Abstract
Cold storage (CS) remains the gold standard for organ preservation worldwide, although it is inevitably associated with ischemia/reperfusion injury (IRI). Molecular hydrogen (H2) is well known to have antioxidative properties. However, its unfavorable features, ie, inflammability, low solubility, and high tissue/substance permeability, have hampered its clinical application. To overcome such obstacles, we developed a novel reconditioning method for donor organs named hydrogen flush after cold storage (HyFACS), which is just an end‐ischemic H2 flush directly to donor organs ex vivo, and, herein, we report its therapeutic impact against hepatic IRI. Whole liver grafts were retrieved from Wistar rats. After 24‐hour CS in UW solution, livers were cold‐flushed with H2 solution (1.0 ppm) via the portal vein (PV), the hepatic artery (HA), or both (PV + HA). Functional integrity and morphological damages were then evaluated by 2‐hour oxygenated reperfusion at 37°C. HyFACS significantly lowered portal venous pressure, transaminase, and high mobility group box protein 1 release compared with vehicle‐treated controls (P < 0.01). Hyaluronic acid clearance was significantly higher in the HyFACS‐PV and ‐PV + HA groups when compared with the others (P < 0.01), demonstrating the efficacy of the PV route to maintain the sinusoidal endothelia. In contrast, bile production and lactate dehydrogenase leakage therein were both significantly improved in HyFACS‐HA and ‐PV + HA (P < 0.01), representing the superiority of the arterial route to attenuate biliary damage. Electron microscopy consistently revealed that sinusoidal ultrastructures were well maintained by portal HyFACS, while microvilli in bile canaliculi were well preserved by arterial flush. As an underlying mechanism, HyFACS significantly lowered oxidative damages, thus improving the glutathione/glutathione disulfide ratio in liver tissue. In conclusion, HyFACS significantly protected liver grafts from IRI by ameliorating oxidative damage upon reperfusion in the characteristic manner with its route of administration. Given its safety, simplicity, and cost‐effectiveness, end‐ischemic HyFACS may be a novel pretransplant conditioning for cold‐stored donor organs.
Abbreviations
- 8‐OHdG
8‐hydroxy‐2‐deoxyguanosine
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- BCA
bicinchoninic acid assay
- CEACAM1
carcinoembryonic antigen‐related cell adhesion molecule 1
- CS
cold storage
- DCD
donation after circulatory death
- ER
endoplasmic reticulum
- GLDH
glutamate dehydrogenase
- GSH
glutathione
- GSSG
glutathione disulfide
- H2
molecular hydrogen
- HA
hepatic artery
- HMGB1
high‐mobility group box protein 1
- HyFACS
hydrogen flush after cold storage
- IPRL
isolated perfused rat liver
- IRI
ischemia/reperfusion injury
- KHB
Krebs‐Henseleit bicarbonate
- LDH
lactate dehydrogenase
- LT
liver transplantation
- MP
machine perfusion
- Mt
mitochondria
- Nu
nucleus
- OsO4
osmic acid fixative
- PV
portal vein
- PVP
portal vein pressure
- ROS
reactive oxygen species
- SEC
sinusoidal endothelial cell
- SEM
scanning electron microscope
- TBARS
thiobarbituric acid reactive substance
- TEM
transmission electron microscope
- UW
University of Wisconsin
Ischemia/reperfusion injury (IRI) remains one of the “unmet medical needs” in solid organ transplantation. For over half a century, the gold standard for organ preservation has long been static cold storage (CS). Although numerous investigations have been attempted to develop effective strategies against this unavoidable process in organ transplantation, few have been applied into clinical practice. One of these successful developments may be machine perfusion (MP) preservation.1, 2, 3 The use of such modern but complex methods is, however, available only in some leading institutions in several industrialized countries. In fact, there are still many obstacles in the way of widespread popularization of such novel techniques, including high costs of purchasing special equipment/machines and hiring a number of professional technicians.4 Thus, the universal standard for organ preservation in the world remains CS. To expand the available donor pool and to alleviate the critical shortage of donor organs, there has been a definite need for developing new strategies to improve CS.
Molecular hydrogen (H2) is a ubiquitous gas that possesses antioxidative properties. Since the first report in 2007,5 accumulating evidence has demonstrated its therapeutic potential against IRI and the underlying mechanisms therein. Currently, H2 is known to have 3 definitive effects: antioxidant,5, 6, 7, 8, 9, 10, 11, 12, 13 anti‐inflammatory,10, 14, 15, 16 and antiapoptosis.8, 17 Though some experimental animal studies have shown its therapeutic potential in organ preservation, eg, lung,11 heart,18 kidney,6 intestine,7 and liver,19, 20 its clinical use is yet to be applied because of its unfavorable features.
Besides its remarkable therapeutic potential, H2 has several characteristic burdens that need to be overcome before clinical application: inflammability,21 low solubility,22 and high tissue/substance permeability.22, 23 First, gaseous H2 is potentially inflammable, and 10% seems to be the safety limit for preventing explosion.24 Ideally, <4% seems preferable to secure the safety of the patient as well as the medical staff.22, 25 Although an aqueous H2 solution is safe and convenient for handling,21 its solubility is very low, and the maximal concentration under 1 atm is just 1.6 ppm.26 In addition, high tissue/substance permeability of H2 is another hurdle. H2 easily diffuses and is rapidly lost by spreading across any substance except for aluminum. Moreover, a recent report demonstrated that neither intravenous, per oral, nor inhalational administration resulted in sufficient H2 delivery to the liver23 because of its easy diffusion to upstream organs (gut) or surrounding tissues before reaching the liver. Such molecular as well as biological characteristics of H2 have hampered its clinical application, and the development of an innovative, game‐changing approach in H2 delivery is necessary to apply its remarkable therapeutic potential into clinical practice.
Donor organs for transplantation are inevitably exposed to the ex vivo environment between procurement from donors and implantation to recipients, consequently providing a unique therapeutic window for extracorporeal treatment of organs. We thus intended to take advantage of this timing and hypothesized that ex vivo administration of H2 solution directly to donor organs may solve the difficulties of H2 delivery to the liver. This study was thus designed to investigate the therapeutic potential of hydrogen flush after cold storage (HyFACS) as a new ex vivo treatment for liver grafts, with special interest in its route of administration via the portal vein (PV), the hepatic artery (HA), or from both.
Materials and Methods
Animals
All experimental protocols were approved by the animal research committee of Kyoto University, and all animals received humane care according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 86‐23, revised 1985). Male Wistar rats (270‐320 g, Japan SLC, Inc., Shizuoka, Japan) were housed under specific pathogen‐free conditions in a temperature‐ and humidity‐controlled environment with a 12‐hour light‐dark cycle, and they were allowed free access to tap water and standard chow pellets ad libitum.
Preparation of H2 Solution
H2 was dissolved in normal saline by the nondestructive hydrogen‐dissolving method, as detailed previously.22 Briefly, a solution bag (polyethylene) and hydrogen generating agent (Ca[OH]2 mixed with aluminum powder; MiZ Co., Ltd., Kamakura, Japan) were put together in an aluminum pouch and sealed tightly (Fig. 1A,B). Importantly, H2 gradually permeates the solution bag, dissolves into the solution inside, and then reaches the plateau concentration of 1.0 ppm by 24 hours. This easy and simple method enables us to prepare H2 solution safely, aseptically, and inexpensively (less than US $3/bag).22 We prepared H2 solution each time, and its concentration was measured via an electrochemical gas sensor (model DHD1‐1; DKK‐TOA Corp., Tokyo, Japan), which was strictly maintained at 1 ppm (0.95 ± 0.02 ppm) throughout the experiments.
