban-teaching).
Statistical analysis
Analyses were performed using the Statistical Analysis System (SAS V.9.4, SAS Institute Inc., Cary, NC, US). In all the statistical models, a P-value of <0.05 was chosen a priori. We reported the effect estimates, P-values and 95% confidence intervals (CI), or the Bonferroni corrected P-values for multiple comparisons. Patients’ clinical characteristics were reported as mean and standard deviation (SD) for continuous variables with normal distributions, and as a median and inter-quartile range (IQR) for either continuous or counting variables without a normal distribution. Similarly, statistical tests were carried out using the chi-square test and percentages for the categorical variables, and Student’s t-test and the Wilcoxon test for numeric variables with normal and non-normal distributions, respectively. The trends were estimated using general linear models with NAFLD as outcome and year as a predictor. Other demographic factors were added to the model and their interaction with year was tested with a P-trend <0.01 set as significance. The adjusted odds ratio (AOR) was calculated with multivariate regressions using generalized estimating equations with the predictors (demographics, patient and hospital characteristics) and each of the 4 outcomes. Binary (mortality and discharge disposition), discrete numeric variables with over-dispersed count distributions (LOS), and continuous variables with a right-skewed spread (THC) were modeled with binary logistic, negative binomial and gamma functions, respectively. As recommended by HCUP, all analysis was performed with the STRATA, CLUSTER and WEIGHT for the SURVEYLOGISTIC, SURVEYFREQ and SURVEYMEANS procedures to account for the complex clustered sampling methodology [29]. For the GENMOD procedures, the CLASS, WEIGHT and REPEATED statements were used to account for these complex and in-hospital correlations [30].
Results
Baseline characteristics of patients hospitalized with NAFLD
The total of 210,660 hospitalized patients with NAFLD were more likely to be female (60.16% vs. 39.84%), with a similar mean age of 54 (Table 1). Compared to males, females were slightly less likely to be White (70.03% vs. 73.51%) but more likely to be Black (9.56% vs. 7.67%) or Hispanic (15.06% vs. 12.94%). Females were more likely to be on governmental health insurance (51.42% vs. 43.81%) but less likely to be on private plans (39.12% vs. 43.84%) or uninsured (9.46% vs. 12.35%). They had a higher frequency of compensated (2.39% vs. 1.93%) and decompensated (2.80% vs. 2.31%) cirrhosis. The most common primary diagnoses during hospitalizations with NAFLD were morbid obesity, acute pancreatitis, and septicemia, in that order (Supplementary Table 2 (102KB, tif) ).
Table 1.
Characteristics of patients hospitalized with nonalcoholic fatty liver disease (NAFLD) in the US from 2007-2014 by sex
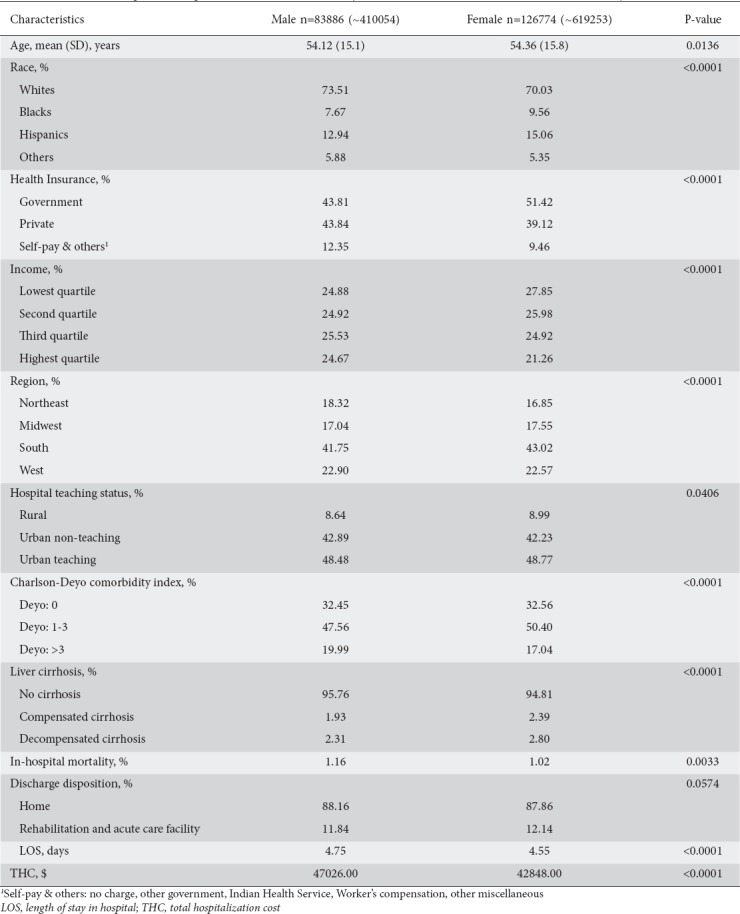
While the in-hospital mortality rate was lower among females (1.02% vs. 1.16%), the discharge disposition was similar across both sexes. The LOS was shorter (4.55 vs. 4.75 days) and THC lower ($42,848.00 vs. $47,026.00) among females compared with males.
