Abstract
The goal of this analysis was to evaluate the ability of insulin resistance, identified by the presence of prediabetes mellitus (PreDM) combined with either an elevated triglyceride (TG >1.7 mmol/l) or body mass index (BMI ≥27.0 kg/m2), to identify increased risk of statin-associated type 2 diabetes mellitus (T2DM). Consequently, a retrospective analysis of data from subjects without diabetes in the Treating to New Targets and the Stroke Prevention by Aggressive Reduction in Cholesterol Levels randomized controlled trials was performed, subdividing participants into 4 experimental groups: (1) normal fasting glucose (NFG) and TG ≤1.7 mmol/l (42%); (2) NFG and TG >1.7 mmol/l (22%); (3) PreDM and TG ≤1.7 mmol/l (20%); and (4) PreDM and TG >1.7 mmol/l (15%). Comparable groupings were created substituting BMI values (kg/m2 <27.0 and ≥27.0) for TG concentrations. Patients received atorvastatin or placebo for a median duration of 4.9 years. Incident T2DM, defined by developing at least 2 fasting plasma glucose (FPG) concentrations ≥126 mg/dl, an increase in FPG ≥37 mg/dl, or a clinical diagnosis of T2DM, was observed in 8.2% of the total population. T2DM event rates (statin or placebo) varied from a low of 2.8%/3.2% (NFG and TG ≤1.7 mmol/l) to a high of 22.8%/7.6% (PreDM and TG >1.7 mmol/l) with intermediate values for only an elevated TG >1.7 mmol/l (5.2%/4.3%) or only PreDM (12.8%/7.6%). Comparable differences were observed when BMI values were substituted for TG concentrations. In conclusion, these data suggest that (1) the diabetogenic impact of statin treatment is relatively modest in general; (2) the diabetogenic impact is accentuated relatively dramatically as FPG and TG concentrations and BMI increase; and (3) PreDM, TG concentrations, and BMI identify people at highest risk of statin-associated T2DM.
Given widespread use of statin therapy and recent concerns as to statin-associated type 2 diabetes (T2DM),1–5 identifying people at the greatest risk of developing T2DM while on statins would provide useful clinical information. Risk of incident T2DM in association with statin therapy is increased in patients with higher blood pressures and fasting plasma glucose (FPG) and triglyceride (TG) concentrations,3 a constellation of abnormalities closely associated with insulin resistance.6 Because insulin resistance is an independent predictor of T2DM,7 having a simple way of identifying an insulin-resistant subpopulation before statin treatment should be clinically useful. Hyper-insulinemia is the surrogate estimate most closely correlated with direct measurements of insulin resistance,8–10 but the absence of a standardized insulin assay limits its clinical utility.11 Prevalence of insulin resistance is increased in patients with prediabetes mellitus (PreDM),12,13 and a diagnosis of PreDM, defined by an FPG concentration ≥5.6 mmol/l (100 mg/dl) and <7.0 mmol/L (126 mg/dl), predicts statin-associated T2DM.14 Although this FPG cut point provides a useful first step in efforts to identify enhanced risk of statin-associated T2DM, PreDM is a heterogeneous entity, and not all subjects with this diagnosis are insulin resistant.12,13 Consequently, we hypothesized that combining a diagnosis of PreDM with an additional index of insulin resistance would more effectively identify a subset of patients with PreDM who were further enriched with insulin resistance and at even greater greatest risk of statin-associated T2DM. For this purpose, we evaluated the impact of combining a diagnosis of PreDM with a measurement of either fasting TG concentration >1.7 mmol/l (>150 mg/dl) or a body mass index (BMI) ≥27.0 kg/m2, either one of which is associated with insulin resistance.15,16 The goal of this analysis was to extend our earlier findings14 by determining the degree to which combining PreDM with either fasting plasma TG concentration >1.7 mmol/l or a BMI ≥27.0 kg/m2 improved identification of subjects most likely to develop statin-associated T2DM.
Methods
The study population consisted of patients without diabetes mellitus (DM) pooled from the Treating to New Targets (TNT) and the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trials, the design and findings of which have been published.17,18 The TNT study17 randomized 10,001 patients with coronary disease to atorvastatin 10 or 80 mg/day, whereas the SPARCL study randomized 4,731 patients with stroke or transient ischemic attack to atorvastatin 80 mg or placebo.18 Both studies had a median follow-up of 4.9 years. Patients with DM (n = 2,843) at baseline were excluded from the combined cohort, and 489 were excluded because fewer than 2 postbaseline FPG measurements were available or because baseline TG (n = 4) or FPG measurements were missing (n = 42).
