Summary
Despite numerous guidelines on the management of anaemia in surgical patients, there is no pragmatic guidance for the diagnosis and management of anaemia and iron deficiency in the postoperative period. A number of experienced researchers and clinicians took part in a two‐day expert workshop and developed the following consensus statement. After presentation of our own research data and local policies and procedures, appropriate relevant literature was reviewed and discussed. We developed a series of best‐practice and evidence‐based statements to advise on patient care with respect to anaemia and iron deficiency in the postoperative period. These statements include: a diagnostic approach to iron deficiency and anaemia in surgical patients; identification of patients appropriate for treatment; and advice on practical management and follow‐up that is easy to implement. Available data allow the fulfilment of the requirements of Pillar 1 of Patient Blood Management. We urge national and international research funding bodies to take note of these recommendations, particularly in terms of funding large‐scale prospective, randomised clinical trials that can most effectively address the important clinical questions and this clearly unmet medical need.
Keywords: anaemia, erythropoiesis stimulating agents, iron deficiency, iron supplementation, postoperative period, transfusion
Recommendations for best clinical practice
All patients who have undergone major surgery (defined as blood loss > 500 ml or lasting > 2 h) and who had pre‐operative anaemia or moderate‐to‐severe blood loss during surgery must be screened for anaemia after surgery.
During recovery from uncomplicated major surgery, haemoglobin concentrations should be monitored, either by standard laboratory or point‐of‐care testing, on a regular daily basis, at least until the third postoperative day, to detect anaemia (haemoglobin < 130 g.l−1 for men, < 120 g.l−1 for women).
Postoperatively, iron deficiency should be defined by ferritin concentration < 100 μg.l−1, ferritin < 100–300 μg.l−1 and transferrin saturation < 20%, or reticulocyte haemoglobin content < 28 pg. High blood loss during surgery may also indicate the need for iron replacement in anaemic patients.
In the postoperative period, when the administration of iron is necessary, early intravenous (i.v.) iron therapy is recommended, after considering contraindications. Where possible, it should be administered using a single high‐dose preparation for the repletion of iron stores.
For non‐cancer patients with severe postoperative anaemia and inflammation‐induced blunted erythropoiesis, or those declining blood transfusion, we suggest considering additional treatment with an erythropoiesis‐stimulating agent.
If patient blood management measures did not prevent the development of severe postoperative anaemia, the adoption of a restrictive transfusion threshold (haemoglobin level: 70–80 g.l−1, depending on patient comorbidities) is recommended in most adult, clinically stable hospitalised patients.
We recommend establishing a patient blood management expert group in every hospital.
Why was this consensus statement developed?
The concept ‘patient blood management’ (PBM) is defined as “the timely application of evidence‐based medical and surgical concepts designed to manage anaemia, optimise haemostasis and minimise blood loss in order to improve patient outcomes after surgery”’ 1. Patient blood management has been shown to reduce transfusion, healthcare costs and morbidity and mortality 2. Treatment of pre‐operative anaemia and isolated iron deficiency are crucial measures for PBM 3, 4. However, detection and early treatment of pre‐operative anaemia and iron deficiency is an accepted logistical challenge and, as a consequence, some patients may undergo surgery without the chance to address their anaemia 3. In addition, there has been increased emphasis on accelerated discharge after surgery and the use of restrictive transfusion thresholds in order to improve outcomes and reduce transfusion requirements, which may have led to overlooking potential opportunities to optimise anaemic patients and improve their functional recovery 5, 6. Therefore, an additional focus on the early detection and treatment of postoperative iron deficiency and anaemia is a novel and complementary measure within the concept of PBM; this allows the attending physician to target patients who lost significant red cell mass during surgery and may require specific attention postoperatively or following discharge 7.
How does this consensus statement differ from other available statements and/or guidelines?
There are a number of statements and guidelines from professional associations recommending a systematic approach to this problem for the management of pre‐operative anaemia 1, 7, 8, 9, 10, 11, 12, 13, 14, 15. Most of these guidelines also recommend the use of a restrictive transfusion threshold for treating acute postoperative anaemia, but recommendations for pharmacological management of anaemia are scarce, or even absent 1, 7, 8, 9, 10, 11, 12, 13, 14, 15. We aimed to update and utilise these few current recommendations to provide a working practice document, based on scientific evidence and the clinical experience of an expert panel, on ‘how to’ feasibly introduce these postoperative anaemia guidelines into clinical practice. Our goal was to provide pragmatic, clear and easy‐to‐follow clinical guidance for the diagnosis and treatment of postoperative anaemia and iron deficiency in order to improve patient recovery, reduce the need for blood transfusion and improve functional outcomes in a cost effective manner. Our recommendations are intended for non‐actively bleeding adult patients in whom all the principles of PBM have been implemented pre‐ and intra‐operatively for the prevention of postoperative iron deficiency and anaemia.
Definition, prevalence and pathophysiology of postoperative anaemia
Postoperative anaemia may be present in up to 80–90% of patients undergoing major surgery, although this prevalence varies widely according to different definitions 16, 17. Anaemia is defined by the World Health Organization (WHO) as a haemoglobin concentration < 130 g.l−1 for men, < 120 g.l−1 for non‐pregnant women and < 110 g.l−1 for pregnant women 18. Although debated 19, and since these definitions are widely accepted, they may be applied to postoperative patients. However, we previously pointed out that the WHO criteria for the definition of anaemia may not be reliable for the classification of non‐pregnant women undergoing surgical procedures with expected moderate‐to‐high blood loss. Women have lower circulating blood volumes and reduced red cell mass when compared with men, but the same procedures performed in either sex often result in comparable volumes of blood loss, resulting in higher transfusion rates in women 4. Therefore, pre‐operative anaemia in non‐pregnant women should be defined, as for men, as a haemoglobin concentration < 130 g.l−1.
According to the WHO, postoperative anaemia could be classified as mild (haemoglobin 110–119 g.l−1 in women and 110–129 g.l−1in men, respectively (110–119/129 g.l−1)), moderate (haemoglobin 80–109 g.l−1) or severe (haemoglobin < 80 g.l−1) 18.
Although multifactorial in origin, pre‐operative anaemia, peri‐operative blood loss (surgical bleeding, coagulopathy, phlebotomies, etc) and postoperative blunted erythropoiesis are the main contributing factors to postoperative anaemia after major surgery. Haemodilution due to excessive fluid administration, which may cause ‘dilutional’ anaemia or aggravate pre‐existing anaemia, and other nutritional deficiencies (e.g. vitamin B12, folic acid) and pharmacological interactions are also contributing factors 20.
Low pre‐operative haemoglobin, female sex and smaller body surface area have been identified as risk factors for the development of postoperative anaemia and increased transfusion needs 21. Additionally, in the general population, the prevalence of anaemia increases with age; older persons are more likely to undergo major surgery and to present with comorbidities, thus increasing the risk of postoperative anaemia, and reducing its tolerability 22, 23.
The end of the surgical procedure does not always signify the end of blood loss. Ongoing postoperative blood loss can continue through drains or into traumatised tissue, or due to repeated phlebotomy during prolonged postoperative hospitalisation. As such, peri‐operative blood loss may result in acute or late postoperative anaemia, especially in patients with the above‐mentioned risks factors. To avoid the detrimental effects of acute anaemia, packed red blood cells are usually transfused as a default measure 20. However, the use of restrictive transfusion thresholds, as emphasised in the third pillar of PBM, also contributes to a higher prevalence of moderate‐to‐severe anaemia on discharge from hospital (haemoglobin concentration < 100 g.l−1), unless pro‐active measures are implemented 20.
