Abstract
A number of pulmonary diseases occur with upper lobe predominance, including cystic fibrosis and smoking-related chronic obstructive pulmonary disease. In the healthy lung, several physiologic and metabolic factors exhibit disparity when comparing the upper lobe of the lung to lower lobe, including differences in oxygenation, ventilation, lymphatic flow, pH, and blood flow. In this study, we asked whether these regional differences in the lung are associated with DNA methylation changes in lung macrophages that could potentially lead to altered cell responsiveness upon subsequent environmental challenge. All analyses were performed using primary lung macrophages collected via bronchoalveolar lavage from healthy human subjects with normal pulmonary function. Epigenome-wide DNA methylation was examined via Infinium MethylationEPIC (850K) array and validated by targeted next-generation bisulfite sequencing. We observed 95 CpG loci with significant differential methylation in lung macrophages, comparing upper lobe to lower lobe (all false discovery rate < 0.05). Several of these genes, including CLIP4, HSH2D, NR4A1, SNX10, and TYK2, have been implicated as participants in inflammatory/immune-related biological processes. Functionally, we identified phenotypic differences in oxygen use, comparing upper versus lower lung macrophages. Our results support a hypothesis that epigenetic changes, specifically DNA methylation, at a multitude of gene loci in lung macrophages are associated with metabolic differences regionally in lung.
INTRODUCTION
Numerous lung diseases display upper lobe predominance, including cystic fibrosis, sarcoidosis, tuberculosis, and smoking-related chronic obstructive pulmonary disease (1). There are well-described physiologic differences in oxygenation and ventilation between the upper and lower lobes of the lung in the upright position (2). The origin of these differences has been largely attributed to gravity’s effects on the ventilation/perfusion ratio (VA/Q) in the lung, which is defined as the amount of inspired air divided by the pulmonary blood flow in an alveolus (3). Metabolic factors, such as lymphatic flow, pH, and blood flow, differ because of these inequalities of the VA/Q in the lung. Additionally, an oxygen gradient occurs in the lung because of this VA/Q from the apex to the base of the lung, with the upper lobe of the lung being notably more oxygen rich than the lower lobe at end expiration in an upright position (1). Hypoxia is known to induce broad effects on various aspects of cell biology, including alteration of the transcription of hundreds of genes that allow cells to adapt to the changing oxygen availability in the local environment (4). However, how metabolic factors contribute to upper lobe predominant lung disease is unclear.
Macrophages are the sentinel innate immune cells of the lung and possess remarkable immune plasticity, with the ability to sense and adapt to the local milieu (5). Lung macrophages play key roles in bacterial recognition and elimination as well as in polarization of innate and adaptive immunity. Depending on the local environment, macrophages sometimes play a role in anti-inflammatory responses, tissue repair, and homeostasis, whereas at other times, they promote inflammatory and phagocytic processes through the complex production of cytokines and cellular interaction (6, 7).
Epigenetics is the study of heritable changes in gene function caused by mechanisms other than changes in the underlying DNA sequence (8). Epigenetic mechanisms have emerged as modulators of host defenses that can lead to a more-prominent immune response and shape the course of inflammation in the host, both driving the production of specific inflammatory mediators and controlling the magnitude of the host response (9). It is now clear that both the innate and adaptive immune responses use epigenetic mechanisms of gene regulation to maintain long-term phenotypes (10). One important mode of epigenetic regulation is DNA methylation (11, 12).
In this study, we investigate whether DNA methylation changes in lung macrophages are associated with the metabolic factors that contribute to the regional phenotypic differences in lung. To examine this question, we initiated an epigenome-wide DNA methylation profiling study of lung macrophages, comparing upper lobe cells to lower lobe–derived cells.
MATERIALS AND METHODS
This study was approved by the Committee for the Protection of Human Subjects at the Geisel School of Medicine at Dartmouth (approval no. 22781). All subjects were healthy adults and nonsmokers without any known underlying lung disease.
Bronchoalveolar lavage and macrophage isolation
Following local anesthesia with nebulized lidocaine and i.v. conscious sedation, subjects underwent flexible bronchoscopy. Briefly, a flexible fiberoptic bronchoscope was inserted transorally and advanced through the vocal cords. Bronchoalveolar lavage (BAL) fluid was obtained from tertiary airways in the right upper lobes (RUL) and right lower lobes (RLL). BAL was performed sequentially in the RUL and RLL with 20 ml of sterile saline followed by 10 ml of air, and this was repeated for a total of five times per airway. Lung macrophages were isolated, as previously described (13, 14). BAL fluid was filtered through two-layer gauze, centrifuged, and washed twice in 0.9% NaCl. Cells were counted with a T10 automated cell counter (Bio-Rad, Hercules, CA). Cytospins were performed using a Shandon Cytospin 3 centrifuge (Thermo Fisher Scientific, Waltham, MA). Briefly, 75,000 cells resuspended in 200 μl of 0.9% NaCl were loaded into a cytology funnel (Fisher Scientific, Pittsburgh, PA) and centrifuged for 10 min. Cells were allowed to air dry and processed for viewing via Hema 3 Stat pack (Fisher). Imaging was done on an Olympus (Waltham, MA) BX41 microscope with DP2-BSW software (version 2007).