Figure 1.
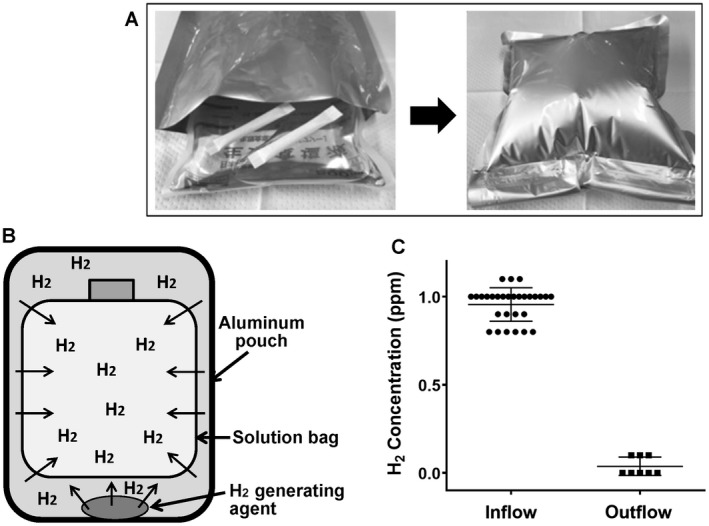
The nondestructive hydrogen dissolving method. (A and B) A small nonwoven fabric pouch containing the hydrogen‐generating agent (Ca[OH]2 mixed with aluminum powder; MiZ Co., Ltd., Kamakura, Japan) is moistened with water. Then a solution bag and 2 pieces of hydrogen‐generating agents are put together into an aluminum pouch and sealed tightly without dead space. Pure hydrogen gas is generated within the aluminum pouch, gradually permeates through the plastic bag, dissolves into the solution inside, and then reaches the plateau concentration of 1.0 ppm by 24 hours. This easy and simple method enables the preparation of H2 solution (maximally 7.0 ppm) safely, aseptically, and inexpensively (less than US $3/bag). We prepared H2 solution each time, and its concentration was measured via the methylene blue platinum colloid reagent–based titration method (dissolved hydrogen concentration measuring reagent; MiZ Co., Ltd.) as well as verified by an electrochemical gas sensor (model DHD1‐1; DKK‐TOA Corp., Tokyo, Japan). The H2 concentration was strictly maintained at 1 ppm (0.95 ± 0.02 ppm) throughout the experiments. (C) The H2 concentration in both the inflow and the outflow of the liver (just before and after the liver, respectively) was determined and is summarized here, indicating that the H2 loss before reaching the liver was minimal and that almost all H2 delivered by HyFACS was absorbed by liver tissues.
Liver Procurement
Whole liver grafts were retrieved as detailed elsewhere.27, 28 Briefly, rats were anesthetized with isoflurane (Escain, Mylan, Osaka, Japan) via a small animal anesthetizer (MK‐A110; Muromachi Kikai Co., Ltd., Tokyo, Japan). After heparinization (200 IU/rat; Mochida Pharmaceutical Co., Ltd., Tokyo, Japan), the common bile duct was cannulated with a 24‐gauge polyethylene tube (TERUMO, Tokyo, Japan). A 14‐gauge catheter (Argyle, COvidIEN, Tokyo, Japan) was inserted into the PV trunk, followed by a blood washout with 40 mL of ice‐cold University of Wisconsin (UW) solution (Viaspan, Astellas, Tokyo, Japan). A 24‐gauge polyethylene tube (TERUMO, Tokyo, Japan) was inserted into the HA through the celiac trunk. The suprahepatic caval vein was cannulated with a 14‐gauge short stent. The liver was immediately flushed with 20 mL of ice‐cold UW solution and then preserved for 24 hours at 4°C in 40 mL of UW solution. Sham‐operated rats underwent the same procedure, but they were not cold‐stored and were directly subjected to isolated ex vivo perfusion for reference.
Experimental Groups
After 24‐hour CS, the livers were randomly assigned into 4 groups and flushed with the respective solution at 4°C through an infusion tube (polyvinyl chloride, 35 cm in length) as follows (n = 10 each):
Control: flushed with 30 mL of saline solution (vehicle) via both the PV and HA.
HyFACS‐PV: flushed with 30 mL of H2 solution via the PV and 30 mL of vehicle via the HA.
HyFACS‐HA: flushed with 30 mL of vehicle via the PV and 30 mL of H2 solution via the HA.
HyFACS‐PV + HA: flushed with 30 mL of H2 solution via both the PV and HA.
Sham livers were also provided for reference, all of which were not cold‐stored but were immediately subjected to a cold flush with 30 mL of vehicle via both the PV and HA. For PV flushing, the pressure was kept at <10 mm Hg by gravity, whereas for HA flushing <50 mm Hg (less than half of physiological arterial pressure) was maintained and monitored by a transducer system (BP‐608; Omron Colin Co., Ltd., Tokyo, Japan).
Ex Vivo Oxygenated Warm Reperfusion
The isolated perfused rat liver (IPRL) system was used to assess liver damage and their functional integrity according to the standardized conditions.28, 29 Briefly, oxygenated reperfusion was performed for 120 minutes via the PV at a constant flow of 3 mL/g liver/minute with 150 mL of oxygenated Krebs‐Henseleit bicarbonate (KHB; K3753, Sigma‐Aldrich Inc., St. Louis, MO) at 37°C. Carbogen (95% O2 + 5% CO2) was used for oxygenation, and the prehepatic partial pressure of oxygen was continuously maintained above 500 mm Hg. Hyaluronic acid (CAS.9067‐32‐7; Wako Pure Chemical Industries, Ltd., Japan) was supplemented into the KHB (1 mg/L), and the perfusate was taken to evaluate transaminase release, oxygen consumption, and hyaluronic acid clearance. Total bile production and portal vein pressure (PVP) were measured throughout reperfusion.28
Liver Enzymes
Hepatic effluent was withdrawn at 10, 30, 60, 90, and 120 minutes of reperfusion and analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels with a standard spectrophotometric method with an automated clinical analyzer (JCA‐BM9030; JEOL, Ltd., Tokyo, Japan). Glutamate dehydrogenase (GLDH) was also measured as a parameter for mitochondrial injury upon reperfusion using a GLDH activity colorimetric assay kit (BioVision, Inc., Milipitas, CA). LDH leakage into the bile was determined as an index for biliary damage.30
Hepatic Oxygen Consumption
Perfusate samples were collected from both the inflow and the outflow of the liver and were then immediately analyzed using a blood gas analyzer (Rapid Point 405, Siemens, Tokyo, Japan). The oxygen consumption rates were calculated by the difference between both samples, given as µL/g liver/minute according to the transhepatic flow and liver mass.28
Oxidative Stress
Thiobarbituric acid reactive substance (TBARS) in liver tissue was measured as an index for lipid peroxidation (Colorimetric TBARS Microplate Assay Kit, Oxford Biomedical Research, Inc., Rochester Mills, MI).28 Total and oxidized glutathione disulfide (GSSG) in liver tissue were both determined (GSSG/glutathione [GSH] quantification kit; Dojindo Molecular Technologies, Inc., Rockville, MD). Reduced GSH was calculated by subtracting GSSG from total GSH. These results were normalized with protein concentration, determined by a bicinchoninic acid assay (BCA) protein assay (Thermo Fisher Scientific, Yokohama, Japan).