Anaemia in the postoperative period, as well as in critical illness, may be aggravated by reduced erythropoietin production and secretion due to inflammatory mediators; blunted bone marrow response to erythropoietin; and decreased iron availability due to down‐regulation of intestinal absorption and impaired mobilisation of iron from body stores 24, 25. Inflammatory cytokines stimulate the secretion of hepcidin, a hormone that targets ferroportin, the only known cellular exporter of iron. This induces the internalisation and degradation of ferroportin, thereby largely inhibiting intestinal iron absorption and greatly reducing iron release from body stores (iron sequestration) 26.
What are the unmet medical needs of postoperative anaemia?
The concerns surrounding postoperative anaemia relate to its potential impact on recovery, rehabilitation, hospital re‐admission or re‐operation, and patient well‐being. Reducing allogeneic blood transfusion improves long‐term outcome and survival 27. However, restrictive transfusion protocols have led to patients being discharged with lower haemoglobin levels than before. With the current paucity of data, it remains unclear whether a lower discharge haemoglobin level may allow optimal functional recovery and quality of life 28, 29, 30, 31, 32, 33, 34. There has been limited research on the consequences of postoperative anaemia in the recovery phase from surgery, with only a small number of studies after cardiac and hip and knee surgery, which demonstrated the association between postoperative anaemia and adverse outcomes such as prolonged recovery, increased mortality and likelihood of re‐admission 31, 32, 33. Postoperative anaemia may also potentially be associated with early postoperative myocardial infarction 34. Correction of postoperative anaemia, as suggested in this consensus statement, is intended to prevent such side‐effects, but studies are urgently needed to prove this.
Diagnosis of postoperative anaemia
When and how to measure haemoglobin concentration?
Measurement of haemoglobin concentration is a routine procedure in postoperative care. Duration of testing for postoperative anaemia depends on the peri‐operative bleeding risk associated with the surgical intervention and patient‐dependent factors. In most cases of uncomplicated recovery from surgery, a nadir in haemoglobin concentration can be observed within the first 3–4 days after surgery. In patients with major complications following major surgery, however, prolonged hospitalisation and exposure to low haemoglobin levels increase the duration of monitoring required.
Usually, blood gas analysis, capillary sampling (e.g. HemoCue, HemoCue AB, Ängelholm, Sweden) or near‐infrared spectroscopy (e.g. Radical‐67, Masimo Corporation, Irvine, CA, USA) are performed as a point‐of‐care assessment, whereas the full blood count is tested in the central laboratory. The use of non‐invasive continuous haemoglobin monitoring devices instead of phlebotomy may reduce blood loss, pain and discomfort for the patient, but concerns about precision limit routine clinical use. Although the debate focuses on accuracy of a single check 35, the reliability of non‐invasive haemoglobin monitoring devices for dynamic changes over time may permit detection of occult bleeding and response to therapy 36.
What are the confounding factors?
In the setting of postoperative care, a number of confounding factors may impact on accurate haemoglobin measurement. Volume overload and haemodilution after major surgery are potential causes for low haemoglobin levels, despite normal and stable red cell mass. Therefore, the diagnosis of anaemia based on simple haemoglobin concentration may be misleading, and is confounded by plasma volume derangements, resulting in significant overdiagnosis 37. Potential volume overload should be taken into account, and may improve after diuresis.
Similar conditions may be present in the peri‐operative setting where prevention of intravascular volume deficit is a cornerstone of peri‐operative management. Here, intravascular volume and fluid therapy are fundamental whenever fasting is indicated for medical reasons; in the event of high‐fluid turnover rates during major surgery, or in cases of reduced enteral resorption due to sustained vomiting, severe diarrhoea or gastro‐intestinal dysfunction following circulatory shock. The primary aim of intravenous fluid therapy (crystalloid and colloid solutions) is the restoration of plasma and blood volume to ensure appropriate cardiac output and tissue perfusion.
Unfortunately, appropriate assessment of volume status is complex. The diagnosis or quantification of moderate‐to‐severe volume deficit and volume responsiveness remains difficult, and may be attempted using laboratory variables (e.g. lactate, base excess), positional manoeuvres (passive lifting of legs), new monitoring devices (measuring pulse variability and stroke volume indexes or other preload variables) or echocardiography. Recent guidelines highlight the importance of avoiding hypervolaemia 7. During postoperative recovery, redistribution and excretion of fluids may lead to rapid recovery of haemodilution‐induced low haemoglobin concentrations.
When and how to measure postoperative iron deficiency?
Although underlying causes of postoperative anaemia are multifactorial, iron deficiency is often present. Although pre‐operative iron deficiency can be diagnosed on the basis of low ferritin concentrations 4, diagnosis of postoperative iron deficiency is more difficult, as ferritin levels may be elevated as part of the acute phase inflammatory response after surgery 38. Thus, patients undergoing major surgery with a high risk of developing moderate‐to‐severe postoperative anaemia should have their haemoglobin and iron status checked on the day of surgery, if it has not been already performed in the pre‐operative assessment. This may also apply to patients with ongoing bleeding and anaemia (e.g. colorectal cancer) that have been treated in the pre‐operative period. As ferritin levels will not be elevated by inflammation immediately after surgery, a postoperative ferritin concentration < 100 μg.l−1 within 24 h after surgery indicates insufficient iron stores to support erythropoiesis with the potential for significant falls in postoperative haemoglobin 8.
Further markers of postoperative iron deficiency are transferrin saturation < 20% with ferritin concentrations 100–300 μg.l−1, or reticulocyte haemoglobin content < 28 pg. These values and parameters may signal the need for intervention in anaemic patients 38, 39, 40, 41.
When should postoperative anaemia be treated?
There are limited supporting data regarding appropriate timing for management of anaemia after surgery, including red cell transfusion. Treatment choice depends on severity and type of anaemia, type of surgery, patient comorbidities and the presence of any surgical complications.
Iron supplementation should be considered in patients with iron deficiency or significant reduction in postoperative haemoglobin, starting early in the postoperative recovery phase where there are no major complications 39, 42, 43, 44, 45, 46. It is important to note that there are no studies identifying the best time to start postoperative iron supplementation.
For non‐cancer patients with severe postoperative anaemia and inflammation‐induced blunted erythropoiesis or those declining blood transfusion, additional treatment with an erythropoiesis‐stimulating agent (e.g. recombinant human erythropoietin [rHuEPO]) may be considered. However, we are aware that for patients without a previous indication this is an off‐label use of rHuEPO, and recommendations vary across countries 10, 12, 15.
Red cell transfusion should be restricted to patients with severe anaemia (haemoglobin < 70–80 g.l−1) and clinical signs and symptoms 7, 10, 11, 12, 47, 48, 49. Red cell transfusion should be considered in patients with active bleeding and in those severely anaemic once bleeding has been stopped 7, 10, 11, 12, 47, 48, 49. However, more research is required on specific transfusion thresholds in specific high‐risk patients.
How should patients be treated postoperatively?
Pharmacological optimisation of postoperative haemoglobin and erythropoiesis should allow correction of iron deficiency and rapid recovery from postoperative anaemia, which may lead to improved postoperative outcomes and improved quality of life. It may also result in a reduction of patient exposure to red cell transfusion and its related risk and complications, thus contributing further to improving surgical outcome and patient safety.
Iron therapy: oral vs. i.v. iron?
The National Institute for Health and Care Excellence in the UK (NICE) recommends offering oral iron after surgery to patients with iron deficiency anaemia 12. However, in the postoperative period, oral iron is often not tolerated or absorbed and has several limitations including frequent gastro‐intestinal side‐effects and, as a consequence, poor treatment adherence. Additionally, the inflammatory response induced by surgery stimulates hepcidin synthesis and release, which in turn inhibits intestinal iron absorption, making oral iron therapy largely ineffective 10, 13, 50. Various randomised placebo‐controlled trials (RCT) in orthopaedic and cardiac surgery patients have demonstrated that oral iron therapy was not better than placebo in correcting postoperative anaemia and reducing transfusion requirements 51, 52, 53, 54, 55, 56, 57.
