Summary
The Lancet Commission on Global Surgery emphasised the importance of access to safe anaesthesia care. Capnography is an essential monitor for safe anaesthesia, but is rarely available in low‐income countries. The aim of this study was twofold: to measure the prevalence of capnography in the operating theatres and in intensive care units; and to determine whether its introduction was feasible and could improve the early recognition of critical airway incidents in a low‐income country. This is the first project to do this. Forty capnographs were donated to eight hospitals in Malawi. Thirty‐two anaesthesia providers received a 1‐day capnography training course with pre‐ and post‐course knowledge testing. Providers kept logbooks of capnography use and recorded their responses to abnormal readings. On follow‐up at 6 months, providers completed questionnaires on any significant patient safety incidents identified using capnography. In January 2017, at the commencement of the project, only one operating theatre had a capnograph. Overall, 97% and 100% ‘capnography gaps’ were identified in the theatres and intensive care units, respectively. The mean (SD) scores of our capnography multiple choice questionnaires improved after training from 15.00 (3.16) to 18.70 (0.99), p = < 0.001. The capnography equipment was appropriately robust and performed well. Six months following implementation, 24 (77%) anaesthesia providers reported recognising 44 oesophageal intubations and 28 (90%) believed that capnography had saved lives. This study has shown it is feasible to introduce capnography in a low‐income country, resulting in early recognition of critical airway incidents and ultimately helping to save lives. Building on the experience of the first trial of pulse oximetry implementation in low‐income countries in 2007, we believe this is one of the most important projects in anaesthesia safety in the last decade.
Keywords: airway, capnography, monitoring, oesophageal intubation, safety
Introduction
The Lancet Commission on Global Surgery concluded that universal access to safe, affordable surgical and anaesthesia care when needed saves lives 1. The introduction of improved patient monitoring in high‐income countries has brought about major improvements in the safety of anaesthesia care [2–4]. This has not taken place in the middle‐ and low‐income countries, and in 2008 the World Federation of Societies of Anaesthesiologists (WFSA) produced international standards for the safe practice of anaesthesia that included pulse oximetry and capnography 5. Pulse oximetry was also included in the World Health Organization (WHO) surgical safety checklist 6 and its successful introduction along with a training package into low‐income countries began with the Global Oximetry Project 7 and later continued with the Lifebox Foundation 8. Capnography is also seen as an essential monitor in high‐income countries 9, and the 4th National Audit Project (NAP4) 10 on major complications of airway management further confirmed the importance of capnography both in the operating theatre and especially in the intensive care unit (ICU) 11. In low‐income countries, anaesthesia providers routinely work without capnography 12, 13, 14 and there is often a 100% gap between the need for capnography and its availability. This ‘capnography gap’ is defined as the difference between the observed and expected numbers of capnographs in the operating theatre or in the ICU.
Pulse oximetry was the first monitor chosen when considering introducing essential anaesthesia monitoring equipment into low‐income countries, due to ease‐of‐use and applicability to all patients. This led to the successful Global Oximetry Project 15 and the Lifebox Foundation initiative. This latter initiative has led to increased availability of pulse oximeters for patients undergoing general anaesthesia in many low‐ and middle‐income countries. In 2016, the Global Capnography Project (GCAP) was established to investigate the feasibility and sustainability of introducing capnography as a standard of care in low‐income and middle‐income countries. A pilot site was identified in Malawi, where anaesthesia links were already established and pulse oximetry had been satisfactorily introduced 8.
The aims of the project were as follows: to quantify the capnography gap; to identify the training and education needs of the anaesthesia providers; and to distribute 40 capnographs. The follow‐up aimed to assess the performance of the capnograph used; to gather the opinions of the anaesthesia providers on the value of the training and the importance of capnography in their routine practice, and to determine if any significant patient safety incidents were identified by using capnography and the resulting action taken.
Methods
Anaesthetists from the Queen Elizabeth Hospital in Blantyre were involved from the earliest planning stage of the project, and relevant local approvals were obtained from the head of anaesthesia. All individual anaesthesia providers gave their consent and some agreed to a video interview of their experiences and opinions on capnography.