DNA methylation array
Epigenome-wide DNA methylation profiling was performed via the Infinium Methylation EPIC Bead Chips (Illumina, San Diego, CA) for the determination of methylation levels of more than 850,000 CpG sites, as previously described (15, 16). DNA was extracted from lung macrophages via Qiagen (Germantown, MD) DNeasy Blood and Tissue Kit. DNA was quantitated on a Qubit 3.0 Fluorometer (Life Technologies, Carlsbad, CA). Bisulfite conversion of DNA was carried out with the Zymo EZ DNA methylation kit (Zymo Research, Irvine, CA), and EPIC array hybridization and scanning were performed at the University of Southern California Molecular Genomics Core.
DNA methylation array data processing
Raw intensity data files from the MethylationEPIC BeadChips were processed by the minfi R/Bioconductor analysis pipeline (version 1.21) (17) with annotation file version ilm10b3.hg19. All samples showed consistent and high-quality profiles. CpGs annotated as single nucleotide polymorphisms, technical probes, and sex chromosome associated, as well as those failing to meet a detection p value < 0.05 in ≥20% samples, were excluded. This preprocessing procedure left 813,096 CpGs with high-quality methylation data in the final data set. DNA methylation microarray data has been deposited into Gene Expression Omnibus under accession no. GSE132547.
Targeted, next-generation bisulfite sequencing of candidate genes
Targeted next-generation bisulfite sequencing (tNGBS) was performed on nine of the original 13 BAL specimens measured in the DNA EPICmethylation array by EpigenDx (Hopkinton, MA). DNA bisulfite modification was done using EZ-96 DNA methylation kit (Zymo), followed by multiplex PCR with Qiagen HotStar Taq and products purified with QIAquick PCR purification kit. Libraries were prepared using the KAPA Library Preparation Kit for Ion Torrent platforms and Ion Xpress Barcode Adapters (Thermo Fisher Scientific). Library products were purified using Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN) and quantified using the Qiagen QIAxcel Advanced System. Barcoded samples were then pooled in an equimolar fashion before template preparation and enrichment were performed on the Ion Chef system (Thermo Fisher Scientific) using Ion 520 and Ion 530 Chef reagents. Enriched, template-positive library products were sequenced on the Ion S5 sequencer using Ion 530 sequencing chips (Thermo Fisher Scientific). FASTQ files from the Ion Torrent S5 server were aligned to the local reference database using open-source Bismark Bisulfite Read Mapper with the Bowtie2 alignment algorithm. Methylation levels were calculated in Bismark by dividing the number of methylated reads by the total number of reads.
Extracellular flux assay
Oxygen consumption rate (OCR) in lung macrophages was measured by Seahorse assay (Agilent, Santa Clara, CA), according to manufacturer’s instructions. Briefly, cells were plated at 50,000 per well and allowed to equilibrate overnight. The following morning, cells were washed twice and placed in XF base medium. The Seahorse instrument sensor cartridge was lowered to 200 μm above the cells, creating the transient microchamber. The sensor contains two fluorophores, one measuring oxygen (O2), the other measuring pH. Changes in oxygen concentration and pH were analyzed via XF Mito Stress Test Report Generator software (version 2.0) and reported as OCR in real time, enabling time-resolved, kinetic data of cell metabolism. Seahorse assays were carried out in DartLab, the Immune Monitoring and Flow Cytometry Shared Resource at the Norris Cotton Cancer Center at Dartmouth-Hitchcock Medical Center.
Statistical analysis
To determine the data structure of methylation profiles with respect to sample characteristics, we performed an unsupervised clustering analysis using the recursively partitioned mixture model (RPMM) method (18), assuming two terminal clusters on 10,341 most variable CpGs (all intersample variance > 0.01). RPMM was implemented in the RPMM R package (version 1.25).