Predictors of inpatient mortality
Only increasing age, sex, health insurance, hospital region and teaching status, Charlson-Deyo comorbidity, and liver cirrhosis were significantly associated with mortality (Table 2). The odds of dying increased by 36% for every 10-year increase in age (AOR 1.36, 95%CI 1.31-1.42; P<0.0001), by 14% among individuals without health insurance/self-pay vs. those with private insurance (AOR 1.14, 95%CI 1.09-1.67; P=0.002). Mortality was also higher among NAFLD hospitalizations in urban centers vs. rural centers and for those with a higher comorbidity burden and liver cirrhosis. However, females had 10% lower odds of mortality (AOR 0.91, 95%CI 0.83-0.99; P=0.03).
Table 2.
Determinants of in-hospital mortality and discharge disposition of patients hospitalized with nonalcoholic fatty liver disease (NAFLD)
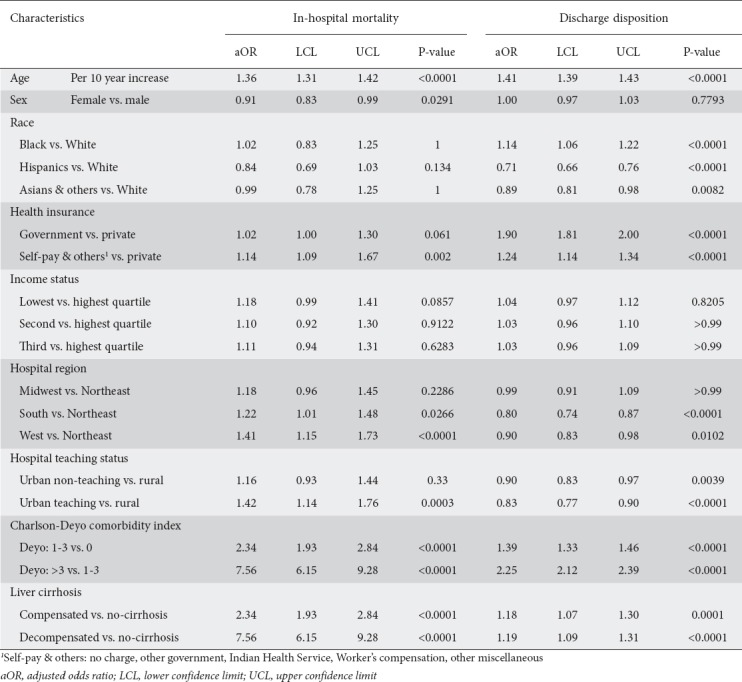
Predictors of discharge disposition
Significant predictors of unfavorable discharge disposition were age, race, health insurance, hospital region, and teaching status, comorbidity burden, and severity of cirrhosis (Table 2). There were 41% greater odds of unfavorable discharge for every 10-year increase in age. Unlike Blacks, who had 14% greater odds of unfavorable discharge compared with Whites (AOR 1.14, 95%CI 1.06-1.22; P<0.0001), Hispanics and Asians had 29% and 11% lower odds, respectively (AOR 0.71, 95%CI 0.66-0.76; P<0.0001 and AOR 0.89, 95%CI 0.81-0.98; P=0.008). Compared to the privately insured, inpatients with government insurance and those who were uninsured/self-pay had 90% and 24% higher odds, respectively, of unfavorable discharge (AOR 1.90, 95%CI 1.81-2.00; P<0.0001 and AOR 1.24, 95%CI 1.14-1.34; P<0.0001). Compared to the Northeast, the Southern and Western regions of the US had lower odds of unfavorable discharges.