Malawi is a small low‐income country in sub‐Saharan Africa with a population of 17.4 million (2017) 16, 17, and life expectancy is 57 years for men and 60 years for women 18, 19. The project was conducted in selected hospitals in the southern region of Malawi. These included the largest hospital in the region, Queen Elizabeth Central Hospital Blantyre (QECH) (WFSA Level 3, referral hospital) 5, Zomba Central Hospital (ZCH), Zomba (WFSA Level 2, district/provincial hospital), and six other government district hospitals (WFSA Level 1, small hospitals) where anaesthesia was undertaken.
A prospective study of anaesthesia mortality in Malawi suggested that the anaesthesia ‘avoidable mortality rate’ was 1:504 anaesthetics, which is 6–100 times higher than the anaesthesia mortality rate in developed countries 20. There are an estimated 109 anaesthesia providers in Malawi, 104 (95%) of whom are non‐physician anaesthetic clinical officers (ACOs) 21. Most anaesthetics are given by these ACOs who have no medical or nursing background, but receive 18 months of anaesthesia training 22. Many had been involved in the introduction of pulse oximetry into Malawi and were known to the GCAP Group through that project and previous training courses.
We obtained information about the capnography gap at all the sites in Malawi from pre‐course questionnaires. Capnography was expected to be present in every operating theatre and at every ICU bed that was used for treating patients who required mechanical ventilation. The GCAP team visited Malawi twice, launching the project in January 2017 and returning to conduct a follow‐up review in August 2017. Contact was maintained by email and telephone calls in the intervening months.
At the project launch, 32 anaesthesia providers attended a training course that included lectures and small group workshop sessions. This covered capnography theory and clinical use of the Nellcor™ N‐85 hand‐held side‐stream monitor (Medtronic Minimally Invasive Therapies, Dublin, Ireland). This equipment provides a capnography waveform display which is simple to operate and has a rechargeable battery that provides power for from 4–7 hr, depending on usage, with backup mains supply. The course also incorporated clinical scenarios with capnography waveform recognition and explained what action was needed to prevent patient harm. The waveform recognition included use of the ‘Hats and Caps’ system, which is a novel method of teaching trainee doctors and ICU nurses about interpretation of capnograph traces (Fig. 1) 23. A multiple choice questionnaire (MCQ) was completed immediately before and after the course.
Figure 1.
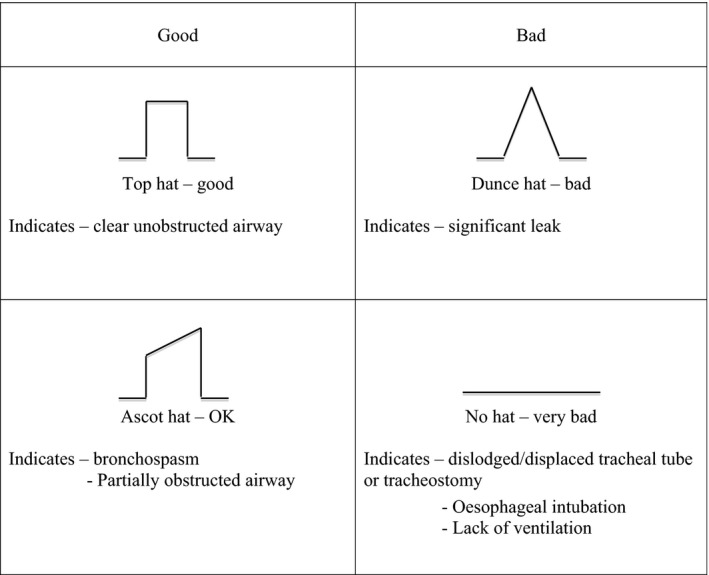
‘Hats and caps’ guide to capnography traces on intensive care; ‘posh hats’ are good 23. Reproduced with permission.
We donated 40 Nellcor N‐85 capnographs and 2000 sets of single‐use disposable gas monitoring lines to the 31 operating theatres and eight ICU beds in the pilot hospitals.
We constructed a GCAP logbook using a format similar to the one that was used during the introduction of pulse oximetry 8. Anaesthesia providers were asked to use the logbook for every case in which capnography was used and to record the following: ASA physical status; type of surgery; type of anaesthesia; and the capnography values and waveform shapes. Any problems encountered with patients or technical problems with the device were recorded.
