Abstract
Activation of the kynurenine pathway is one of the described mechanisms by which inflammation can induce depression. It involves multiple pathways including interference with the bioavailability of tryptophan central to the synthesis of the neurotransmitter serotonin.
In this systematic review, we examine the relationship between kynurenine metabolites (kynurenine, kynurenic acid, tryptophan, quinolinic acid, the ratio of kynurenine and tryptophan) and mood disorders by conducting a meta-analysis.
Fifty-six studies were identified, 21 met inclusion criteria and 14 were deemed suitable (9 investigating unipolar depression and 5 bipolar disorder).
We found decreased levels of kynurenine in unipolar major depression vs. healthy controls but studies were significantly heterogeneous in nature. No significant differences were found in tryptophan levels or kynurenine/tryptophan ratios. Kynurenine metabolites are likely to play a role in major depression but an exact etiological role in mood disorder seem complex and requires further research.
Keywords: Kynurenine, Mood disorder, Depression, Bipolar disorder, tryptophan, inflammation, quinolinic acid
INTRODUCTION
Major depressive disorder (MDD) and bipolar disorder (BD) are leading causes of mental disorders worldwide (Whiteford et al., 2013). They are a major public health concern due to their chronicity, risk of suicide and high co-morbidity with other disabling diseases such as diabetes and cardiovascular diseases (Angst et al., 2013; Barth et al., 2004; Fornaro et al., 2017; Greenberg et al., 2015; Hesdorffer et al., 2000; Knol et al., 2006; Simon, 2003). The underlying pathophysiology of these disorders is complex, possibly involving multiple biological systems including immune, endocrine, and metabolic processes impacting the functional and structural aspects of the brain. Understanding this pathophysiology is critical for the development of new treatment and effective relapse prevention strategies.
A consistent body of evidence from both preclinical and clinical studies shows that inflammation in both periphery and the central nervous system plays a role in the pathophysiology of mood disorders. Elevated peripheral pro-inflammatory markers have been associated with depression and bipolar disorders (Miller et al., 2009; Strawbridge et al., 2015). Further evidence originates from the clinical observation that one-third of patients treated for cancer or hepatitis C with the pro-inflammatory cytokine interferon-alpha (IFNα) develop an MDD (Widner et al., 2000) whereas anti-inflammatory agents such as cyclooxygenase-2 (COX-2) inhibitors and N-acetyl cysteine could contribute to alleviating depressive symptoms (Müller, 2010). In addition, the prevalence of MDD is higher in patients with chronic medical conditions such as rheumatoid arthritis and type 2 diabetes which are often characterized by low-grade chronic inflammation (Katon et al., 2010).
Communication between the immune system and the brain takes place through multiple pathways, including neural afferents and circulating immune mediators which activate brain endothelial and innate immune cells (perivascular and meningeal macrophages, microglia) (Dantzer et al., 2008). Activated microglia release pro-inflammatory cytokines such as IL-1, IL-6, TNF, and prostaglandins (Perry et al., 2010). Dysregulation of this reciprocal interaction between the nervous and immune systems may lead to neuronal damage or neurodegeneration (Miller et al., 2009).
Serotonin is biochemically derived from tryptophan (TRP). Decreased brain serotoninergic neurotransmission forms the basis of the serotonin hypothesis of depression (Coppen, 1968). Activation of the kynurenine pathway (KP) is one of the potential mechanisms by which inflammation can induce depression by interfering with the metabolism of TRP (Dantzer et al., 2011; Oxenkrug, 2010). Two major enzymatic pathways metabolize TRP. One pathway utilizes the liver enzyme tryptophan 2, 3 dioxygenase (TDO2) of which the activity is regulated by glucocorticoids. The other pathway is regulated by the ubiquitous indoleamine 2, 3 dioxygenase (IDO1) enzyme. IDO1 is activated by proinflammatory cytokines especially interferon gamma (IFNγ). Downstream of IDO1, two separate metabolic pathways transform kynurenine (KYN), the main metabolite of TRP, to the neuroprotective kynurenic acid (KYNA) in astrocytes or the neurotoxic 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN) in microglia (Müller and Schwarz, 2008; Schwarcz et al., 2012). This latter pathway is regulated by activation of microglial kynurenine mono-oxygenase (KMO). KYN/TRP ratio provides a measure of peripheral tryptophan breakdown and is used as an index of IDO1 activity when observed with other immune activation markers such as neopterin (Dantzer et al., 2011).
Current pathogenic models suggest that elevated pro-inflammatory cytokines activate IDO1 and KMO resulting in increased susceptibility to MDD (Campbell et al., 2014) by switching the kynurenine metabolites balance towards neurotoxicity (Müller and Schwarz, 2008; Myint and Kim, 2003). Moreover, preclinical studies have demonstrated that inflammation-induced depression is mediated by increased brain levels of QUIN activating NMDA receptors (Walker et al., 2013). This is in agreement with the therapeutic role of ketamine in the treatment of MDD and the central role played by astrocytes and microglia in glutamine/glutamate dysregulation (Arnone et al., 2015), potentially enhancing neurotoxicity of quinolinic acid (Dantzer and Walker, 2014). Thus, KYNA/QA ratio is proposed as putative neuroprotection index in that decreases in the ratio may contribute to inflammation-induced pathology (Savitz et al., 2015a; Savitz et al., 2015b).
Results from prospective cohort studies in which patients with cancer or hepatitis C virus infection are administered IFN-alpha and mood symptoms are assessed longitudinally together with KP metabolites reveal consistent increases in plasma KYN metabolites associated with symptoms of depression and support the ‘inflammatory hypothesis’ for depression pathogenesis (Baranyi et al., 2015; Bonaccorso et al., 2002; Capuron et al., 2003). Activation of KP is associated with decreased TRP, increased KYN, increased KYN/TRP ratio and possibly decrease in the neuroprotective index KYNA/QA ratio. A meta-analysis of studies measuring plasma tryptophan levels in patients with MDD showed reduced plasma TRP levels (Ogawa et al., 2014) (Ogawa et al 2014). However, the results from studies measuring peripheral blood-based KP metabolites in mood disorders are not always consistent, as increases, decreases or no changes in KP metabolites have been reported.