Predictors of LOS
Sex, age, Black race, health insurance, hospital region and teaching status, comorbidity burden, and liver cirrhosis were associated with LOS (Table 3). Females had a 5% shorter LOS than males (4.35 [95%CI, 4.26-4.45] vs. 4.18 [95%CI 4.08-4.27] days; P<0.0001) (Table 3). Unlike other races, when compared to Whites, Blacks had a 7% longer LOS (4.48 [95%CI 4.34-4.62] vs. 4.20 [95%CI 4.11-4.29] days; P<0.0001). Government-insured and uninsured/self-pay patients had 19% and 10% longer stays, respectively (4.65 [95%CI 4.56-4.75] and 4.28 [95%CI 4.14-4.42] vs. 3.90 [95%CI 3.81-3.99] days; P<0.0001). Individuals in the lowest and second quartile had 4% and 3% longer LOS, respectively, compared to those from the highest income quartile. Compared to inpatients in the Northeastern regions, those in the Midwest and West had 5% and 7% shorter LOS (4.20 [95%CI 4.10-4.36] and 4.11 [95%CI 3.99-4.23] vs. 4.40 [95%CI 4.27-4.54] days; P=0.0385 and P=0.001, respectively). NAFLD hospitalizations in urban centers and those with higher comorbidity indices were associated with a longer LOS (Table 3).
Table 3.
Determinants of total cost and duration of hospitalization of patients admitted with nonalcoholic fatty liver disease (NAFLD)
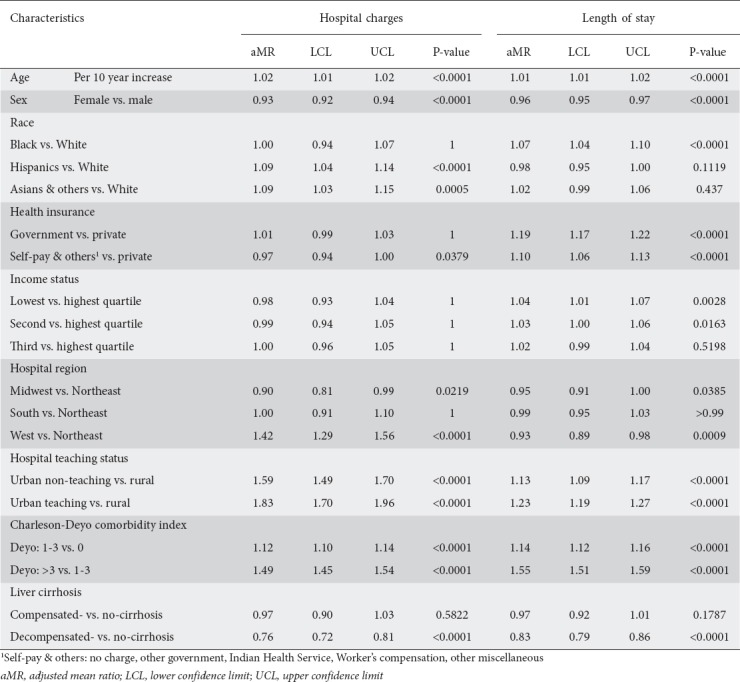
Predictors of THC
Male sex, Hispanic and Asian races, hospital region and teaching status, and comorbidity burden were associated with a higher THC among inpatients with NAFLD (Table 3). Females had 7% lower THC than males ($35,662 [95%CI 34,349-37,026] vs. $38,299 [95%CI 36,872-39,7768]; P<0.0001). While Blacks ($35,850 [95%CI 34,290-37,482]) showed no difference in THC compared to Whites ($35,267 [95%CI 33,905-36,684]; P>0.99), Hispanics and Asians had a 9% higher THC ($38,397 [95%CI 36,812-40,049]; P<0.0001 and $38,414 [95%CI 36,485-40,445]; P=0.0005). Inpatients in the Western regions ($49,304 [95%CI 46,970-51,755]) had 42% higher THC compared to the Northeastern regions ($34,795 [95%CI 32,502-37,249]; P<0.0001), unlike those in the Midwest ($31,244 [95%CI 29,647-32,927]; P=0.022) and the South ($34,795 [95%CI 32,502-37,249]; P>0.99). Urban centers (non-teaching and teaching) had a higher THC compared to rural centers ($41,177 [95%CI 39,463-42,965] and $47,298 [95%CI 45,139-49,560] vs. $25,911 [95%CI 24,551-27,348]; P<0.0001). THC increased with the number of comorbidities (Table 3).