On the other hand, NICE recommends considering i.v. iron after surgery for patients who have iron deficiency anaemia and cannot tolerate or absorb oral iron, or are unable to adhere to oral iron treatment, as well as for those who are diagnosed with functional iron deficiency 12. Thus, patients presenting with postoperative iron deficiency and/or moderate‐to‐severe postoperative anaemia (haemoglobin < 100 g.l−1) may benefit from i.v. iron supplementation, which has proven to be more effective than oral iron in a number of surgical settings (Supporting Information online, Appendix S1) 39, 42, 43, 44, 45, 58, 59, 60, 61, 62, 63. Most recent RCTs have shown improved outcomes following high‐dose postoperative i.v. iron (e.g. 1000 mg) as demonstrated by increased haemoglobin and/or reduction in transfusion requirements, and no i.v. iron‐related serious adverse events were reported (Supporting Information, Appendix S1) 39, 42, 44, 45. Importantly, in orthopaedic surgical patients with postoperative haemoglobin < 100 g.l−1 and/or uncorrected pre‐operative iron deficiency (ferritin < 100 μg.l−1), a greater rise in haemoglobin and better scores for ‘usual activities’ were observed with high‐dose i.v. iron compared with oral iron 42.
Similarly, compared with oral iron, the use of i.v. iron for treating postpartum anaemia has been shown to result in greater and faster haemoglobin increases, improved replenishment of iron stores, a lower incidence of adverse side‐effects and greater improvement in quality of life 15, 46.
Therefore, should postoperative iron therapy be indicated, i.v. formulations are recommended. This is in line with recent management guidelines in surgical patients experiencing severe bleeding (GRADE 2C for i.v. iron preparations postoperatively) 7. When calculating the total iron dose, it is important to take into account the pre‐operative haemoglobin level and iron status, the magnitude of postoperative haemoglobin drop and whether patients have received postoperative red cell transfusion (Fig. 1). The use of i.v. iron formulations which allow rapid (15–60 min) infusion of high doses of iron (1000 mg or more) offers added convenience to both physicians and patients and should be preferred, despite their higher cost (Table 1) 40, 64.
Figure 1.
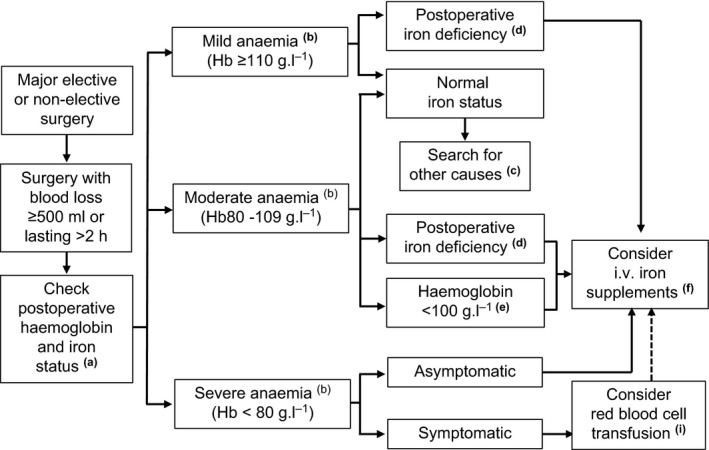
Postoperative anaemia management. (a) Whenever possible, assess iron status within 24 h postoperatively, if it has not been already performed in the pre‐operative assessment. Monitor haemoglobin for 3–4 days postoperatively. (b) According to WHO classification. (c) Appropriate treatment should be considered. (d) Postoperative ferritin < 100 μg.l−1, ferritin < 300 μg.l−1 and transferrin saturation < 20% or reticulocyte haemoglobin content < 28 pg. (e) Due to pre‐operative anaemia or heavy surgical bleeding, irrespective of iron status. (f) Total iron deficiency = (target haemoglobin − actual haemoglobin) × weight (kg) × 0.24. Add another 10 mg.kg−1 for replenishing iron stores, especially in patients with pre‐operative iron deficiency. Consider adding recombinant human erythropoietin (40,000 IU) for patients with severe anaemia or declining transfusion. For i.v. iron dosing schedule, see Table 1. (i) Transfuse one red blood cell unit at the time, with post‐transfusion re‐assessment of further needs. Consider i.v. iron supplementation after transfusion, using post‐transfusion haemoglobin as actual haemoglobin for total iron deficiency calculation.
Table 1.
Dosing characteristics for intravenous iron formulations available in Europe
| Iron gluconatea | Iron Sucroseb | LMWIDc | Ferric carboxymaltosed | Iron isomaltosidee | |
|---|---|---|---|---|---|
| Brand name | Ferrlecit® | Venofer® | Cosmofer® | Ferinject® | Monofer® Monoferro® |
| Maximal single dose (mg) | 125 | 200 (max 600 mg/week) | 20 mg/kg | 20 mg/kg (max 1000 mg) | 20 mg/kg |
| Suggested postoperative dosage: | |||||
| Dose (mg)/frequency (days) | 125/2 | 200/1–2 | 500–1000/7f | 500–1000/7 | 500–1500/7 |
| Infusion time (min) | 60 | 30 | ≥ 60 | ≥ 10–15 | ≥ 15–30 |
| Maximal total dose (mg) | 2000 | 2000 | 2000 | 2000 | 2000 |
Ferrlecit summary of product characteristics. http://www.products.sanofi-aventis.us/ferrlecit/ferrlecit.pdf (accessed 18/02/2018).
Venofer summary of product characteristics. http://www.luitpold.com/documents/22.pdf (accessed: 18/02/2018).
LMWID, low molecular weight iron dextran; Cosmofer summary of product characteristics. http://www.cosmofer.com/product/cosmofer-spc/cosmofer-spc.aspx. (accessed: 18/02/2018).
Ferinject summary of product characteristics. http://www.ferinject.co.uk/smpc/ (accessed: 18/02/2018).
Monofer summary of product characteristics. http://www.monofer.com/spc.aspx. (accessed: 18/02/2018).
Although it is not an approved dosing by European Medicines Agency, Auerbach et al. (Am J Hematol 2011; 86: 860) have not observed any serious adverse events in over 5000 administrations of LMWID at doses of 1000 mg in 250 ml of normal saline over 1 h.
What are the true risks and contraindications of i.v. iron?
Many clinicians and health authorities still consider that i.v. iron is strongly associated with major side‐effects such as anaphylaxis, infection or oxidative stress. However, these side‐effects appear not to be significant with the newer i.v. iron preparations, such as ferric carboxymaltose, iron isomaltoside and low molecular weight iron dextran 64, 65, 66.
After an extensive review of the data, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) concluded that “all i.v. iron medicines have a small risk of causing allergic reactions which can be life‐threatening if not treated promptly” 67. However, the reported incidence of potentially life‐threatening hypersensitivity reactions (< 1:250,000 administrations) is vastly overestimated; the pathophysiological mechanism is poorly understood, although a substantial proportion is thought to be mediated by complement activation, resulting in complement activation‐related pseudo allergy (CARPA) 68, 69. Minor infusion reactions due to ‘labile’ iron may occur, but are usually self‐limiting without intervention and should not be misinterpreted as acute hypersensitivity events 65, 70.