In this review, we examine the relationship between activation of the kynurenine pathway and mood disorders by systematically reviewing the literature and by running a meta-analysis. We specifically included studies that investigated tryptophan and kynurenine metabolites (tryptophan, kynurenine, kynurenic acid, quinolinic acid, ratio of kynurenine to tryptophan) in patients with MDD or BD. We predicted that decreased TRP, increased KYN, increased KYN/TRP ratio and decreased KYNA/QA ratio would be associated with MDD and BD.
METHODS
Search strategy
The review process adhered to the principles of the PRISMA-Statement (Liberati et al., 2009; Moher et al., 2009; Shamseer et al., 2015). We intended to study the relationship between activation of the kynurenine pathway and mood disorders. Eligibility criteria included: 1) English language articles, 2) Studies published in peer-reviewed journals, and 3) Matched case-control studies comparing patients with mood disorders (unipolar and bipolar disorders) with healthy controls and measuring the circulating levels of kynurenine pathway metabolites reported as means and standard deviation. We also considered longitudinal studies which provided a baseline evaluation. As a secondary analysis, we considered the possibility of measuring longitudinal changes in response to pharmacological or psychological treatment.
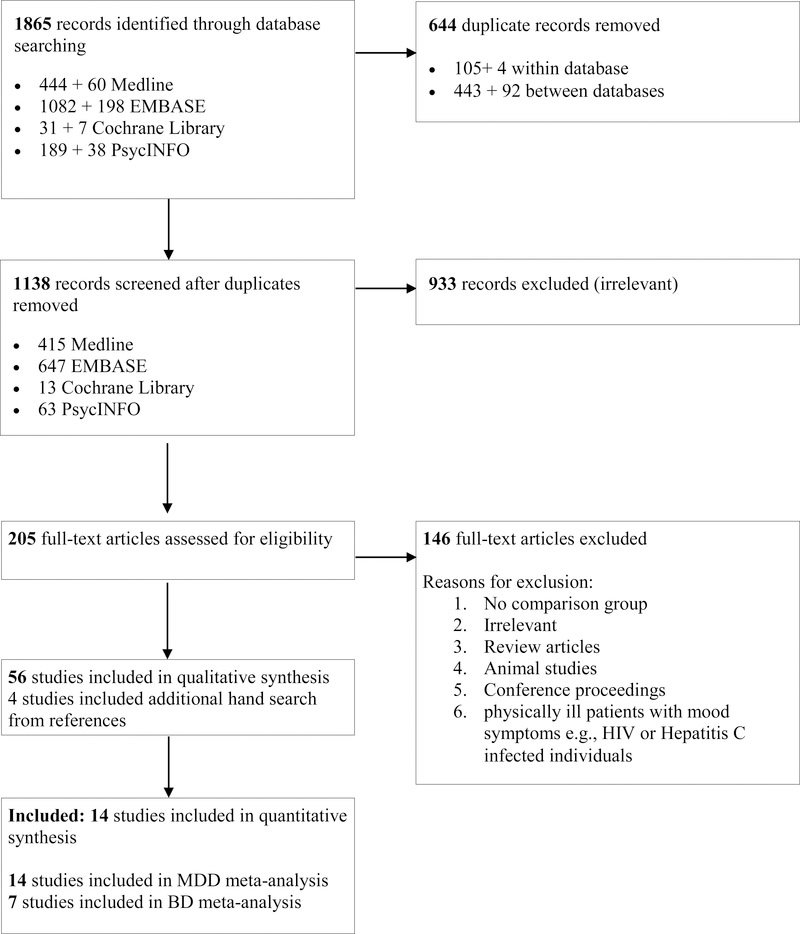
We excluded studies carried out in physically ill patients with mood symptoms, e.g., HIV or Hepatitis C infected individuals. We developed a protocol to review study eligibility, information sources, and data elements for abstraction (available upon request). Ovid Medline, Elsevier EMBASE, Cochrane library, and Ovid PsycINFO were systematically researched from inception to June 2017. The search terms included – “Kynurenine” [Mesh] OR “Kynurenine” [tw] OR “Quinolinic Acid” [Mesh] OR “Quinolinic Acid” [tw] OR “picolinic acid” [Supplementary Concept] OR “picolinic acid” [tw] OR “3-Hydroxyanthranilic Acid” [Mesh] OR “3-Hydroxyanthranilic Acid” [tw] OR “kynurenine metabolites” [tw] OR “kynurenine metabolite” [tw] AND “Affect” [Mesh] OR “Affect” [tw] OR “Mood Disorders” [Mesh] OR “Mood Disorders” [tw] OR “mood” [tw] OR “mood symptoms” [tw]. The detailed search strategy is provided as online Appendix A. Study eligibility was assessed following the criteria are shown in the flowchart in Figure 1. Two trained reviewers (SS, HS) independently reviewed the unduplicated records retrieved from the search to assess eligibility and resolved uncertainty by consensus (SS, HS, and DA). Basic demographic information and clinical variables were systematically extracted including all available levels of metabolites.
Figure 1:
Study flow for the systematic review – Kynurenine Pathway and Mood Disorders
Quality assessment, Data synthesis, and analysis
Study quality assessment and scoring was carried out by using the ‘Study Quality Form’ from the ‘Guide to Community Preventive Services’ (Zaza et al., 2000) and varied based on the ‘number of limitations’ from ‘good’ (0–1) to ‘fair’ (2–4) and ‘limited’ (≥ 5).
