Abstract
Objectives
Maternal folic acid supplementation is considered mandatory in almost every country in the world to prevent congenital malformations. However, little is known about the association of maternal folic acid intake with the occurrence of childhood cancer. Hence, this study aimed to determine the effects of maternal folic acid consumption on the risk of childhood cancer.
Methods
A total of 158 related articles were obtained from PubMed, Google Scholar, Scopus, and ProQuest using standardized keywords, of which 17 were included in the final review.
Results
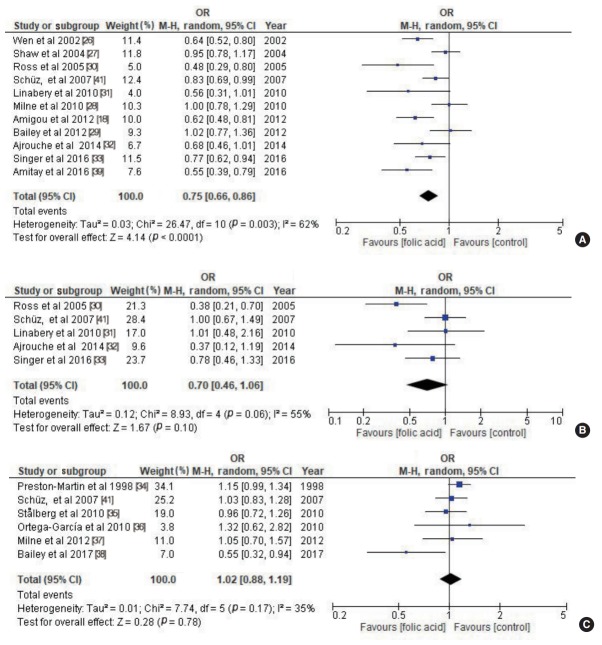
Eleven of the 17 articles showed a significant protective association between maternal folic acid supplementation and childhood cancer. Using a random-effects model, pooled odds ratios (ORs) showed a protective association between maternal folic acid supplementation and childhood acute lymphoblastic leukaemia (OR, 0.75; 95% confidence interval [CI], 0.66 to 0.86). However, there was no significant association between maternal folic acid supplementation and acute myeloid leukaemia (OR, 0.70; 95% CI, 0.46 to 1.06) or childhood brain tumours (OR, 1.02; 95% CI, 0.88 to 1.19).
Conclusions
Maternal folic acid supplementation was found to have a protective effect against childhood acute lymphoblastic leukaemia. Thus, healthcare professionals are recommended to provide regular health education and health promotion to the community on the benefits of folic acid supplementation during pregnancy.
Keywords: Childhood cancer, Maternal, Folate, Vitamin supplementation
INTRODUCTION
Childhood cancer is defined as cancer occurring before 19 years of age. Although rare, it is the most common cause of death among children and adolescents, with approximately 300 000 new cases diagnosed each year worldwide [1]. The burden of childhood cancer is mostly felt in low-income and middle-income countries, where the risk of mortality from childhood cancer is almost 4 times higher than in high-income countries [2,3]. This is due to the low diagnostic rate, as patients usually do not seek treatment, and even those who do receive treatment often discontinue it due to an inability to afford the high costs of treatment. Moreover, the health professionals caring for such patients also lack specialized training [4].
There are 12 different categories of childhood cancer according to the International Classification of Childhood Cancer, with the most common including leukaemia, lymphomas, brain tumours, and solid tumours [1]. Although childhood cancer is mostly attributable to genetic and hereditary factors, environmental and lifestyle factors may also contribute to the occurrence of cancer among children and adolescents. However, in the majority of cases, the precise cause remains unknown. Studies on risk factors for childhood cancer have suggested that maternal reproductive factors are the major contributors, including a history of miscarriage, elderly primigravida status, and the intake of processed meats, alcohol, and tobacco. Paternal factors such as smoking and occupational exposure to hydrocarbons and paints have also been studied, but with unclear findings [5,6].