The CHMP's review also concluded that “the benefits of these medicines are greater than their risks, provided that adequate measures are taken to minimise the risk of allergic reactions” 67. To this end, i.v. iron preparations should only be given in an environment where resuscitation facilities are available, so that patients who develop an allergic reaction can be treated immediately, and patients should be closely observed for signs and symptoms of hypersensitivity reactions during and for at least 30 min following each injection of i.v. iron. Guidelines for the diagnosis and management of these reactions have been recently published 71. In addition, the CHMP's report contraindicates the use of i.v. in patients with hypersensitivity to the active substance or excipients; with serious hypersensitivity to other parenteral iron products; or in the first trimester of pregnancy 67.
Elemental iron is an essential growth factor for bacteria, with many species expressing iron transport proteins that compete with transferrin, and it has long been suggested that patients with iron overload are at increased risk of infection 72. However, data from meta‐analyses and large observational studies showed that peri‐operative i.v. iron did not increase postoperative infection or 30‐day mortality rates in surgical patients 64, 66. In contrast, red cell transfusion delivers haem and labile iron which readily supports bacterial growth 73. Nevertheless, in the absence of definitive clinical data, it would seem logical to refrain from i.v. iron administration in the setting of acute infection 8.
The available evidence relating i.v. iron administration to oxidative stress leading to atherogenesis and vascular remodelling, is sparse and indirect. It is mostly derived from retrospective observational studies addressing long‐term i.v. iron therapy 70, whereas in the postoperative period, very short‐term i.v. iron courses are administered (one or two large doses) 64. Thus, it does not seem to be of concern in the postoperative setting.
Should we treat iron deficiency without anaemia?
A normal haemoglobin level does not exclude iron deficiency. In fact, the WHO recognises that ‘mild’ anaemia (haemoglobin 110–119/129 g.l−1) is a misnomer, as iron deficiency is already advanced by the time anaemia is detected, and has consequences even when anaemia is not clinically apparent 18.
Non‐anaemic patients with reduced or absent iron stores may have symptoms such as fatigue or reduced exercise tolerance, as iron is required for optimal mitochondrial function, and is essential for respiration and energy production 40, 74. Current guidelines do not recommend routine iron screening in the absence of anaemia. However, the benefits of oral or i.v. iron replacement for non‐anaemic iron deficiency‐associated fatigue have been demonstrated in menstruating women, runners and blood donors 75, 76, 77, 78.
In congestive heart failure, a frequent comorbidity among surgical patients, non‐anaemic iron deficiency was independently associated with compromised physical performance and quality of life, and an increase in all‐cause and cardiovascular mortality; treatment of non‐anaemic iron deficiency with i.v. iron may improve functional status within four weeks, and reduces hospitalisations for cardiovascular reasons and mortality 79, 80. In addition, improvements are maintained after 24 and 52 weeks 79, 80, 81.
In observational studies of patients undergoing abdominal or cardiac surgery, pre‐operative non‐anaemic iron deficiency was associated with poor outcomes, including: increased rates of postoperative infection; transfusion; fatigue; and prolonged hospital stay 82, 83, 84. Although it is presently unknown whether pre‐operative correction of non‐anaemic iron deficiency may offset the excess of risk of postoperative complications, some guidelines recommend peri‐operative iron supplementation for patients with non‐anaemic iron deficiency 14, 85.
Secondary thrombocytosis can also be seen after major surgery, as platelets behave as an acute phase reactant. Iron deficiency has also been shown to induce secondary thrombocytosis in several clinical settings. Correction of iron deficiency usually lowers platelet count and platelet activation in patients with chronic kidney disease, cancer or inflammatory bowel disease‐associated secondary thrombocytosis, and may contribute to reduced risk of thrombo embolic events 86, 87, 88, 89.
Is there a role for erythropoiesis‐stimulating agents?
In patients without a previous indication, postoperative administration of rHuEPO is an off‐label use of this medicinal product. The effects of postoperative rHuEPO have been evaluated in case series, mostly in Jehova's Witnesses and in two RCTs, yielding inconclusive results due to selection bias 58 or premature interruption 59.
In women with moderate‐to‐severe postpartum anaemia, five RCTs evaluated the effects of iron sucrose (300–1600 mg) or iron sucrose plus rHuEPO (20,000–40,000 IU) on haemoglobin recovery and transfusion needs 15. A trend to faster haemoglobin increment was observed with rHuEPO plus i.v. iron compared with i.v. iron alone, but no significant differences in transfusion rates which were very low, were observed. The benefits seemed to be greatest in the rHuEPO‐treated sub‐group with elevated C‐reactive protein levels after Caesarean section 15.
Although it does not strictly refer to surgical patients, a recent meta‐analysis also found a reduction in mortality rates (risk ratio (RR) 0.63, 95%CI 0.49–0.79, p < 0.0001) in critically ill trauma patients who received rHuEPO (nine studies, 2607 patients), without increasing the risk of thromboembolic complications 90. In cardiac surgery patients, rHuEPO seems to exert neurological and renal protective effect 91, 92. The mechanisms underlying these non‐erythropoietic effects of rHuEPO need to be elucidated before recommending its use in patients without an approved indication.
Short‐term pre‐operative (1–4 days before operation) administration of rHuEPO to anaemic patients, with or without i.v. iron, has been shown to reduce postoperative transfusion in elective orthopaedic and cardiac surgery 93, 94. In hip fracture repair surgery, there are conflicting data. One RCT failed to show a reduction in red cell transfusion in patients receiving rHuEPO plus i.v. iron 95. However, they included patients with haemoglobin < 100 g.l−1 but did not study women with haemoglobin ≥ 120 g.l−1, and transfused fixed amounts of red cells according to pre‐defined transfusion thresholds (e.g. patients with haemoglobin ≤ 70 g.l−1 received three units of red cells, and those with haemoglobin 71–89 g.l−1 and severe symptoms received two units). In contrast, in an observational study of 196 anaemic hip fracture patients managed with peri‐operative i.v. iron and a restrictive transfusion protocol, administration of rHuEPO on admission was associated with reduced transfusion requirements and higher haemoglobin levels on discharge and at postoperative day 30 96. An analysis including 544 women with haemoglobin < 130 g.l−1 undergoing hip fracture repair showed that the blood‐sparing effect of this strategy was restricted to those presenting with haemoglobin concentrations between 120 and 130 g.l−1 (n = 305) 97.
Thus, in non‐cancer patients with severe postoperative anaemia and blunted erythropoiesis due to infection and/or inflammation, as well as in those who refuse blood transfusion, we suggest considering treatment with rHuEPO. However, as stated above, some guidelines do not support the off‐label use of this medicinal product 12.
Red blood cell transfusion: transfusion thresholds for whom and when?
Allogeneic red cell transfusion is associated with a significant increase in peri‐operative morbidity and mortality 2, 98, 99, 100. In addition, there is a worldwide shortage of blood, with substantial associated costs to the manufacturer and health systems 101. Moreover, red cell transfusion risks infectious, immunological, haemolytic, non‐haemolytic adverse reactions, cardiac and pulmonary complications 2, 98. Despite successful implementation of PBM programmes, red cell transfusion is still widely used as a default treatment for the majority of patients with acute postoperative anaemia 102.
Historically, the standard for red cell transfusion was a liberal transfusion threshold, namely haemoglobin level < 100 g.l−1 (or haematocrit < 30%). This arbitrary transfusion threshold has been gradually lowered towards haemoglobin 70–80 g.l−1, according to data derived from a number of RCTs evaluating the effect on patient outcomes of restrictive vs. more liberal red cell transfusion strategies in a variety of clinical settings. When subjected to pooled analysis in several systematic reviews and meta‐analyses (Supporting Information online, Appendix S2) 103, 104, 105, 106, 107, 108, data from these RCTs show that, in terms of morbidity and mortality, a restrictive red cell transfusion strategy is equivalent to or more beneficial than a liberal strategy 101, 109.