We visited the QECH and ZCH sites to see that the capnographs were correctly set up and being used properly in the operating theatres and ICU, and to answer any queries about the project, logbooks or equipment. The ACO who was responsible for anaesthetic equipment in the region was given training in the onsite maintenance of the capnographs.
In August 2017, we conducted follow‐up visits to QECH, ZCH and other district hospitals, checked the capnography arrangements, addressed any queries about the project and collected information through questionnaires and interviews. We also carried out further workshop training, and all the anaesthesia providers who had been using the capnographs completed questionnaires. These asked about their use of capnography (including location and frequency), the incidents recognised or problems diagnosed during use, and their opinions on the technical aspects of the Nellcor N‐85 and its value in their practice. It provided an opportunity to expand on personal clinical cases in which they found capnography had been particularly helpful. Many providers gave video interviews of their experience of the training and their use of capnography. Anaesthesia providers who had also attended the January teaching courses completed an additional questionnaire that assessed the training package they had received 6 months earlier.
We performed data analysis of the MCQs using Stata 14.2. As the data from the pre‐ and post‐training MCQs were normally distributed, we undertook a two‐sample t‐test. Data from the logbooks and questionnaires were entered using Microsoft Excel software (Microsoft, Redmond, WA, USA).
Results
At the start of the GCAP project in January 2017, there was only one capnograph in the southern region of Malawi at the QECH, which has 13 operating theatres giving a hospital capnography gap of 92% ((13–1)/13). None of the other seven hospitals (total 18 theatres) had any capnographs, a gap of 100% ((18–0)/18). The overall capnography gap in all the operating theatres was 97% ((31–1)/31). Only QECH and ZCH had ICUs, each with four beds but no capnographs, giving a capnography gap in ICU of 100% ((8–0)/8).
We assessed knowledge of capnography by MCQs completed by 32 anaesthesia providers immediately before and after the January training course. All the anaesthesia providers spoke good English and there were no language barriers to this activity. There was an improvement in the MCQ scores after the one‐day training course, with a pre‐ training mean (SD) of 15.00 (3.16) and post‐training mean of 18.70 (0.99) out of 20. The mean difference between pre‐ and post‐training groups was 3.70 (p < 0.001, 95%CI 2.51–4.88). The 95%CIs for each group did not cross – 95%CI (13.85–16.14) pre‐ and (18.34–19.05) post‐course and therefore correlate with the reported p value.
Anaesthesia providers who attended the training in January also completed a course feedback questionnaire in August. All the anaesthesia providers thought that the one‐day course and follow‐up visits had been sufficient for them to use the capnography monitors, understand the end‐tidal carbon dioxide (ETCO2) values and interpret the capnography waveforms. The manual provided was a useful reference. They had all remembered the ‘Hats and caps’ system and found it useful to recognise capnography waveforms. Suggestions for improvement included online teaching materials and teaching for nurses on ICUs (Table 1).
Table 1.