Subjects were classified as having normal fasting glucose (NFG; FPG <5.6 mmol/l: n = 7,085) or PreDM (n = 4,269) on the basis of their FPG as defined by American Diabetes Association criteria.19 Follow-up visits occurred at week 12 and at months 6 and 9 (in TNT), and at week 12 in the first year and every 6 months thereafter. FPG was measured before statin treatment, annually, and at the end of the studies. Incident T2DM was defined prospectively as ≥2 postbaseline FPG measurements ≥7.0 mmol/l or at least 1 postbaseline FPG ≥2.0 mmol/l above baseline.20 Subjects were also considered to have developed T2DM on the basis of adverse event reporting or new medication for diabetes.
The analysis involved comparison of incident T2DM on the basis of baseline FPG plus either TG concentration or BMI value. Thus, comparisons were between patients subdivided into 4 subgroups on the basis of their FPG and TG concentration: NFG and TG ≤1.7 mmol/l; NFG and TG >1.7 mmol/l; PreDM and TG ≤1.7 mmol/l; and PreDM and TG >1.7 mmol/l. In addition, comparisons were made between patients subdivided into 4 subgroups on the basis of their baseline FPG and BMI values (NFG and BMI <27.0 kg/m2; NFG and BMI ≥ 27.0 kg/m2; PreDM BMI < 27.0 kg/m2, and PreDM and BMI ≥ 27.0 kg/m2). Comparisons were also made using these same groupings in SPARCL to compare incident T2DM in statin-treated versus placebo-treated subjects.
Univariate and multivariate adjusted Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to compare incident T2DM between the groups. Models were adjusted for factors that may have been independently associated with development of T2DM including hypertension, high-density lipoprotein cholesterol (HDL-C), and study.
Results
Baseline demographic and metabolic characteristics of the 4 statin-treated groups, subdivided on the basis of combining glucose tolerance status with either TG concentration or BMI, are compared in Table 1 (TG concentration) and Table 2 (BMI). In Table 1, the largest group comprised people with NFG and TG ≤1.7 mmol/l (42%), with the fewest subjects having PreDM and TG >1.7 mmol/l (15%). The 4 groups were reasonably comparable in terms of age, gender, and race. BMI was highest in those with PreDM and TG >1.7 mmol/l and lowest in subjects with NFG and TG ≤1.7 mmol/l. By selection, FPG was highest in those with PreDM, with higher TG concentrations in those selected for that characteristic. Low-density lipoprotein cholesterol (LDL-C) concentration was reasonably comparable across the 4 groups, and HDL-C concentrations were lowest in the 2 groups with elevated TG concentrations.
Table 1.
Baseline characteristics of the combined TNT and SPARCL population by glycemic status and TG concentration
| Fasting glucose (mmol/L) | <5.6 | <5.6 | ≥5.6 - <7.0 | ≥5.6 - <7.0 | |
|---|---|---|---|---|---|
