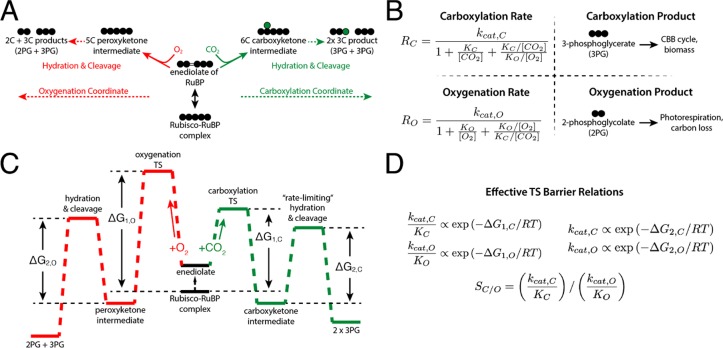
Figure 1.
Description of the catalytic mechanism of Rubisco. The “middle-out” diagram in panel A shows the ordered mechanisms of carboxylation and oxygenation. Circles represent carbon atoms. RuBP is isomerized to an enediolate before carboxylation or oxygenation. Addition of CO2 or O2 to the enediolate of RuBP is considered irreversible as are the subsequent hydration and cleavage steps of the carboxylation and oxygenation arms. (B) Carboxylation displays effective Michaelis–Menten kinetics (maximum catalytic rate kcat,C, half-maximum CO2 concentration KM = KC) with competitive inhibition by O2 (assuming half-maximum inhibitory O2 concentration Ki = KO). Carboxylation results in net addition of one carbon to the five-carbon RuBP, producing two 3PG molecules. 3PG is part of the CBB cycle and can therefore be used to continue the cycle and produce biomass. Oxygenation also displays effective Michaelis–Menten kinetics (kcat,O, KM = KO, half-maximum inhibitory CO2 concentration KI = KC). Oxygenation of RuBP produces one 3PG and one 2PG. Rates of carboxylation (RC) and oxygenation (RO) are calculated from kinetic parameters and the CO2 and O2 concentrations. The reaction coordinate diagram in panel C describes carboxylation and oxygenation as a function of two “effective” barriers.6 The first effective barrier includes enolization and gas addition, while the second includes hydration and cleavage. (D) Given standard assumptions (Supporting Information), catalytic efficiencies (kcat/KM) are related to the height of the first effective barrier while kcats are related to the second. The first barrier to oxygenation is drawn higher than for carboxylation because oxygenation is typically slower than carboxylation. Net reactions of RuBP carboxylation and oxygenation are both quite thermodynamically favorable.9