Examples of critical incidents recognised by using capnography and corrective action taken in the operating theatres
| Clinical scenario | Capnograph waveform ‘Hats and caps’ (23) | Critical incident | Corrective action taken |
|---|---|---|---|
| Adult, female, ruptured ectopic pregnancy, emergency laparotomy, intubated with tracheal tube | No hat | Oesophageal intubation | Tracheal tube removed, re‐intubated – confirmed with top hat waveform |
| Child, cleft palate repair, uncuffed tracheal tube, difficulty in intubation, three attempts | No hat | Oesophageal intubation | Tracheal tube removed, re‐intubation – confirmed with top hat waveform on final attempt |
| Neonate, tracheo‐oesophageal fistula, uncuffed tracheal tube |
Top hat to no hat sudden change in the capnography waveform |
Tracheal tube displacement | Careful repositioning of tracheal tube – confirmed with top hat waveform |
| Female, ectopic pregnancy, general anaesthetic | Ascot hat | Severe bronchospasm, caused by anaphylaxis | Adrenaline intravenous and salbutamol nebulisers |
| Adult, laminectomy, anticipated 8‐h procedure, intubated, tracheal tube position confirmed |
Top hat rising baseline before start of surgery |
Rebreathing of CO2, soda lime exhausted | No soda lime available, woke patient up, case postponed until soda lime available |
| Neonate, hydrocephalus, external ventricular drain, uncuffed tracheal tube | Top hat to no hat | Tracheal tube kinked | Tracheal tube unkinked and secured – confirmed with top hat waveform |
| Child, six years old, thoracotomy, sudden high airway pressures | Ascot hat to no hat | Blocked tracheal tube | Tracheal tube suctioned and repositioned |
| Obstetric case, spinal converted to general anaesthetic due to massive haemorrhage |
Top hat progressively worsening rise in end‐tidal CO2 noted on waveform |
Hypoventilation – ventilator error, machine leak | Manual ventilation, mechanical ventilator replaced |
Logbook data were collected from 699 episodes of monitoring with the capnographs. It demonstrated capnography use across a wide range of procedures; 49% of patients were younger than 16 years and 87% were ASA physical status 1 or 2. These data showed clinical interpretation of the capnography waveforms and provided details of scenarios and critical incidents, summarised in Tables 1 and 2.
Table 2.
Examples of critical incidents recognised by using capnography and corrective action taken in the ICU
| Clinical scenario | Capnograph waveform ‘Hats and Caps’ (22) | Critical incident | Corrective action taken |
|---|---|---|---|
| Adult, male, severe traumatic brain injury (TBI), polytrauma, admitted to ICU from theatre post emergency laparotomy |
No hat on arrival in ICU |
Blocked tracheal tube/endo‐bronchial intubation | Suctioned tracheal tube and pulled back – correct position confirmed by top hat waveform |
| Adult, female, ICU admission with septic shock and DIC post normal delivery and General anaesthetic for retained products |
No hat changed to top hat with return of spontaneous circulation |
Severe hypotension/cardiac arrest | CPR commenced. Observed ROSC with return of waveform |
| Adults with TBI, intubated and ventilated in ICU |
Top hat rising and/or falling ETCO2 values |
Hypo‐ and hyperventilation | Helpful to adjust ventilator settings in ICU as no arterial blood gas sampling available |
| Paediatric ICU | Top hat to no hat | Accidental extubation and tracheal tube displacements | Capnography alarm immediately alerted staff, patient re‐intubated or tracheal tube repositioned – confirmed with Top Hat waveform |
TBI, traumatic brain injury; ICU, intensive care unit; CPR, cardiopulmonary resuscitation; DIC, disseminated intravascular coagulopathy; ROSC, return of spontaneous circulation.
Twenty‐eight (90%) anaesthesia providers reported that they believed that the use of capnography had saved lives. This group said that a minimum number of 57 lives had been saved during the 6‐month period of use. Twenty‐nine out of 31 (94%) anaesthesia providers reported capnography to be useful in all the different areas of their practice. Twenty‐seven out of 31 (87%) said it had been extremely useful in ICU.
Out of the 40 capnographs, 38 were fully functional after 6 months. Two capnographs did not switch on and were never used. Several had been dropped, but continued to work satisfactorily.
Discussion
This is, to our knowledge, the first project to study the implementation of capnography and its impact in a low‐income country. The 97% gap in capnography provision in the operating theatres and the 100% gap in capnography provision in intensive care reflects the results of surveys in other low‐income countries 13, 14, 24. The oximetry gap in operating theatres at the start of the Global Oximetry Project in Uganda was 64% 7. These findings confirm our belief that there is a substantial global deficit in monitoring using capnography for patients undergoing anaesthesia, despite waveform capnography being a recommended international standard for anaesthesia monitoring since 2010 5.
This study was a quality improvement project and had a number of limitations. It was run with minimum financial resources and the data collected were mostly descriptive and qualitative.
The area of Malawi was chosen due to existing clinical contacts, the known lack of capnography and the previous successful experience with the implementation of pulse oximetry. The hospitals were sufficiently diverse to provide information about relevant issues and this was one of the study strengths.