Meta-analysis was conducted using STATA 9.0 (Stata Corp, College Station, Texas) supplemented by ‘Metan’ software downloadable from the Centre for Statistics in Medicine, Oxford, UK as previously described (Arnone et al., 2009; Arnone et al., 2012; Lavagnino et al., 2016; Selvaraj et al., 2014). Standardised mean differences were calculated using Cohen’s d statistic. The presence of heterogeneity was tested using the Q-test. Its magnitude was estimated using I2, which can be interpreted as the proportion of effect size variance due to heterogeneity (Higgins et al., 2003). If the Q-test was significant, Galbraith plot served to identify studies contributing to heterogeneity. We explored heterogeneity with meta-regression by using all the available confounders including a year of publication, mean age, the percentage of depressed men, and illness severity for both symptoms of depression and elation whenever possible. Publication bias was examined using the Egger’s test (Egger et al., 1997) with a significance level of p < 0.05.
RESULTS
Study selection
The initial database search identified 1,456 eligible studies, 56 survived the initial suitability assessment and additional hand search identified 2 more studies each in MDD and BD respectively. Twenty-one were included in the meta-analysis (14 investigating MDD and 7 BD) (Figure 1: Flow diagram). Table 1 and 2 summarizes the details of the studies included in the meta-analysis and the Supplementary Table describes details of the selection process and quality assessment.
Table 1:
Kynurenine Pathway metabolites in patients with major depressive disorder compared to healthy controls
| Author, Year | Depressed patients | Controls | Depressed patients | Controls | Main findings | Inflammatory makers | Anti-depressant Medication | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (M/F) | Age mean (SD) | N (M/F) | Age mean (SD) | TRP mean (SD) | KYN mean (SD) | K/T Ratio mean (SD) | KYNA/QA Ratio mean (SD) | TRP mean (SD) | KYN mean (SD) | K/T Ratio mean (SD) | KYNA/QA Ratio mean (SD) | ||||
| Wood K, 1978a | 27 (10/17) | 58.3 (3.9) | 30 (14/16) | 52.8 (2.6) | NR | 2.3±0.2 nMol/ml | NR | NR | NR | 2.3 ± 0.1 | NR | NR | No difference in KYN | NR | No |
| Sublette M, 2011 | 30 (16/14) | 37.8 (13.1) | 31 (15/16) | 35.7(13.9) | 59.2 (10.4) (μmol/L) | 1.5 (0.3) | 25.6 (5.7) | NR | 60.2 (7.7) | 1.3 (0.4) | 22.2 (5.5) | NR | Increased K/T ratio in patients No difference in KYN and TRY KYN levels were higher in MDD suicide attempters compared with MDD non-attempters |
Neopterin correlated positively with K/T ratio in a sub-group of suicide attempters | No |
| Quak J, 2014 | 1042 (334/708) | 40.9 (12.1) | 1007 (379/628) | 41.8 (14.3) | 64.1 (13.4) μmol/l | 2.2 (0.7) | 35.1 (9.1) | NR | 63.9 (14.3) | 2.3 (0.7) | 36.0 (9.2) | NR | No difference in KYN, TRY and KYN/TRY ratio | CRP and IL-6 were associated with K/T in all subjects and in subjects with current MDD | Yes(N=462) |
| Bradley K, 2015 | 50 (35/15) | 15.9 (2.1) | 22 (9/13) | 16 (2.6) | 898.9 (685.8) ng/ml | 374.2 (138.2) ng/ml | 0.58 (0.34) | NR | 982.9 (406.3) ng/ml | 408.7(148.1) ng/ml | 0.49 (0.27) | NR | No difference in KYN, TRY and KYN/TRY | NR | Yes (n=17) |
| Dahl J, 2015 | 50 (12/38) | 40 (12.0) | 34 (15/19) | 38.3 (13.9) | 56.6 (11.4) μM/l | 2.0 (0.6) | 0.04 (0.03–0.038) | 54.6 (12.4) | 2.2 (0.82) | 0.04 (0.029–0.04) | No difference in KYN, TRY, KYN/TRY and KYN/QA ratios | K/T ratio positively correlated with the plasma conc. of cytokines in HC but not in MDD patients. | No | ||
| Meier T, 2015 | 73 (16/57) | 34.2 (9.3) | 91 (36/55) | 31.6 (9.3) | 54.4 (11.4) μM | 1.90 (0.5) | 0.04 (0.01) | 0.11 (0.04) | 60.4 (17.4) | 1.95 ± 0.48 | 0.03 (0.01) | 0.12 (0.04) | No difference in KYN, TRY and KYN/TRY KYN/QA ratios reductions not present after corrections |
log normalized CRP correlated with TRY, KYN/TRY and KYN/QA ration in both groups | No |
| Baranyi, 2017 | 71 (47/24) | 49.2 (11.4) | 48 (31/17) | 46.1 (18.3) | 60.1 (10.9) μmol/L | 2.4 (0.62) μmol/L | 0.04 (0.01) | NR | 67.7 (9.5) μmol/L | 2.5 (0.62) μmol/L | 0.04 (0.01) | NR | No difference in KYN, KYN/TRY and QA Decreased TRY and KYNA in patients |
NR | Yes (n=59) |
| Krause, 2017 | 32 (16/16) | 44.6 (11.6) | 20 (15/5) | 40 (10.4) | NR | 1.78 (0.35) | NR | 0.17 (0.05) *Quin/KYN ratio |
NR | 2.04 (0.35) | NR | 0.14 (0.03) *Quin/KYN ratio |
Decreased KYN Increased *Quin/KYN ratio |
NR | No |
| Umehara, 2017 | 33 (10/23) | 47.1 (13.2) | 33 (10/23) | 46.5 (10.1) | NR | 0.0004 (0.0001) | 0.026 (0.001) | NR | NR | 0.0005 (0.0001) | 0.031 (0.001) | NR | Decreased KYN and no difference in KYN/TRY ratio KYN levels normalized after antidepressant treatment |
NR | No |
NR- Not Reported; NS – Non significant