In addition, evidence‐based guidelines have translated the results of RCTs and meta‐analyses into clinical practice 7, 10, 11, 12, 47, 48, 49. One of the most recently published guidelines on red cell transfusion thresholds recommends a restrictive red cell transfusion threshold (haemoglobin < 70 g.l−1) for hospitalised adult patients who are haemodynamically stable, including critically ill patients 49. However, a transfusion threshold of at least 80 g.l−1 is suggested for patients undergoing orthopaedic surgery, cardiac or oncological surgery, and those with pre‐existing cardiovascular disease 12, 49, 110, 111. Nevertheless, transfusion of red cells for higher haemoglobin levels should be evaluated case by case, considering acute ongoing blood loss, comorbidities and signs of organ ischaemia or symptoms indicative of hypoxia. In any case, published guidelines agree that red cell transfusion is not beneficial when haemoglobin is > 100 g.l−1 7, 10, 11, 12, 47, 48, 49, 112. Confounding factors on haemoglobin levels have to be considered, as discussed above.
Cost assessment implications
There are very few studies on the cost implications of the management of postoperative anaemia. Most such studies have evaluated pre‐operative interventions, and since the pre‐operative haemoglobin value is strongly associated with the postoperative haemoglobin, interventions aimed to improve pre‐operative anaemia also influence postoperative well‐being and its related costs 113.
Costs of anaemia management and PBM may vary from institution to institution and depend on the extent to which different aspects of PBM have been implemented. The following costs per patient were recently calculated in a single German University Hospital: diagnosis of anaemia €49–124; treatment of anaemia (including iron deficiency anaemia and megaloblastic anaemia) €13–128 114.
Similarly, data from > 600,000 patients (2008–2014) who were enrolled in a PBM, peri‐operative management programme targeting anaemia and iron deficiency, demonstrated a risk‐adjusted reduction in postoperative red cell transfusion, infection and mortality rates, shortened length of hospital stay and an estimated US$ 78–97 million in activity‐based savings 2.
A recent RCT that investigated the use of i.v. iron vs. standard care in the management of postoperative anaemia did not include a formal cost analysis; however, transfusion rates, infections and length of hospital stay were decreased, suggesting cost effectiveness 39. A retrospective, matched cohort reported on costs of postoperative i.v. iron therapy in total lower limb arthroplasty, and found that use of iron formulations was cost neutral (−25.5 to 62.1 €/patient for iron sucrose and −51.1 to 64.4 €/patient for ferric carboxymaltose) compared with red cell transfusion 43. In contrast, although NICE guidelines still recommend oral iron in the pre‐operative and postoperative settings 12, available evidence on the lack of efficacy of oral iron in the postoperative period suggests that cost analysis of this intervention is not meaningful 51, 52, 53, 54, 55, 56, 57.
Suggestions for further research
During the writing of this consensus statement, it became apparent that there are areas of postoperative anaemia management for which further research is required:
Monitoring. It is really important to emphasise the need for detailed anaemia studies following discharge with periodic monitoring of haemoglobin and iron parameters in relation to functional recovery.
Interventions. More data and clinical trials are required to firmly establish the impact of postoperative anaemia management strategies (i.v. iron vs. oral iron, rHuEPO, red cell transfusion and nutritional support) on functional recovery and quality of life; on end‐points in addition to laboratory end‐points, such as haemoglobin increase; and interventional end‐points, such as reduction or avoidance of red cell transfusion. Further research is required to assess the effects of correction of iron deficiency, with or without anaemia, on platelet counts, platelet activation and thromboembolic events, especially in the elderly. Timing of interventions in the postoperative course needs to be addressed in future trials. Dosing of anti‐anaemia treatment and combination of means of treatment must be systematically investigated.
Patients. It is also necessary to define which patient groups are most likely to benefit from such treatments.
Mechanisms of action. The mechanisms underlying non‐erythropoietic effects of rHuEPO, such as neurological and renal protective effects, and iron need to be elucidated.
Cost. The cost effectiveness of postoperative anaemia correction must be investigated at the different time‐points for its administration, using formal cost evaluation. Until such data are available, the predominant signal from available publications and peer reviewed recommendation support the concept of postoperative anaemia screening, diagnosis and appropriate treatment.
Supporting information
Appendix S1. Studies evaluating the effect of postoperative intravenous iron administration (11 studies, 1855 patients).
Appendix S2. Recent systematic reviews and meta‐analyses comparing restrictive versus liberal packed red cell transfusion strategy.
Acknowledgements
This consensus statement was developed after some of the authors attended a workshop on patient blood management sponsored by Pharmacosmos. At the end of the workshop, the authors met voluntarily at the instigation of the senior author (AK), without any involvement by the company in the meeting. EB, AAK, AS and SGR were later invited to join the panel. Once the full panel was constituted, the authors discussed and agreed with the title of the consensus statement, the different subject areas to be covered and which of the authors would write each segment (two per each one). Following completion of the first draft, the recommendations were selected using a Delphic process, and all authors agreed on the final version of the manuscript. Pharmacosmos had no involvement in the decision to write the consensus statement, the inclusion of the authors, or the drafting or final version of this consensus statement.
MM, AB, AGA, HK, SK, RRB, TR, CS‐O and AK have received funding for research and/or honoraria from Pharmacosmos. In addition, MM has received honoraria from Vifor Pharma, Pharmanutra, Zambon and Celgene. AGA's research department has received grant support from Syner‐Med, UK and Vifor Pharma, Switzerland; AGA has received honoraria or travel support for consulting or lecturing from Ethicon Endosurgery, Johnson and Johnson, Olympus and Vifor Pharma. EB has received funding and/or honoraria from Vifor Pharma. AB's department has received grants from Vifor Pharma. SK has received honoraria for lecturing and funding for the academic, non‐profit educational e‐learning platform http://www.peri-operative bleeding.org, from companies involved in peri‐operative coagulation monitoring (Roche, TEM International). PM has received research grants from B. Braun, CSL Behring, Fresenius Kabi and Vifor Pharma for the implementation of Frankfurt's Patient Blood Management Program in four German university hospitals, and honoraria from B.Braun, CSL Behring, Ferring, Pharmacosmos and Vifor Pharma. AS received research grants from CSL Behring, Gauss Surgical, Masimo and HbO2 Therapeutics; he has also received honoraria from CSL Behring, Masimo and Merck, and acted as a consultant for CSL Behring, Gauss Surgical, Masimo Corporation and Vifor Pharma. DS's academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Ministry of Health (Gesundheitsdirektion) of the Canton of Zurich, Switzerland for Highly Specialized Medicine, the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland and Vifor SA, Villarssur‐ Glane, Switzerland. DS was the chair of the ABC Faculty and is the co‐chair of the ABC‐Trauma Faculty, which both are managed by Physicians World Europe and sponsored by unrestricted educational grants from Novo Nordisk, CSL Behring and LFB Biomedicaments. DS has received honoraria or travel support for consulting or lecturing from the following companies: Baxter; Bayer; B. Braun; Boehringer; Bristol‐Myers‐Squibb; CSL Behring; Curacyte; Daiichi Sankyo; Ethicon Biosurgery; Fresenius; Galenica; LFB Biomedicaments,; Merck Sharp & Dohme; Octapharma; PAION; Pharmacosmos; Photonics Healthcare; Ratiopharm; Roche Diagnostics International Ltd; Roche Pharma; Sarstedt; Schering‐Plough International; Tem International; Verum Diagnostica and Vifor Pharma. AK or his institution has received educational grant funding or honoraria from Pharamcosmos, Vifor Pharma, Haemonetics, Fisher and Paykel and Masimo. AK is the Editor‐in‐Chief of Anaesthesia and this manuscript has undergone an additional external review as a result. AAK, GML and SGR have nothing to declare.