The evidence regarding recognition of critical incidents after the capnographs had been used for 6 months is compelling. Seventy‐seven percent of anaesthesia providers reported a total of 44 oesophageal intubations, and 81% reported a total of 81 breathing circuit disconnections (Table 3). Critical incidents are often under‐reported in questionnaires 25, but oesophageal intubation and disconnection are two of the most important incidents that capnography monitoring can detect; both can lead to significant patient harm and mortality if not identified early and corrected quickly 10.
Table 3.
Critical incidents identified with capnography from anaesthetic provider questionnaires covering 1418 cases in August 2017. Values are number (proportion)
| Critical incident/clinical scenario | Anaesthesia providers reporting incident/scenario | Incidents/scenarios recognised |
|---|---|---|
| Oesophageal intubations | 24/31 (77%) | 44 |
| Breathing circuit disconnections | 25/31 (81%) | 81 |
| Significant leak | 14/31 (45%) | 94 |
| Readiness for extubation | 20/31 (65%) | 247 |
| Hypoventilation | 25/31 (81%) | 174 |
| Hyperventilation | 24/31 (77%) | 186 |
| Severe hypotension | 12/31 (39%) | 20 |
| Bronchospasm | 13/31 (42%) | 27 |
| Kinked tracheal tube or sample line | 17/31 (55%) | 55 |
| Secretions | 21/31 (68%) | 53 |
Anaesthesia providers gave more detailed accounts of these oesophageal intubations when interviewed (see Tables 1 and 2). For example, after placing the tracheal tube, and despite breath sounds being perceived as normal, the absence of a capnography trace (no hat) was noted, provoking early re‐intubation and successful placement of the tracheal tube in the trachea. Many reflected that without the recognition and correction of these critical incidents, the adverse outcomes could have been very serious. This corroborates the experience of when routine capnography was first introduced into high‐income countries from 1986, the incidence of undetected oesophageal intubation causing catastrophic injury notably decreased, and was eventually virtually eliminated 26, 27. Capnography became mandatory in the US from 1991 (Fig. 2).
Figure 2.
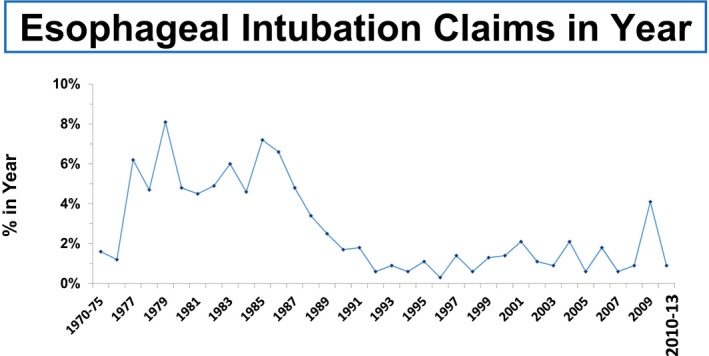
Trends in oesophageal intubation over time. The proportion of claims for delayed detection of oesophageal intubation by year of event in the Anesthesia Closed Claims Project Database 27. The years 1970–1975 and 2010–2013 were collapsed due to the small total number of claims in the database for those years. Reproduced with permission.
During the 6‐month study period, 44 oesophageal intubations were reported in Southern Malawi, which has a population of 7.5 million 16, giving a rate of 11.7 oesophageal intubations per million population per year. Assuming intubation rates and capnography use in Malawi to be representative of sub‐Saharan Africa, with a population of 1022 million 16, we estimate that over 11,000 oesophageal intubations could occur per year. These pose a very significant patient safety risk that would most effectively be mitigated by the implementation of capnography. Oesophageal intubation, undetected because capnography is not used, is now labelled a ‘Never Event’ in the NHS 28. Since the introduction of capnography in Malawi, 90% of the anaesthesia providers thought lives have been saved.
The recent African Surgical Outcomes Study commented that globally an average of 1% of patients die after surgery, but this number rises to 2.1% for patients in Africa. Of these, 5.9% died on the day of surgery, and the lack of capnography leading to acute airway deaths may have been a contributing factor 29.
