Abstract
Objective
Among patients with chronic pain, risk of opioid use is elevated with high opioid dose or concurrent benzodiazepine use. This study examined whether these clinical factors, or sociodemographic factors of race and gender, are associated with opioid dose reduction.
Design and Setting
A retrospective cohort study of outpatients prescribed chronic opioid therapy between 2007 and 2012 within a large, academic health care system in Bronx, New York, using electronic medical record data. Included patients were prescribed a stable dose of chronic opioid therapy over a one-year “baseline period” and did not have cancer.
Methods
The primary outcome was opioid dose reduction (≥30% reduction from baseline) within two years. Multivariable logistic regression tested the associations of two clinical variables (baseline daily opioid dose and concurrent benzodiazepine prescription) and two sociodemographic variables (race/ethnicity and gender) with opioid dose reduction.
Results
Of 1,097 patients, 463 (42.2%) had opioid dose reduction. High opioid dose (≥100 morphine-milligram equivalents [MME]) was associated with lower odds of opioid dose reduction compared with an opioid dose <100 MME (adjusted odds ratio [AOR] = 0.69, 95% confidence interval [CI] = 0.54–0.89). Concurrent benzodiazepine prescription was not associated with opioid dose reduction. Black (vs white) race and female (vs male) gender were associated with greater odds of opioid dose reduction (AOR = 1.82, 95% CI = 1.22–2.70; and AOR = 1.43, 95% CI = 1.11–1.83, respectively).
Conclusions
Black race and female gender were associated with greater odds of opioid dose reduction, whereas clinical factors of high opioid dose and concurrent benzodiazepine prescription were not. Efforts to reduce opioid dose should target patients based on clinical factors and address potential biases in clinical decision-making.
Keywords: Disparities, Racial, Disparities, Gender, Opioids, Chronic Pain
Introduction
Despite limited evidence of its effectiveness, opioid pain reliever (OPR) use is common among individuals with chronic noncancer pain [1–6]. Accompanying the increase in OPR prescriptions in the past decade [5–8] has been a steep rise in overdose-related hospitalizations and deaths [9–11]. In addition to the risk of overdose [8], other risks of prescription OPR use in chronic pain include opioid use disorder [6,12,13], physiological dependence and withdrawal symptoms [6,12,14], and worsening of physical functioning [15].
The risks associated with OPR use for chronic pain are disproportionately high among patients prescribed high OPR dose or concurrent benzodiazepines. OPR dose has been positively associated with risk of opioid use disorder [13,16], fatal and nonfatal overdose [1,17,18], hospitalization [19], and suicide mortality [20]. OPR dose greater than 100 morphine-milligram equivalents (MME) per day is associated with at least a sevenfold increase in risk of fatal overdose, compared with OPR doses of less than 100 MME/d [21–23]. Concurrent benzodiazepine use is also a risk factor for OPR-related adverse events, such as emergency department visits and hospitalizations due to fatal or nonfatal overdose [24]. Benzodiazepines were involved in nearly a third of opioid-related overdose deaths in 2014 and 2015 [25].
Given the risks associated with OPR use, particularly with high OPR dose or concurrent benzodiazepine use, recent guidelines recommended that clinicians reduce the dose or discontinue OPRs in circumstances where the risks outweigh the benefits of continuing treatment [26,27]. Indeed, emerging data indicate that OPR prescribing is beginning to decrease at the population level [28]. However, a clinician’s assessment of a patient’s risk–benefit ratio relies on subjective determinations, such as patients’ self-report of perceived benefits and clinicians’ judgment about potential misuse. In this context, there is room for clinician bias to play a role in decisions about OPR dose reduction. Previous studies have found that race and gender influence OPR prescribing for pain. For example, as compared with white patients, black patients are less likely to receive OPR prescriptions [29–31], less likely to receive high-dose OPRs [32], and more likely to receive urine drug tests [33]. Additionally, although females report more frequent and severe pain [34,35], male gender is associated with higher likelihood of OPR dose escalation [36]. Sociodemographic factors such as race and gender could be associated with OPR dose reduction as well.
To date, little has been reported about patient factors influencing OPR dose reduction among patients with chronic noncancer pain. The goal of this study was to examine whether clinical factors associated with high risk or sociodemographic factors associated with disparities in OPR management were associated with OPR dose reduction in a cohort of patients treated with chronic OPR therapy (COT) in a large, urban academic health care system. We hypothesized that the clinical factors high OPR dose and concurrent benzodiazepine use and the sociodemographic factors black race and female gender would be associated with greater likelihood of OPR dose reduction.