Trends in hospitalizations for NAFLD
Hospitalizations with NAFLD almost tripled, with an increase of 79 NAFLD diagnoses per 100,000 hospitalizations per year, from 319/100,000 in 2007 to 902/100,000 hospitalizations in 2014 (Fig. 2A, Supplementary Table 3 (646.3KB, tif) ). All through the study period, males had both a higher frequency of NAFLD and a slightly steeper increase in the frequency of NAFLD hospitalization per year (83 vs. 76 per 100,000 hospitalizations, P<0.0001) (see slopes in Fig. 2B). After the trend was stratified by race, Hispanics had the highest prevalence of NAFLD hospitalizations, followed by Whites, slightly higher than Asians, while Blacks had the lowest prevalence. The rate of NAFLD hospitalizations per year followed a similar trend, with Hispanics having an average of 107 new cases of NAFLD per 100,000 hospitalizations per year compared to 48 in Blacks, and to 79.5 and 80.4 in Whites and Asians (see slopes in Fig. 2C). Privately-insured and uninsured individuals had a higher rate and trend of NAFLD hospitalizations than those with government insurance (see slopes in Fig. 2D). There was no statistically significant difference in the frequency and trends of hospitalizations for NAFLD (Fig. 2E). Finally, there was a regional trend in the frequencies of NAFLD hospitalizations (see trends and slopes in Fig. 2F), with the Western region having the highest frequencies and rate of increase in NAFLD hospitalizations (90.26/100,000), followed by the South (81.10/100,000), then the Midwest (74.58/100,000), with the least being in the Northeastern region (67.92/100,000). The graph (Fig. 2F) reveals that the Western regions of the US have both a higher burden of NAFLD among hospitalizations and a sharper increase in this burden per year.
Figure 2.
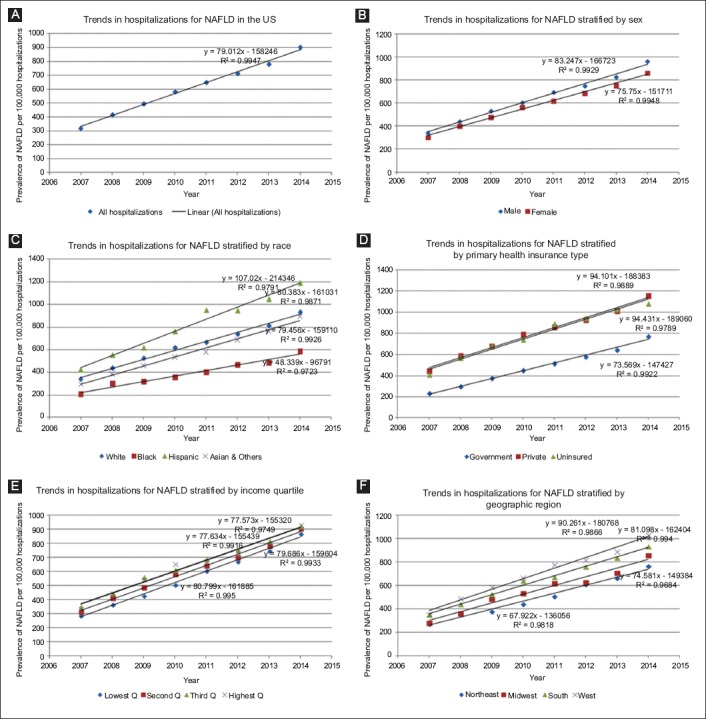
Trends in hospitalizations for nonalcoholic fatty liver disease (NAFLD) in the US from 2007-2014 (A), and categorized by sex (B), race (C), health insurance (D), income quartile (E), and hospital region (F)
Discussion
In this nationally representative study, we showed that the frequency of NAFLD among hospitalized patients tripled from 2007-2014, and that this increase varied significantly across demographic groups. Males, Hispanics, individuals with non-private health insurance, and those residing in the Western and Southern regions of the US were disproportionately affected, with a higher prevalence of NAFLD and poorer outcomes.