Capnography is also useful outside the operating theatre 30, 31, and the eight capnographs supplied to two ICUs detected problems with secretions and blocked tracheal tubes. Capnography had also been used to monitor resuscitation during six cardiac arrests. The ICU nurse‐to‐patient ratios are always less than 1:1, and blood gas analysis is not available in Malawi. Measuring blood gases involves expensive equipment that requires regular maintenance, costly agents and disposables; capnography can provide completely new information to assist in managing ventilated patients. Hypoventilation had been recognised on 174 occasions and hyperventilation on 186 occasions, again improving the safety and quality of the patient care delivered in theatre and ICU.
At the 6‐month follow‐up, all the capnographs were fully functional, except for the two that had never been switched on. All of the anaesthesia providers said the capnographs were easy to use, and 77% were now using them on every intubated patient. Producing a change in practice of this magnitude within 6 months in this challenging environment is noteworthy. The fact that a high proportion of providers experienced oesophageal intubations and breathing circuit disconnections that were detected early and ‘saved’ by capnography, may have driven this change.
The capnographs performed well, and were appropriately robust for this environment. Anaesthesia providers gave their opinions (see Tables 4and 5) and many suggested ways of securing the capnographs to prevent them falling to the floor, including clamps, cases and drip stand attachments. Nevertheless, all those that had fallen were still working satisfactorily. The capnographs used required very little maintenance, and a member of staff received appropriate training in January and was given the small amount of equipment necessary for a simple annual service. Two thousand disposable gas sampling line sets had been provided for the project; in the future, replacing these presents an additional supply issue and running cost. The Nellcor N‐85 capnograph also has the facility to provide pulse oximetry; this may be advantageous, and on occasions some providers used it for both indications, for instance during cardiac arrests on the wards. As additional patient monitoring becomes available in low‐income countries it is preferable to combine as many modalities as possible in one unit, as is done in high‐income countries 5.
Table 4.
Opinions of anaesthesia providers about capnography. Values are number (proportion)
| Opinions of anaesthesia providers | Providers agreeing |
|---|---|
| ‘Capnography changed their anaesthetic practice’ | 31/31 (100%) |
| ‘Capnography helped in preventing complications’ | 31/31 (100%) |
| ‘They will continue to use capnography’ | 31/31 (100%) |
| ‘They would recommend the use of capnography to colleagues’ | 31/31 (100%) |
| ‘They would want capnography used on self or a family member’ | 31/31 (100%) |
Table 5.
Opinions of 31 anaesthesia providers on capnography and equipment issues. Values are proportion
| Anaesthesia providers | |
|---|---|
| Practicalities of use | |
| The device is always easy to find | 86% |
| Both waveform and ETCO2 values are useful | 86% |
| Used the device on every intubated patient | 77% |
| Did not have storage issues | 76% |
| Did not have cleaning issues | 81% |
| Did not have gas line issues | 70% |
| Design issues | |
| Did not have issues with the size of waveform | 81% |
| Did not have an issue with the size of the screen | 71% |
| Did not have issues with the connection to tracheal tube | 90% |
| Did not have issues with the capnography device not working during a case | 78% |
| Found the device easy to use | 97% |
| Battery life | |
| Battery ran out during use | 38% |
| Battery life should be 4 h | 8% |
| Battery life should be 8 h | 25% |
| Battery life should be 12 h | 67% |
| Used capnography connected to mains | 21% |
New technology should only be introduced with an appropriate training package. Those responsible for the delivery of this package found it practical and straightforward to teach. The feedback showed that the manual provided had been a useful ongoing reference, and that anaesthesia providers had all remembered and used the ‘Hats and caps’ system for distinguishing capnograph waveforms. Suggested improvements included online teaching materials, more locally‐based case scenarios and ICU nurse training. Some ICU nurses were particularly enthusiastic about capnography, and on the two ICUs visited, it is now used to monitor every patient who requires mechanical ventilation.
Only one operating theatre had a capnograph at the start of this project, effectively demonstrating an absolute ‘gap’ in capnograph provision in this low‐income country, despite international standards recommending it. This study has shown that it is feasible to produce an appropriate training package for anaesthesia providers to help them successfully introduce capnography to monitor almost every patient who required tracheal intubation and mechanical ventilation in theatre and ICU over a period of 6 months. Increased recognition of airway incidents was judged to have saved lives.