Methods
Setting and Overview
We conducted a retrospective observational cohort study within a large, urban academic health care system. The Montefiore Health System is located in Bronx, New York, with more than 30 primary care practices. The majority of patients (approximately 75%) are publicly insured by Medicaid and/or Medicare plans. Using electronic medical record (EMR) data, we identified a cohort of patients prescribed COT between July 2007 and December 2012 and examined clinical and sociodemographic variables associated with OPR dose reduction within two years of follow-up after study entry. During the study time frame (2007 to 2014), there were limited and inconsistent initiatives to improve the safety of opioid prescribing for chronic pain; however, no initiatives specifically addressed opioid tapering. The study was approved by the affiliated institutional review board.
Use of EMR Prescription Data
To identify the cohort and determine OPR dose reduction, OPR prescription data were extracted from the EMR, and individual OPR prescriptions were organized into distinct six-month periods. Then, the daily OPR dose was calculated for each six-month period by determining the MME units prescribed during the period [26,37] and dividing the total MME by 180 days.
Prescriptions for all formulations of OPR medications were included in calculations, with the following exceptions: 1) buprenorphine and liquid methadone, because they were likely to be used for treatment of opioid use disorder rather than for treatment of chronic pain, and 2) intravenous or injection OPR medications, because they were likely to be used for acute rather than chronic pain treatment. Prescriptions that lacked the data necessary to calculate the daily OPR dose (e.g., date or quantity prescribed) were excluded from daily OPR dose calculations, as were outlier prescriptions where the total dose exceeded 75,000 MME (<1% of all eligible prescriptions).
Study Cohort
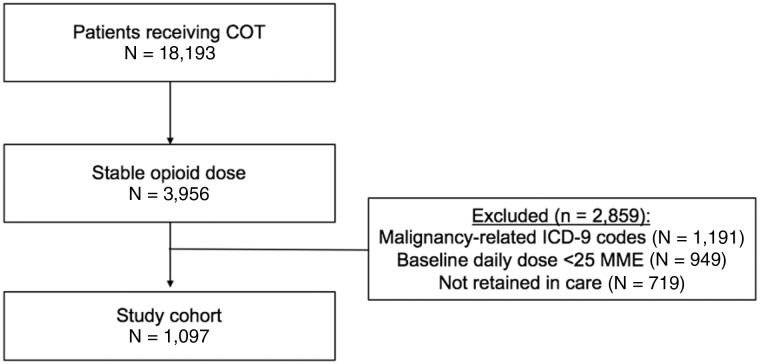
Patients were eligible for inclusion if they were: 1) age 18 years or older, 2) prescribed COT between January 2007 and December 2012, 3) prescribed a stable OPR dose, and 4) retained in care (definitions below). We excluded patients with cancer ((International Classification of Diseases, Ninth Revision [ICD-9] codes 140-209, 230-239) 140–209, 230–239) or a baseline daily OPR dose of less than 25 MME/d.
We defined COT as having three or more OPR prescriptions at least 21 days apart in each of two consecutive six-month periods [23], between July 2007 and December 2012. We considered the OPR dose to be stable if there was less than a 20% difference in daily OPR dose between the two consecutive six-month periods, and the second of the two periods was considered the “baseline” for the purposes of determining the baseline daily OPR dose. If a patient met our inclusion criteria during multiple time periods, the earliest defined study entry. Lastly, we defined retained in care as having one or more outpatient visits with any physician (primary care or specialist) in the Montefiore Health System in each six-month period from baseline through two years of follow-up. We employed conservative definitions of our inclusion criteria to maximize the specificity of our exposure variables (by isolating a cohort of individuals on stable doses of chronic monthly OPR medications), as well as the specificity of our primary outcome variable (to reduce misclassification of patients with either large fluctuations in OPR dose or inconsistent follow-up rather than actual OPR dose reductions).
Primary Outcome
The primary outcome was OPR dose reduction (dichotomous; yes/no) within two years following the end of each patient’s baseline period. No standard definition for OPR dose reduction exists [38,39], and we chose to define it as a reduction in daily OPR dose of at least 30% in any six-month follow-up period relative to baseline. We chose this definition because it had face validity as a clinically meaningful change and corresponded to a minimum reduction of 7.5 MME/d in our cohort (equivalent to 5 mg of Oxycodone). However, given the lack of a standard definition, we also conducted sensitivity analyses using two secondary OPR dose reduction definitions, described below.
Main Exposure Variables
We examined two clinical variables (baseline daily OPR dose and concurrent benzodiazepine prescription) and two sociodemographic variables (race/ethnicity and gender). Baseline daily OPR dose was calculated as a continuous variable (number of MME/d). Patients were classified as having been prescribed concurrent benzodiazepines (yes/no) if they had at least two unique benzodiazepine prescriptions (of any duration, with or without refills) during the baseline period. Patients were classified into one of four mutually exclusive race/ethnicity categories in the EMR data: 1) black, 2) white, 3) Hispanic (any race), or 4) other/unknown (American Indian or Alaskan Native, Asian, multiracial, Native Hawaiian or Pacific Islander, other, or unknown). Other/unknown was combined because the majority of these patients were reported as “other” or “declined,” and each of the remaining categories comprised <2% of the study cohort. Patients were classified by gender according to EMR documentation (female vs male).