TRP - Tryptophan; KYN - Kynurenine; K/T ratio – Kynurenine/Tryptophan ratio; KYNA - Kynureninc Acid; QA - Quinolinic acid
MDD – Major Depressive Disorder; CRP – c-reactive protein; IL-6 – Interleukin-6
Table 2:
Kynurenine Pathway metabolites in patients with bipolar disorder compared to healthy controls
| Author, Year | Bipolar patients | Controls | Bipolar patients | Control patients | Main findings | Inflammatory makers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (M/F) | Age mean (SD) | N | Age mean (SD) | TRP mean (SD) | KYN mean (SD) | K/T ratio mean (SD) | KYNA/QA Ratio mean (SD) | TRP mean (SD) | KYN mean (SD) | K/T ratio mean (SD) | KYNA/QA Ratio mean (SD) | |||
| Myint 2007a | 39 (15/24) | 37.6 (11.6) | 80 (40/40) | 39.1 (8.8) | 61.0 (15.9) (μmol/l) | 1.72±0.65 (μmol/l) | 0.028±0.011 | NR | 70.6±12.7 (μmol/l) | 1.88±0.46 (μmol/l) | 0.027±0.007 | NR | Bipolar mania Decreased TRP in patients with mania No differences in KYN, K/T ratio |
NR |
| Reininghaus 2014 | 78 | 46.9 (14.2) | 156 | 35.2 (11.6) | NR | 3.1 ± 0.9 | 52.8 (12.9) | NR | NR | 2.6 ± 0.7 | 45.23 ± 11.26 | NR | Euthymic patients Increased KYN and KYN/TRY in patients Overweight euthymic patients with BD had a significantly increased serum kynurenine concentration and KYN/TRY |
Neopterin levels were decreased in patients with BD when compared to healthy controls |
| Savitz, 2015a | 63 (13/50) | 38.8 (1.4) | 48 (19/29) | 32.6 (1.5) | 57.7 (1.3) μM | 1.90 (0.1) nM | 0.03 (0.001) | 0.12 (0.01) | 64.4 (2.9) | 1.99 (0.07) | 0.03 (0.001) | 0.14 (0.01) | Bipolar depressed Decreased KynA/QAin patients |
CRP levels was not significantly different among the groups. |
| Poletti 2016 | 22 (8/14) | 46.5 (13.7) | 14 (6/8) | 27.2 (8.3) | 11.97 (2.6) | 367.82 (112.1) | 31.17 (8.5) | NR | 14.08 (1.9) | 430.54 (148.2) | 30.33 (7.6) | NR | Bipolar depressed Decreased TRY, KYN and KYNA levels in patients No differences in K/T ratio |
NR |
| Platzer 2017 | 68 (42/26) | 43.6 (14.3) | 93 (36/57) | 38.1 (15.1) | 11739.07 (2714.62) ng/ml | 477.29 (230.32) ng/ml | NR | NR | 12805.84 (9444.7) ng/ml | 445.49 (152.15) ng/ml | NR | NR | Euthymic patients No significant difference in KYN and TRY in patients vs controls Increased KYN in males compared to females in both groups |
No correlations was found between patients and inflammatory markers |
NR- Not Reported; NS – Non significant
TRP - Tryptophan; KYN - Kynurenine; K/T ratio – Kynurenine/Tryptophan ratio; KYNA - Kynurenic Acid; QA - Quinolinic acid
MDD – Major Depressive Disorder; BD - Bipolar Disorder; Pts – patients; HC - Healthy Controls
MDD in comparison with healthy controls
Fourteen studies were identified and eleven met inclusion criteria (Baranyi et al., 2017; Bradley et al., 2015; Dahl et al., 2015; Krause et al., 2017; Meier et al., 2016; Myint et al., 2013; Myint et al., 2007b; Neupane et al., 2015; Quak et al., 2014; Savitz et al., 2015b; Sorgdrager et al., 2017; Sublette et al., 2011; Umehara et al., 2017; Wood et al., 1978) (Table 1) having excluded the study by Myint and colleagues, 2013 (Myint et al., 2013) because cases and controls were not fully matched at baseline, Myint and colleagues, 2007 (Myint et al., 2007b) which enrolled patients with BD, and Neupane and colleagues (Neupane et al., 2015) which included participants with alcohol-related disorders. The studies by Meier and colleagues (2015) and by Savitz and colleagues (2015) (Savitz et al., 2015b) contained the same participants and were included in separate analyses. Quak et al. (Quak et al., 2014) and Sorgdrager et al. (Sorgdrager et al., 2017) shared study participants and the study with the largest sample size (Quak et al., 2014) was included (supplementary data were made available by the authors). The majority of the studies included unmedicated patients aside Quak and others (Quak et al., 2014), Bradley and others (Bradley et al., 2015) and Baranyi and colleagues (Baranyi et al., 2017).
a). Circulating levels of kynurenine
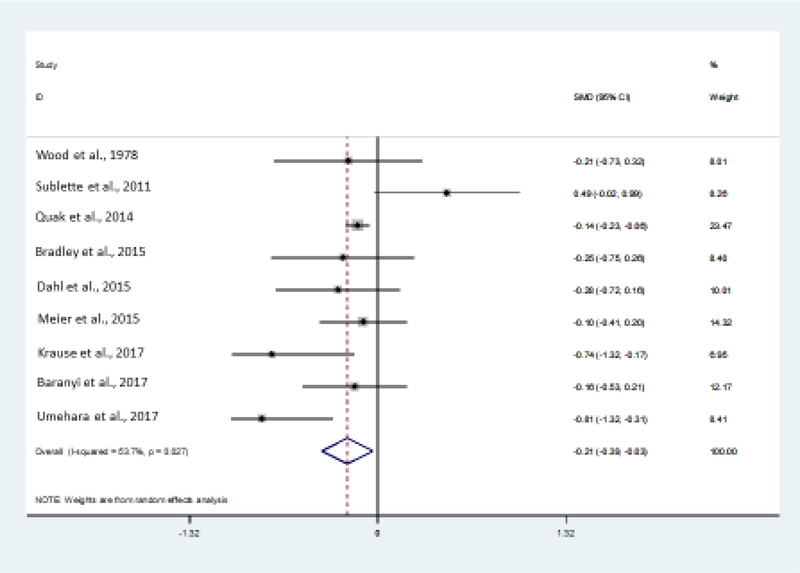
Analysis of the nine studies available (Baranyi et al., 2017; Bradley et al., 2015; Dahl et al., 2015; Krause et al., 2017; Meier et al., 2016; Quak et al., 2014; Sublette et al., 2011; Umehara et al., 2017; Wood et al., 1978) showed a significant reduction in kynurenine in MDD vs. healthy controls with an effect size of −0.21 (CI: −0.39, −0.03) (Figure 2a). Significant heterogeneity (p=0.027) was systematically explored with meta-regression analyses. None of the available variables extracted from the studies proved to explain the variance of the effect size (year of publication, mean age, %men, illness severity, number of medicated individuals, all ps>0.05). There was no evidence of publication bias (p=0.47).