Eight‐hydroxy‐2‐deoxyguanosine (8‐OHdG) release into the perfusate was also measured by an enzyme‐linked immunosorbent assay (8‐OHdG check; Nikken Seil Co., Ltd., Shizuoka, Japan).
Hmgb1 Release
To quantify comprehensive tissue damage after 2‐hour oxygenated reperfusion, high‐mobility group box protein 1 (HMGB1), one of the most hazardous damage‐associated molecular patterns, deviated into perfusate and was determined using a HMGB1 enzyme‐linked immunosorbent assay kit II (Shino‐Test, Sagamihara, Japan).
Hyaluronic Acid Clearance
To evaluate the functional integrity of the sinusoidal endothelial cells (SECs), hyaluronic acid clearance was determined. SECs are the main site for uptaking and degrading hyaluronic acid,31, 32 and in the current isolated setting, hyaluronic acid production from other organs is completely excluded. Thus, its clearance in IPRL is one of the best indicators for estimating SEC function. Hyaluronic acid was measured with an enzyme‐linked protein assay (Corgenix, Inc., Westminster, CO), and the clearance rate was calculated from the difference between the inflow and outflow, provided as ng/g liver/hour.
Tissue ATP Contents
At the end of oxygenated reperfusion, liver samples were snap‐frozen and stored in liquid nitrogen. Tissue adenosine triphosphate (ATP) contents were determined by luciferin‐luciferase reaction with an ATP bioluminescence kit (Toyo B‐Net CO., Ltd., Tokyo, Japan). The results were normalized to protein concentration, measured by a BCA protein assay kit (Thermo Fisher Scientific).
Immunohistochemistry For Ceacam1
Immunohistochemical staining for carcinoembryonic antigen‐related cell adhesion molecule 1 (CEACAM1) was performed to assess the integrity of bile canaliculi.33, 34 As described previously, CEACAM1 is highly expressed in bile canaliculi in the liver of rodents and humans and recognized as one of the most sensitive parameters for early diagnosis of cholangiopathy in various pathological conditions.34 After antigen retrieval, quenching, and blocking, the paraffin sections were incubated with the primary mouse anti‐rat CEACAM1 antibody (clone11‐1H; Merck, Darmstadt, Germany), followed by incubation with the second antibody. The stains were visualized with 3,3'‐diaminobenzidine tetrahydrochloride solution, counterstained with hematoxylin‐eosin, and observed with a BZ‐9000 microscope (Keyence, Osaka, Japan). Negative controls were incubated with nonimmunized first antibodies. The stained area was quantified by using ImageJ software (National Institutes of Health, Bethesda, MD).
Electron Microscopy
At the end of reperfusion, additional livers were perfused though the PV with 2% glutaraldehyde/4% paraformaldehyde in a 0.1‐M phosphate buffer, pH 7.4. After fixation, the samples were cut into 2‐mm cubes for transmission electron microscope (TEM) analysis and 1‐mm slices for scanning electron microscope (SEM) analysis. TEM samples were postfixed with osmic acid fixative (OsO4), dehydrated, embedded in epoxy resin, and heat‐polymerized at 60°C before observation with H‐7650 TEM (Hitachi High Technologies, Tokyo, Japan). After postfixation with OsO4, SEM samples were dehydrated, substituted, freeze‐dried with t‐butyl‐alcohol, ion‐sputter‐coated, and then examined by 2 independent pathologists in a blinded fashion with S‐4700 SEM (Hitachi High Technologies).
Statistical Analysis
All statistical analyses were performed among the 4 groups (control, HyFACS‐PV, ‐HA, and ‐PV + HA). The sham group was prepared just for reference to estimate the inhibitory/recovery rate caused by prolonged CS or by HyFACS treatments. All results are expressed as the mean ± standard error of the mean, unless otherwise indicated. Comparisons among the 4 groups were performed with 1‐way analysis of variance (ANOVA). Two‐way repeated‐measures ANOVA followed by Bonferroni’s posttest were used to assess time‐dependent alterations and differences among the groups at each time point. P value of <0.05 was considered statistically significant. All calculations were performed with Prism 6 (GraphPad Software, Inc., San Diego, CA).
Results
Confirmation of H2 Concentration in the Flushing Solution
First, we determined the H2 concentration in the flushing solution just before and after the liver (inflow and outflow, respectively). Figure 1C shows the H2 concentration in both the inflow and the outflow in the HyFACS‐PV group, indicating the following:
The H2 loss before reaching the liver was minimal.
Almost all H2 delivered by HyFACS was absorbed by liver tissues.
Hepatocellular Damage Upon Reperfusion
AST release was significantly lower in all of the HyFACS groups when compared with the control group (P < 0.001; Fig. 2A). ALT (P < 0.01; Fig. 2B) as well as LDH (P < 0.01; Fig. 2C) showed a significant decrease throughout reperfusion indicating significantly less hepatocellular damage achieved by any route of HyFACS.
Figure 2.
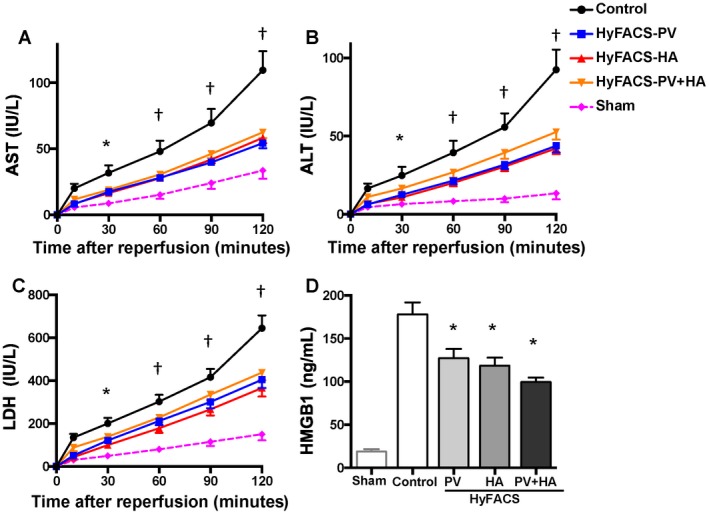
Transaminase and HMGB1 release upon reperfusion. (A) AST, (B) ALT, and (C) LDH release into the perfusate, as indices for hepatocellular damage upon reperfusion. All differences among the groups were assessed via 2‐way repeated‐measures ANOVA (AST, P < 0.001; ALT, P < 0.01; LDH, P < 0.01). Time point assessments were performed by Bonferroni’s posttest (*P < 0.05; † P < 0.001; versus control group). Error bars are sometimes invisible due to small standard errors of the mean (n = 10 each). (D) HMGB1 release into the perfusate served as an index for comprehensive tissue damage after 2 hours of oxygenated reperfusion as well as for a hazardous proinflammatory signal thereafter. All data are presented as the mean ± standard error of the mean (n = 10 each). All differences among the groups were assessed via 1‐way ANOVA (P < 0.001) followed by Bonferroni’s posttest. *P < 0.05 versus other groups.
Cumulative HMGB1 release during oxygenated reperfusion was significantly reduced in all of the HyFACS groups when compared with the control group (P < 0.001; Fig. 2D), demonstrating significantly less comprehensive tissue damage achieved by HyFACS.