Covariates
The following other variables were generated and adjusted for: 1) baseline age (continuous); 2) language spoken (English vs non-English); 3) medical complexity (continuous, using a modified Elixhauser Index score algorithm) [40]; 4) year of cohort entry (continuous); 5) tobacco use disorder (yes/no); 6) alcohol use disorder (yes/no); 7) nonopioid drug use disorder (yes/no); 8) opioid abuse (yes/no); 9) any mental health disorder (anxiety, depression, bipolar disorder, PTSD, or schizophrenia; yes/no); and 10) 11 independent pain diagnosis category variables (yes/no for each) [41]; all but age, language, and year of cohort entry were determined using ICD-9 codes from encounters before or during the baseline period. Pain diagnoses were categorized into 11 clinically relevant, non–mutually exclusive categories of painful conditions that have been used previously [41]. For alcohol, tobacco, and nonopioid drug use disorders, we included ICD-9 codes for both abuse and dependence. However, opioid dependence was not included as a covariate because the ICD-9 codes for opioid dependence among patients on chronic opioid therapy are prone to misclassification; that is, physicians may indicate “opioid dependence” to signify the patient has physical dependence on opioids rather than an opioid use disorder [42,43].
Statistical Analysis
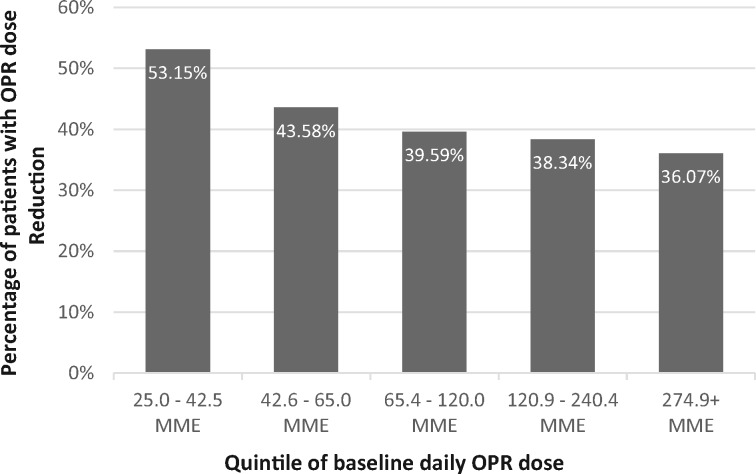
We first examined bivariate associations of each variable with the outcome of OPR dose reduction. We conducted t tests or Mann-Whitney U tests for continuous variables (baseline daily OPR dose) and chi-square tests for categorical variables (concurrent benzodiazepine prescription, race/ethnicity, gender). To further examine the relationship between baseline daily OPR dose and OPR dose reduction, quintiles of baseline daily OPR dose were tested for trend using the homogeneity of odds and Score test.
To estimate the odds of OPR dose reduction with each main exposure variable while adjusting for others and covariates, we built a multivariable logistic regression model using a backward elimination procedure with forced entry of all main exposure variables. A cutoff P value of ≤0.20 on bivariate analysis was used for inclusion in the model, and a cutoff P value of >0.05 on the Wald statistic, verified using a likelihood ratio (LR) test, was used for exclusion in the model. An adjusted odds ratio (AOR) was determined for each variable in the final model.
Sensitivity Analyses
Due to the lack of a standard definition for OPR dose reduction [38,39], we conducted sensitivity analyses using two secondary outcome definitions for OPR dose reduction: 1) a “large dose reduction”: a reduction in daily OPR dose of at least 50% (if baseline daily OPR dose <240 MME/d) or to 120 MME/d (if baseline daily OPR dose ≥240 MME/d) in any follow-up period [44]; and 2) a “sustained dose reduction”: a reduction in daily OPR dose of at least 30% in two consecutive follow-up periods relative to baseline. Additionally, to examine whether a commonly used definition of high-dose OPR use was associated with OPR dose reduction, we conducted a sensitivity analysis using baseline daily OPR dose as a dichotomous variable (high OPR dose [≥100 MME/d]; yes/no). Separate multivariable logistic regression models were built for each sensitivity analysis conducted.
Results
A total of 1,097 patients met eligibility criteria for the study cohort (Figure 1). Most were female (55.2%), the mean age was 51.8 (±11.3) years, and the race/ethnicity distribution was 32% black, 33% Hispanic, 20% other/unknown, and 15% white. The median baseline daily OPR dose (interquartile range [IQR]) was 90 (47–198) MME, and 46% had an OPR dose of ≥100 MME. Nineteen percent had concurrent benzodiazepine prescriptions (Table 1). Overall, 42.2% had OPR dose reduction in the two-year follow-up using our primary definition.