Maternal vitamin consumption, especially folic acid supplementation during pregnancy, has consistently been shown to reduce the risk of neural tube defects [7,8]. It may also reduce the risk of other congenital malformations such as cardiovascular defects [9,10], oral clefts [11,12], urinary tract defects [13,14] and limb-reduction defects [10,12]. Folic acid is the fully oxidized monoglutamyl form of folate, a water-soluble B vitamin [15]. The World Health Organisation (WHO) has recommended daily supplementation with 400 μg of folic acid for women before and during pregnancy [16]. A few studies have suggested that maternal supplementation of folic acid can prevent childhood cancers, such as acute lymphoblastic leukaemia (ALL) [17,18], brain tumours [19] and Wilms tumour [20]. Hence, this systematic review aimed to determine the protective effects of maternal vitamin supplementation containing folic acid on childhood cancer, as a more thorough understanding of its protective effects will be important for future preventive strategies.
METHODS
Search Protocol
Articles were searched systematically between September 1, 2018 and November 1, 2018, following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline. Four search engines—PubMed, Google Scholar, ProQuest, and Scopus—were utilised to search for articles. The search was carried out using keywords agreed upon in advance. The following keywords were derived from the PICO search guide: population (P): child* OR infant* OR adolescent* OR “young people” OR paediatric OR “age 0 to 19 years old”; intervention (I): “maternal vitamin supplementation” OR “prenatal supplementation” OR “folate* supplementation” OR “maternal medication”; comparison (C): compare all studies done in other countries; and outcome (O): cancer* OR tumor* OR neoplasms OR malignancy* OR “acute lymphoblastic leukemia” OR lymphoma* OR “acute myeloid leukemias” OR “central nervous neoplasms” OR “malignant bone tumors” OR neuroblastoma OR retinoblastoma OR “renal tumors.”
Study Selection
Four reviewers were involved in this systematic review in order to have multiple rounds of relevance screening and to reduce bias [21]. They were paired randomly as reviewer 1 (R1) and reviewer 2 (R2). In the first stage of the article search, 158 article titles and abstracts contained the search keywords. These article titles and abstracts were given to the paired reviewers at random. Each pair of reviewers received 79 article titles and abstracts to screen. The article titles and abstracts were reviewed by both reviewers, R1 and R2, and when there was disagreement, a third reviewer, R3, who was from another pair of reviewers, made a final determination of whether to accept or reject the article title or abstract. In total, 120 duplicates and irrelevant article titles and abstracts were removed at this stage. In the second stage of screening, 38 articles were retrieved for a full-text assessment. All 4 reviewers were given the retrieved articles to assess their relevance to the study. Finally, only 17 articles were found to be related to maternal supplementation with vitamins containing folic acid and childhood cancer (Figure 1). Data extraction was performed after a full review of the selected articles. The inclusion criteria were articles analysing the relationship of maternal folic acid supplementation during pregnancy with childhood cancer, case-control studies, original articles, articles written in English, and articles published after 1998. Unpublished studies, dissertations, reviews, studies investigating the maternal use of other vitamins and iron supplementation, and studies investigating the maternal use of medications for pain relief or medical conditions were excluded from the review.
Figure. 1.
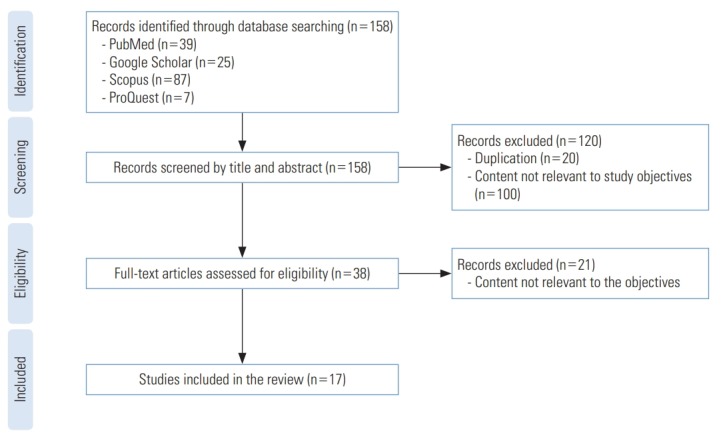
Selection process for eligible studies.