This article is accompanied by an editorial by Hatton and Smith, Anaesthesia 2018; 73: 1313–1316.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. SABM . Anemia prevention and management program implementation guide. 2015. https://www.sabm.org/sites/default/files/anemia_prevention_management_program_implementation_guide.pdf (accessed 11/10/2017).
- 2. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health‐system‐wide patient blood management program: a retrospective observational study in four major adult tertiary‐care hospitals. Transfusion 2017; 57: 1347–58. [DOI] [PubMed] [Google Scholar]
- 3. Muñoz M, Gómez‐Ramírez S, Kozek‐Langeneker S, et al. ‘Fit to fly’: overcoming barriers to preoperative haemoglobin optimization in surgical patients. British Journal of Anaesthesia 2015; 115: 15–24. [DOI] [PubMed] [Google Scholar]
- 4. Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri‐operative management of anaemia and iron deficiency. Anaesthesia 2017; 72: 233–47. [DOI] [PubMed] [Google Scholar]
- 5. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfusion Medicine Reviews 2017; 31: 62–71. [DOI] [PubMed] [Google Scholar]
- 6. Spahn DR. Patient blood management: the new standard. Transfusion 2017; 57: 1325–7. [DOI] [PubMed] [Google Scholar]
- 7. Kozek‐Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. First update 2016. European Journal of Anaesthesiology 2017; 43: 332–95. [DOI] [PubMed] [Google Scholar]
- 8. Beris P, Muñoz M, García‐Erce JA, Thomas D, Maniatis A, van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. British Journal of Anaesthesia 2008; 100: 599–604. [DOI] [PubMed] [Google Scholar]
- 9. National Blood Authority . Patient blood management guidelines: Module 2 ‐ Perioperative. https://www.blood.gov.au/pbm-module-2 (accessed 11/10/2017).
- 10. Leal‐Noval SR, Muñoz M, Asuero M, et al. Spanish expert panel on alternatives to allogeneic blood transfusion. Spanish consensus statement on alternatives to allogeneic blood transfusion: the 2013 update of the ‘Seville Document’. Blood Transfusion 2013; 11: 585–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Society of Anesthesiologists Task Force on Perioperative Blood Management . Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015; 122: 241–75. [DOI] [PubMed] [Google Scholar]
- 12. NICE guideline [NG24] . Blood transfusion. https://www.nice.org.uk/guidance/ng24/chapter/Recommendations#alternatives-to-blood-transfusion-for-patients-having-surgery-2 (accessed 27/06/2017).
- 13. Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfusion 2016; 14: 23–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotzé A, Harris A, Baker C, et al. British committee for standards in haematology guidelines on the identification and management of pre‐operative anaemia. British Journal of Haematology 2015; 171: 322–31. [DOI] [PubMed] [Google Scholar]
- 15. Muñoz M, Peña‐Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the post‐partum period: NATA consensus statement. Transfusion Medicine 2017; 28: 22–39. [DOI] [PubMed] [Google Scholar]
- 16. Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. American Journal of Medicine 2004; 116: 58S–69S. [DOI] [PubMed] [Google Scholar]
- 17. Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010; 113: 482–95. [DOI] [PubMed] [Google Scholar]
- 18. WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO/NMH/NHD/MNM/11.1. http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed 28/04/2017).
- 19. Butcher A, Richards T, Stanworth SJ, Klein AA. Diagnostic criteria for pre‐operative anaemia–time to end sex discrimination. Anaesthesia 2017; 72: 811–814. [DOI] [PubMed] [Google Scholar]
- 20. Muñoz M, Shander A, Rijhwani T, Dyga R, Waters JH. Postoperative blood management strategies In: Frank SM, Waters JH, eds. Patient blood management: multidisciplinary approaches to optimizing care. Bethesda, MD: AABB Press, 2016: 233–58. [Google Scholar]
- 21. Hayn D, Kreiner K, Kastner P, et al. Data driven methods for predicting blood transfusion needs in elective surgery. Studies in Health Technology and Informatics 2016; 223: 9–16. [PubMed] [Google Scholar]
- 22. Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Current Opinion in Hematology 2005; 12: 123–8. [DOI] [PubMed] [Google Scholar]
- 23. Gómez‐Ramírez S, Remacha‐Sevilla ÁF, Muñoz‐Gómez M. Anaemia in the elderly. Medicina Clinica (Barcelona) 2017; 149: 496–503. [DOI] [PubMed] [Google Scholar]
- 24. van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Critical Care Medicine 2000; 28: 2773–8. [DOI] [PubMed] [Google Scholar]
- 25. van Iperen CE, Kraaijenhagen RJ, Biesma DH, Beguin Y, Marx JJ, van de Wiel A. Iron metabolism and erythropoiesis after surgery. British Journal of Surgery 1998; 85: 41–5. [DOI] [PubMed] [Google Scholar]
- 26. Muñoz M, García‐Erce JA, Remacha AF. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. Journal of Clinical Pathology 2011; 64: 287–296. [DOI] [PubMed] [Google Scholar]
- 27. Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion 2014; 54: 2753–9. [DOI] [PubMed] [Google Scholar]
- 28. So‐Osman C1, Nelissen R, Brand R, Brand A, Stiggelbout AM. Postoperative anemia after joint replacement surgery is not related to quality of life during the first two weeks postoperatively. Transfusion 2011; 51: 71–81. [DOI] [PubMed] [Google Scholar]
- 29. Vuille‐Lessard E, Boudreault D, Girard F, Ruel M, Chagnon M, Hardy JF. Postoperative anemia does not impede functional outcome and quality of life early after hip and knee arthroplasties. Transfusion 2012; 52: 261–70. [DOI] [PubMed] [Google Scholar]
- 30. Jans O, Bandholm T, Kurbegovic S, et al. Postoperative anemia and early functional outcomes after fast‐track hip arthroplasty: a prospective cohort study. Transfusion 2016; 56: 917–25. [DOI] [PubMed] [Google Scholar]
- 31. Koch CG, Li L, Sun Z, et al. Magnitude of anemia at discharge increases 30‐day hospital readmissions. Journal of Patient Safety 2017; 13: 202–6. [DOI] [PubMed] [Google Scholar]
- 32. Choi YJ, Kim SO, Sim JH, Hahm KD. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesthesia and Analgesia 2016; 122: 1923–8. [DOI] [PubMed] [Google Scholar]
- 33. Pitter FT, Jorgensen CC, Lindberg‐Larsen M, Kehlet H. Postoperative morbidity and discharge destinations after fast‐track hip and knee arthroplasty in patients older than 85 years. Anesthesia and Analgesia 2016; 122: 1807–15. [DOI] [PubMed] [Google Scholar]
- 34. Jorgensen CC, Kehlet H. Early thromboembolic events </=1 week after fast‐track total hip and knee arthroplasty. Thrombosis Research 2016; 138: 37–42. [DOI] [PubMed] [Google Scholar]