Figure 1.
Study flow diagram. COT = chronic opioid pain reliever therapy; ICD-9 = International Classification of Diseases, Ninth Revision; MME = morphine-milligram equivalents.
Table 1.
Patient characteristics
| Characteristic | Total Cohort (N = 1,097) |
|---|---|
| Age, mean ± SD, y | 51.80 ± 11.34 |
| Race/ethnicity, No. (%)* | |
| White | 166 (15.1) |
| Black | 351 (32.0) |
| Hispanic | 357 (32.5) |
| Other | 223 (20.3) |
| Female, No. (%) | 606 (55.2) |
| Baseline dose, median (IQR), MME | 90.0 (46.9–197.5) |
| Concurrent benzodiazepines, No. (%) | 211 (19.2) |
| Medical comorbidities, No. (%)† | |
| 0–1 | 216 (19.7) |
| 2–3 | 369 (33.6) |
| 4+ | 512 (46.7) |
| English speaking, No. (%) | 940 (85.7) |
| Year of cohort entry, median (IQR) | 2009 (2008–2011) |
| Tobacco use disorder, No. (%) | 376 (34.3) |
| Alcohol use disorder, No. (%) | 100 (9.1) |
| Nonopioid drug use disorder, No. (%) | 289 (26.3) |
| Opioid abuse, No. (%) | 24 (2.2) |
| Mental health disorder, No. (%)‡ | 653 (59.5) |
| Pain diagnosis category, No. (%)§ | |
| Extremity | 853 (77.8) |
| Neck | 146 (13.3) |
| Headache | 296 (27.0) |
| Back | 834 (76.0) |
| Neuropathic | 415 (37.8) |
| Abdomen | 386 (35.2) |
| Chest | 287 (26.2) |
IQR = interquartile range; MME = morphine-milligram equivalents.
Mutually exclusive categories based on separate self-identified race and ethnicity variables.
Calculated using modified Elixhauser category diagnosis coding algorithm developed by Quan et al. [40].
Based on provider ICD-9 coding, including codes for anxiety, depression, bipolar disorder, post-traumatic stress disorder, or schizophrenia.
Based on provider ICD-9 coding, categorizations based on the approach of Larochelle et al. [41]. Patients may have multiple pain diagnoses.
Clinical Variables Associated with OPR Dose Reduction
A lower baseline daily OPR dose was observed in those who had OPR dose reduction (median MME = 75.0, IQR = 41.3–165.0) compared with those who did not (median MME = 100.0, IQR = 52.5–220.0, P < 0.001), and there was a linear trend of decreasing odds of OPR dose reduction with increasing quintile of baseline daily OPR dose (P < 0.001) (Figure 2). Variables retained in the multivariable logistic regression model were baseline daily OPR dose, concurrent benzodiazepine prescription, race/ethnicity, gender, age, and neck pain diagnosis. In this model, the odds of OPR dose reduction were 2% lower for each increase in baseline daily OPR dose of 20 MME (adjusted odds ratio [AOR] = 0.98, 95% confidence interval [CI] = 0.96–0.99) (Table 2). Concurrent benzodiazepine prescription was not associated with OPR dose reduction in bivariate analysis or multivariable modeling.
Figure 2.
Percentage with opioid pain reliever (OPR) dose reduction by quintile of baseline daily OPR dose. MME = morphine-milligram equivalents; OPR = opioid pain reliever.
Table 2.