| Triglycerides (mmol/L) | ≤1.7 | >1.7 | ≤1.7 | >1.7 | |
| Total (n=11354) | (N=4782) | (N = 2303) | (N = 2544) | (N = 1725) | |
| Male | 8495 (74.8%) | 3521 (73.6%) | 1632 (70.9%) | 2039 (80.2%) | 1303 (75.5%) |
| White | 10778 (94.9%) | 4489 (94.0%) | 2219 (96.4%) | 2431 (95.6%) | 1639 (95.0%) |
| Age (years) | 61.2 ± 9.9 | 61.5 ± 10.2 | 59.2 ± 10.0 | 62.8 ± 9.3 | 60.8 ± 9.5 |
| BMI (kg/m2) | 27.8 ± 4.3 | 26.7 ± 4.0 | 28.3 ± 4.2 | 28.1 ± 4.2 | 29.6 ± 4.6 |
| Fasting glucose | |||||
| (mmol/L) | 5.38 ± 0.59 | 5.01 ± 0.38 | 5.03 ± 0.37 | 5.95 ± 0.34 | 6.01 ± 0.37 |
| (mg/dl) | 96.8 ± 10.6 | 90.18 ± 6.84 | 90.54 ± 6.66 | 107.10 ± 6.12 | 108.18 ± 6.66 |
| Systolic BP (mmHg) | 132 ± 18 | 131 ± 18 | 131.4 ± 17 | 134 ± 19 | 133.9 ± 17 |
| Diastolic BP (mmHg) | 79 ± 10 | 79 ± 10 | 79.6 ± 10 | 79.3 ±10 | 80.3 ± 10 |
| Smoker | |||||
| Never | 3252 (28.6%) | 1554 (32.5%) | 631 (27.4%) | 691 (27.2%) | 376 (21.8%) |
| Ex | 6345 (55.9%) | 2517 (52.6%) | 1223 (53.1%) | 1536 (60.4%) | 1069 (62.0%) |
| Current | 1757 (15.5%) | 711 (14.9%) | 449 (19.5%) | 317 (12.5%) | 280 (16.2%) |
| Hypertension | 6033 (53.1%) | 2329 (48.7%) | 1256 (54.5%) | 1426 (56.1%) | 1022 (59.3%) |
| Total Cholesterol | |||||
| (mmol/L) | 4.84 ± 0.80 | 4.74 ± 0.80 | 5.12 ± 0.81 | 4.66 ± 0.80 | 5.08 ± 0.75 |
| (mg/dl) | 187.16 ± 30.94 | 183.29 ± 30.93 | 197.99 ± 31.32 | 180.20 ± 30.94 | 196.44 ± 29.00 |
| LDL-C | |||||
| (mmol/L) | 2.85 ± 0.67 | 2.85 ± 0.67 | 2.87 ± 0.70 | 2.80 ± 0.67 | 2.85 ± 0.65 |
| (mg/dl) | 110.21 ± 25.91 | 110.20 ± 25.91 | 110.98 ± 27.07 | 108.27 ± 25.91 | 110.21 ± 25.14 |
| HDL-C | |||||
| (mmol/L) | 1.27 ± 0.31 | 1.35 ± 0.34 | 1.17 ± 0.28 | 1.32 ± 0.31 | 1.14 ± 0.26 |
| (mg/dl) | 49.11 ± 11.99 | 52.20 ± 131.5 | 45.24 ± 10.83 | 51.04 ± 11.99 | 44.08 ± 10.05 |
| TG | |||||
| (mmol/L) | 1.64 ± 0.82 | 1.19 ± 0.30 | 2.42 ± 0.99 | 1.22 ± 0.29 | 2.44 ± 0.71 |
| (mg/dl) | 145.26 ± 72.63 | 105.40 ± 26.57 | 214.35 ± 87.69 | 108.10 ± 25.69 | 216.12 ± 62.89 |
All values depicted as mean ± SD, unless otherwise specified.
BMI = body mass index; BP = blood pressure; HDL-C = high density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; TG = triglycerides.
Table 2.
Baseline characteristics of the combined TNT and SPARCL population by glycemic status and BMI
| Fasting Glucose (mmol/L) | <5.6 | <5.6 | ≥5.6 - <7.0 | ≥5.6 - <7.0 | |
|---|---|---|---|---|---|
| BMI (kg/ m2) | <27 | ≥27 | <27 | ≥27 | |
| Total (n= 11281) | (N=3697) | (N = 3348) | (N = 1592) | (N = 2644) | |
| Male | 8450 (74.9%) | 2691 (72.8%) | 2439 (72.6%) | 1251 (78.6%) | 2069 (78.2%) |
| White | 10709 (94.4%) | 3522 (95.3%) | 3150 (94.1%) | 1530 (96.1%) | 2507 (94.8%) |
| Age (years) | 61.2 ± 9.9 | 61.9 ± 10.3 | 59.6 ± 9.9 | 63.3 ± 9.6 | 61.1 ± 9.2 |
| BMI (kg/m2) | 27.8 ± 4.3 | 24.3 ± 2.0 | 30.4 ± 3.5 | 24.7 ± 1.7 | 31.1 ± 3.7 |
| Fasting glucose | |||||
| (mmol/L) | 5.38 ± 0.59 | 5.0 ± 0.38 | 5.03 ± 0.37 | 5.94 ± 0.33 | 6.01 ± 0.33 |