Data Extraction and Data Synthesis
A standardized form was used for data extraction from the studies that had been selected, including country, year published, study period, age of the children, the number of participants, the use of matching controls, matching variables, primary outcomes, and the quality of the study. All analyses were performed using Review Manager version 5.3 [22]. The heterogeneity between studies in the meta-analysis was assessed using forest plots. The I2 and chi-square tests were used to formally check for the presence of heterogeneity. Heterogeneity was classified as low, medium and high for I2 values of 25%, 50%, and 75%, respectively, and for the chi-square test, a p-value <0.05 was considered to indicate significance. The combined risk estimate was calculated using a random-effects model because the true effect size may not have been constant across all the included studies, and the effect measures were odds ratios (ORs).
Study Quality and Publication Bias
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the selected studies. The NOS evaluates 3 categories using 8 items to quantify study quality: selection of the participants, comparability of the participant group, and exposure ascertainment. A maximum of 1 star is given for each item within the selection and exposure categories, while a maximum of 2 stars can be given in the comparability category. The total maximum score of these 3 categories is 9. A study with a score of ≥7 was considered to be a high-quality study [23]. Two authors independently assessed the quality of the selected studies. All potential confounders, such as the duration of folic acid consumption during pregnancy, dietary intake, and maternal medication history, were considered. Publication bias was evaluated using funnel plots, followed by the regression-based approach proposed by Egger et al. [24] and the rank correlation test provided by Begg and Mazumdar [25], which are formal and objective tests for publication bias.
Ethics Statement
This paper is a systematic review and meta-analysis study so it did not need ethical consideration.
RESULTS
A summary of the selected studies is presented in Table 1. All studies had a case-control design. Five studies reported findings for childhood ALL [18,26-29], 4 studies investigated both childhood ALL and acute myeloid leukaemia (AML) [30-33], 5 studies dealt with childhood brain tumours (CBT) [34-38], 1 study reported findings for both childhood ALL and lymphoma [39], 1 study investigated germ cell tumours [40] and 1 study reported findings for childhood ALL, AML, CBT, lymphoma, neuroblastoma, Wilms tumour, bone tumours, soft tissue sarcoma, and non-Hodgkin lymphoma [41]. We only conducted a meta-analysis on childhood ALL, AML, and CBT. Childhood ALL and AML were analysed separately, as these diseases have different origins. As there was only 1 study that reported information regarding germ cell tumours, lymphoma, neuroblastoma, Wilms tumour, bone tumours, soft tissue sarcoma, and non-Hodgkin lymphoma, it was not possible to perform a meta-analysis for these tumours. However, the authors reported that maternal use of vitamins containing folic acid during pregnancy was associated with a reduction in the risk of germ cell tumours (OR, 0.7; 95% confidence interval [CI], 0.4 to 1.2) [40] and non-Hodgkin lymphoma (OR, 0.68; 95% CI, 0.48 to 0.97) [41].
Table 1.