Variables associated with OPR dose reduction
| Independent Variables | OPR Dose Reduction (N = 463) | No OPR Dose Reduction (N = 634) | Bivariate P Value* | Adjusted Odds Ratio† (95% CI) |
|---|---|---|---|---|
| Clinical | ||||
| Baseline OPR dose, median (IQR), MME‡ | 75.0 (41.3–165.0) | 100.0 (52.5–220.0) | <0.001 | 0.98 (0.96–0.99) |
| Concurrent benzodiazepines, No. (%) | 79 (37.4) | 132 (62.6) | 0.12 | 0.85 (0.61–1.17) |
| Sociodemographic | ||||
| Race/ethnicity, No. (%) | 0.003 | |||
| White | 60 (36.1) | 106 (63.9) | Ref. | |
| Black | 175 (49.9) | 176 (50.1) | 1.82 (1.22–2.70) | |
| Hispanic | 147 (41.2) | 210 (58.8) | 1.21 (0.82–1.79) | |
| Other/unknown | 81 (36.3) | 142 (63.7) | 0.97 (0.63–1.49) | |
| Gender, No. (%) | 0.003 | |||
| Male | 183 (37.3) | 308 (62.7) | Ref. | |
| Female | 280 (46.2) | 326 (53.8) | 1.43 (1.11–1.83) | |
| Covariates | ||||
| Age, No. (%) | 0.007 | |||
| <60 y | 349 (40.2) | 520 (59.8) | Ref. | |
| ≥60 y | 114 (50.0) | 114 (50.0) | 1.44 (1.06–1.95) | |
| No. of medical comorbidities (%)§ | 0.60 | |||
| 0–1 | 93 (43.1) | 123 (56.9) | – | |
| 2–3 | 148 (40.1) | 221 (59.9) | – | |
| 4+ | 222 (43.4) | 290 (56.6) | – | |
| English speaking, No. (%) | 402 (42.8) | 538 (57.2) | 0.56 | – |
| Year of cohort entry, median (IQR) | 2009 (2008–2011) | 2009 (2008–2011) | 0.87 | – |
| Tobacco use disorder, No. (%) | 155 (41.2) | 221 (58.8) | 0.63 | – |
| Alcohol use disorder, No. (%) | 45 (45.0) | 55 (55.0) | 0.55 | – |
| Nonopioid drug use disorder, No. (%) | 113 (39.1) | 176 (60.9) | 0.21 | – |
| Opioid abuse, No. (%) | 9 (37.5) | 15 (62.5) | 0.64 | – |
| Mental health disorder, No. (%)¶ | 272 (41.7) | 381 (58.3) | 0.65 | – |
| Pain diagnosis category, No. (%)‖ | ||||
| Extremity | 369 (43.3) | 484 (56.7) | 0.19 | – |
| Neck | 76 (52.1) | 70 (47.8) | 0.01 | 1.59 (1.10–2.29) |
| Headache | 136 (46.0) | 160 (54.0) | 0.13 | – |
| Back | 352 (42.2) | 482 (57.8) | 1.00 | – |
| Neuropathic | 165 (39.8) | 250 (60.2) | 0.20 | – |
| Abdomen | 178 (46.1) | 208 (53.9) | 0.05 | – |
| Chest | 130 (45.3) | 157 (54.7) | 0.22 | – |
CI = confidence interval; IQR = interquartile range; MME = morphine-milligram equivalents; OPR = opioid pain reliever.
Bivariate P values calculated using t test (if normally distributed) or Mann-Whitney U test (if not normally distributed) for continuous variables; chi-square test for categorical variables.
Multivariable logistic regression adjusted for age, race/ethnicity, gender, baseline OPR dose (continuous), concurrent benzodiazepines, and neck pain diagnosis.
Unadjusted and adjusted odds ratio calculated for increments of 20 MME.
Calculated using modified Elixhauser category diagnosis coding algorithm developed by Quan et al. [40].
Based on provider ICD-9 coding, including codes for anxiety, depression, bipolar disorder, post-traumatic stress disorder, or schizophrenia.
Based on ICD-9 coding, based on the approach of Larochelle et al. [41]. Patients may have multiple pain diagnoses.
Sociodemographic Variables Associated with OPR Dose Reduction
Fifty percent of black patients had OPR dose reduction compared with 37% of white patients (Table 2). The odds of OPR dose reduction were 82% higher in black patients compared with white patients (AOR = 1.82, 95% CI = 1.22–2.70). No significant differences in the odds of OPR dose reduction were observed between Hispanic and white patients. Forty-six percent of women had OPR dose reduction compared with 37% of men (Table 2). The odds of OPR dose reduction were 43% higher in women compared with men (AOR = 1.43, 95% CI = 1.11–1.83).
Results of Sensitivity Analyses
Although 42.2% of patients met the primary definition of OPR dose reduction, 31.2% had a “large dose reduction” and 28.7% had a “sustained dose reduction” (Table 3). The association between baseline daily OPR dose and OPR dose reduction was consistent in direction, magnitude, and statistical significance among all outcome definitions (P < 0.05 for all). Concurrent benzodiazepine prescription was not associated with OPR dose reduction using any (primary or secondary) outcome definition. The association between black race and OPR dose reduction was also consistent in direction, magnitude, and significance among all outcome definitions (P < 0.05 for all). The association of female gender with OPR dose reduction was similar for all outcome definitions but was not statistically significant for the “large dose reduction” secondary outcome definition (Table 3). Finally, using the dichotomous variable for baseline OPR dose (“high OPR dose”; yes/no), patients with a high OPR dose had 31% lower odds of OPR dose reduction compared with those without a high OPR dose (AOR = 0.69, 95% CI = 0.54–0.89).
Table 3.