Mitochondrial Integrity and ATP Restoration Upon Reperfusion
Tissue ATP concentration after 2‐hour oxygenated reperfusion was significantly higher in the HyFACS‐PV + HA group than in the control group (P < 0.05; Fig. 3A). However, the oxygen consumption rate did not differ among the 4 groups (Fig. 3B), indicating that the HyFACS‐PV + HA group produced significantly greater ATP upon reperfusion with an equal amount of oxygen uptake compared with untreated controls. Intra‐mitochondrial enzyme, GLDH release was also significantly lowered in all of the HyFACS groups compared with that in the control group (P < 0.01; Fig. 3C), showing that HyFACS significantly reduced mitochondrial damage upon reperfusion. Ultrastructual observation with TEM demonstrated mitochondrial swelling with less electron density in the control livers. The endoplasmic reticulum (ER) was also disrupted. In contrast, such deleterious alterations were significantly alleviated in all of the HyFACS groups (Fig. 3E‐G).
Figure 3.
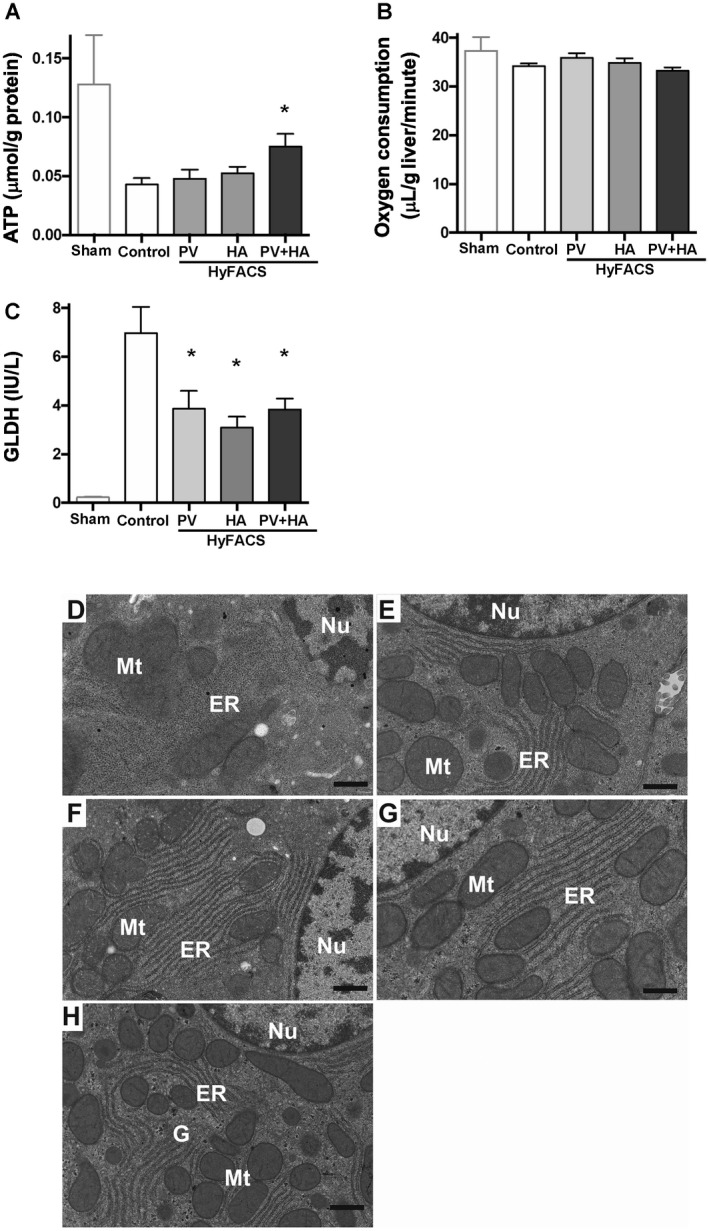
Mitochondrial integrity and ATP restoration upon reperfusion. (A) Tissue ATP concentration after 24 hours of static CS and a subsequent 2 hours of oxygenated reperfusion served as a parameter for microcirculatory integrity, as well as for mitochondrial viability. HyFACS‐PV + HA produced significantly better ATP charge upon reperfusion with an equal amount of oxygen uptake compared with untreated livers (mean ± standard error of the mean; n = 10 each). Intergroup difference by 1‐way ANOVA (P < 0.05) and Bonferroni’s posttest. *P < 0.05 versus control group. (B) Oxygen consumption rates of liver grafts at 120 minutes of oxygenated reperfusion. There was no difference among the groups (1‐way ANOVA; P = 0.14). (C) GLDH release into the perfusate as a parameter for hepatic mitochondrial injury (mean ± standard error of the mean, n = 10 each). All differences among the groups were assessed via 1‐way ANOVA (P < 0.001) followed by Bonferroni’s posttest. *P < 0.05 versus other groups. (D‐H) Ultrastructural photomicrographs of representative tissue sections observed with transmission electron microscopy (TEM). The original magnification was ×8000. The scale bar in each panel represents 500 nm. (D) In control livers, hepatocellular mitochondria (Mt) showed severe swelling with less electron density. ER were also disrupted. In contrast, such deleterious alterations were significantly alleviated in HyFACS‐treated livers: (E) ‐PV, (F) ‐HA, and (G) ‐PV + HA. (H) Ultrastructural observation of hepatocytes in sham livers, not cold‐stored but subjected to 2‐hour oxygenated reperfusion, were used as a reference for baseline comparison with the 4 experimental groups. (G) Glycogen granules were observed in noncold‐stored livers.
Functional/Morphological Integrity of Sinusoidal Endothelia
End‐ischemic HyFACS significantly lowered PVP (an index for total vascular resistance) in all the treated livers, which was maintained throughout reperfusion (P < 0.001; Fig. 4A).
Figure 4.