To investigate the putative cell-type proportions in all samples, the RefFreeEWAS algorithm (R package version 2.1) (19) was applied to 10,000 most variable CpGs across all samples. The proportion of putative cell types was calculated iteratively for the number of cell types K from 2 to 10. The optimal number of putative cell types K = 2 was selected, as it minimized the variance of the bootstrapped deviance matrix. Subsequently, the two putative cell types by sample were displayed as stratified boxplots for visualization purposes. Putative cell-type 1 proportion was included as a fixed-effect term in the differential methylation analysis linear model, as described below.
The distribution of intersample variance in methylation β value was examined prior to differential methylation analysis: 27,107 CpGs with intersample variance in β values exceeding 0.005 were selected for further investigation. To account for the upper and lower lobe measurements collected from the same subject, the correlation coefficient (0.935) for the 27,107 CpG β values among the unique subjects (n = 13) was first calculated by the duplicateCorrelation function in the limma R/Bioconductor package (version 3.38.3). Differential methylation analysis was implemented by passing logit-transformed β values (i.e., M values), the consensus correlation coefficient for matched subject pairs, into the lmFit and eBayes functions in limma, with adjustment of subject age, sex, and RefFree cell-type 1 as fixed effects and the subject as a random effect in the model, such that Y = β0 + XRUL βRUL + βage Xage + βsex Xsex + βcell type 1 Xcell type 1 + Random Effect (Subject Pair), where Y is the methylation β value, β0 is the intercept, X is a given covariate, β is the respective model coefficient, XRUL is an indicator variable of sample from the right upper lobe, and βRUL is the linear model coefficient associated with it. Data processing, statistical analysis and data visualization R code can be found at https://github.com/Christensen-Lab-Dartmouth/CF_Epigenetics.
RESULTS
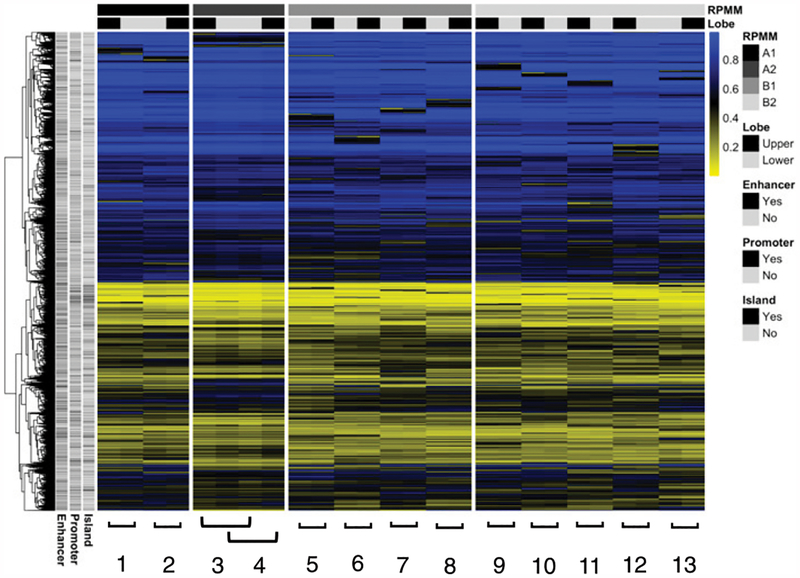
Healthy subjects (n = 13) underwent flexible bronchoscopy to obtain BAL fluid from tertiary airways in both the RUL and RLL, and lung macrophages were isolated as described above. Seven female subjects and six male subjects had a mean age of 27.4 y (SD = 5.3). Genome-scale DNA methylation was measured in lung macrophages from both RUL and RLL, and the most variably methylated 10,341 CpGs from the EPIC methylation array were selected for unsupervised model-based clustering with RPMM (Fig. 1). In nearly all patients, matched RUL and RLL samples cluster immediately adjacent to each other, indicating that intersample variance in DNA methylation is larger than intra-sample variance. For all subjects, both the RUL and RLL sample share RPMM cluster membership. The genomic context for the most variable probes was evenly distributed across promoters/nonpromoters, enhancers/nonenhancers, and CpG islands/non-CpG islands.
FIGURE 1. Unsupervised clustering analysis, upper lobe versus lower lobe macrophages.
RPMM of the 10,000 CpGs (rows) with greatest sample variance across subjects. Individual subjects 1–13 are shown in columns with sample status bar at top: black (upper) and gray (lower). Blue color represents increased sample methylation.