Summary of the reviewed articles
| Study | Country | Study period | Age of the children (y) | No. of participants |
Matching control | Matching variables | Primary outcome | NOS score | |
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Preston-Martin et al., 1998 [34] | USA, France, Israel, and Europe | 1976-1994 | 0-19 | 1051 | 1919 | Varied by study centre | Age, sex, geographic region | CBT | 8 |
| Wen et al., 2002 [26] | USA and Canada | 1989-1993 | <15 | 1842 | 1986 | Random-digit dialling | Age, sex | ALL | 8 |
| Shaw et al., 2004 [27] | Canada | 1980-2000 | <15 | 789 | 789 | Population-based registry | Age, sex | ALL | 7 |
| Ross et al., 2005 [30] | North America | 1997-2002 | <20 | 97 (ALL), 51 (AML) | 173 | Roster and birth registry | Age, sex | ALL, AML | 7 |
| Schüz et al., 2007 [41] | Germany | 1992-1997 | 0-14 | 650 (ALL), 105 (AML), 399 (CBT), 157 (neuroblastoma), 147 (Wilms tumour), 97 (bone tumours), 137 (soft tissue sarcoma) | 2057 | Population-based registry | Sex, date of birth | ALL, AML, CBT, neuroblastoma, Wilms tumour, bone tumour, soft tissue sarcoma | 7 |
| Johnson et al., 2009 [40] | USA and Canada | 1993-2001 | 0-15 | 278 | 423 | Random-digit dialling | Birth year, sex | Germ cell tumours | 8 |
| Milne et al., 2010 [28] | Australia | 2003-2007 | 0-15 | 393 | 1249 | Random-digit dialling | Age, sex, state of residence | ALL | 7 |
| Linabery et al., 2010 [31] | USA | 1996-2006 | <1 | 264 (ALL), 172 (AML) | 324 | State birth registry | Birth year, location of residence | ALL, AML | 7 |
| Stålberg et al., 2010 [35] | Sweden | 1990-1999 | 0-15 | 512 | 525 | Medical birth register | Location, sex, birth year | CBT | 7 |
| Ortega-García et al., 2010 [36] | Spain | 2004-2006 | 0-15 | 67 | 155 | Hospital record registry | Birth year | CBT | 8 |
| Amigou et al., 2012 [18] | France | 2003-2004 | 0-15 | 764 | 1681 | Population-based registry | Age, sex, region | ALL | 7 |
| Bailey et al., 2012 [29] | Australia | 2003-2006 | 0-14 | 333 | 695 | Random-digit dialling | Age, sex, state of residence | ALL | 8 |
| Milne et al., 2012 [37] | Australia | 2005-2011 | 0-14 | 335 | 1363 | Random-digit dialling | Age, sex, state of residence | CBT | 7 |
| Ajrouche et al., 2014 [32] | France | 2010-2011 | <15 | 636 (ALL), 100 (AML) | 1421 | Population-based registry, quota sampling method | Age, sex | ALL, AML | 8 |
| Amitay et al., 2016 [39] | Israel | 2005-2013 | 1-19 | 121 (ALL), 69 (lymphoma) | 384 | Home communities | Age, sex | ALL, lymphoma | 7 |
| Singer et al., 2016 [33] | USA (California) | 1995-2008 | 0-14 | 681 (ALL), 103 (AML) | 1076 | California birth certificates | Date of birth, sex, Hispanic ethnicity, maternal race | ALL, AML | 8 |
| Bailey et al., 2017 [38] | France | 2010-2011 | <15 | 301 | 1421 | Population-based registry | Age, sex, region | CBT | 8 |
NOS, Newcastle-Ottawa Scale; CBT, childhood brain tumour; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
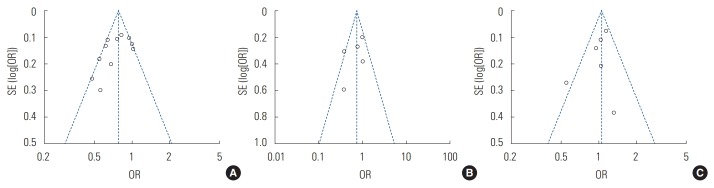
Maternal folic acid supplementation was associated with a protective effect against childhood ALL (OR, 0.75; 95% CI, 0.66 to 0.86; p<0.001) (Figure 2A). Maternal folic acid supplementation also showed a protective effect against childhood AML, but that effect was statistically insignificant (OR, 0.70; 95% CI, 0.46 to 1.06; p=0.10) (Figure 2B). No significant association was found between maternal folic acid supplementation and CBT (OR, 1.02; 95% CI, 0.88 to 1.19; p=0.78) (Figure 2C). There was moderate heterogeneity among all the studies. The studies’ quality scores were high, ranging from 7 to 8. The quality scores of each study are summarized in Table 1. No publication bias was detected in the childhood ALL studies (Egger test: p= 0.126; Begg rank correlation: p=0.161), childhood AML studies (Egger test: p=0.334; Begg rank correlation: p=0.462), or CBT studies (Egger test: p=0.290; Begg rank correlation: p= 0.707) (Figure 3).