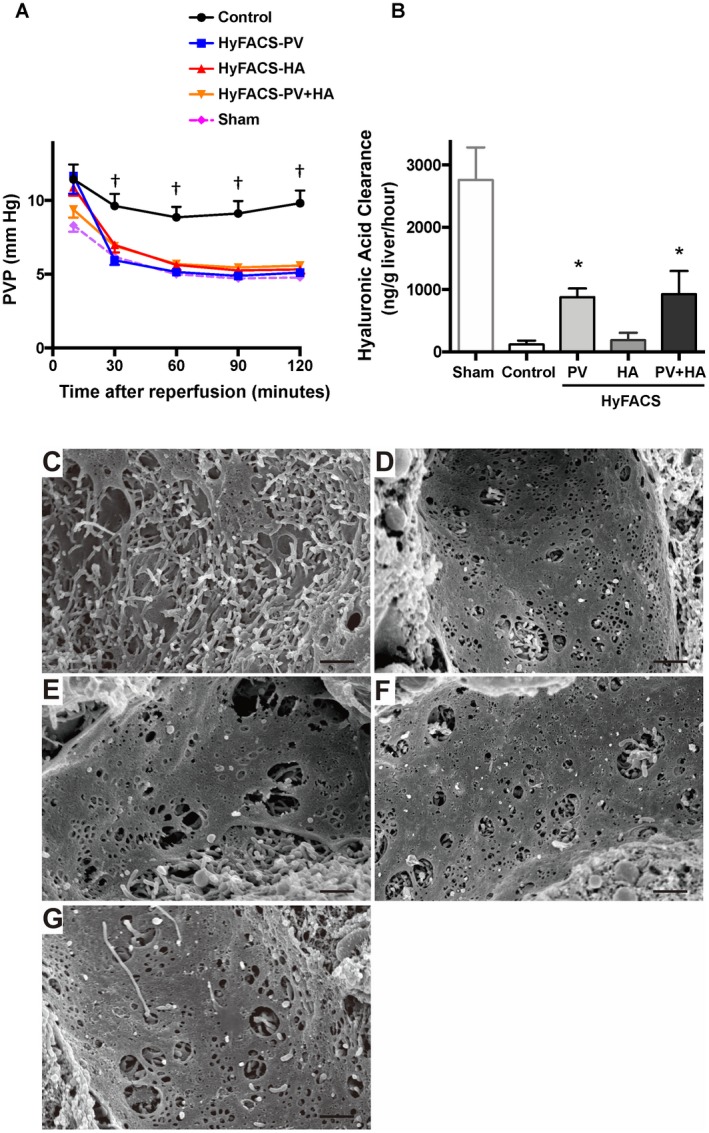
The functional/morphological integrity of the sinusoidal endothelia. (A) Chronological alteration of PVP during 2 hours of oxygenated reperfusion served as an index for total vascular resistance of liver grafts. All differences among the groups were assessed via 2‐way ANOVA (P < 0.001) followed by Bonferroni’s posttest. †P < 0.001 versus control group. Error bars are sometimes invisible because of small standard errors of the mean (mean ± standard error of the mean, n = 10 each). (B) Hyaluronic acid clearance as a parameter for functional integrity of SECs. One‐way ANOVA (P < 0.01) followed by Bonferroni’s posttest. *P < 0.05 versus control group (mean ± standard error of the mean, n = 10 each). (C‐G) SEM for the sinusoidal endothelia. The original magnification was ×4000. The scale bar in each panel represents 50 μm. (C) In the control livers, the sinusoidal fenestrae were fused and enlarged, and the sinusoidal linings were almost disrupted after 24‐hour cold preservation followed by 2‐hour oxygenated reperfusion. (D, E, and F) In contrast, sinusoidal linings and fenestrae therein were well preserved in all of the HyFACS groups, particularly in (D) HyFACS‐PV and (F) ‐PV + HA, demonstrating significant protection of sinusoidal endothelia by portal HyFACS. (G) The sinusoidal endothelia in sham livers, not cold‐stored but subjected to 2‐hour oxygenated reperfusion, served as a reference for baseline comparison with the 4 experimental groups.
Regarding the functional integrity of the sinusoidal endothelia, hyaluronic acid clearance was more than 7 times higher in HyFACS‐PV (880.4 ± 138.6 ng/g liver/hour) and ‐PV + HA (928.4 ± 370.5) than in the control group (119.1 ± 63.4; P < 0.01; Fig. 4B). Of interest, HyFACS‐HA (188.0 ± 117.0) could not efficiently protect SEC function, indicating HyFACS via PV route maintained the viability of SECs more effectively rather than via HA.
Consistently, SEM observation revealed apparent damage of fused and enlarged fenestrae on SECs in control livers, whereas sinusoidal linings and fenestrae therein were well maintained in all of the HyFACS groups, particularly in HyFACS‐PV and ‐PV + HA (Fig. 4C‐F).
Bile Production and Biliary Damage Upon Reperfusion
In contrast to hyaluronic acid clearance, arterial HyFACS (‐HA and ‐PV + HA) significantly increased bile production during 2 hours of reperfusion when compared with the others (P < 0.001; Fig. 5A).
Figure 5.
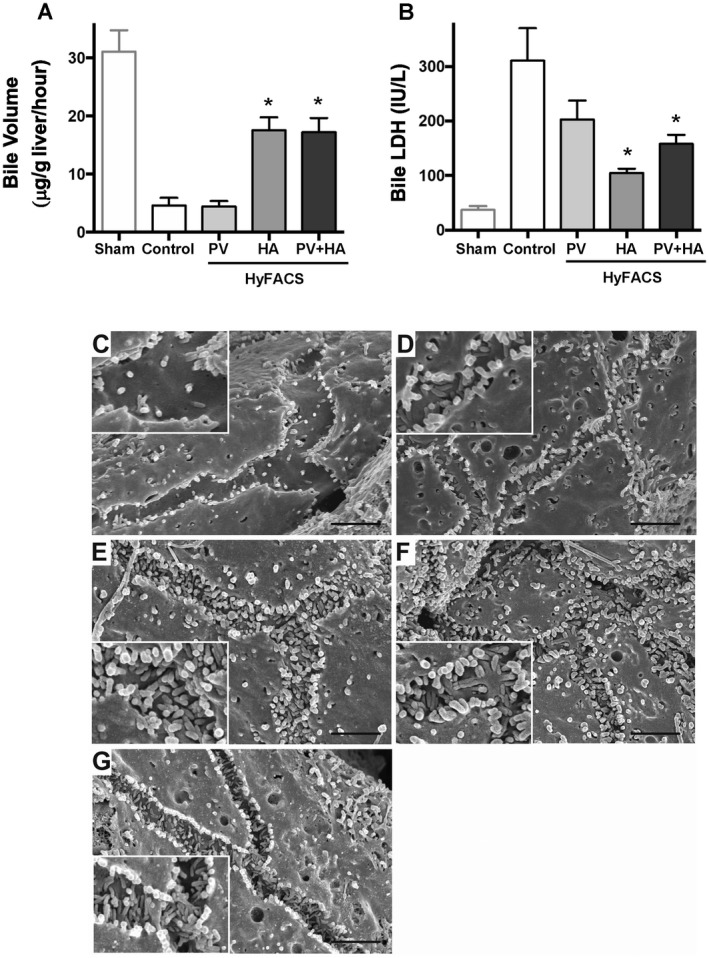
Bile production and biliary damage upon reperfusion. (A) Bile production during 2 hours of reperfusion was used as a comprehensive parameter for liver graft function. One‐way ANOVA (P < 0.001) followed by Bonferroni’s posttest. *P < 0.05 versus control group (mean ± standard error of the mean, n = 10 each). (B) LDH leakage into bile as an indicator for biliary damage. Intergroup difference by 1‐way ANOVA (P < 0.01) and Bonferroni’s posttest. *P < 0.05 versus control group. (C‐G) The ultrastructural observation for the bile canaliculi using SEM. The original magnification was ×13,000. The scale bar in each panel represents 2 μm. (C) In the controls, ultrastructural architecture of microvilli formation in the bile canaliculi was severely injured, and only sparse microvilli were left after prolonged CS and oxygenated reperfusion. The height of the microvilli was also lowered. (D) Portal HyFACS also better maintained microvilli than in the controls but worse than in arterial HyFACS. (E and F) In contrast, microvilli formation was well preserved by arterial HyFACS, where both density and height looked almost intact. (G) Bile canaliculi in sham livers, not cold‐stored but subjected to 2‐hour oxygenated reperfusion, served as a reference for baseline comparison with the 4 experimental groups.
In line, arterial HyFACS significantly lowered LDH leakage into bile compared with those in the portal HyFACS and the control groups (P < 0.01; Fig. 5B), demonstrating arterial HyFACS, ie, direct H2 infusion to the biliary plexus, significantly protects intrahepatic bile ducts from IRI.
As shown in Fig. 5C, SEM observation clearly manifested that only sparse microvilli were left in the bile canaliculi, and their height was also very lowered. In contrast, microvilli were well preserved by arterial HyFACS, where both density and height looked almost intact (Fig. 5E,F). Portal HyFACS also better maintained microvilli than in the controls but was worse compared with arterial HyFACS (Fig. 5D).