Considering the large capnography gap in Malawi, and the likelihood that those operating theatres that do not have pulse oximetry worldwide will certainly not have capnography, it is reasonable to assume that there must be at least 70,000 operating theatres in the world without capnography 32, 33, a safety issue that represents both risk and opportunity. This GCAP study has shown the capnograph used to be appropriately robust and demonstrated that, following a short course on capnography, a very significant change in practice could be achieved, increasing patient safety. We contend that if comparable equipment were available in other low‐income countries, similar improvements could be reproduced there.
For these reasons, we believe that this is one of the most important projects in anaesthesia safety in the last decade. The results support the development of an international project to help make global capnography provision a reality, so that like pulse oximetry, it can be included in the WHO surgical safety checklist and improve patient safety worldwide. All relevant organisations should consider taking this forward.
Acknowledgements
All the medical anaesthetists, ACOs and ITU nurses in Malawi who took part in the project, and Dr T Schnittger and Dr A Tobin who took part in the first visit to Malawi. The authors are grateful for funding received from Medtronic for travel and associated costs, and in particular for donating and delivering all the capnographs and disposables used in the project. Medtronic had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication. RJ and FR contributed equally to the project. RJ, FR, EO and DW all received travel expenses incurred for this project from Medtronic. DW has received lecture fees from Aguettant Ltd and Medtronic, all donated to Lifebox. All other authors declare no competing interests.
This article is accompanied by an editorial by Lipnick et al., Anaesthesia 2019; 74: 146‐149.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015; 386: 569–624. [DOI] [PubMed] [Google Scholar]
- 2. Eichhorn JH. Prevention of intraoperative anesthesia accidents and related severe injury through safety monitoring. Anesthesiology 1989; 70: 572–7. [DOI] [PubMed] [Google Scholar]
- 3. Eichhorn JH, Cooper JB, Cullen DJ, Maier WR, Philip JH, Seeman RG. Standards for patient monitoring during anesthesia at Harvard Medical School. Journal of the American Medical Association 1986; 256: 1017–20. [PubMed] [Google Scholar]
- 4. Association of Anaesthetists of Great Britain and Ireland . Recommendations for standards of monitoring during anaesthesia and recovery. London, UK: Association of Anaesthetists of Great Britain and Ireland; 1987. [Google Scholar]
- 5. Merry AF, Cooper JB, Soyannwo O, Wilson IH, Eichhorn JH. International standards for a safe practice of anesthesia 2010. Canadian Journal of Anesthesia 2010; 57: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organisation . Guidelines for safe surgery 2009. http://apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf (accessed 11/12/2017).
- 7. Walker IA, Merry AF, Wilson IH, et al. Global oximetry: an international anaesthesia quality improvement project. Anaesthesia 2009; 64: 1051–60. [DOI] [PubMed] [Google Scholar]
- 8. Albert V, Mndolo S, Harrison EM, O'Sullivan E, Wilson IH, Walker IA. Lifebox pulse oximeter implementation in Malawi: evaluation of educational outcomes and impact on oxygen desaturation episodes during anaesthesia. Anaesthesia 2017; 72: 686–93. [DOI] [PubMed] [Google Scholar]
- 9. Whitaker DK. Time for capnography – everywhere. Anaesthesia 2011; 66: 544–9. [DOI] [PubMed] [Google Scholar]
- 10. Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. British Journal of Anaesthesia 2011; 106: 617–31. [DOI] [PubMed] [Google Scholar]
- 11. Cook TM, Woodall N, Harper J, Benger J. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. British Journal of Anaesthesia 2011; 106: 632–42. [DOI] [PubMed] [Google Scholar]
- 12. Dart PJ, Kinnear J, Bould MD, Mwansa SL, Rakhda Z, Snell D. An evaluation of inpatient morbidity and critical care provision in Zambia. Anaesthesia 2017; 72: 172–80. [DOI] [PubMed] [Google Scholar]
- 13. Baxter LS, Ravelojaona VA, Rakotoarison HN, et al. An observational assessment of anesthesia capacity in Madagascar as a prerequisite to the development of a national surgical plan. Anesthesia and Analgesia 2017; 124: 2001–7. [DOI] [PubMed] [Google Scholar]
- 14. Koka R, Chima AM, Sampson JB, et al. Anesthesia practice and perioperative outcomes at two tertiary care hospitals in freetown, Sierra Leone. Anesthesia and Analgesia 2016; 123: 213–27. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organisation . Global pulse oximetry project, first international consultation meeting; 29th and 30th October 2008 WHO Headquarters, Geneva, Switzerland. Geneva, Switzerland 2008. http://www.who.int/patientsafety/events/08/1st_pulse_oximetry_meeting_background_doc.pdf (accessed 11/12/2017).