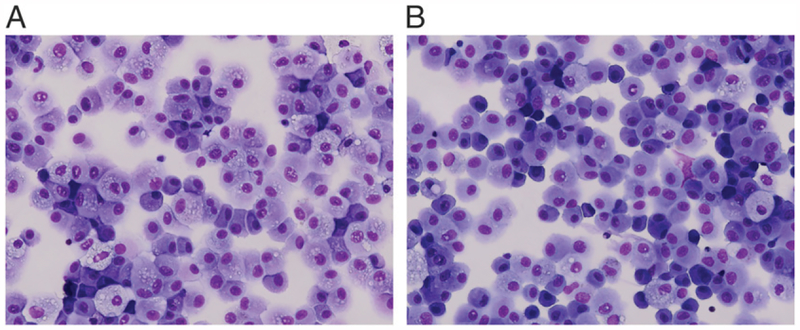
To determine the putative cell-type composition, we applied the RefFreeEWAS algorithm (R package v.2.1, Linux R version 3.5) to 10,000 most variable CpGs, with 500 bootstraps, 10 iterations, and nine presumptive cell types (i.e., K = 2–10), as previously described (16). We chose K = 2 as this value minimized the column variance of the deviance matrix. The distribution of the two putative cell types was very similar between matched RUL and RLL samples (Supplemental Fig. 1). We also explored phenotypic differences between lung macrophages of the upper and lower lobes. Visual evaluation of lung macrophage morphology via cytospin revealed cells similar in presentation from both lobes: typically, a monocytoid appearance with reniform nuclei and ample cytosol (Fig. 2A, 2B), comprising what appeared to be >85% of the total cell population.
FIGURE 2. BAL cell cytospins.
BAL cell samples were obtained from tertiary airways from both the RUL (A) and RLL (B) of healthy subjects (n = 13). BAL cells from healthy subjects are visually a mostly homogeneous population of cells, primarily lung macrophages with oval to reniform nuclei and abundant cytosol. Images shown are representative of multiple subjects. BAL cells were stained with H&E. Original magnification ×400.
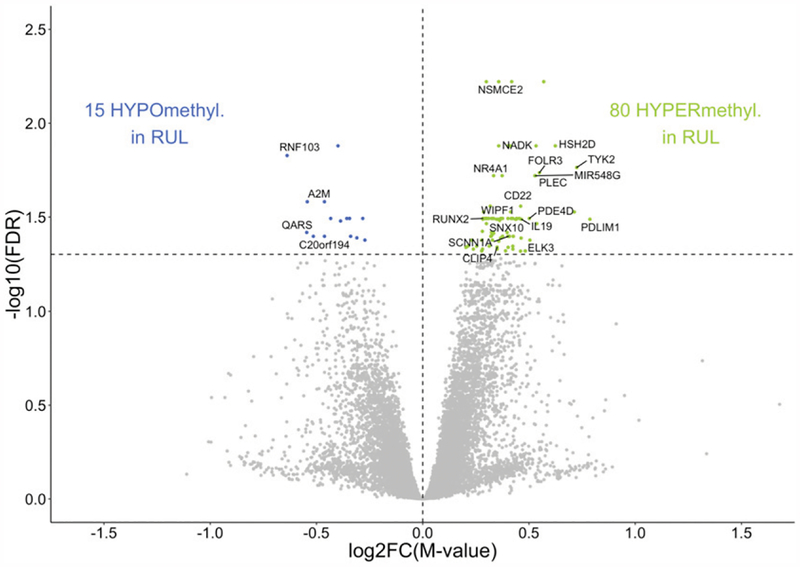
Next, we investigated the relationship between DNA methylation in upper lobe versus lower lobe lung macrophages. Differential analysis comparing RUL and RLL revealeda total of 95 CpGs at a false discovery rate (FDR) < 0.05 (Fig. 3) and 372 CpGs at an FDR < 0.10 (Supplemental Table I). Of the 95 CpGs with an FDR < 0.05, the top 15 CpGs associated with genes of known function are shown in Table I. CpG location varied across multiple University of California, Santa Cruz reference gene groups, including 5′ untranslated region (UTR), TSS1500, TSS200, and body. The observed change in methylation values ranged from −8.2 to 10.7% for upper lobe cells. Upper lobe cells exhibited increased methylation at CpGs tracking to several genes previously implicated in host–pathogen interaction or in inflammatory/immune-related biological processes, including CAP-Gly domain-containing linker protein family member 4 (CLIP4), HSH2D, NR4A1, SNX10, and TYK2.
FIGURE 3. Epigenome-wide differential methylation in RUL macrophages.
Comparative analysis of DNA methylation in upper versus lower lobe macrophages in healthy subjects identified 95 differentially methylated CpGs (FDR p < 0.05). CpGs hypermethylated in RUL (green), and hypomethylated CpGs (blue) are plotted as log2-fold increase or decrease in methylation M value (x-axis) versus log10 FDR-adjusted p value (y-axis). Statistically significant CpGs associated with specific genes are labeled, and unlabeled points represent CpGs associated with no known gene at that location.