Immunohistochemical staining for CEACAM1 showed specific staining along with bile canaliculi. In particular, arterial HyFACS (‐HA and ‐HA + PV) maintained dense and thick staining, appearing like “chicken wire” (Fig. 6C,D, respectively). In the livers treated by HyFACS‐PV, CEACAM1 staining was better preserved than in the controls but worse than in arterial HyFACS (Fig. 6B). The positively stained area was significantly larger in HyFACS‐HA and ‐PV + HA than in control livers (P < 0.001; Fig. 6F), demonstrating significantly better preserved bile canaliculi by arterial HyFACS.
Figure 6.
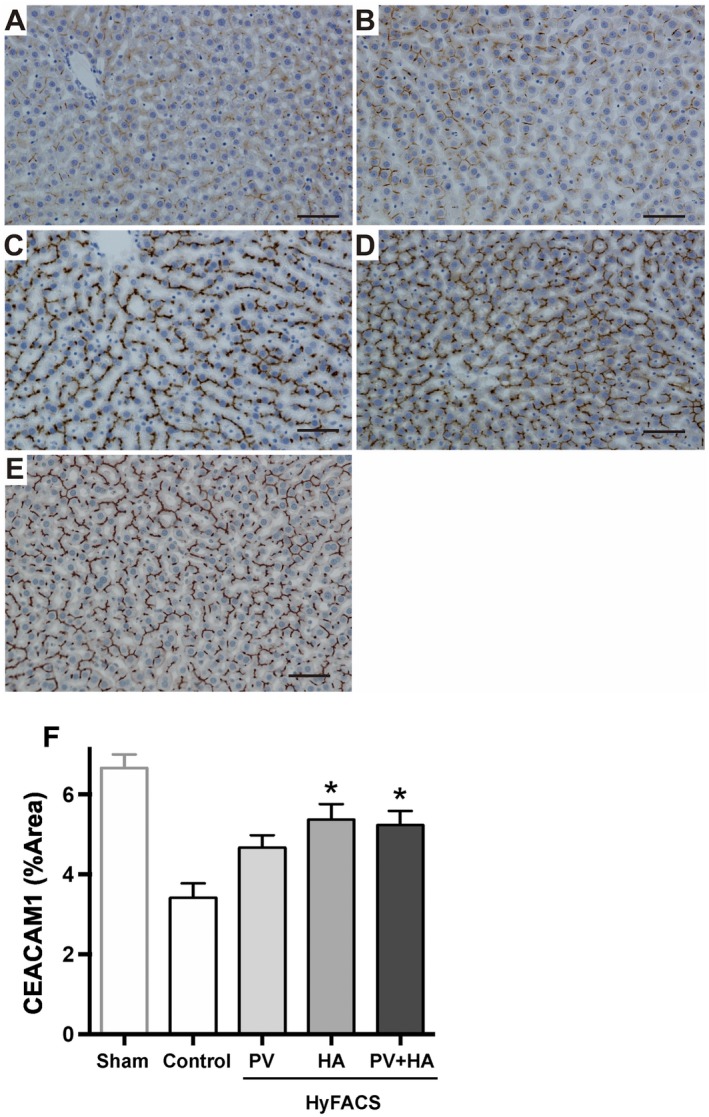
Immunohistochemistry for CEACAM1. Light microscopic appearance of immunohistochemical staining for CEACAM1. Scale bar in each panel indicates 100 μm. (A) Control livers showed weak staining in almost all liver lobules. (B, C, and D) In contrast, arterial HyFACS—(C) ‐HA; (D) ‐PV + HA—exhibited dense and thick staining along with bile canaliculi, which appeared like “chicken wire.” Similarly, in the SEM observation (Fig. 5), (B) HyFACS‐PV resulted in better staining than in the controls, but (C and D) worse than in arterial HyFACS, demonstrating the superiority of the arterial route for H2 administration to protect biliary epithelia. (E) CEACAM1 staining in sham livers, not cold‐stored but subjected to 2‐hour oxygenated reperfusion, served as a reference for baseline comparison with the 4 experimental groups. (F) The quantification of the CEACAM1‐stained area by using the ImageJ software. All differences among the groups were assessed via 1‐way ANOVA (P < 0.001) followed by Bonferroni’s posttest. *P < 0.05 versus control group.
Oxidative Stress and Redox Status
Though all of the HyFACS groups exhibited a lower trend of TBARS contents, only HyFACS‐HA and ‐PV + HA showed statistically significant improvement when compared with the control (P < 0.01; Fig. 7A). Similarly, 8‐OHdG release was significantly lowered by any route of HyFACS when compared with the control group (P < 0.01; Fig. 7B).
Figure 7.
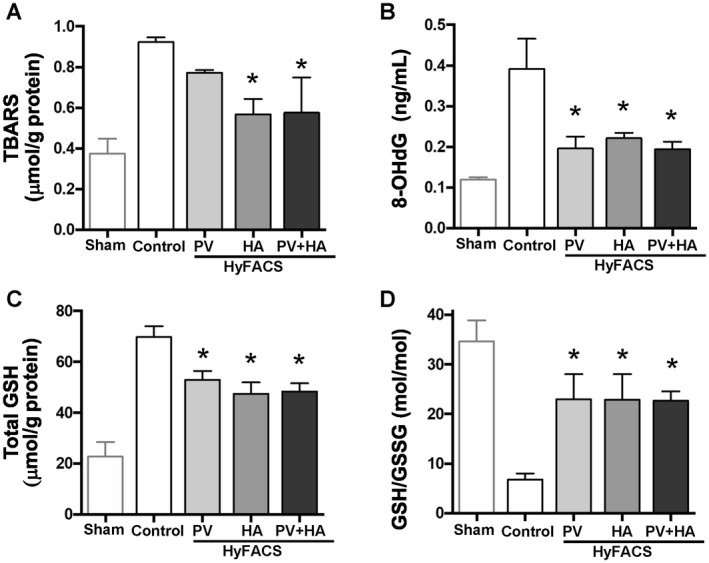
The oxidative stress and redox status. (A) TBARS level in liver tissue; (B) 8‐OHdG release into the perfusate; and (C) total GSH contents in liver tissue served as parameters for tissue oxidative stresses upon reperfusion (mean ± standard error of the mean, n = 10 each). One‐way ANOVA (P < 0.01 in all 3 parameters) followed by Bonferroni’s posttest. *P < 0.05 versus control group. (D) GSH/GSSG ratio (mol/mol) given as an index for cellular redox status. All differences among the groups were assessed via 1‐way ANOVA (P < 0.01) followed by Bonferroni’s posttest. *P < 0.05 versus control group.
Total tissue GSH was significantly reduced in all of the HyFACS groups compared with the control group (P < 0.01; Fig. 7C). Thus, the GSH/GSSG ratio (mol/mol) was significantly higher in all of the HyFACS groups when compared with the control group (P < 0.01; Fig. 7D).