- 35. Gayat E, Aulagnier J, Matthieu E, Boisson M, Fischler M. Non‐invasive measurement of hemoglobin: assessment of two different point‐of‐care technologies. PLoS ONE 2012; 7: e30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barker SJ, Shander A, Ramsay MA. Continuous noninvasive hemoglobin monitoring: a measured response to a critical review. Anesthesia and Analgesia 2016; 122: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noumi BL, Teruya S, Salomon S, Helmke S, Maurer MS. Blood volume measurements in patients with heart failure and a preserved ejection fraction: implications for diagnosing anemia. Congestive Heart Failure 2011; 17: 14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muñoz M, García‐Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. Journal of Clinical Pathology 2011; 64: 281–6. [DOI] [PubMed] [Google Scholar]
- 39. Khalafallah AA, Yan C, Al‐Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open‐label, randomised controlled trial. Lancet Haematology 2016; 3: e415–25. [DOI] [PubMed] [Google Scholar]
- 40. Cappellini MD, Comin‐Colet J, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis and management. American Journal of Hematology 2017; 92: 1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas L, Thomas C. Detection of iron restriction in anaemic and non‐anaemic patients: new diagnostic approaches. European Journal of Haematology 2017; 99: 262–8. [DOI] [PubMed] [Google Scholar]
- 42. Bisbe E, Moltó L, Arroyo R, Muniesa JM, Tejero M. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. British Journal of Anaesthesia 2014; 113: 402–9. [DOI] [PubMed] [Google Scholar]
- 43. Muñoz M, Gómez‐Ramírez S, Martín‐Montañez E, Naveira E, Seara J, Pavía J. Cost of post‐operative intravenous iron therapy in total lower limb arthroplasty: a retrospective, matched cohort study. Blood Transfusion 2014; 12: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non‐anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double‐blind placebo‐controlled clinical trial (the PROTECT trial). Vox Sanguinis 2015; 109: 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim YW, Bae JM, Park YK, et al. and study group . Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemiafollowing gastrectomy: the FAIRY randomized clinical trial. Journal of the American Medical Association 2017; 317: 2097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holm C, Thomsen LL, Norgaard A, Langhoff‐Roos J. Single‐dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: a randomized controlled trial. Vox Sanguinis 2017; 112: 219–28. [DOI] [PubMed] [Google Scholar]
- 47. Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P. Rossetti G; Italian Society of Transfusion Medicine and Immunohaematology Working Party. Recommendations for the transfusion management of patients in the peri‐operative period. III. The post‐operative period. Blood Transfusion 2011; 9: 320–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klein AA, Arnold P, Bingham RM, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia 2016; 71: 829–42. [DOI] [PubMed] [Google Scholar]
- 49. Carson JL, Guyatt G, Heddle NM, et al. Red blood cell transfusion: 2016 clinical practice guideline from the AABB. Annals of Internal Medicine 2016; 316: 2025–35. [Google Scholar]
- 50. Vaglio S, Gentili S, Marano G, et al. The Italian regulatory guidelines for the implementation of patient blood management. Blood Transfusion 2017; 15: 325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crosby L, Palarski VA, Cottington E, Cmolik B. Iron supplementation for acute blood loss anemia after coronary artery bypass surgery: a randomized, placebo‐controlled study. Heart and Lung 1994; 23: 493–9. [PubMed] [Google Scholar]
- 52. Sutton PM, Cresswell T, Livesey JP, Speed K, Bagga T. Treatment of anaemia after joint replacement. A double‐blind, randomised, controlled trial of ferrous sulphate versus placebo. Journal of Bone and Joint Surgery Britain 2004; 86: 31–3. [PubMed] [Google Scholar]
- 53. Weatherall M, Maling TJ. Oral iron therapy for anaemia after orthopaedic surgery: randomized clinical trial. ANZ Journal of Surgery 2004; 74: 1049–51. [DOI] [PubMed] [Google Scholar]
- 54. Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. Journal of Bone and Joint Surgery Britain 2005; 87: 213–7. [DOI] [PubMed] [Google Scholar]
- 55. Zauber NP, Zauber AG, Gordon FJ, et al. Iron supplementation after femoral head replacement for patients with normal iron stores. Journal of the American Medical Association 1992; 267: 525–7. [PubMed] [Google Scholar]
- 56. Prasad N, Rajamani V, Hullin D, Murray JM. Post‐operative anaemia in femoral neck fracture patients: does it need treatment? A single blinded prospective randomised controlled trial. Injury 2009; 40: 1073–6. [DOI] [PubMed] [Google Scholar]
- 57. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. Journal of Bone and Joint Surgery America 2010; 92: 265–9. [DOI] [PubMed] [Google Scholar]
- 58. Madi‐Jebara SN, Sleilaty GS, Achouh PE, et al. Postoperative intravenous iron used alone or in combination with low‐dose erythropoietin is not effective for correction of anemia after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004; 18: 59–63. [DOI] [PubMed] [Google Scholar]
- 59. Karkouti K, McCluskey SA, Ghannam M, Salpeter MJ, Quirt I, Yau TM. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Canadian Journal of Anesthesia 2006; 53: 11–9. [DOI] [PubMed] [Google Scholar]
- 60. Mudge DW, Tan KS, Miles R, et al. A randomized controlled trial of intravenous or oral iron for posttransplant anemia in kidney transplantation. Transplantation 2012; 93: 822–6. [DOI] [PubMed] [Google Scholar]
- 61. Titos‐Arcos JC, Soria‐Aledo V, Carrillo‐Alcaraz A, Ventura‐López M, Palacios‐Muñoz S, Pellicer‐Franco E. Is intravenous iron useful for reducing transfusions in surgically treated colorectal cancer patients? World Journal of Surgery 2012; 36: 1893–7. [DOI] [PubMed] [Google Scholar]
- 62. Garrido‐Martín P, Nassar‐Mansur MI, de la Llana‐Ducrós R, et al. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interactive Cardiovascular Thoracic Surgery 2012; 15: 1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeong O, Park YK. Effect of intravenous iron supplementation for acute postoperative anemia in patients undergoing gastrectomy for gastric carcinoma: a pilot study. Annals of Surgical Oncology 2014; 21: 547–52. [DOI] [PubMed] [Google Scholar]
- 64. Muñoz M, Gómez‐Ramírez S, Besser M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfusion 2017; 15: 422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet 2007; 369: 1502–4. [DOI] [PubMed] [Google Scholar]
- 66. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clinic Proceedings 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 67. European Medicines Agency . New recommendations to manage risk of allergic reactions with intravenous iron containing medicines. EMA/579491/2013. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (accessed: 18/06/2017).