| (mg/dl) | 96.84 ± 10.62 | 90 ± 6.84 | 90.54 ± 6.66 | 106.90 ± 5.93 | 108.10 ± 6.0 |
| Systolic BP (mmHg) | 132.42 ± 17.8 | 131 ± 18.0 | 131.96 ± 17.1 | 133.1 ± 18.6 | 134.59 ± 17.7 |
| Diastolic BP (mmHg) | 79.26 ± 10.0 | 78.06 ± 9.8 | 80.1 ± 10.0 | 78.5 ± 10.2 | 80.4 ± 9.8 |
| Smoker | |||||
| Never | 3225 (28.6%) | 1174 (31.8%) | 997 (29.8%) | 428 (26.9%) | 626 (23.7%) |
| Ex | 6318 (56.0%) | 1853 (50.1%) | 1873 (55.9%) | 903 (56.7%) | 1689 (63.9%) |
| Current | 1738 (15.4%) | 670 (18.1%) | 478 (14.3%) | 261 (16.4%) | 329 (12.4%) |
| HTN | 5991 (53.1%) | 1651 (44.7%) | 1915 (57.2%) | 786 (49.4%) | 1639 (62.0%) |
| Total Cholesterol | |||||
| (mmol/L) | 4.84 ± 0.81 | 5.28 ± 0.14 | 4.82 ± 0.80 | 4.87 ± 0.83 | 4.79 ± 0.76 |
| (mg/dl) | 187.16 ± 31.32 | 204.18 ± 5.41 | 186.39 ± 30.94 | 188.32 ± 32.10 | 185.23 ± 29.39 |
| LDL-C | |||||
| (mmol/L) | 2.83 ± 0.67 | 2.87 ± 0.70 | 2.82 ± 0.66 | 2.87 ± 0.70 | 2.78 ± 0.63 |
| (mg/dl) | 109.43 ± 25.91 | 110.98 ± 27.07 | 109.05 ± 25.52 | 110.98 ± 27.07 | 107.50 ± 24.36 |
| HDL-C | |||||
| (mmol/L) | 1.27 ± 0.32 | 1.34 ± 0.35 | 1.24 ± 0.30 | 1.30 ± 0.31 | 1.20 ± 0.27 |
| (mg/dl) | 49.11 ± 12.37 | 51.82 ± 13.53 | 47.95 ± 11.60 | 50.27 ± 11.99 | 46.40 ± 10.44 |
| TG | |||||
| (mmol/L) | 1.62 ± 0.81 | 1.45 ± 0.65 | 1.71 ± 0.98 | 1.54 ± 0.71 | 1.79 ± 0.79 |
| (mg/dl) | 143.49 ± 71.74 | 128.43 ± 57.57 | 151.46 ± 86.80 | 136.40 ± 62.89 | 158.55 ± 69.97 |
All values depicted as mean ± SD, unless otherwise specified.
BMI = body mass index; BP = blood pressure; HDL-C = high density lipoprotein-cholesterol; HTN = hypertension; LDL-C = low-density lipoproteincholesterol; TG = triglycerides.
Similar comparisons are provided in Table 2, evaluating differences in the 4 statin-treated groups that varied in terms of glucose tolerance status and BMI. In this instance, the largest group consisted of people with NFG and a BMI ≤27 kg/m2 (33%), with the smallest group comprising those with PreDM and a BMI ≤27.0 kg/m2 (14%). The 4 groups were again comparable in terms of age, gender, and race. People with NfG and BMI ≥27 kg/m2 and PreDM and BMI ≥27 kg/m2 had fairly comparable average BMI values. Baseline total cholesterol and HDL-C levels were comparable across the 4 groups. Baseline TG concentrations were highest in the PreDM and BMI ≥27 kg/m2 group and lowest in people with NFG and BMI <27 kg/m2.
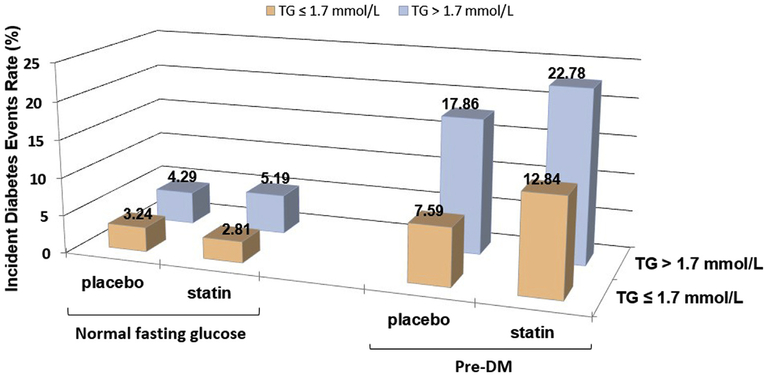
There were 939 incident cases (8.2%) of T2DM identified during the median follow-up of 4.9 years. Results in Figure 1 indicate that the incidence of T2DM was lowest in statin-treated patients with NFG and TG ≤1.7 mmol/l (2.8%), increasing progressively to 5.2%, 12.8%, and 22.8%, in those with only a TG concentration >1.7 mmol/l, with only PreDM, and with combined PreDM and a TG concentration >1.7 mmol/l, respectively.