TABLE I.
Top EPIC CpGs with differential methylation in upper lobe macrophages associated with genes of known function
| Name | Chromosome | Coordinate | Strand | UCSC Ref Gene Group | Gene | Δβ value | p Value | FDR p Value |
|---|---|---|---|---|---|---|---|---|
| cg22265796 | chr19 | 16254043 | + | 5′-UTR | HSH2D | 0.1070 | 4.05 × 10−6 | 0.013 |
| cgl9307883 | chr19 | 10484624 | + | Body | TYK2 | 0.1038 | 8.89 × 10−6 | 0.017 |
| cg07676849 | chr11 | 71845665 | − | TSS1500 | FOLR3 | 0.0747 | 1.02 × 10−5 | 0.018 |
| cgl7323256 | chr12 | 52445897 | − | 5′-UTR/Body | NR4A1 | 0.0507 | 1.33 × 10−5 | 0.019 |
| cg00889217 | chr12 | 9233083 | − | Body | A2M | −0.082 | 1.98 × 10−5 | 0.026 |
| cgl3427753 | chr6 | 45406009 | − | Body | RUNX2 | 0.049 | 4.76 × 10−5 | 0.032 |
| cg25648980 | chr12 | 51719079 | + | TSS1500 | BIN2 | 0.0484 | 4.77 × 10−5 | 0.032 |
| cg02290197 | chr7 | 26370519 | + | 5′-UTR | SNX10 | 0.0508 | 3.90 × 10−5 | 0.032 |
| cgl1259727 | chr2 | 175465297 | − | 5′-UTR | W1PF1 | 0.0516 | 4.33 × 10−5 | 0.032 |
| cgl8255141 | Chr12 | 6487113 | − | TSS1500 | SCNN1A | 0.0742 | 4.79 × 10−5 | 0.032 |
| cgl6711835 | chr5 | 59558467 | − | 5′-UTR | PDE4D | 0.0747 | 3.80 × 10−5 | 0.032 |
| cg14975420 | Chr10 | 91096289 | + | 5′-UTR/Body | IFIT3 | 0.0479 | 5.95 × 10−5 | 0.033 |
| cg06086177 | chr16 | 73090646 | + | 5′-UTR | ZFHX3 | 0.0465 | 7.92 × 10−5 | 0.038 |
| cgl3126638 | Chr12 | 6487080 | + | TSS1500 | SCNN1A | 0.0545 | 1.01 × 10−4 | 0.040 |
| cg26118047 | chr2 | 29320360 | + | TSS200 | CLIP4 | 0.0590 | 1.40 × 10−5 | 0.046 |
Δβ, increase in the proportion of methylated alleles in upper lobe cells; TSS, transcription start site; UCSC, University of California, Santa Cruz.
Subsequently, we used tNGBS for validation of the results from Infinium MethylationEPIC array. Assays were designed and run for NR4A1 (cg17323256), A2M (cg00889217), RUNX2 (cg13427753), and CLIP4 (cg26118047). A number of the original Infinium EPIC array CpGs were situated within repeat elements (LINE or SINE), and tNGBS assay design was unsuccessful (i.e., HSH2D, TYK2, SNX10, and SCNN1A). Methylation values from tNGBS were consistent with the EPIC array. Percent methylation difference (Δβ) values for select gene-associated CpGs are presented in Table II. Additionally, an example of tNGBS assay design for CLIP4 is illustrated in Supplemental Fig. 2.
TABLE II.