- 16. Brinkhoff T. City population, malawi: regions, major cities, towns & villages – population statistics 2017. http://www.citypopulation.de/Malawi.html (accessed 11/12/2017).
- 17. The world bank data: Malawi, 2016. https://data.worldbank.org/country/malawi (accessed 11/12/2017).
- 18. World Health Organization . Malawi country statistics 2016. http://www.who.int/countries/mwi/en/(accessed 11/12/2017).
- 19. United Nations Development Programme . Human development reports. 2016. http://hdr.undp.org/en/countries/profiles/MWI (accessed 11/12/2017).
- 20. Hansen D, Gausi SC, Merikebu M. Anaesthesia in Malawi: complications and deaths. Tropical Doctor 2000; 30: 146–9. [DOI] [PubMed] [Google Scholar]
- 21. Henry JAFE, Borgstein E, Mkandawire N, Goddia C. Surgical and anaesthetic capacity of hospitals in Malawi: key insights. Health and Policy Planning 2015; 2015: 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biccard BM, Green‐Thompson L. Socially accountable anaesthesia: matching human resources with community need for safe care. Anaesthesia 2018; 73: 271–4. [DOI] [PubMed] [Google Scholar]
- 23. Cook TM, Kelly FE, Goswami A. ‘Hats and caps’ capnography training on intensive care. Anaesthesia 2013; 68: 421. [Google Scholar]
- 24. Bruno E, White MC, Baxter LS, et al. An evaluation of preparedness, delivery and impact of surgical and anesthesia care in madagascar: a framework for a national surgical plan. World Journal of Surgery 2017; 41: 1218–24. [DOI] [PubMed] [Google Scholar]
- 25. Institute of Medicine (US) . To err is human: building a safer health system. Washington, DC: National Academies Press (US); 2000. [PubMed] [Google Scholar]
- 26. Eichhorn JH. Monitoring standards: role of monitoring in reducing risk of anesthesia In: LA Fleisher PD, ed. Problems in anesthesia: 13 quality, safety, risk, and outcomes. Philadelphia, PA: Lippincott Williams & Wilkins, 2001: 430–43. [Google Scholar]
- 27. Honardar MR, Posner KL, Domino KB. Delayed detection of esophageal intubation in anesthesia malpractice claims: brief report of a case series. Anesthesia and Analgesia 2017; 125: 1948–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NHS Improvement . Revised never events policy and framework and never events list 2018. London, UK: NHS Improvement; 2018. https://improvement.nhs.uk/resources/never-events-policy-and-framework/(accessed 20/01/2018). [Google Scholar]
- 29. Biccard BM, Madiba TE, Kluyts HL, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7‐day prospective observational cohort study. Lancet 2018; 391: 1589–98. [DOI] [PubMed] [Google Scholar]
- 30. Whitaker DK, Benson JP. Capnography standards for outside the operating room. Current Opinion in Anaesthesiology 2016; 29: 485–92. [DOI] [PubMed] [Google Scholar]
- 31. Kodali BS. Capnography outside the operating rooms. Anesthesiology 2013; 118: 192–201. [DOI] [PubMed] [Google Scholar]
- 32. Funk LM, Weiser TG, Berry WR, et al. Global operating theatre distribution and pulse oximetry supply: an estimation from reported data. Lancet 2010; 376: 1055–61. [DOI] [PubMed] [Google Scholar]
- 33. Foundation L. What is the pulse oximetry gap? London, UK: Lifebox Foundation ; 2017. http://www.lifebox.org/about-lifebox/faq-2/what-is-the-pulse-oximetry-gap/ (accessed 11/12/2017). [Google Scholar]