Figure. 2.
Maternal folic acid supplementation and risk of childhood (A) acute lymphoblastic leukaemia, (B) acute myeloid leukaemia, and (C) childhood brain tumour. OR, odds ratio; CI, confidence interval; M-H, Mantel-Haenszel.
Figure. 3.
Distribution of studies included in the meta-analysis (A) acute lymphoblastic leukaemia, (B) acute myeloid leukaemia, and (C) childhood brain tumour. SE, standard error; OR, odds ratio.
DISCUSSION
The WHO has recommended prophylactic folic acid supplementation of 300 μg/d since 1968 to prevent neural tube defects, and in 1998, the recommended amount increased to 400 μg daily during pregnancy [16]. Folic acid supplementation should be started before conception to obtain an optimal preventive effect against neural tube defects and other congenital abnormalities [16]. Apart from folic acid, maternal vitamins given routinely during pregnancy include iron, vitamin B12, and vitamin C, especially in developing countries [16], and vitamin D in certain countries [42].
The present systematic review revealed that maternal folic acid supplementation was associated with a protective effect against childhood ALL (OR,0.75; 95% CI, 0.66 to 0.86; p<0.001). This finding is similar to that of a previous meta-analysis conducted in the USA in 2007 (OR, 0.64; 95% CI, 0.53 to 0.78) [43]. However, Goh et al. [43] did not specify which types of vitamins showed a protective effect against childhood cancer. Metayer et al. [44] reported that maternal intake of both vitamins and folic acid at any time during pregnancy was associated with a reduced risk of ALL in the offspring, with ORs of 0.85 (95% CI, 0.78 to 0.92) and 0.80 (95% CI, 0.71 to 0.89), respectively. Metayer et al. [44] conducted a study based on a large international collaboration, known as the Childhood Leukemia International Consortium Study (CLIC). The CLIC studies involved 10 countries with a total of 19 183 participants. The large sample sizes in the CLIC studies enabled the researchers to better determine the average values of their data and to minimise errors [45,46].
For childhood AML, this systematic review revealed that maternal folate supplementation showed a protective effect against childhood AML, but without statistical significance (OR, 0.70; 95% CI, 0.46 to 1.06; p=0.10). The statistical insignificance of the association between maternal folate supplementation and AML most likely occurred because AML is a relatively rare disease compared to ALL among children [47]. This finding is similar to that of Metayer et al. [44], who reported no significant association between AML and maternal folic acid supplementation (OR, 0.92; 95% CI, 0.75 to 1.14).
In this study, there was no significant association between maternal folic acid supplementation and CBT (OR, 1.02; 95% CI, 0.88 to 1.19; p=0.78). This finding was different from the recently published meta-analysis by Chiavarini et al. [48], who reported that maternal folic acid intake was inversely associated with the risk of childhood brain and spinal cord tumours (OR, 0.77; 95% CI, 0.67 to 0.88).
Apart from these protective effects, other studies have been conducted to evaluate the possible effects of folic acid supplementation on cancer risk. A meta-analysis of data from 50 000 subjects found that folic acid supplementation with a duration of 5 years showed no significant association with cancer (relative risk, 1.06; 95% CI, 0.99 to 1.13) compared to placebo [49]. However, folic acid fortification programs implemented in Canada and USA have led to a reduction in the incidence of cancers such as Wilms tumour, neuroblastoma, and primitive neuroectodermal tumours among children according to ecological studies [20].
The mechanism underlying this protective effect is still debated. Folic acid is essential for nucleotide synthesis [50]. Therefore, folic acid deficiency causes chromosomal breaks due to the massive incorporation of uracil into human DNA. This mechanism is similar to that of DNA damage caused by radiation, which is likely a major cause of cancer in children, as well as in adults [51]. Deficiencies of other micronutrients, such as vitamin B12, vitamin B6, niacin, vitamin C, vitamin E, iron, and zinc can also cause DNA damage due to DNA strand breaks and oxidative reactions [50]. However, several animal studies have suggested that folic acid supplementation may stimulate DNA methylation, potentially playing a role in carcinogenesis [50], which contradicts the findings of a protective effect in most epidemiological studies among humans [20,44,49].