Comparison of percent methylation changes (Δβ) between EPIC array and tNGBS platforms at gene-specific CpGs
| Gene | Location | EPIC Δβ Value |
tNGBS Δβ Value |
|---|---|---|---|
| NR4A1 | cgl7323256 | 0.051 | 0.011 |
| CLIP4 | cg26118047 | 0.059 | 0.073 |
| RUNX2 | cgl3427753 | 0.049 | 0.044 |
| A2M | cg00889217 | −0.082 | −0.086 |
| PDE4D | cgl6711835 | 0.075 | 0.052 |
| ZFHX3 | cg06086177 | 0.046 | 0.017 |
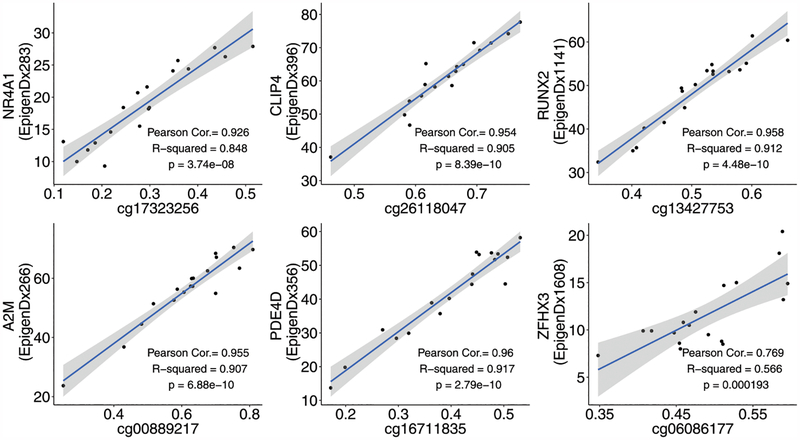
At all targeted gene loci, the two methylation platforms, Illumina EPIC array versus tNGBS, showed significant correlations: NR4A1, CLIP4, RUNX2, A2M, PDE4D, and ZFHX3 (Fig. 4). Correlation and p values ranged from 0.769 and p = 1.93e−04 (ZFHX3) to 0.96 and p = 2.79e−10 (PDE4D).
FIGURE 4. Correlation of DNA methylation analysis between Illumina EPIC methylation array and tNGBS platforms.
Correlation coefficients and linear regression p values were determined for CpGs associated with the genes NR4A1, CLIP4, RUNX2, A2M, PDE4D, and ZFHX3 as methodology validation, directly comparing the Illumina EPIC DNA methylation array and tNGBS assays.
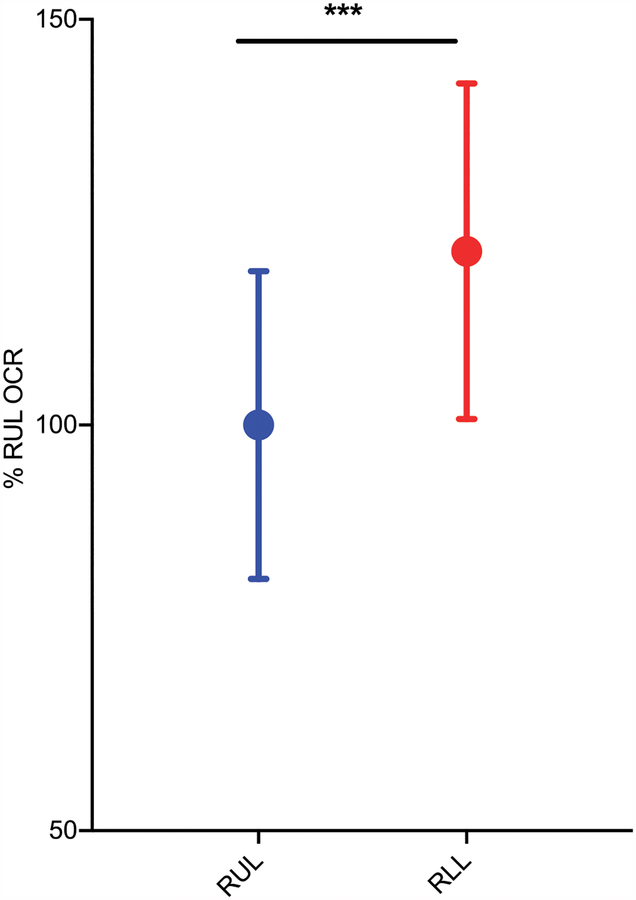
Lung macrophages were also evaluated for differences in metabolic function by measuring basal respiration levels. OCR was assayed as a measure of basal mitochondrial function in cells comparing upper versus lower. The XF Cell Mito Stress Test performed on the Seahorse extracellular flux analyzer revealed a 21.4% difference (p < 0.001) in basal respiration between RUL versus RLL cells, with lower lobe cells exhibiting a higher OCR (Fig. 5).
FIGURE 5. Oxygen consumption is higher in lower lobe macrophages.
Primary lung macrophages isolated from BAL fluid were plated and allowed to equilibrate in vitro for 16 h. OCR was measured via a two-fluorophore sensor cartridge via the Seahorse Mito Stress assay. Data are pooled from six to eight wells per experiment, with lung macrophages from three separate donors. Values were normalized to percent RUL OCR. ***p < 0.001 by unpaired Student t test.