More than 60% of the patients in our study were female, suggesting that primary admissions for NAFLD were more burdensome among women; however, the HCUP-NIS had more female hospitalizations (female 59.73% vs. male 40.27%) before selecting for NAFLD. In contrast, there was a higher frequency of NAFLD hospitalizations among men than women (Fig. 2A), consistent with many studies showing that both NAFLD and nonalcoholic steatohepatitis are more prevalent among males than females [8,31-36]. We extend these studies in many ways. First, we reported that the male-predominant distribution of NAFLD observed in the community continues to the hospital setting. Second, we showed that males also had a higher rate of NAFLD hospitalization per year than women, further widening the gap in the burden of NAFLD between the sexes. The higher prevalence of NAFLD among males has been attributed to higher frequencies of insulin resistance and greater consumption of alcohol and non-diet soda among males compared to females [37]. Third, we showed that males have poorer in-hospital outcomes among NAFLD subjects: higher mortality, greater THC, and longer LOS. The cause of these poorer indexes among males may be related to health-seeking attitudes. Women utilize primary care more frequently [38-40], and may have had better management of their comorbidities, resulting in less severe presentations on admissions [41]. In addition, our data suggest that males have more comorbidities with a Charlson-Deyo score >3 (male 19.99% vs. female 17.04%). To curtail these poor trends among males, public health studies of optimal measures should be instituted, targeted towards encouraging better control of comorbidities in males in the community.
We also report that hospitalization for NAFLD was higher among Hispanics than in other racial groups (Fig. 2B), consistent with many studies [42-44]. As with the sexual disparities mentioned above, our findings extend these studies in many ways [42,43]. We demonstrated that a similar Hispanic-predominant distribution of NAFLD occurs among hospitalized patients and that Hispanics show a greater increase in the rate of NAFLD hospitalizations than other races, suggesting that an epidemic of NAFLD among the Hispanic population of the US might be imminent. The higher prevalence of NAFLD among Hispanics is an active focus for research [44]. Racial/ethnic variation in NAFLD prevalence has been partly attributed to diet, lifestyle, and genetic differences. One of the most studied genes is the Patatin-like phospholipase domain-containing protein 3 (PNPLA3) which encodes a membrane-bound phospholipase protein that regulates energy storage and usage. Hispanics have an allele of PNPLA3 (rs738409[G]) that favors increased fat accumulation in the liver, unlike Blacks who have a different allele of PNPLA3 (rs6006460[T]) that results in lower hepatic fat content [5,45]. Furthermore, Hispanics and Asians had a higher cost but better discharge disposition outcomes. The higher cost corroborates other studies by revealing the escalating cost of healthcare among Hispanics, who tend to use emergency departments more than office visits and therefore do not benefit from the preventive medical services prioritized by primary care physicians. The better discharge dispositions among Hispanics and Asians with NAFLD compared to Whites mirror other diseases. The causes remain unclear [46,47], but may be related to the availability of support at home, financial resources or the ethnic beliefs of patients and their families. Hispanic culture treats elders with respect and views discharge to home as a more positive outcome [48]. Similarly to reports from other studies on blacks, we showed that they had a longer LOS and poorer discharge disposition, which might be related to their higher comorbidity burden [49,50]. On further analysis, our results showed that Blacks had the highest frequency of comorbidities (Deyo ≥1: 71.8%) vs. other races: Whites (68.34%), Hispanics (60.87%), and Asians and other races (65.4%). To slow down this increasing burden of NAFLD among Hispanics, aggressive public health measures are needed, directed at Hispanic-specific risk factors such as diet, lifestyle, and health-seeking behaviors. More importantly, public health outreaches should be performed within the Hispanic community, to sensitize Hispanics to their higher susceptibility to NAFLD. Furthermore, primary care physicians could have a higher suspicion of NAFLD among their Hispanic and White patients.
Health insurance determines the type of healthcare available to the holder. Our data reports higher odds of mortality, poorer discharge disposition, and longer LOS among other groups compared to the privately-insured. Our results are similar to those of studies in 1993 and 2009, which showed that the uninsured population has higher mortality than the insured among community dwellers in the US [51,52]. Findings from another study among patients hospitalized in the US for myocardial infarction, stroke, and pneumonia are also consistent with our study [53]. These outcome disparities have been attributed to numerous factors, including difficulty in arranging discharge disposition, poor or absent outpatient management of comorbidities, lack of a primary care physician, less frequent use of subspecialists, and lower use of invasive and expensive procedures, amongst others [53,54]. We also demonstrated higher hospitalization rates and a greater number of hospitalizations/year among government-insured and uninsured/self-pay vs. privately-insured, implying that the causes of higher hospitalizations among the non-privately insured persist in the US and continue to widen the gap between privately and non-privately insured patients.