Figure 2:
Meta-analysis output (funnel plot) in the unipolar major depression of circulating levels of kynurenine metabolites
b). Circulating levels of tryptophan
Six studies were included in this analysis (Bradley et al., 2015; Dahl et al., 2015; Meier et al., 2016; Quak et al., 2014; Sublette et al., 2011; Wood et al., 1978) which showed a non-significant reduction in levels of TRP in patients with MDD. The effect size was −0.20 (CI: −048, 0.07). There was significant heterogeneity (p=0.001). Meta-regressions indicated that prescribed medication influenced the effect size (Coef: 0.0007, Z=3.02, p=0.003) as well as % men (Coef: −0.01, Z=−2.49, p=0.013), and year of publication (Coef: −0.17, Z=−3.27, p=0.001), whereas severity trended to significance (Coef: −0.18, Z=−1.94, p=0.053) and mean age was not significant (p=0.57). There was no evidence of publication bias (p=0.25).
c). Kynurenine/tryptophan ratio
Six studies were included in this analysis (Baranyi et al., 2017; Bradley et al., 2015; Dahl et al., 2015; Meier et al., 2016; Quak et al., 2014; Sublette et al., 2011) and indicated a trend towards an increase in the kynurenine/tryptophan ratio in MDD with an effect size of 0.18 (CI: −0.06, 0.42). This analysis was characterized by high level of heterogeneity (p=0.006). The presence of publication bias (p=0.018) indicated that results from these studies are not necessarily generalizable. Exploration of heterogeneity indicated that the effect size correlated with increasing number of medicated patients (coeff: −0.0002, Z=3.62, p<0.001), whereas a year of publication, mean age of the depressed group, % of men and severity did not affect the effect size (all Ps>0.05).
d). Kynurenic acid/quinolinic acid ratio
There were no sufficient studies in MDD to carry out this analysis.
Bipolar disorder in comparison with healthy controls
Seven studies were identified of which five studies were included (Myint et al., 2007a; Platzer et al., 2017; Poletti et al., 2016; Reininghaus et al., 2014; Savitz et al., 2015a) (Table 2) having excluded the post-mortem study by Miller and colleagues (2008) and the study by Birner and colleagues (Birner et al., 2017) overlapping with Platzer and colleagues (Platzer et al., 2017) already included in the same analyses. Overlapping data from Reininghaus and colleagues (2014) and Platzer et al. (2017) were considered in separate sub-analyses. All patients were medicated aside the study by Myint and others (Myint et al., 2007a) resulting in very little variance for a meaningful analysis.
a). Circulating levels of kynurenine
Four studies were included in this analysis (Myint et al., 2007a; Platzer et al., 2017; Poletti et al., 2016; Savitz et al., 2015a) which showed a non-significant marginal decrease in levels of kynurenine with an effect size of −0.42 (CI: −1.18, 0.34). There was evidence of a high level of heterogeneity (p<0.001) in the absence of publication bias (p=0.51). Exploration of heterogeneity with meta-regression analyses based on the available variables showed that a higher percentage of men (Coeff: 3.75, Z=6.23, p<0.001) positively correlated with the effect size, but not the year of publication, mean age and severity of illness (all ps>0.05).
b). Circulating levels of tryptophan
Four studies were included in this analysis (Myint et al., 2007a; Platzer et al., 2017; Poletti et al., 2016; Savitz et al., 2015a) which showed a non-significant tendency to a reduction in levels of TRP in patients with BD. The effect size was −1.15 (CI: −2.43, 0.1). There was evidence of significant heterogeneity (p<0.001) in the absence of publication bias (p=0.3). Meta-regressions indicated that the effect size positively correlated with increasing percentage of men (Coeff: 6.93, Z=3.04, p=0.002). Year of publication, mean age of the patients, and severity of illness did not affect the effect size (ps>0.05).
c). Kynurenine/tryptophan ratio
Four studies were included in this analysis (Myint et al., 2007a; Poletti et al., 2016; Reininghaus et al., 2014; Savitz et al., 2015a) which indicated a non-significant tendency to an increased ratio 0.24 (CI: −0.10, 0.59) with significant heterogeneity (P=0.025) and no evidence of publication bias (p=0.35). Meta-regressions indicated that the effect size positively correlated with increasing age (Coeff: 0.056, Z=2.62, p=0.009). Year of publication, %men, and severity of illness did not affect the effect size (ps>0.05).
d). Kynurenic acid/quinolinic acid ratio
There were no sufficient studies in BD to carry out this analysis.
Longitudinal studies evaluating the effect of therapeutic interventions and clinical outcome on kynurenine pathways
There were only two studies evaluating the effect of pharmacological interventions on kynurenine pathways in patients with MDD (Krause et al., 2012; Wood et al., 1978), but none in BD meeting our inclusion criteria. Hence it was not possible to conduct meta-analyses. Wood and colleagues (Wood et al., 1978) found that clinical outcome could not be accurately predicted by measurements of plasma kynurenine levels. They also reported that amitriptyline did not significantly increase plasma kynurenine concentration contrary to lithium and mianserin. Krause and others reported that higher kynurenine/tryptophan ratio at baseline predicted remission in reboxetine-treated patients with MDD with or without adjuvant treatment with the cyclooxygenase-2 inhibitor celecoxib. Remitters were also characterized by lower kynurenic acid/kynurenine and quinolinic acid/kynurenine ratios. Authors proposed that lower downstream kynurenine metabolites formation were likely to be predictive of better outcome (Krause et al., 2012).