This meta-analysis has certain strengths and limitations. The strengths are that the search strategy used in this systematic review and meta-analysis was extensive, and that the articles were retrieved from 4 databases and no publication bias was noted. A limitation is that all studies included in this study had a case-cohort design, since limited cohort studies have investigated the association between folic acid and childhood cancers. Studies of correlations between maternal folic acid intake and childhood cancers have mostly been performed using case-control studies, most likely because case-control studies are relatively less costly and more suitable for rare diseases such as childhood cancers [52].
In contrast to cohort studies, case-control studies create a hypothesis regarding the association between the exposure and disease or condition of interest, but do not provide evidence for a causal relationship [52]. Furthermore, case-control studies are also associated with recall bias. As compared to controls, cases are likely to think harder in terms of recalling the exposure of interest [53]. Maternal recall of folic acid use during specific periods of pregnancy may be subject to error. The effect estimates for folic acid supplementation used in this study might have been biased by exposure misclassification. Another disadvantage of case-control studies is the need to find an appropriate control group that does not have the disease or condition of interest, while still being similar to the case group with regard to other factors, such as age, race, and residential area [53].
CONCLUSION
Maternal intake of folic acid during pregnancy has a significant protective effect against childhood ALL. Mandatory prescriptions of folic acid for women during pregnancy, which have been implemented in most countries, are a good initiative that can prevent neural tube defects and reduce the risk of childhood cancer. Thus, we recommend that healthcare professionals provide regular health education and health promotion to raise awareness in the community regarding the benefits of taking folic acid during pregnancy.
Acknowledgments
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
AUTHOR CONTRIBUTIONS
Conceptualization: AMN, WRWI. Data curation: WRWI, RAR, NAAR, AA. Formal analysis: WRWI. Funding acquisition: None. Methodology: WRWI, RAR, NAAR, AA. Project administration: AMN, WRWI. Visualization: AMN, WRWI. Writing - original draft: WRWI, RAR, NAAR, AA. Writing - review & editing: AMN, WRWI.
REFERENCES
- 1.Steliarova-Foucher E, Colombet M, Ries LA, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001- 10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi: 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S, Howard SC, Hunger SP, Antillon FG, Metzger ML, Israels T, et al. Treating childhood cancer in low- and middle-income countries. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: disease control priorities. 3rd ed. Washington, DC: World Bank; 2015. chapter 7. [PubMed] [Google Scholar]
- 3.Howard SC, Zaidi A, Cao X, Weil O, Bey P, Patte C, et al. The My Child Matters programme: effect of public–private partnerships on paediatric cancer care in low-income and middle-income countries. Lancet Oncol. 2018;19(5):e252–e266. doi: 10.1016/S1470-2045(18)30123-2. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Linet MS, Wacholder S, Zahm SH. Interpreting epidemiologic research: lessons from studies of childhood cancer. Pediatrics. 2003;112(1 Pt 2):218–232. [PubMed] [Google Scholar]
- 6.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8(6):541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 7.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 8.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341(20):1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 9.Shaw GM, O’Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet. 1995;59(4):536–545. doi: 10.1002/ajmg.1320590428. [DOI] [PubMed] [Google Scholar]
- 10.Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. Am J Med Genet. 1996;62(2):179–183. doi: 10.1002/(SICI)1096-8628(19960315)62:2<179::AID-AJMG12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Tolarova M, Harris J. Reduced recurrence of orofacial clefts after periconceptional supplementation with high-dose folic acid and multivitamins. Teratology. 1995;51(2):71–78. doi: 10.1002/tera.1420510205. [DOI] [PubMed] [Google Scholar]