Our study observed significant variations in prevalence rate and yearly increase in NAFLD within the geographic distribution of the US. Interestingly, our observations follow the geographic trends in obesity and type 2 diabetes mellitus within the US, which are both risk factors for NAFLD [55]. Similar geographic distributions have been reported for race, income, and health insurance types [56]. Trending from Northeast to Midwest, to West and South regions of the US, there is generally higher proportion of ethnic minorities with fewer personal resources, poorer access to healthcare and fewer high-quality healthcare facilities, which might all be responsible for the higher prevalence and mortality among hospitalizations with NAFLD [56]. Furthermore, these regional disparities could reflect systematic problems at different levels of healthcare delivery, and they warrant further investigations.
Our study should be interpreted cautiously, bearing in mind the limitations of a cross-sectional study in general and those of ICD-9 coding in particular. There may have been coding errors and imprecision in the ICD-9 implementation, resulting in underestimation of the cases. The NIS does not contain information on how NAFLD was diagnosed, including magnetic resonance imaging, liver biopsy, ultrasound, and liver function tests. Furthermore, we were unable to confirm the diagnosis by requesting the records, as the NIS is completely de-identified. The prevalence of NAFLD can differ significantly based on the diagnostic modality, and this might have affected our results. Furthermore, the difference in diagnostic modalities may contribute to variations in prevalence of NAFLD across income and region categories in our study. However, because physicians and centers in the US comply with similar practice guidelines, we do not expect significant regional variations in the diagnostic modalities of choice for NAFLD. The absence of laboratory data made it impossible to calculate the model for end-stage liver disease score and other indices of liver severity. However, we used the Charlson-Deyo and Baveno IV indexes, which are well-researched parameters, to account respectively for various non-hepatic and hepatic-specific comorbidities. Although these comorbidities were captured in ICD-9-CM, it does not capture the severity of each illness. Very few individuals from other races were in the NIS dataset, so we could not investigate the presence of disparities among those groups. Although NAFLD is a spectrum of liver disease with different outcomes, unfortunately the ICD-9-CM nomenclature does not distinguish among the subtypes, so we were unable to study how they vary with sex and race. Furthermore, the NIS does not specify the immediate cause of death, thus making it impossible for us to study the possible factors responsible for the higher death rate among males and the uninsured population. Despite these shortcomings, we believe that since the NIS encompasses numerous hospitals across various states in the US, it provides an excellent nationally representative sample and results in reliable estimates.
In conclusion, our novel findings revealed a rising frequency of hospitalizations for NAFLD in the US. There are demographic and regional variations in the trends and clinical outcomes of hospitalizations with NAFLD from 2007-2014 in the US among males, Hispanics, Blacks, non-privately insured, and individuals in the Southern and Western regions of the US. Systemic factors at multiple levels of healthcare perpetuate these inequalities. Future studies are needed to identify and eliminate these inequalities, and aggressive public health measures are required to arrest this increasing trend in NAFLD.
Summary Box.