DISCUSSION
It has been recognized for more than a half century that differences in physiologic and metabolic factors, such as lymphatic flow, ventilation, pH, and oxygenation levels, exist regionally within the lung (2, 3, 20–22). However, the impact of these physiologic differences on disease pathology or the immune response in upper lobe predominant lung diseases is unclear. We hypothesized that regional physiologic differences may contribute to epigenetic variability in lung macrophages isolated from the upper and lower lobes of the lung, as these cells are long lived and would be prone to epigenetic modifications in response to environmental differences. The goal of this study was to determine if epigenetic changes in lung macrophages, specifically DNA methylation changes, exist regionally in the lung (i.e., upper versus lower lobe) that may ultimately predispose upper lobe lung macrophages to respond differently than lower lobe macrophages.
Limited studies to date have examined lung-related DNA methylation profiling or differences in regional innate immune cells of the lung. Many such DNA methylation studies are lung cancer related (23–27). Additionally, several groups have looked at the effects of cigarette smoking on lung-related DNA methylation (28, 29), and various studies have investigated changes in the DNA methylome in the single cell–type setting (30, 31). One of our recent studies has identified differential methylation in cystic fibrosis subjects at 109 gene-associated CpGs in lung macrophages, using the Illumina Infinium MethylationEPIC (850 K) array (16). In addition, we have demonstrated variant regional lung microRNA and cytokine expression associated with hypoxia in lung macrophages and BAL fluid (upper versus lower lobe) (32).
In this study, we report DNA methylation differences from regional lung macrophages, providing a direct comparison of the epigenetic molecular signature of upper lobe versus lower lobe innate immune cells from healthy individuals. Our cell collection methodology (flexible bronchoscopy) is a rare approach used in research studies and affords us a unique opportunity to analyze innate immune cells isolated directly from the airway and alveolar space.
Our initial strategy was to collect lung macrophages of both the upper lobe and lower lobe in healthy subjects to analyze epigenome-wide DNA methylation patterns. DNA methylation profiling via unsupervised clustering analysis showed that matched upper lobe and lower lobe samples cluster immediately adjacent to each other in nearly all subjects. Additionally, we identified 95 differentially methylated CpGs (all FDR < 0.05), of which 80 were hypermethylated in upper lobe cells and 15 were hypomethylated in upper lobe cells as well as 372 differential CpGs (all FDR < 0.10). Differentially methylated CpGs included those associated with genes such as NR4A1, SNX10, SCNN1A, and A2M. The orphan nuclear receptor NR4A1, which has been shown to be rapidly induced in response to stimuli, such as LPS (33), has been linked to the regulation of glucose metabolism and inflammation as well as implicated in hypoxia-induced apoptosis (34, 35). SNX10, a member of the family of sorting nexins, is a protein involved in intracellular protein sorting, trafficking, and signal transduction (36). Moreover, SNX10 has been reported to regulate endosomal morphology, which might be crucial for macrophage function, including phagocytosis and digestion of pathogens, inflammatory response, and Ag presentation (37). The SCNN1A gene encodes for the α subunit of the epithelial sodium channel (ENaC), which plays a critical role in the ion and fluid regulation of the lung. Altered activity of ENaC, which regulates the airway surface liquid layer, might lead to airway dehydration, mucus stasis, and bacterial overgrowth, as can be seen in cystic fibrosis and chronic bronchitis (38); however, an exact role for ENaC in lung macrophages, specifically, has yet to be fully established.
In addition to epigenetic changes in lung macrophages, we have also identified altered oxygen use in upper versus lower lobe cells. Lower lobe macrophages consistently exhibit a higher OCR than upper lobe macrophages. This increased OCR may represent a compensation mechanism to counter the decreased oxygen present in the lower lobe. Mitochondrial and cellular metabolic processes are central to immune cell responses, hypoxia sensing, and apoptosis in addition to their well-known roles of substrate oxidation and ATP production (39–41). Additionally, glucose metabolism is pivotal during macrophage pathogen phagocytosis (42). Although we do not currently have a direct causal link, one gene we have identified as differentially methylated, NR4A1, has been shown to be a transcriptional regulator of multiple glycolytic enzymes (35) and hence could ultimately be connected to changes in oxygen use levels in lung macrophages.
This study has several major strengths. To the best of our knowledge, this is the first study to demonstrate gene-associated DNA methylation differences in lung macrophages, comparing upper versus lower lobe. We have used the Infinium MethylationEPIC array, spanning 850,000 sites across the human genome, and validated these observations via tNGBS. In addition, we provide evidence of phenotypic differences of upper versus lower lobe cells, namely, a divergence in basal metabolism. However, a limitation of this study is that although we have identified epigenetic and a metabolic phenotypic difference between upper lobe and lower lobe cells in healthy subjects, a causative relationship between the DNA methylation changes and cellular phenotype is yet to be ascertained. Future work with larger sample size and power should investigate such a relationship.