Results of sensitivity analyses using primary and secondary outcome definitions
| Primary OPR Dose Reduction, AOR (95% CI)* | P Value | “Large Dose Reduction,” AOR (95% CI)* | P Value | “Sustained Dose Reduction,” AOR (95% CI)* | P Value | |
|---|---|---|---|---|---|---|
| Total No. (%) with outcome | 463 (42.21) | – | 300 (31.18) | – | 315 (28.71) | – |
| Independent variables | ||||||
| Clinical | ||||||
| Baseline OPR dose, MME, increments of 20 | 0.98 (0.96–0.99) | 0.001 | 0.95 (0.93–0.97) | <0.001 | 0.98 (0.96–0.99) | 0.01 |
| Concurrent benzodiazepines | 0.85 (0.61–1.17) | 0.32 | 0.93 (0.65–1.31) | 0.71 | 1.00 (0.71–1.43) | 0.99 |
| Sociodemographic | ||||||
| Race/ethnicity | ||||||
| White | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black | 1.82 (1.22–2.70) | 0.003 | 1.73 (1.13–2.65) | 0.01 | 1.58 (1.02–2.45) | 0.04 |
| Hispanic | 1.21 (0.82–1.79) | 0.35 | 1.08 (0.71–1.65) | 0.73 | 1.26 (0.82–1.95) | 0.30 |
| Other/unknown | 0.97 (0.63–1.49) | 0.89 | 0.97 (0.61–1.56) | 0.91 | 1.12 (0.70–1.81) | 0.64 |
| Gender | ||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Female | 1.43 (1.11–1.83) | 0.006 | 1.15 (0.88–1.51) | 0.30 | 1.31 (1.00–1.72) | 0.05 |
| Covariates | ||||||
| Age | ||||||
| <60 y | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥60 y | 1.44 (1.06–1.95) | 0.02 | 1.61 (1.17–2.21) | 0.003 | 1.44 (1.04–1.98) | 0.03 |
| Pain diagnosis category† | ||||||
| Neck | 1.59 (1.10–2.29) | 0.01 | 1.37 (0.93–2.02) | 0.11 | 1.67 (1.15–2.44) | 0.008 |
CI = confidence interval; IQR = interquartile range; MME = morphine-milligram equivalents; OPR = opioid pain reliever.
Multivariable logistic regression adjusted for age, race/ethnicity, gender, baseline OPR dose (continuous), concurrent benzodiazepines, and neck pain diagnosis.
Based on provider ICD-9 coding, categorizations based on the approach of Larochelle et al. [41] Patients may have multiple pain diagnoses.
Discussion
In this cohort of patients who were prescribed stable doses of COT for chronic noncancer pain in a large, urban academic health care system, high baseline daily OPR doses and concurrent benzodiazepines were not associated with OPR dose reduction as hypothesized; rather, baseline daily OPR dose was negatively associated with OPR dose reduction. Further, both black race and female gender were associated with greater odds of OPR dose reduction, compared with white race and male gender, respectively.
The finding that black and female patients had greater odds of OPR dose reduction in our cohort is consistent with prior studies of race and gender differences in OPR prescribing. Previous studies have consistently found that black patients received fewer OPRs, lower doses of OPRs, and tighter oversight when prescribed OPRs compared with white patients [29–33]. Although females were more likely to report pain [34,35] and recent OPR use compared with males (2.2% vs 1.7% prevalence) in national population-based surveys [45], studies from ambulatory care and outpatient visits found that health care providers wrote fewer prescriptions to females compared with males [31], and specifically among patients with chronic noncancer pain, females received lower doses of pain medication compared with males [31,36]. Importantly, the finding that OPR dose reduction was more likely for black or female patients (compared with white or male patients) is discordant with epidemiologic data suggesting that they have lower, not higher, risk for OPR overdose death [8,31,36].
Although this study did not assess provider bias, it is possible that provider biases underlie the race and gender differences observed. Although it is also possible that patients requesting an OPR dose reduction might differ by race or gender, we believe that this is a less likely explanation. Differential OPR management by race or gender might reflect differences in providers’ appraisal of patients’ severity of pain, or OPR-related risk by patients’ sociodemographic characteristics. In fact, prior studies have reported differences in physician–patient communication and pain assessment consistent with racial and ethnic biases [46,47]. For example, in some studies, physicians tended to view black (vs white) patients as more contentious, less effective communicators with lower levels of positive affect [48,49], and they tended to underestimate pain severity in African American and Hispanic patients [47]. Racial differences in urine drug testing for patients with chronic pain suggest that provider bias may influence assessment of risk of opioids as well [50]. Current guidelines recommend OPR dose reduction when the risks exceed benefits of continuing the same dose [26,27], so it is important to consider the potential for bias in assessment of the benefits and risks. Our findings of race and gender differences in OPR dose reduction highlight the importance of implementing equitable OPR dose reduction practices.
Although our study time period preceded the release of the Centers for Disease Control and Prevention guidelines, the finding that baseline OPR dose was negatively associated with OPR dose reduction is unexpected in the context of current recommendations to consider and offer dose reduction to patients prescribed high OPR doses [26]. However, the finding is consistent with a prior study that found that patients with an OPR dose of at least 120 MME were less likely to discontinue OPR compared with those with an OPR dose of less than 120 MME [51]. This finding may reflect unmeasured confounders that act as barriers to OPR dose reduction among patients on high-dose OPRs, including a higher pain severity, longer duration of OPR therapy, physical dependence, tolerance, or an opioid use disorder. This finding may also partly reflect our outcome definition, which was based on a percent reduction and therefore corresponded to a higher absolute reduction for patients at higher doses.