Figure 1.
Incident T2DM according to baseline presence of PreDM and TG concentrations. Comparison of the rates of incident T2DM as a function of glycemic status (NFG vs PreDM) and plasma TG concentration (≤1.7 vs >1.7 mmol/1) in the 4 experimental groups, with each group stratified by treatment, placebo or atorvastatin.
There was little or no increased risk of statin treatment in those with NFG compared to placebo. However, in those with PreDM, there was a substantial increased baseline risk for incident T2DM even with placebo treatment, for example, incident T2DM occurred in 7.6% of placebo-treated patients with PreDM and TG ≤1.7 mmol/l and 17.9% of placebo patients with PreDM and TG >1.7 mmol/l. Statin treatment markedly accentuated this risk in those with PreDM compared to placebo. For instance, 22.8% of subjects with PreDM and TG >1.7 mmol/l developed incident T2DM, a 27% increased risk versus those getting placebo therapy.
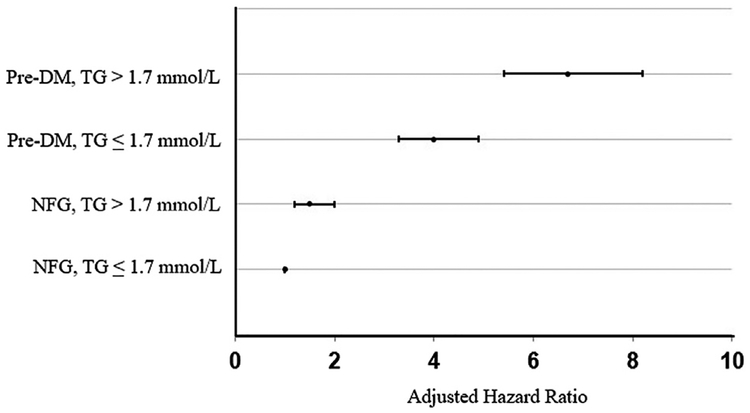
HRs in statin-treated subjects were calculated to evaluate the statistical significance of the findings in Figure 1 and are summarized in Figure 2. Statin treatment in patients with either a TG concentration >1.7 mmol/L or PreDM or PreDM and TG concentration >1.7 mmol/l was associated with a significantly (p <0.001) greater HR for developing T2DM compared to those with NFG and TG ≤1.7 mmol/l. Furthermore, the HRs increased progressively (p <0.001), from those with only a high TG, to only PreDM, to the highest HR when both PreDM and TG >1.7 mmol/l were present at baseline (HRs [95% CIs] are 1.5 [1.2 to 2.0], 4.0 [3.3 to 4.9], and 6.7 [5.4 to 8.2], respectively).
Figure 2.
Hazard for T2DM according to baseline presence of PreDM and TG concentrations. Compared to reference (NFG, TG ≤1.7 mmol/l), all the other groups are significantly different (p <0.001). HRs (95% CIs) are 1.5 (1.2 to 2.0), 4.0 (3.3 to 4.9), and 6.7 (5.4 to 8.2), respectively. Models were adjusted for the following characteristics: BMI, HDL-C concentration, history of hypertension, and study.