What is already known:
In the United States (US) community, the prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising, especially among Hispanics and males
Among hospitalized patients, little is known about the prevalence, trends and outcomes of hospitalizations with NAFLD
What the new findings are:
Hospitalizations for NAFLD tripled from 2007-2014 in the US
Hospitalization rate was higher and increased at a quicker rate/year among males, Hispanics, non-privately insured, and individuals residing in the Western region of the US
Males had poorer outcomes compared to females
Blacks, Hispanics and Asians had poorer outcomes compared to Whites
ICD-9-CM codes used to identify clinical conditions in the study
Ten most common primary diagnoses during hospitalizations with nonalcoholic fatty liver disease (NAFLD)
Trends in hospitalizations for nonalcoholic fatty liver disease (NAFLD): Crude (A), by Sex (B), Race (C), Health Insurance (D), Income status (E) and Region (F)
Biography
North Shore Medical Center, Salem, MA; Tufts University Medical School, Boston, MA; University of Massachusetts Medical School, Worcester, MA; University of Massachusetts Lowell, MA; Englewood Hospital and Medical Center, NJ; University of Kentucky College of Medicine; St. Cloud State University, Plymouth, MN, USA
Footnotes
Conflict of Interest: None
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review:the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver:a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 4.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 5.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects:a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40:475–483. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease:a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease:a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States:impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 9.Arab JP, Barrera F, Gallego C, et al. High prevalence of undiagnosed liver cirrhosis and advanced fibrosis in type 2 diabetic patients. Ann Hepatol. 2016;15:721–728. doi: 10.5604/16652681.1212434. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49:222–227. doi: 10.1097/MCG.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs HF, Broderick RC, Harnsberger CR, et al. Benefits of bariatric surgery do not reach obese men. J Laparoendosc Adv Surg Tech A. 2015;25:196–201. doi: 10.1089/lap.2014.0639. [DOI] [PubMed] [Google Scholar]
- 12.Mainous AG, 3rd, Johnson SP, Saxena SK, Wright RU. Inpatient bariatric surgery among eligible black and white men and women in the United States 1999-2010. Am J Gastroenterol. 2013;108:1218–1223. doi: 10.1038/ajg.2012.365. [DOI] [PubMed] [Google Scholar]
- 13.Charlton M. Cirrhosis and liver failure in NAFLD:molehill or mountain? Hepatology. 2008;47:1431–1433. doi: 10.1002/hep.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [[Accessed 2 July 2019]];HCUP-US NIS Overview. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp#data . [Google Scholar]
- 15.Loomis AK, Kabadi S, Preiss D, et al. Body mass index and risk of nonalcoholic fatty liver disease:two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101:945–952. doi: 10.1210/jc.2015-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai TF, Wang TS, Hung ST, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63:40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Adejumo AC, Alliu S, Ajayi TO, et al. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease:a cross-sectional study. PLoS One. 2017;12:e0176416. doi: 10.1371/journal.pone.0176416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koebnick C, Getahun D, Reynolds K, et al. Trends in nonalcoholic fatty liver disease-related hospitalizations in US children, adolescents, and young adults. J Pediatr Gastroenterol Nutr. 2009;48:597–603. doi: 10.1097/MPG.0b013e318192d224. [DOI] [PubMed] [Google Scholar]
- 19.Sayiner M, Otgonsuren M, Cable R, et al. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51:254–260. doi: 10.1097/MCG.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adejumo AC, Adegbala OM, Adejumo KL, Bukong TN. Reduced incidence and better liver disease outcomes among chronic HCV infected patients who consume cannabis. Can J Gastroenterol Hepatol. 2018 doi: 10.1155/2018/9430953. Article ID 9430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.Greenleaf EK, Hollenbeak CS, Wong J. Trends in the use and impact of neoadjuvant chemotherapy on perioperative outcomes for resected gastric cancer:evidence from the American College of Surgeons National Cancer Database. Surgery. 2016;159:1099–1112. doi: 10.1016/j.surg.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Akanbi O, Adejumo AC, Saleem N, Francisque F, Soliman M, Ogunbayo GO. Sickle cell disease is associated with higher mortality among patients hospitalized with ischemic bowel disease. Eur J Gastroenterol Hepatol. 2018;30:1027–1032. doi: 10.1097/MEG.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 24.Adejumo AC, Akanbi O, Pani L. Among inpatients, ischemic bowel disease predisposes to Clostridium difficile infection with concomitant higher mortality and worse outcomes. Eur J Gastroenterol Hepatol. 2019;31:109–115. doi: 10.1097/MEG.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 25.Adejumo AC, Adejumo KL, Adegbala OM, et al. Protein-energy malnutrition and outcomes of hospitalizations for heart failure in the USA. Am J Cardiol. 2018;123:9290935. doi: 10.1016/j.amjcard.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Akanbi O, Adejumo AC. Early endoscopy is associated with better clinical outcomes in patients hospitalized with ischemic bowel disease. Dig Dis Sci. 2019 Mar 30; doi: 10.1007/s10620-019-05598-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Thabut D, Rudler M, Dib N, et al. French Club for the Study of Portal Hypertension (CFEHTP) Multicenter prospective validation of the Baveno IV and Baveno II/III criteria in cirrhosis patients with variceal bleeding. Hepatology. 2015;61:1024–1032. doi: 10.1002/hep.27407. [DOI] [PubMed] [Google Scholar]
- 28.May FP, Rolston VS, Tapper EB, Lakshmanan A, Saab S, Sundaram V. The impact of race and ethnicity on mortality and healthcare utilization in alcoholic hepatitis:a cross-sectional study. BMC Gastroenterol. 2016;16:129. doi: 10.1186/s12876-016-0544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [[Accessed 2 July 2019]];HCUP Methods Series Calculating National Inpatient Sample (NIS) Variances for Data Years 2012 and Later. Available from: https://www.hcup-us.ahrq.gov/reports/methods/2015_09.jsp#appa . [Google Scholar]
- 30.Hale JJ, Thompson DM, Darden PM. Calculating subset weighted analysis using PROC SURVEYFREQ and GENMOD. [[Accessed 2 July 2019]]. Available from: http://support.sas.com/resources/papers/proceedings13/272-2013.pdf .