Importantly, there is no standard or consensus definition of a clinically meaningful OPR dose reduction, and there is substantial heterogeneity in the measuring and reporting of OPR dose reductions [39]. For the purposes of this study, we defined OPR dose reduction as a reduction in daily OPR dose of at least 30%, a reduction equivalent to at least 5 mg of oxycodone per day. However, given the heterogeneity of prior definitions and lack of a standard, we also report two secondary outcome definitions requiring either a large dose reduction or sustained dose reduction. Analyses were similar among all outcome definitions, and therefore support the validity of our primary outcome definition. Nevertheless, the lack of a standardized definition of an OPR dose reduction is a barrier to advancing knowledge about best practices regarding dose reduction. Future studies should aim to determine clinically meaningful dose reductions and standardize ways to identify reductions using prescription data [39].
The limitations of our study include that it was conducted using a single institution during a time of evolving OPR prescribing practices, which may limit generalizability to other settings and time periods. EMR prescription data may differ from pharmacy dispensing data. Additionally, we limited the cohort to patients retained in care in order to ensure that there was opportunity to have an OPR dose reduction, and we are therefore unable to draw conclusions about OPR dose reductions among patients not retained in care. Twenty percent of patients in the cohort had race/ethnicity reported as “other/unknown,” which could indicate misclassification; however, no significant difference was found between odds of OPR dose reduction in other/unknown vs white patients. Lastly, because we used EMR data, we cannot draw conclusions about the indications for OPR dose reduction.
In conclusion, our findings raise concern that clinical decisions about OPR dose reduction may be influenced more by social factors such as race and gender than by clinical factors that indicate actual risk. Further research is needed to examine these associations in other settings, using prospective primary data collection, and to more deeply examine providers’ clinical decision-making regarding OPR dose reduction. Additionally, research is needed to examine the relationship between age and OPR dose reduction. Future research and clinical initiatives aimed at OPR dose reduction should focus on patients at high risk for OPR-related adverse events and aim for equity among all patient populations.
Acknowledgments
We would like to thank Dr. Ellie Schoenbaum (Albert Einstein College of Medicine, Bronx, NY, USA) and the Substance Use Affinity Group of the Division of General Internal Medicine at Montefiore Medical Center for their feedback on this project.
Funding sources: Support for this project was provided by National Institutes of Health grants: R01DA039046 and K24DA046309 (PI: Starrels), K24DA036955 (PI: Cunningham), and K23DA044327 (PI: Perez).
Conflicts of interest: JS receives research support from the Opioid Post-Marketing Requirement Consortium, an FDA-mandated consortium of opioid manufacturers, for an observational study of the risks of opioids. The other authors have no conflicts of interest to disclose, and no commercial entities were involved in any way with this study.
Prior presentations: Preliminary findings from this study were presented at the Society of General Internal Medicine (SGIM) Annual Meeting in Washington DC on April 21, 2017, and at the Association for Medical Education and Research in Substance Abuse (AMERSA) Annual National Conference in Washington DC on November 2, 2017.
References
- 1. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015;162(4):276–86. [DOI] [PubMed] [Google Scholar]
- 2. Ballantyne JC. Is lack of evidence the problem? J Pain 2010;11(9):830–2. [DOI] [PubMed] [Google Scholar]
- 3. Ballantyne JC, Shin NS.. Efficacy of opioids for chronic pain: A review of the evidence. Clin J Pain 2008;24(6):469–78. [DOI] [PubMed] [Google Scholar]
- 4. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: Prediction and identification of aberrant drug-related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009;10(2):131–46. [DOI] [PubMed] [Google Scholar]
- 5. Sites BD, Beach ML, Davis MA.. Increases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among users. Reg Anesth Pain Med 2014;39(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martell BA, O'Connor PG, Kerns RD, et al. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Ann Intern Med 2007;146(2):116–27. [DOI] [PubMed] [Google Scholar]
- 7. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011;60(43):1487–92. [PubMed] [Google Scholar]
- 9. Martins SS, Sampson L, Cerda M, Galea S.. Worldwide prevalence and trends in unintentional drug overdose: A systematic review of the literature. Am J Public Health 2015;105(11):e29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han B, Compton WM, Jones CM, Cai R.. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003-2013. JAMA 2015;314(14):1468–78. [DOI] [PubMed] [Google Scholar]
- 11. Rudd RA, Seth P, David F, Scholl L.. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep 2016;65(5051):1445–52. [DOI] [PubMed] [Google Scholar]
- 12. Boscarino JA, Rukstalis MR, Hoffman SN, et al. Prevalence of prescription opioid-use disorder among chronic pain patients: Comparison of the DSM-5 vs. DSM-4 diagnostic criteria. J Addict Dis 2011;30(3):185–94. [DOI] [PubMed] [Google Scholar]
- 13. Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: The role of opioid prescription. Clin J Pain 2014;30(7):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atluri S, Sudarshan G, Manchikanti L.. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician 2014;17(2):E119–28. [PubMed] [Google Scholar]