Discussion
H2 is a ubiquitous but quite characteristic molecule, which effectively scavenges reactive oxygen species (ROS), just yielding harmless H2O.5, 35 Moreover, H2 selectively erases hydroxyl radicals and peroxynitrites, both of which are the most harmful ROS generated upon tissue reoxygenation after ischemia.5, 36 From this perspective, H2 is likely one of the most efficient, safe, and economical ROS scavengers that exist in nature, without any possible adverse effects to treated cells, tissues, and organs. Hence, numerous studies have tried its application for organ preservation,7, 18, 37 most of which, however, adopted H2 dissolving into preservation solution6, 18, 37 or inhalation by recipients.7, 19, 35 As aforementioned, H2 easily penetrates any substance and disappears instantly. Continuous H2 dissolution or production by water electrolysis6, 37 during the whole preservation period may possibly solve such problems; however, complex equipment is necessary to avoid the risk of explosion. Moreover, the liver is the largest solid organ in the human body, deeply located in the right subphrenic space; therefore, sufficient H2 delivery to transplanted livers in situ seems rather difficult compared with other organs.23 Thus, we finally conceived of the direct injection of an aqueous H2 solution into liver grafts ex vivo, named HyFACS. This single flush with H2 solution into cold‐stored organs eliminates the explosion risk of gaseous H2, and approximately 1.0 ppm concentration (less than the saturated concentration of 1.6 ppm at 1 atm) could prevent a potential risk for gas embolism within hepatic microvasculature. Furthermore, direct administration into donor organs ex vivo could solve the difficulty of delivering a sufficient amount of H2, even in deeply located organs, such as the liver. In fact, almost all H2 delivered by HyFACS was successfully absorbed in liver tissues, demonstrating the superiority and feasibility of HyFACS as a new delivery method for using H2 in liver transplantation (LT).
Now, the last question arises: When is the suitable timing for this novel treatment? We focused on ROS burst upon blood reflow and hypothesized that oxidative stress during the first several hours upon reperfusion should have a substantial impact on the viability of transplanted organs thereafter. As expected, we demonstrated for the first time that end‐ischemic HyFACS significantly decreased oxidative tissue damage, such as TBARS and 8‐OHdG upon reperfusion, thereby maintaining morphological as well as functional integrity of liver grafts. Considering that control livers consumed an equal amount of oxygen while exhibiting less ATP restoration upon reperfusion, HyFACS enabled efficient oxygen utilization to restore ATP charge, whereas in controls, a substantial amount of oxygen was consumed to promote ROS production. A significant decrease of GLDH release as well as a better‐maintained mitochondrial ultrastructure directly reflects significant protection of hepatocellular mitochondria achieved by HyFACS, undoubtedly contributing to better ATP restoration. These results suggest a remarkable potential of end‐ischemic HyFACS as a novel therapeutic strategy to reduce initial oxidative damage to transplanted organs upon reperfusion. Although hepatocytes possess a certain amount of antioxidants, including superoxide dismutase, GSH, and glutathione peroxidase as “self‐defense systems” against oxidative stresses, we should take into account the characteristic feature in hepatic IRI, ie, sinusoidal space is the center of Kupffer cell activation at the earliest phase of reperfusion. Thus, HyFACS, direct H2 delivering into sinusoidal space, significantly attenuated reperfusion injury of liver grafts by neutralizing ROS burst from Kupffer cells.
Of interest, this simple ex vivo treatment results in characteristic protection depending on its route of administration. In addition to hepatocellular protection, HyFACS via PV predominantly protects SECs, whereas via HA effectively preserves biliary systems. Such characteristic protection depending on the administration route, however, seems to be reasonable, considering the anatomical feature of the liver. The PV directly flows into sinusoidal microcirculation, while only the HA enters the biliary plexus to feed the whole biliary system in the liver.38, 39
Recently, a critical shortage of donor organs has led to worldwide acceptance of extended criteria donors (ECDs), despite the higher risk for primary nonfunction (PNF). Donation after circulatory death (DCD) organs are the primary target to expanding potential donor pools, and in fact, DCD LT has recently increased in Western countries,40 coupled with current remarkable developments of MP techniques.1, 41 In DCD LT, however, ischemic cholangiopathy is one of the main causes of graft loss.42, 43 Arterial HyFACS successfully maintained microvilli formation in bile canaliculi even after prolonged CS as long as 24 hours. Though not yet tested, arterial HyFACS may be a promising strategy to reduce biliary damage in DCD LT, particularly in a large majority of transplant centers in the world where MP is not always available.
A limitation of this study is that we adopted an ex vivo reperfusion model called IPRL. However, this isolated setting enables us to avoid interference from immunological alloreactions or from technical dispersions, thereby facilitating a precise and detailed assessment of organ preservation itself.29 However, longterm observation of this novel technique in a LT model is a prerequisite before its clinical application.
In conclusion, just a single end‐ischemic flush of H2 solution significantly protected liver grafts from IRI by alleviating oxidative damages in the characteristic manner with its route of administration. Portal HyFACS predominantly protects SECs, while arterial HyFACS effectively preserves intrahepatic bile ducts. Given its safety, simplicity, and cost‐effectiveness, HyFACS may provide a major advance in cell, tissue, and organ transplantation/preservation.
Acknowledgments
The authors thank MiZ Co., Ltd. (Kanagawa, Japan), for their skillful cooperation in preparing the H2 solution as well as measuring its concentration. We are also grateful for the technical assistance with electron microscopy by the Center for Anatomical, Pathological, and Forensic Medical Research, Graduate School of Medicine, Kyoto University.
This work was supported by a Grant‐in‐Aid for Scientific Research B (Koichiro Hata and Shinji Uemoto, No. 17H04271) and by another Scientific Research B (Shinji Uemoto and Koichiro Hata, No. 15H04019) from the Japan Society for the Promotion of Science, Tokyo, Japan.
Potential conflict of interest: Nothing to report.
Correction Statement: The copyright line for this article was changed on July 26, 2019 after original online publication.