DISCUSSION
In this manuscript, we set out to review the range of kynurenine metabolites variations in MDD/BD in comparison with healthy controls by selecting the most robust evidence available in the literature to generate summary effect sizes. Our results indicate that although kynurenine metabolites show variations in affected individuals, these changes are of a small magnitude and reach significance in the analysis of kynurenine metabolites in unipolar major depression. Based on the data analyzed, alterations of the balance between neuroprotective and neurotoxic kynurenine metabolites could still play a pathogenic role in subgroups of patients with mood disorders. The direction of changes in kynurenine metabolites which indicates a reduction in major depression vs. healthy controls rather than an increase as we anticipated suggests that major depression in chronic inflammatory conditions may etiologically differ from other depressive disorders.
In both MDD and BD the high level of heterogeneity suggests that a number of confounders and clinical variables are likely to contribute to the effect size so that variables such as year of publication, age of participants, sex, and illness severity for both symptoms of depression and mania appear to influence variations in the effect size. Furthermore, in the case of kynurenine/tryptophan ratio in MDD, the presence of publication bias seems to suggest that studies might be of a too small magnitude to detect meaningful differences without incurring into type I/II error.
There were differences in design across all the studies reviewed. For example, a large number of studies were observational and presented naturalistic uncontrolled data. Case-control studies, which represent the main source of this review, did not offer any information regarding the temporal and/or directional relationship between mood disorders and kynurenine metabolites variations. The results from the only two prospective cohort studies that were included in the present meta-analysis provided insufficient data to allow conclusive statements about the generalizability of the results. Other putative factors shown to affect levels of kynurenine metabolites include antidepressants and other treatments like electroconvulsive therapy (Cervenka et al., 2017). However, the variations in the effect size of reduction of kynurenine levels seen in MDD patients vs. controls was not explained by the antidepressant medication status in our analyses.
Other potential limitations include the effects of weight and obesity, physical activity, use of tobacco and other addictive drugs. TDO and IDO are two major enzymatic pathways that metabolize TRP with the TDO’s activity regulated by glucocorticoids and IDO activated by proinflammatory cytokines such as IFNγ. Effect of dietary modifications, body weight, and physical activity can affect tryptophan levels and therefore kynurenine pathway and its metabolites both in patients with mood disorders and in healthy controls (Cervenka et al., 2017; Strasser, 2017). However, these factors are not controlled in all studies. For example, Reininghaus et al report increased KYN and KYN/TRY ratio levels in overweight bipolar disorder patients. Future studies should take these factors into consideration when evaluating KP pathway in mood disorders.
The question also remains whether peripheral measurements are the optimal approach to evaluate kynurenine metabolites and whether effectively reflect brain concentrations. Raison et al. 2010 (Raison et al., 2010) is the only study that evaluated the effect of IFN-alpha/ribavirin therapy on both plasma and cerebrospinal fluid (CSF) levels of TRP, KYN and QUIN as well as the KYN/TRP ratio in HCV patients (n=16). IFN-alpha administration increased KYN in peripheral blood and KYN, QUIN and KYNA in the CSF after ~12 weeks compared to control patients. CSF KYN and QUIN correlated with the severity of depressive symptoms and, in addition, plasma KYN and QUIN concentrations correlated with the CSF KYN and QUIN concentrations. Thus, Raison et al. study, though with limited sample size, provides evidence that changes in peripheral KYN metabolism are linked to central nervous system cytokine responses in this specific IFN-alpha-induced depression. Future prospective studies including both peripheral and CSF measures of KP metabolites in mood disorders are required to better define if peripheral and CSF levels are indeed correlated and if they are relevant to the course of illness and treatment.
It is also unknown if any specific kynurenine metabolite could efficiently reflect symptom changes in mood disorders. It has been suggested that downstream metabolites such as KYNA and/or QUIN could possibly allow a more refined understanding of the underlying mechanisms of mood regulation in view of their interaction with inflammatory responses (Myint, 2012). However, this hypothesis remains to be tested as it is not possible based on the results presented here to comment on whether alterations in kynurenine pathways are a consequence of inflammation or are driven by alterations in cortisol levels possibly modulated by susceptibility to stress (Dantzer et al., 2011).
In addition to depression, inflammation has been linked to suicidal risk. When plasma KYN and TRP were assayed in patients with MDD with and without a history of suicide attempts, KYN and K/T ratio were significantly higher in MDD patients with suicide attempt while higher KYN, K/T ratio predicted suicide attempt (Sublette et al., 2011). Cerebrospinal fluid levels of QUIN and KYNA measured in suicide attempters and controls showed that QUIN was significantly elevated in suicidal patients and QUIN correlated with suicide intent scale (Erhardt et al., 2013). Together these findings suggest that the pharmacologic manipulation of KYN levels might reduce suicide risk.
High KYN levels were associated with increased immune activation, while pre and post-treatment KYN levels significantly predicted changes in depression, suggesting that heightened KP activation may contribute to depressive symptoms in patients treated for cancer. Findings from a pancreatic cancer study yielded similar results, reflecting tumor-induced immunomodulation in the pathophysiology of depression (Botwinick et al., 2014). However, patients with renal cell carcinoma (Van Gool et al., 2008), receiving IFN-α showed an increase in K/T ratio during the treatment, but no correlation was observed between psychiatric symptoms and KP metabolites. Thus, KP pathway measures associated with immune activation do not always relate to mood changes.