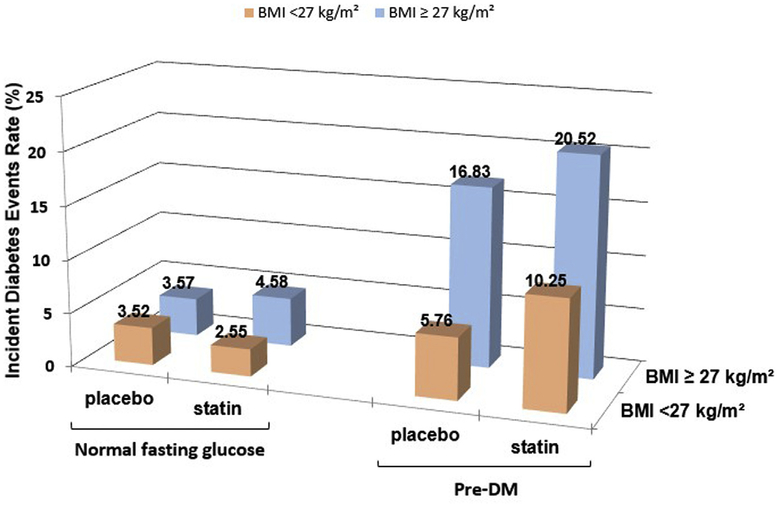
Figure 3 depicts rates of incident T2DM according to fasting glucose concentration and BMI, with results comparable to those seen in Figure 1. Incident T2DM also increased in a parallel fashion in the same 4 experimental groups treated with placebo, and these changes were accentuated with statin therapy. In subjects with NFG, the overall rate of incident T2DM was relatively low and fairly consistent (ranging from 2.6% to 4.6%) whether patients were treated with statins and/or BMI was ≥27 kg/m2. The lowest rate of T2DM incidence in statin-treated patients was seen in people with NFG and BMI <27 kg/m2, was higher in those with NFG and BMI ≥27 kg/m2, and progressively increased in the PreDM and BMI <27 kg/m and PreDM and BMI ≥ 27 kg/m2 groups. The highest absolute incidence of T2DM (20.5%) was seen in the subjects with PreDM and BMI ≥27 kg/m2, which was 22% higher than those in the same category treated with placebo.
Figure 3.
Incident T2DM according to baseline presence of PreDM and BMI. Comparison of the rates of incident T2DM as a function of glycemic status (NFG vs PreDM) and BMI (<27 vs ≥27 kg/m2) in the 4 experimental groups, with each group stratified by treatment, placebo or atorvastatin.
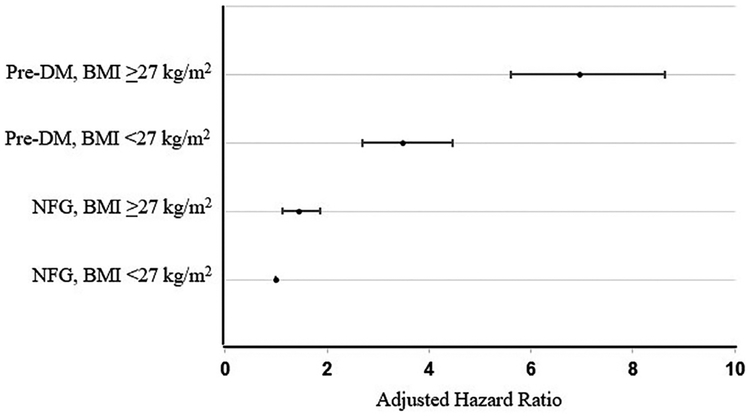
Adjusted HRs of incident T2DM in the 4 groups of patients in whom BMI values were combined with PreDM are demonstrated in Figure 4, with an overall pattern mirroring the HR trend seen with TG levels in Figure 2. The HR (95% CI) was lowest in people with NFG and BMI <27 kg/m2, then progressively increased to 1.4 (1.1 to 1.9) with NFG and bMi ≥27 kg/m2 and 3.5 (2.7 to 4.5) in those with PreDM and BMI <27 kg/m2 (p <0.001). People with both PreDM and BMI ≥27 kg/m had the highest HR of 7.0 (5.6 to 8.6).
Figure 4.
Hazard for T2DM according to baseline presence of PreDM and BMI. Compared to reference (NFG, BMI <27 kg/m2), all the other groups are significantly different (p <0.001). HRs (95% CIs) are 1.4 (1.1 to 1.9), 3.5 (2.7 to 4.5), and 7.0 (5.6 to 8.6), respectively. Models were adjusted for the following characteristics: HDL-C concentration, history of hypertension, and study.
Discussion
These results provide new information of both conceptual and clinical relevance concerning the relation among insulin resistance, statin treatment, and T2DM. At the conceptual level, perhaps the most informative finding was the comparison of incident T2DM in patients treated with placebo versus statin. Focusing on Figures 1 and 3, the event rates of T2DM, when treated with either statin or placebo, were low in subjects with NFG, who had a TG concentration ≤1.7 mmol/l or a BMI <27.0 kg/m2. T2DM event rates were somewhat greater in subjects with NFG and either an elevated TG concentration or BMI, even more so in those with PreDM alone, and the differences between statin and placebo accentuated in these groups. However, incident T2DM was substantially higher in those with PreDM plus either an elevated BMI or TG concentration, associated with the greatest difference between statin and placebo treatment. These data support the conclusion that the diabetogenic impact of statin treatment is relatively modest in the most general sense but is accentuated relatively dramatically as plasma glucose, TG concentrations and BMI increase.