- 12.Werler MM, Hayes C, Louik C, Shapiro S, Mitchell AA. Multivitamin supplementation and risk of birth defects. Am J Epidemiol. 1999;150(7):675–682. doi: 10.1093/oxfordjournals.aje.a010070. [DOI] [PubMed] [Google Scholar]
- 13.Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Periconceptional multivitamin use in relation to the risk of congenital urinary tract anomalies. Epidemiology. 1995;6(3):212–218. doi: 10.1097/00001648-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Khoury MJ, Olney RS, Mulinare J. Does periconceptional multivitamin use reduce the risk for limb deficiency in offspring? Epidemiology. 1997;8(2):157–161. doi: 10.1097/00001648-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80(5):1123–1128. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Guideline: daily iron and folic acid supplementation in pregnant women. 2012 [cited 2019 Jan 23]. Available from: https://apps.who.int/iris/handle/10665/77770. [PubMed]
- 17.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study. Lancet. 2001;358(9297):1935–1940. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 18.Amigou A, Rudant J, Orsi L, Goujon-Bellec S, Leverger G, Baruchel A, et al. Folic acid supplementation, MTHFR and MTRR polymorphisms, and the risk of childhood leukemia: the ESCALE study (SFCE) Cancer Causes Control. 2012;23(8):1265–1277. doi: 10.1007/s10552-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 19.Ford E, Catt S, Chalmers A, Fallowfield L. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. 2012;14(4):392–404. doi: 10.1093/neuonc/nor229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grupp SG, Greenberg ML, Ray JG, Busto U, Lanctôt KL, Nulman I, et al. Pediatric cancer rates after universal folic acid flour fortification in Ontario. J Clin Pharmacol. 2011;51(1):60–65. doi: 10.1177/0091270010365553. [DOI] [PubMed] [Google Scholar]
- 21.Sargeant JM, O’Connor AM. Conducting systematic reviews of intervention questions II: relevance screening, data extraction, assessing risk of bias, presenting the results and interpreting the findings. Zoonoses Public Health. 2014;61 Suppl 1:39–51. doi: 10.1111/zph.12124. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane Collaboration RevMan information. [cited 2019 Jan 23]. Available from: http://community.cochrane.org/help/tools-and-software/revman-web.
- 23.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2019 Jan 20]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 26.Wen W, Shu XO, Potter JD, Severson RK, Buckley JD, Reaman GH, et al. Parental medication use and risk of childhood acute lymphoblastic leukemia. Cancer. 2002;95(8):1786–1794. doi: 10.1002/cncr.10859. [DOI] [PubMed] [Google Scholar]
- 27.Shaw AK, Infante-Rivard C, Morrison HI. Use of medication during pregnancy and risk of childhood leukemia (Canada) Cancer Causes Control. 2004;15(9):931–937. doi: 10.1007/s10552-004-2230-6. [DOI] [PubMed] [Google Scholar]
- 28.Milne E, Royle JA, Miller M, Bower C, de Klerk NH, Bailey HD, et al. Maternal folate and other vitamin supplementation during pregnancy and risk of acute lymphoblastic leukemia in the offspring. Int J Cancer. 2010;126(11):2690–2699. doi: 10.1002/ijc.24969. [DOI] [PubMed] [Google Scholar]
- 29.Bailey HD, Miller M, Langridge A, de Klerk NH, van Bockxmeer FM, Attia J, et al. Maternal dietary intake of folate and vitamins B6 and B12 during pregnancy and the risk of childhood acute lymphoblastic leukemia. Nutr Cancer. 2012;64(7):1122–1130. doi: 10.1080/01635581.2012.707278. [DOI] [PubMed] [Google Scholar]
- 30.Ross JA, Blair CK, Olshan AF, Robison LL, Smith FO, Heerema NA, et al. Periconceptional vitamin useand leukemia risk in children with Down syndrome: a Children’s Oncology Group study. Cancer. 2005;104(2):405–410. doi: 10.1002/cncr.21171. [DOI] [PubMed] [Google Scholar]