- 68. Auerbach M, Adamson J, Bircher A, et al. On the safety of intravenous iron, evidence trumps conjecture. Haematologica 2015; 100: e214–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. British Journal of Pharmacology 2015; 172: 5025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Macdougall IC, Bircher AJ, Eckardt KU, et al. Conference Participants . Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) Controversies Conference. Kidney International 2016; 89: 28–39. [DOI] [PubMed] [Google Scholar]
- 71. Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014; 99: 1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ganz T. Iron in innate immunity: starve the invaders. Current Opinion in Immunology 2009; 21: 63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andrews SC, Robinson AK, Rodríguez‐Quiñones F. Bacterial iron homeostasis. FEMS Microbiology Reviews 2003; 27: 215–37. [DOI] [PubMed] [Google Scholar]
- 74. Pratt JJ, Khan KS. Non‐anaemic iron deficiency – a disease looking for recognition of diagnosis: a systematic review. European Journal of Haematology 2016; 96: 618–28. [DOI] [PubMed] [Google Scholar]
- 75. Sharma R, Stanek JR, Koch TL, et al. Intravenous iron therapy in non‐anemic iron‐deficient menstruating adolescent females with fatigue. American Journal of Hematology 2016; 91: 973–7. [DOI] [PubMed] [Google Scholar]
- 76. Burden RJ, Morton K, Richards T, et al. Is iron treatment beneficial in, iron‐deficient but non‐anaemic (IDNA) endurance athletes? A systematic review and meta‐analysis. British Journal of Sports Medicine 2015; 49: 1389. [DOI] [PubMed] [Google Scholar]
- 77. Pittori C, Buser A, Gasser UE, et al. A pilot iron substitution programme in female blood donors with iron deficiency without anaemia. Vox Sanguinis 2011; 100: 303–11. [DOI] [PubMed] [Google Scholar]
- 78. Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011; 118: 3222–7. [DOI] [PubMed] [Google Scholar]
- 79. Anker SD, Comin Colet J, Filippatos G, et al. FAIR‐HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. New England Journal of Medicine 2009; 361: 2436–48. [DOI] [PubMed] [Google Scholar]
- 80. Jankowska EA, Tkaczyszyn M, Suchocki T, et al. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. European Journal of Heart Failure 2016; 18: 786–95. [DOI] [PubMed] [Google Scholar]
- 81. Goodnough LT, Comin‐Colet J, Leal‐Noval S, et al. Management of anemia in patients with congestive heart failure. American Journal of Hematology 2017; 92: 88–93. [DOI] [PubMed] [Google Scholar]
- 82. Harju E. Empty iron stores as a significant risk factor in abdominal surgery. Journal of Parenteral and Enteral Nutrition 1988; 12: 282–5. [DOI] [PubMed] [Google Scholar]
- 83. Piednoir P, Allou N, Driss F, et al. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: a prospective observational study. European Journal of Anaesthesiology 2011; 28: 796–801. [DOI] [PubMed] [Google Scholar]
- 84. Miles LF, Kunz SA, Na LH, Braat S, Burbury K, Story DA. Postoperative outcomes following cardiac surgery in non‐anaemic iron‐replete and iron‐deficient patients ‐ an exploratory study. Anaesthesia 2017; 73: 450–458. [DOI] [PubMed] [Google Scholar]
- 85. Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. British Journal of Anaesthesia 2011; 106: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kulnigg‐Dabsch S, Schmid W, Howaldt S, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflammatory Bowel Diseases 2013; 19: 1609–16. [DOI] [PubMed] [Google Scholar]
- 87. Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. American Journal of Hematology 2012; 87: 308–10. [DOI] [PubMed] [Google Scholar]
- 88. Hazara AM, Bhandari S. Intravenous iron infusion reduces platelet counts in patients with chronic kidney disease. Journal of Clinical Pharmacology Therapeutic 2015; 40: 20–23. [DOI] [PubMed] [Google Scholar]
- 89. Kaiafa G, Savopoulos C, Kanellos I, et al. Anemia and stroke: where do we stand? Acta Neurologica Scandinavica 2017; 135: 596–602. [DOI] [PubMed] [Google Scholar]
- 90. French CJ, Glassford NJ, Gantner D, et al. Erythropoiesis‐stimulating Agents in Critically Ill Trauma Patients: a Systematic Review and Meta‐analysis. Annals of Surgery 2017; 265: 54–62. [DOI] [PubMed] [Google Scholar]
- 91. Penny‐Dimri JC, Cochrane AD, Perry LA, Smith JA. Characterising the role of perioperative erythropoietin for preventing acute kidney injury after cardiac surgery: systematic review and meta‐analysis. Heart Lung Circulation 2016; 25: 1067–76. [DOI] [PubMed] [Google Scholar]
- 92. Lakič N, Mrak M, Šušteršič M, Rakovec P, Bunc M. Perioperative erythropoietin protects the CNS against ischemic lesions in patients after open heart surgery. Wiener Klinische Wochenschrift 2016; 128: 875–81. [DOI] [PubMed] [Google Scholar]
- 93. Weltert L, Rondinelli B, Bello R, et al. A single dose of erythropoietin reduces perioperative transfusions in cardiac surgery: results of a prospective single‐blind randomized controlled trial. Transfusion 2015; 55: 1644–54. [DOI] [PubMed] [Google Scholar]
- 94. Cuenca J, García‐Erce JA, Martínez F, Pérez‐Serrano L, Herrera A, Muñoz M. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion 2006; 46: 1112–9. [DOI] [PubMed] [Google Scholar]
- 95. Bernabeu‐Wittel M, Romero M, Ollero‐Baturone M, et al. PAHFRAC‐01 Investigators. Ferric carboxymaltose with or without erythropoietin in anemic patients with hip fracture: a randomized clinical trial. Transfusion 2016; 56: 2199–211. [DOI] [PubMed] [Google Scholar]
- 96. García‐Erce JA, Cuenca J, Haman‐Alcober S, Martínez AA, Herrera A, Muñoz M. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sanguinis 2009; 97: 260–7. [DOI] [PubMed] [Google Scholar]
- 97. Muñoz M, Gómez‐Ramírez S, Auerbach M. Stimulating erythropoiesis before hip fracture repair for reducing blood transfusion: should we change the hemoglobin cutoff level for defining anemia in females? Transfusion 2016; 56: 2160–3. [DOI] [PubMed] [Google Scholar]
- 98. Meybohm P, Herrmann E. Steinbicker AU; PBM‐study Collaborators. Patient Blood Management is associated with a substantial reduction of red blood cell utilization and safe for patient's outcome: a prospective, multicenter cohort study with a non‐inferiority design. Annals of Surgery 2016; 264: 203–11. [DOI] [PubMed] [Google Scholar]
- 99. Goodnough LT, Shieh L, Hadhazy E, et al. Improved blood utilization using real‐time clinical decision support. Transfusion 2014; 54: 1358–65. [DOI] [PubMed] [Google Scholar]
- 100. Gross I, Seifert B, Hofmann A, et al. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion 2015; 55: 1075–81. [DOI] [PubMed] [Google Scholar]
- 101. Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfusion 2017; 15: 307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Isbister JP, Shander A, Spahn DR, Erhard J, Farmer SL, Hofmann A. Adverse blood transfusion outcomes: establishing causation. Transfusion Medicine Reviews 2011; 25: 89–101. [DOI] [PubMed] [Google Scholar]
- 103. Curley GF, Shehata N, Mazer CD, Hare GM, Friedrich JO. Transfusion thresholds for guiding RBC transfusion for cardiovascular surgery: a systematic review and meta‐analysis. Critical Care Medicine 2014; 42: 2611–24. [DOI] [PubMed] [Google Scholar]
- 104. Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a metaanalysis and systematic review. American Journal of Medicine 2014; 127: 124–31. [DOI] [PubMed] [Google Scholar]
- 105. Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database of Systematic Reviews 2015; 4: CD009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta‐analysis and trial sequential analysis. British Medical Journal 2015; 350: h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ripollés Melchor J, Casans Francés R, Espinosa A, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion in critically ill patients and in patients with acute coronary syndrome: s systematic review, meta‐analysis and trial sequential analysis. Minerva Anestesiologica 2016; 82: 582–98. [PubMed] [Google Scholar]
- 108. Carson JL, Stanworth SJ, Roubinian NR, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2016; 10: CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liumbruno GM, Vaglio S, Biancofiore G, et al. Transfusion thresholds and beyond. Blood Transfusion 2016; 14: 123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. de Almeida JP, Vincent JL, Galas FR, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology 2015; 122: 29–38. [DOI] [PubMed] [Google Scholar]
- 111. Bergamin FS, Almeida JP, Landoni G, et al. Liberal Versus restrictive transfusion strategy in critically Ill oncologic patients: the transfusion requirements in critically Ill oncologic patients randomized controlled trial. Critical Care Medicine 2017; 45: 766–73. [DOI] [PubMed] [Google Scholar]
- 112. Shander A, Gross I, Hill S, Javidroozi M, Sledge S. A new perspective on best transfusion practices. Blood Transfusion 2013; 11: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Muñoz M, Gómez‐Ramírez S, Kozek‐Langeneker S. Pre‐operative haematological assessment in patients scheduled for major surgery. Anaesthesia 2016; 71(Suppl 1): 19–28. [DOI] [PubMed] [Google Scholar]
- 114. Kleinerüschkamp AG, Zacharowski K, Ettwein C, et al. Cost analysis of patient blood management. Anaesthesist 2016; 65: 438–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Studies evaluating the effect of postoperative intravenous iron administration (11 studies, 1855 patients).
Appendix S2. Recent systematic reviews and meta‐analyses comparing restrictive versus liberal packed red cell transfusion strategy.