Limitations of this meta-analysis include the small number of studies included, the nature of case-control studies and absence of randomization, the occurrence of study bias so that studies were generally similar with relatively small sample sizes resulting in relatively low statistical power and the very high level of between studies heterogeneity. There are limitations in the estimation of effect sizes based on the inverse-variance methodology we used in this work especially in relation to the variance introduced by confounders. Hence, we explored all the possible causes of heterogeneity by using meta-regression analyses based on the variables we could consistently extract from the studies. Other approaches such as the Mantel-Haenszel methods offer advantages over the method we used here although constrained in the presence of several confounders and the relatively small sample sizes characterizing the studies included in this review (Tripepi et al., 2010). Mood disorders are often heterogeneous clinical presentations which limit our ability to generalize the results from this meta-analysis largely based on studies which did not specifically include linear effects. This is particularly relevant in view of the high levels of heterogeneity noted in the analyses, the low power of meta-regressions (Arnone et al., 2012) and the discrepancies in the confounders reported in the original studies. Future work would benefit from focusing on kynurenine changes linked to between-subject heterogeneity to more specifically improve precision and generalizability of the results.
In conclusion, the main finding of our work suggests a reduction in kynurenine metabolites in major depression vs. healthy controls. The direction of the change contravenes the postulated theoretical increase based on findings of low mood in chronic and inflammatory diseases and would require replication and exploration in future studies with particular attention to linear effects. If confirmed, this result suggests different etiologies for mood dysregulation. Our findings support a role for kynurenine metabolites variations in the pathogenesis of mood disorders, although it does not allow definitive conclusions regarding its role in mood disorders due to the complex interaction among epidemiological and clinical factors. This finding is important in guiding further research which would benefit from larger studies preferably comparing kynurenine metabolites in individual experiencing mood disorders with and without inflammatory conditions in comparison with healthy controls. Furthermore, systematic measurements of inflammatory markers, cortisol levels, stress responses and a comprehensive exploration of clinical and epidemiological variables would allow to evaluate the impact of these potential variables on kynurenine metabolites measurements and increase the precision of the findings.
Supplementary Material
Acknowledgment
The authors would like to thank the support by Mrs. Emily Couvilon, librarian at Texas Medical Center Library, Houston, who helped to obtain the manuscripts. Danilo Arnone has received support from the Academy of Medical Sciences, UK (grant no. AMS-SGCL8).
Conflict of interest: Danilo Arnone has received travel grants from Janssen-Cilag and Servier and sponsorship from Lundbeck. Robert Dantzer received honoraria from Danone Nutricia Research and is funded by NIH (R01NS073939, R21MH104694, R01CA193522). Sudhakar Selvaraj has received intravenous citalopram for clinical research from Lundbeck UK. Smita Saraykar, Haitham Salem, and Antonio L. Teixeira do not have any conflict of interest to report.
REFERENCES
- Angst J, Hengartner MP, Gamma A, von Zerssen D, Angst F, 2013. Mortality of 403 patients with mood disorders 48 to 52 years after their psychiatric hospitalisation. Eur Arch Psychiatry Clin Neurosci 263, 425–434. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM, 2009. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 195, 194–201. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM, 2012. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol 22, 1–16. [DOI] [PubMed] [Google Scholar]
- Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J, 2015. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol 25, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Amouzadeh-Ghadikolai O, von Lewinski D, Breitenecker RJ, Rothenhausler HB, Robier C, Baranyi M, Theokas S, Meinitzer A, 2017. Revisiting the tryptophan-serotonin deficiency and the inflammatory hypotheses of major depression in a biopsychosocial approach. PeerJ 5, e3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A, Meinitzer A, Breitenecker RJ, Amouzadeh-Ghadikolai O, Stauber R, Rothenhausler HB, 2015. Quinolinic Acid Responses during Interferon-alpha-Induced Depressive Symptomatology in Patients with Chronic Hepatitis C Infection - A Novel Aspect for Depression and Inflammatory Hypothesis. PloS one 10, e0137022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth J, Schumacher M, Herrmann-Lingen C, 2004. Depression as a Risk Factor for Mortality in Patients With Coronary Heart Disease: A Meta-analysis. Psychosomatic Medicine 66, 802–813. [DOI] [PubMed] [Google Scholar]
- Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R, Pilz R, Rauch P, Maget A, Hamm C, Herzog-Eberhard S, Mangge H, Fuchs D, Moll N, Zelzer S, Schütze G, Schwarz M, Reininghaus B, Kapfhammer HP, Reininghaus EZ, 2017. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PloS one 12, e0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M, 2002. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. Journal of affective disorders 72, 237–241. [DOI] [PubMed] [Google Scholar]
- Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, Chabot JA, 2014. A biological basis for depression in pancreatic cancer. HPB : the official journal of the International Hepato Pancreato Biliary Association 16, 740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V, 2015. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res 227, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, Möller T, 2014. Kynurenines in CNS disease: regulation by inflammatory cytokines. Frontiers in Neuroscience 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH, 2003. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry 54, 906–914. [DOI] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, Ruas JL, 2017. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357. [DOI] [PubMed] [Google Scholar]
- Coppen AJ, 1968. Depressed states and indolealkylamines. Adv Pharmacol 6, 283–291. [DOI] [PubMed] [Google Scholar]
- Dahl J, Andreassen OA, Verkerk R, Malt UF, Sandvik L, Brundin L, Ormstad H, 2015. Ongoing episode of major depressive disorder is not associated with elevated plasma levels of kynurenine pathway markers. Psychoneuroendocrinology 56, 12–22. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW, 2011. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Walker AK, 2014. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J Neural Transm (Vienna) 121, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Träskman-Bendz L, Guillemin GJ, Brundin L, 2013. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Solmi M, Veronese N, De Berardis D, Buonaguro EF, Tomasetti C, Perna G, Preti A, Carta MG, 2017. The burden of mood-disorder/cerebrovascular disease comorbidity: essential neurobiology, psychopharmacology, and physical activity interventions. Int Rev Psychiatry 29, 425–435. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC, 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76, 155–162. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Annegers JF, Cascino G, 2000. Major depression is a risk factor for seizures in older adults. Annals of Neurology 47, 246–249. [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. Bmj 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D, 2010. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 363, 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Twisk JWR, Beekman ATF, Heine RJ, Snoek FJ, Pouwer F, 2006. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 49, 837–845. [DOI] [PubMed] [Google Scholar]