- 31.Linabery AM, Puumala SE, Hilden JM, Davies SM, Heerema NA, Roesler MA, et al. Maternal vitamin and iron supplementation and risk of infant leukaemia: a report from the Children’s Oncology Group. Br J Cancer. 2010;103(11):1724–1728. doi: 10.1038/sj.bjc.6605957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajrouche R, Rudant J, Orsi L, Petit A, Baruchel A, Nelken B, et al. Maternal reproductive history, fertility treatments and folic acid supplementation in the risk of childhood acute leukemia: the ESTELLE study. Cancer Causes Control. 2014;25(10):1283–1293. doi: 10.1007/s10552-014-0429-8. [DOI] [PubMed] [Google Scholar]
- 33.Singer AW, Selvin S, Block G, Golden C, Carmichael SL, Metayer C. Maternal prenatal intake of one-carbon metabolism nutrients and risk of childhood leukemia. Cancer Causes Control. 2016;27(7):929–940. doi: 10.1007/s10552-016-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston-Martin S, Pogoda JM, Mueller BA, Lubin F, Holly EA, Filippini G, et al. Prenatal vitamin supplementation and risk of childhood brain tumors. Int J Cancer Suppl. 1998;11:17–22. [PubMed] [Google Scholar]
- 35.Stålberg K, Haglund B, Strömberg B, Kieler H. Prenatal exposure to medicines and the risk of childhood brain tumor. Cancer Epidemiol. 2010;34(4):400–404. doi: 10.1016/j.canep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Ortega-García JA, Ferrís-Tortajada J, Claudio L, Soldin OP, Sanchez-Sauco MF, Fuster-Soler JL, et al. Case control study of periconceptional folic acid intake and nervous system tumors in children. Childs Nerv Syst. 2010;26(12):1727–1733. doi: 10.1007/s00381-010-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne E, Greenop KR, Bower C, Miller M, van Bockxmeer FM, Scott RJ, et al. Maternal use of folic acid and other supplements and risk of childhood brain tumors. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1933–1941. doi: 10.1158/1055-9965.EPI-12-0803. [DOI] [PubMed] [Google Scholar]
- 38.Bailey HD, Rios P, Lacour B, Guerrini-Rousseau L, Bertozzi AI, Leblond P, et al. Factors related to pregnancy and birth and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France) Int J Cancer. 2017;140(8):1757–1769. doi: 10.1002/ijc.30597. [DOI] [PubMed] [Google Scholar]
- 39.Amitay EL, Dubnov Raz G, Keinan-Boker L. Breastfeeding, other early life exposures and childhood leukemia and lymphoma. Nutr Cancer. 2016;68(6):968–977. doi: 10.1080/01635581.2016.1190020. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KJ, Poynter JN, Ross JA, Robison LL, Shu XO. Pediatric germ cell tumors and maternal vitamin supplementation: a Children’s Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2661–2664. doi: 10.1158/1055-9965.EPI-09-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schüz J, Weihkopf T, Kaatsch P. Medication use during pregnancy and the risk of childhood cancer in the offspring. Eur J Pediatr. 2007;166(5):433–441. doi: 10.1007/s00431-006-0401-z. [DOI] [PubMed] [Google Scholar]
- 42.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 43.Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81(5):685–691. doi: 10.1038/sj.clpt.6100100. [DOI] [PubMed] [Google Scholar]
- 44.Metayer C, Milne E, Dockerty JD, Clavel J, Pombo-de-Oliveira MS, Wesseling C, et al. Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: a Childhood Leukemia International Consortium study. Epidemiology. 2014;25(6):811–822. doi: 10.1097/EDE.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 46.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 47.Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 48.Chiavarini M, Naldini G, Fabiani R. Maternal folate intake and risk of childhood brain and spinal cord tumors: a systematic review and meta-analysis. Neuroepidemiology. 2018;51(1-2):82–95. doi: 10.1159/000490249. [DOI] [PubMed] [Google Scholar]
- 49.Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381(9871):1029–1036. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barua S, Kuizon S, Junaid MA. Folic acid supplementation in pregnancy and implications in health and disease. J Biomed Sci. 2014;21:77. doi: 10.1186/s12929-014-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475(1-2):7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 52.Caruana EJ, Roman M, Hernández-Sánchez J, Solli P. Longitudinal studies. J Thorac Dis. 2015;7(11):E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]