The results of this analysis also provide important clinical information to guide efforts aimed at minimizing the adverse effects of statin therapy. In essence, there appears to be 4 hierarchical categories of risk to develop statin-associated T2DM. We have created a simple 4-tier category system for classification of risk, with category 1 being lowest risk and category 4 being highest risk. Category 1 includes the majority (~40%) of statin-treated subjects who had a normal FPG, were not overweight or obese, and had a TG concentration ≤1.7 mmol/l or BMI < 27 kg/m2. The diabetic event rate was low (~3%) in these patients (Figures 1 and 3) and essentially identical to the rates in placebo-treated subjects. At the other extreme, category 4 consists of statin-treated participants who had PreDM plus either an elevated TG (~15%) concentration or excess adiposity (~20%) The diabetic event rate was more than 5-fold higher in these patients compared to the low-risk group, category 1, and was accentuated in statin-treated patients. When presented alone, the other 3 indexes (PreDM, TG >1.7 mmol/l, or BMI ≥27.0 kg/m2) identified patients whose diabetic event rates were intermediate between the high-and low-risk groups. However, because PreDM alone seemed to be associated with a higher diabetic event rate compared to increases in either BMI or TG concentration, it seemed reasonable to assign it to category 3, with the other 2 isolated abnormalities making up category 2.
How best to use this information is at the discretion of the individual health care provider. However, several generalizations can be made. Obtaining these simple measurements before initiating statin treatment provides considerable insight as to degree of risk of incident T2DM in a given patient. Once the risk category has been determined, it is possible to tailor an appropriate clinical plan to follow patients in whom statin therapy has been initiated. Thus, it would seem prudent to monitor glycemic status more frequently in those at greatest risk of T2DM (category 4) and to consider other pharmacological and non-pharmacological interventions to reduce risk. For example, aggressive effort to overcome excess adiposity and encourage more physical activity was shown in the Diabetes Prevention Program to delay or prevent incident T2DM in high-risk groups.21 The importance of having the ability to initiate interventions aimed at decreasing risk of T2DM in statin-treated patients identified at enhanced risk is underlined by the finding that weight gain predicted incident T2DM in the TNT study.22
Although the results of our analysis provide straightforward clinical information concerning the relative risk of T2DM following initiation of statin treatment, their pathophysiological implications are less clear. T2DM occurs when insulin-resistant subjects cannot maintain the degree of hyperinsulinemia required to prevent decompensation of glucose tolerance.8,9 Cederberg et al.,23 using surrogate estimates, reported that both insulin sensitivity and insulin secretion decreased in statin-treated patients. If we accept the view that statins have an untoward effect on insulin action and secretion, 2 recent reports provide an intriguing mechanistic explanation. Swerdlow et al.,24 based on evidence from genetic analysis and randomized trials, concluded that the increased risk of T2DM noted with statin usage is at least “partially explained by hydroxymethylglutaryl coenzyme A reductase inhibition.” These findings suggest that the adverse effects of statins on carbohydrate metabolism is somehow tied in to their mechanism of action on LDL-C concentration, an observation that complements the recent finding that the prevalence of type 2 diabetes was reduced in subjects with familial hy-percholesterolemia.25 In the latter study, it was suggested that decreased LDL-receptor—mediated transmembrane transport in patients with familial hypercholesterolemia decreases likelihood of the detrimental accumulation of cholesterol in the pancreatic β-cell, thus maintaining insulin secretory function. Conversely, increased transmembrane uptake of cholesterol associated with statin treatment might play an important role in decreasing insulin action and/or insulin secretory function. The possibility that statin-associated T2DM is mechanistically intrinsic to the therapeutic effect of statins on decreasing plasma LDL-C is both intriguing and worthy of further evaluation.
Niacin usage in the treatment of dyslipidemic patients has also been associated with an increase in incident T2DM.26 Furthermore, based on this work of Goldberg and Jacobson, it appears that incident T2DM in niacin-treated patients is also associated with a loss of insulin sensitivity. Thus, measurement of FPG and TG concentrations would also seem to be useful in identifying those patients at the greatest risk of incident in T2DM when treated with niacin.