- 15. Goesling J, Moser SE, Lin LA, et al. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med 2018;19(2):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mack KA, Zhang K, Paulozzi L, Jones C.. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved 2015;26(1):182–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohnert AS, Logan JE, Ganoczy D, Dowell D.. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care 2016;54(5):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016;17(1):85–98. [DOI] [PubMed] [Google Scholar]
- 19. Liang Y, Turner BJ.. National cohort study of opioid analgesic dose and risk of future hospitalization. J Hosp Med 2015;10(7):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ilgen MA, Bohnert AS, Ganoczy D, et al. Opioid dose and risk of suicide. Pain 2016;157(5):1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gwira Baumblatt JA, Wiedeman C, Dunn JR, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med 2014;174(5):796–801. [DOI] [PubMed] [Google Scholar]
- 22. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- 23. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun EC, Dixit A, Humphreys K, et al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: Retrospective analysis. BMJ 2017;356(j760). doi: 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kandel DB, Hu MC, Griesler P, Wall M.. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend 2017;178:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 27. McCarberg B. Washington state opioid prescribing guidelines. Pain Med 2015;16(8):1455–6. [DOI] [PubMed] [Google Scholar]
- 28. Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ.. Secular trends in opioid prescribing in the USA. J Pain Res 2017;10:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R.. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 2008;299(1):70–8. [DOI] [PubMed] [Google Scholar]
- 30. Joynt M, Train MK, Robbins BW, et al. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med 2013;28(12):1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wisniewski AM, Purdy CH, Blondell RD.. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis 2008;27(1):1–11. [DOI] [PubMed] [Google Scholar]
- 32. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK.. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010;151(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hausmann LR, Gao S, Lee ES, Kwoh CK.. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain 2013;154(1):46–52. [DOI] [PubMed] [Google Scholar]
- 34. Hurley RW, Adams MC.. Sex, gender, and pain: An overview of a complex field. Anesth Analg 2008;107(1):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Munce SE, Stewart DE.. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics 2007;48(5):394–9. [DOI] [PubMed] [Google Scholar]
- 36. Kaplovitch E, Gomes T, Camacho X, et al. Sex differences in dose escalation and overdose death during chronic opioid therapy: A population-based cohort study. PLoS One 2015;10(8):e0134550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Center for Injury Prevention and Control. CDC Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics with Oral Morphine Milligram Equivalent Conversion Factors, 2016 Version. Atlanta, GA: Centers for Disease Control and Prevention; 2016. Available at: https://www.cdc.gov/drugoverdose/media/ (Accessed May 1, 2018).
- 38. Berna C, Kulich RJ, Rathmell JP.. Tapering long-term opioid therapy in chronic noncancer pain: Evidence and recommendations for everyday practice. Mayo Clin Proc 2015;90(6):828–42. [DOI] [PubMed] [Google Scholar]
- 39. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: A systematic review. Ann Intern Med 2017;167(3):181–91. [DOI] [PubMed] [Google Scholar]
- 40. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 41. Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF.. Opioid prescribing after nonfatal overdose and association with repeated overdose. Ann Intern Med 2016;165(5):376–7. [DOI] [PubMed] [Google Scholar]
- 42. Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain 2013;154(11):2287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Academy of Pain Medicine, American Pain Society, American Society of Addition Medicine. Definitions related to the use of opioids for the treatment of pain. WMJ 2001;100(5):28–9. [PubMed] [Google Scholar]
- 44. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: A randomized controlled trial. J Pain 2017;18(3):308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parsells Kelly J, Cook SF, Kaufman DW, et al. Prevalence and characteristics of opioid use in the US adult population. Pain 2008;138(3):507–13. [DOI] [PubMed] [Google Scholar]
- 46. Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med 2003;4(3):277–94. [DOI] [PubMed] [Google Scholar]
- 47. Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: Pain management attitudes and practice. Cancer 2000;88(8):1929–38. [PubMed] [Google Scholar]
- 48. Johnson RL, Roter D, Powe NR, Cooper LA.. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health 2004;94(12):2084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Street RL Jr, Gordon H, Haidet P.. Physicians' communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc Sci Med 2007;65(3):586–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Becker WC, Starrels JL, Heo M, et al. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med 2011;9(3):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin BC, Fan MY, Edlund MJ, et al. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med 2011;26(12):1450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]