REFERENCES
- 1. Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international‐matched case analysis. Ann Surg 2015;262:764‐771. [DOI] [PubMed] [Google Scholar]
- 2. Bruinsma BG, Sridharan GV, Weeder PD, Avruch JH, Saeidi N, Özer S, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci Rep 2016;6:22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selzner M, Goldaracena N, Echeverri J, Kaths JM, Linares I, Selzner N, et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: first North American results. Liver Transpl 2016;22:1501‐1508. [DOI] [PubMed] [Google Scholar]
- 4. Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int 2015;28:690‐699. [DOI] [PubMed] [Google Scholar]
- 5. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688‐694. [DOI] [PubMed] [Google Scholar]
- 6. Abe T, Li XK, Yazawa K, Hatayama N, Xie L, Sato B, et al. Hydrogen‐rich University of Wisconsin solution attenuates renal cold ischemia‐reperfusion injury. Transplantation 2012;94:14‐21. [DOI] [PubMed] [Google Scholar]
- 7. Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant 2008;8:2015‐2024. [DOI] [PubMed] [Google Scholar]
- 8. Diao M, Zhang S, Wu L, Huan L, Huang F, Cui Y, Lin Z. Hydrogen gas inhalation attenuates seawater instillation‐induced acute lung injury via the Nrf2 pathway in rabbits. Inflammation 2016;39:2029‐2039. [DOI] [PubMed] [Google Scholar]
- 9. Huang T, Wang W, Tu C, Yang Z, Bramwell D, Sun X. Hydrogen‐rich saline attenuates ischemia‐reperfusion injury in skeletal muscle. J Surg Res 2015;194:471‐480. [DOI] [PubMed] [Google Scholar]
- 10. Iuchi K, Imoto A, Kamimura N, Nishimaki K, Ichimiya H, Yokota T, Ohta S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction‐dependent generation of oxidized phospholipid mediators. Sci Rep 2016;6:18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawamura T, Huang CS, Peng X, Masutani K, Shigemura N, Billiar TR, et al. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery 2011;150:240‐249. [DOI] [PubMed] [Google Scholar]
- 12. Shigeta T, Sakamoto S, Li XK, Cai S, Liu C, Kurokawa R, et al. Luminal injection of hydrogen‐rich solution attenuates intestinal ischemia‐reperfusion injury in rats. Transplantation 2015;99:500‐507. [DOI] [PubMed] [Google Scholar]
- 13. Yu J, Zhang W, Zhang R, Jiang G, Tang H, Ruan X, et al. Molecular hydrogen attenuates hypoxia/reoxygenation injury of intrahepatic cholangiocytes by activating Nrf2 expression. Toxicol Lett 2015;238:11‐19. [DOI] [PubMed] [Google Scholar]
- 14. Tao B, Liu L, Wang N, Tong D, Wang W, Zhang J. Hydrogen‐rich saline attenuates lipopolysaccharide‐induced heart dysfunction by restoring fatty acid oxidation in rats by mitigating C‐jun N‐terminal kinase activation. Shock 2015;44:593‐600. [DOI] [PubMed] [Google Scholar]
- 15. Liu L, Xie K, Chen H, Dong X, Li Y, Yu Y, et al. Inhalation of hydrogen gas attenuates brain injury in mice with cecal ligation and puncture via inhibiting neuroinflammation, oxidative stress and neuronal apoptosis. Brain Res 2014;1589:78‐92. [DOI] [PubMed] [Google Scholar]
- 16. Tao B, Liu L, Wang N, Wang W, Jiang J, Zhang J. Effects of hydrogen‐rich saline on aquaporin 1, 5 in septic rat lungs. J Surg Res 2016;202:291‐298. [DOI] [PubMed] [Google Scholar]
- 17. Du H, Sheng M, Wu L, Zhang Y, Shi D, Weng Y, et al. Hydrogen‐rich saline attenuates acute kidney injury after liver transplantation via activating p53‐mediated autophagy. Transplantation 2016;100:563‐570. [DOI] [PubMed] [Google Scholar]
- 18. Tan M, Sun X, Guo L, Su C, Sun X, Xu Z. Hydrogen as additive of HTK solution fortifies myocardial preservation in grafts with prolonged cold ischemia. Int J Cardiol 2013;167:383‐390. [DOI] [PubMed] [Google Scholar]
- 19. Li H, Chen O, Ye Z, Zhang R, Hu H, Zhang N, et al. Inhalation of high concentrations of hydrogen ameliorates liver ischemia/reperfusion injury through A2A receptor mediated PI3K‐Akt pathway. Biochem Pharmacol 2017;130:83‐92. [DOI] [PubMed] [Google Scholar]
- 20. Shimada S, Wakayama K, Fukai M, Shimamura T, Ishikawa T, Fukumori D, et al. Hydrogen gas ameliorates hepatic reperfusion injury after prolonged cold preservation in isolated perfused rat liver. Artif Organs 2016;40:1128‐1136. [DOI] [PubMed] [Google Scholar]
- 21. Seo T, Kurokawa R, Sato B. A convenient method for determining the concentration of hydrogen in water: use of methylene blue with colloidal platinum. Med Gas Res 2012;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurokawa R, Seo T, Sato B, Hirano S, Sato F. Convenient methods for ingestion of molecular hydrogen: drinking, injection, and inhalation. Med Gas Res 2015;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu C, Kurokawa R, Fujino M, Hirano S, Sato B, Li XK. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep 2014;4:5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas GO. Flame acceleration and the development of detonation in fuel‐oxygen mixtures at elevated temperatures and pressures. J Hazard Mater 2009;163:783‐794. [DOI] [PubMed] [Google Scholar]
- 25. Kawamura T, Wakabayashi N, Shigemura N, Huang CS, Masutani K, Tanaka Y, et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol 2013;304:L646‐L656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asada R, Kageyama K, Tanaka H, Matsui H, Kimura M, Saitoh Y, Miwa N. Antitumor effects of nano‐bubble hydrogen‐dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis‐like cell death. Oncol Rep 2010;24:1463‐1470. [DOI] [PubMed] [Google Scholar]
- 27. Hata K, Tolba RH, Wei L, Doorschodt BM, Büttner R, Yamamoto Y, Minor T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transpl 2007;13:114‐121. [DOI] [PubMed] [Google Scholar]
- 28. Okamura Y, Hata K, Tanaka H, Hirao H, Kubota T, Inamoto O, et al. Impact of subnormothermic machine perfusion preservation in severely steatotic rat livers: a detailed assessment in an isolated setting. Am J Transplant 2017;17:1204‐1215. [DOI] [PubMed] [Google Scholar]
- 29. Bessems M, 't Hart NA, Tolba R, Doorschodt BM, Leuvenink HG, Ploeg RJ, et al. The isolated perfused rat liver: standardization of a time‐honoured model. Lab Anim 2006;40:236‐246. [DOI] [PubMed] [Google Scholar]
- 30. Vajdová K, Smreková R, Kukan M, Lutterová M, Wsólová L. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia–reperfusion of rat livers. Cryobiology 2000;41:145‐152. [DOI] [PubMed] [Google Scholar]
- 31. McCourt PA, Smedsrød BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology 1999;30:1276‐1286. [DOI] [PubMed] [Google Scholar]
- 32. Sano N, Tamura T, Toriyabe N, Nowatari T, Nakayama K, Tanoi T, et al. New drug delivery system for liver sinusoidal endothelial cells for ischemia‐reperfusion injury. World J Gastroenterol 2015;21:12778‐12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundberg U, Obrink B. CEACAM1 isoforms with different cytoplasmic domains show different localization, organization and adhesive properties in polarized epithelial cells. J Cell Sci 2002;115(part 6):1273‐1284. [DOI] [PubMed] [Google Scholar]
- 34. Miyao M, Ozeki M, Abiru H, Manabe S, Kotani H, Tsuruyama T, Tamaki K. Bile canalicular abnormalities in the early phase of a mouse model of sclerosing cholangitis. Dig Liver Dis 2013;45:216‐225. [DOI] [PubMed] [Google Scholar]
- 35. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 2007;361:670‐674. [DOI] [PubMed] [Google Scholar]
- 36. Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia‐reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada T, Uchida K, Onuma K, Kuzuno J, Ujihira M, Inoue G, et al. Hydrogen supplementation of preservation solution improves viability of osteochondral grafts. ScientificWorldJournal 2014;2014:109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken) 2008;291:653‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, et al. Cholangiocytes and blood supply. World J Gastroenterol 2006;12:3546‐3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson RJ, Bradbury LL, Martin K, Neuberger J. for UK Transplant Registry. Organ donation and transplantation in the UK‐the last decade: a report from the UK national transplant registry. Transplantation 2014;97(suppl 1):S1‐S27. [DOI] [PubMed] [Google Scholar]
- 41. Perera T, Mergental H, Stephenson B, Roll GR, Cilliers H, Liang R, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl 2016;22:120‐124. [DOI] [PubMed] [Google Scholar]
- 42. Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta‐analysis. Ann Surg 2011;253:259‐264. [DOI] [PubMed] [Google Scholar]
- 43. Mallik M, Callaghan CJ, Hope M, Gibbs P, Davies S, Gimson AE, et al. Comparison of liver transplantation outcomes from adult split liver and circulatory death donors. Br J Surg 2012;99:839‐847. [DOI] [PubMed] [Google Scholar]