Important questions concerning the relation between statin usage and T2DM remain unanswered. For example, do statins impair insulin action, secretion, or both? Is the putative adverse effect of statins on insulin action or secretion accentuated in subjects with PreDM compared to those with NFG, or is the impact of statins the same, and incident T2DM more likely to occur in those with PreDM because of their inherent increased risk? Interesting as these are, the untoward effects on glucose homeostasis associated with statin use should not obscure the substantial and unequivocal benefits of statin treatment on decreasing cardiovascular disease.3–5,14 Hopefully, the availability of a simple means to identify people at high risk to develop statin-associated T2DM will lead to targeted interventions (such as intensive weight loss) to decrease this risk.
Acknowledgment:
The TNT and SPARCL clinical trials were funded by Pfizer, Inc. Statistical analysis for this study was provided by Rana Fayyad, Pfizer Inc.
Drs. Kohli and Waters had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures
Dr. Kohli is in the advisory board of Amgen and receives travel reimbursement from Pfizer, Inc. Dr. Waters receives remuneration for participating in clinical trial committees from Aastrom, Cerenis, CSL, Pfizer, Sanofi-Aventis; honoraria for lectures from Pfizer and Zydus Medica; and consulting fees from Novo Nordisk and Pfizer. Dr. Knowles receives Grants 5IRG222930034 from American Heart Association and is supported by a Clinical Scientist Development Award from the Doris Duke Charitable Trust. Other authors have no conflicts to report.
References
- 1.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 2.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 3.Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, Kastelein JJ, Colhoun H, Barter P. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011;57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, Taskinen MR. Statins are diabetogenic—myth or reality? Atheroscler Suppl 2012;13:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 7.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329: 1988–1992. [DOI] [PubMed] [Google Scholar]
- 8.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care 2000;23:171–175. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005;96:399–404. [DOI] [PubMed] [Google Scholar]
- 10.Abbasi F, Okeke Q, Reaven GM. Evaluation of fasting plasma insulin concentration as an estimate of insulin action in nondiabetic individuals: comparison with the homeostasis model assessment of insulin resistance (HOMA-IR). Acta Diabetol 2014;51:193–197. [DOI] [PubMed] [Google Scholar]
- 11.Reaven G Wanted!: a standardized measurement of plasma insulin concentration. Arterioscler Thromb Vasc Biol 2011;31:954–955. [DOI] [PubMed] [Google Scholar]
- 12.Novoa FJ, Boronat M, Saavedra P, Díaz-Cremades JM, Varillas VF, La Roche F, Alberiche MP, Carrillo A. Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care 2005;28:2388–2393. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Reaven GM. Isolated impaired fasting glucose and peripheral insulin sensitivity: not a simple relationship. Diabetes Care 2008;31:347–352. [DOI] [PubMed] [Google Scholar]
- 14.Kohli P, Waters DD, Nemr R, Arsenault BJ, Messig M, DeMicco DA, Laskey R, Kastelein JJ. Risk of new-onset diabetes and cardiovascular risk reduction from high-dose statin therapy in pre-diabetics and non-pre-diabetics: an analysis from TNT and IDEAL. J Am Coll Cardiol 2015;65:402–404. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi F, Brown BW Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 2002;40:937–943. [DOI] [PubMed] [Google Scholar]
- 16.Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med 1992;231:25–30. [DOI] [PubMed] [Google Scholar]
- 17.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 18.Karam JG, Loney-Hutchinson L, McFarlane SI. High-dose atorvastatin after stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J Cardiometab Syndr 2008;3:68–69. [DOI] [PubMed] [Google Scholar]
- 19.Standards of medical care in diabetes-2015: summary of revisions. Diabetes Care 2015;38(Suppl):S4. [DOI] [PubMed] [Google Scholar]
- 20.Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103:357–362. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong KL, Waters DD, Messig M, DeMicco DA, Rye KA, Barter PJ. Effect of change in body weight on incident diabetes mellitus in patients with stable coronary artery disease treated with atorvastatin (from the treating to new targets study). Am J Cardiol 2014;113:1593–1598. [DOI] [PubMed] [Google Scholar]
- 23.Cederberg H, Stancakova A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia 2015;58:1109–1117. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der A DL, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, DIAGRAM Consortium; MAGIC Consortium; InterAct Consortium; Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg-Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimäki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 2015;313:1029–1036. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc 2008;83:470–478. [DOI] [PubMed] [Google Scholar]