- 31.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 32.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States:the Third National Health and Nutrition Examination Survey 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider ALC, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity (Silver Spring) 2014;22:292–299. doi: 10.1002/oby.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 36.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease:practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 37.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274–283. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–152. [PubMed] [Google Scholar]
- 39.Carrière G. Consultations with doctors and nurses. Health Rep. 2005;16:45–48. [PubMed] [Google Scholar]
- 40.Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour:a QUALICOPC study. BMC Fam Pract. 2016;17:38. doi: 10.1186/s12875-016-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 43.Ko CW, Kelley K, Meyer KE. Physician specialty and the outcomes and cost of admissions for end-stage liver disease. Am J Gastroenterol. 2001;96:3411–3418. doi: 10.1111/j.1572-0241.2001.05343.x. [DOI] [PubMed] [Google Scholar]
- 44.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic fatty liver disease in Latinos. Clin Gastroenterol Hepatol. 2016;14:5–12. doi: 10.1016/j.cgh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang PF, Ostir GV, Kuo YF, Granger CV, Ottenbacher KJ. Ethnic differences in discharge destination among older patients with traumatic brain injury. Arch Phys Med Rehabil. 2008;89:231–236. doi: 10.1016/j.apmr.2007.08.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergés I-M, Kuo Y-F, Ostir GV, Granger CV, Graham JE, Ottenbacher KJ. Gender and ethnic differences in rehabilitation outcomes following hip replacement surgery. Am J Phys Med Rehabil. 2008;87:567–572. doi: 10.1097/PHM.0b013e31817c143a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roush CV, Cox JE. The meaning of home:how it shapes the practice of home and hospice care. Home Healthc Nurse. 2000;18:388–394. doi: 10.1097/00004045-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Oramasionwu CU, Hunter JM, Skinner J, et al. Black race as a predictor of poor health outcomes among a national cohort of HIV/AIDS patients admitted to US hospitals:a cohort study. BMC Infect Dis. 2009;9:127. doi: 10.1186/1471-2334-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlestein WE, Akagi DS, Chotai S, Chambless LB. The impact of race on discharge disposition and length of hospitalization following craniotomy for brain tumor. World Neurosurg. 2017;104:24–38. doi: 10.1016/j.wneu.2017.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. Health insurance and mortality in US adults. Am J Public Health. 2009;99:2289–2295. doi: 10.2105/AJPH.2008.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franks P, Clancy CM, Gold MR. Health insurance and mortality. Evidence from a national cohort. JAMA. 1993;270:737–741. [PubMed] [Google Scholar]
- 53.Hasan O, Orav EJ, Hicks LS. Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459. doi: 10.1002/jhm.687. [DOI] [PubMed] [Google Scholar]
- 54.DeNavas-Walt C. Income, poverty, and health insurance coverage in the United States (2005) DIANE Publishing. 2010 [Google Scholar]
- 55.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 56.Chandra A, Skinner JS. National Research Council, &Committee on Population. [[Accessed 2 July 2019]];Critical perspectives on racial and ethnic differences in health in late life. National Academies Press (US) 2004 Available from: https://www.ncbi.nlm.nih.gov/books/NBK25524/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-9-CM codes used to identify clinical conditions in the study
Ten most common primary diagnoses during hospitalizations with nonalcoholic fatty liver disease (NAFLD)
Trends in hospitalizations for nonalcoholic fatty liver disease (NAFLD): Crude (A), by Sex (B), Race (C), Health Insurance (D), Income status (E) and Region (F)