- Krause D, Myint AM, Schuett C, Musil R, Dehning S, Cerovecki A, Riedel M, Arolt V, Schwarz MJ, Müller N, 2017. High Kynurenine (a Tryptophan Metabolite) Predicts Remission in Patients with Major Depression to Add-on Treatment with Celecoxib. Front Psychiatry 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DL, Riedel M, Muller N, Weidinger E, Schwarz MJ, Myint AM, 2012. Effects of antidepressants and cyclooxygenase-2 inhibitor on cytokines and kynurenines in stimulated in vitro blood culture from depressed patients. Inflammopharmacology 20, 169–176. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S, 2016. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neuroscience and biobehavioral reviews 68, 714–726. [DOI] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, 2010. COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs 11, 31–42. [PubMed] [Google Scholar]
- Müller N, Schwarz MJ, 2008. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci 258 Suppl 2, 97–106. [DOI] [PubMed] [Google Scholar]
- Myint AM, 2012. Kynurenines: from the perspective of major psychiatric disorders. The FEBS journal 279, 1375–1385. [DOI] [PubMed] [Google Scholar]
- Myint AM, Bondy B, Baghai TC, Eser D, Nothdurfter C, Schüle C, Zill P, Müller N, Rupprecht R, Schwarz MJ, 2013. Tryptophan metabolism and immunogenetics in major depression: a role for interferon-γ gene. Brain Behav Immun 31, 128–133. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, 2003. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses 61, 519–525. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW, Leonard BE, 2007a. Tryptophan breakdown pathway in bipolar mania. J Affect Disord 102, 65–72. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B, 2007b. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98, 143–151. [DOI] [PubMed] [Google Scholar]
- Neupane SP, Lien L, Martinez P, Hestad K, Bramness JG, 2015. The relationship of alcohol use disorders and depressive symptoms to tryptophan metabolism: cross-sectional data from a Nepalese alcohol treatment sample. Alcohol Clin Exp Res 39, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Fujii T, Koga N, Hori H, Teraishi T, Hattori K, Noda T, Higuchi T, Motohashi N, Kunugi H, 2014. Plasma L-tryptophan concentration in major depressive disorder: new data and meta-analysis. J Clin Psychiatry 75, e906–915. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF, 2010. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. The Israel journal of psychiatry and related sciences 47, 56–63. [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C, 2010. Microglia in neurodegenerative disease. Nat Rev Neurol 6, 193–201. [DOI] [PubMed] [Google Scholar]
- Platzer M, Dalkner N, Fellendorf FT, Birner A, Bengesser SA, Queissner R, Kainzbauer N, Pilz R, Herzog-Eberhard S, Hamm C, Hörmanseder C, Maget A, Rauch P, Mangge H, Fuchs D, Zelzer S, Schütze G, Moll N, Schwarz MJ, Mansur RB, McIntyre RS, Reininghaus EZ, 2017. Tryptophan breakdown and cognition in bipolar disorder. Psychoneuroendocrinology 81, 144–150. [DOI] [PubMed] [Google Scholar]
- Poletti S, Myint AM, Schüetze G, Bollettini I, Mazza E, Grillitsch D, Locatelli C, Schwarz M, Colombo C, Benedetti F, 2016. Kynurenine pathway and white matter microstructure in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, Penninx BW, Kema IP, de Jonge P, 2014. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 45, 202–210. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: Relationship to CNS immune responses and depression. Molecular psychiatry 15, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus EZ, McIntyre RS, Reininghaus B, Geisler S, Bengesser SA, Lackner N, Hecht K, Birner A, Kattnig F, Unterweger R, Kapfhammer HP, Zelzer S, Fuchs D, Mangge H, 2014. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: a preliminary report. Bipolar disorders 16, 432–440. [DOI] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, Bellgowan PS, Teague TK, Drevets WC, 2015a. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 52, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R, 2015b. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 40, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Cappai A, Howes O, 2014. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev 45, 233–245. [DOI] [PubMed] [Google Scholar]
- Simon GE, 2003. Social and economic burden of mood disorders. Biol Psychiatry 54, 208–215. [DOI] [PubMed] [Google Scholar]
- Sorgdrager FJH, Doornbos B, Penninx B, de Jonge P, Kema IP, 2017. The association between the hypothalamic pituitary adrenal axis and tryptophan metabolism in persons with recurrent major depressive disorder and healthy controls. Journal of affective disorders 222, 32–39. [DOI] [PubMed] [Google Scholar]
- Strasser B, 2017. Immune-mediated inflammation as a driver of obesity and comorbid conditions. Obesity (Silver Spring) 25, 987–988. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, John Mann J, Postolache TT, 2011. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, behavior, and immunity 25, 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripepi G, Jager KJ, Dekker FW, Zoccali C, 2010. Stratification for confounding--part 1: the Mantel-Haenszel formula. Nephron Clin Pract 116, c317–321. [DOI] [PubMed] [Google Scholar]
- Umehara H, Numata S, Watanabe SY, Hatakeyama Y, Kinoshita M, Tomioka Y, Nakahara K, Nikawa T, Ohmori T, 2017. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci Rep 7, 4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gool AR, Verkerk R, Fekkes D, Bannink M, Sleijfer S, Kruit WH, van der Holt B, Scharpe S, Eggermont AM, Stoter G, Hengeveld MW, 2008. Neurotoxic and neuroprotective metabolites of kynurenine in patients with renal cell carcinoma treated with interferon-alpha: course and relationship with psychiatric status. Psychiatry Clin Neurosci 62, 597–602. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, 2013. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38, 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Widner B, Ledochowski M, Fuchs D, 2000. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr Drug Metab 1, 193–204. [DOI] [PubMed] [Google Scholar]
- Wood K, Harwood J, Coppen A, 1978. The effect of antidepressant drugs on plasma kynurenine in depressed patients. Psychopharmacology 59, 263–266. [DOI] [PubMed] [Google Scholar]
- Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kulis VG, Teutsch SM, Pappaioanou M, 2000. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Task Force on Community Preventive Services. Am J Prev Med 18, 44–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.