In conclusion, through epigenome-wide DNA methylation profiling, we have identified 95 differential methylated CpGs in lung macrophages from the RUL relative to the RLL. Additionally, we have demonstrated energy metabolome-related phenotypical difference (i.e., oxygen consumption) in regional macrophages, upper versus lower. These observations support a hypothesis that epigenetic changes, specifically DNA methylation, at a multitude of gene loci in lung macrophages are associated with the well-established metabolic regional differences in lung and may, at least in part, be contributing to a differential innate immune response in alternate regions of the lung.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01HL122372 (to A.A.) and R01CA216265 (to B.C.C.). Subject enrollment and clinical sample collection were achieved with the assistance of the Cystic Fibrosis Translational Research Core at Dartmouth, which is jointly funded by NIH Grant P30GM106394 (to Bruce A. Stanton, principal investigator) and Cystic Fibrosis Foundation Award STANTO11R0 (to Bruce A. Stanton, principal investigator). This work was also supported by a Burroughs Wellcome Big Data in Life Sciences training grant and DartLab–National Cancer Institute Cancer Center Support Grant 5P30 CA023108–37.
Abbreviations used in this article:
- BAL
bronchoalveolar lavage
- CLIP4
CAP-Gly domain-containing linker protein family member 4
- ENaC
epithelial sodium channel
- FDR
false discovery rate
- OCR
oxygen consumption rate
- RLL
right lower lobe
- RPMM
recursively partitioned mixture model
- RUL
right upper lobe
- tNGBS
targeted next-generation bisulfite sequencing
- UTR
untranslated region
- VA/Q
ventilation/perfusion ratio
Footnotes
The DNA methylation microarray data presented in this article have been submitted to the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132547) under accession number GSE132547.
The online version of this article contains supplemental material.
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Nemec SF, Bankier AA, and Eisenberg RL. 2013. Upper lobe-predominant diseases of the lung. AJR Am. J. Roentgenol 200: W222–W237. [DOI] [PubMed] [Google Scholar]
- 2.West JB 1962. Regional differences in gas exchange in the lung of erect man. J. Appl. Physiol 17: 893–898. [DOI] [PubMed] [Google Scholar]
- 3.West JB 1963. Blood-flow, ventilation, and gas exchange in the lung. Lancet 2: 1055–1058. [DOI] [PubMed] [Google Scholar]
- 4.Cummins EP, and Taylor CT. 2005. Hypoxia-responsive transcription factors. Pflugers Arch 450: 363–371. [DOI] [PubMed] [Google Scholar]
- 5.Bruscia EM, and Bonfield TL. 2016. Cystic fibrosis lung immunity: the role of the macrophage. J. Innate Immun 8: 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirayama D, Iida T, and Nakase H. 2017. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci 19: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi G, Cavazza A, Spagnolo P, Bellafiore S, Kuhn E, Carassai P, Caramanico L, Montanari G, Cappiello G, Andreani A, et al. 2017. The role of macrophages in interstitial lung diseases: Number 3 in the series “pathology for the clinician.” Dorfmüller P and Cavazza A, eds. Eur. Respir. Rev 26: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CT, and Morris JR. 2001. Genes, genetics, and epigenetics: a correspondence. Science 293: 1103–1105. [DOI] [PubMed] [Google Scholar]
- 9.Morandini AC, Santos CF, and Yilmaz Ö. 2016. Role of epigenetics in modulation of immune response at the junction of host-pathogen interaction and danger molecule signaling. Pathog. Dis 74: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNardo AR, Nishiguchi T, Mace EM, Rajapakshe K, Mtetwa G, Kay A, Maphalala G, Secor WE, Mejia R, Orange JS, et al. 2018. Schistosomiasis induces persistent DNA methylation and tuberculosis-specific immune changes. J. Immunol 201: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsit CJ, Brummel SS, Kacanek D, Seage III GR, Spector SA, Armstrong DA, Lester BM, and Rich K, Pediatric HIV/AIDS Cohort Studies Network. 2015. Infant peripheral blood repetitive element hypomethylation associated with antiretroviral therapy in utero. Epigenetics 10: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selman M, Martinez FJ, and Pardo A. 2019. Why does an aging smoker’s lung develop idiopathic pulmonary fibrosis and not chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med 199: 279–285. [DOI] [PubMed] [Google Scholar]
- 13.Bessich JL, Nymon AB, Moulton LA, Dorman D, and Ashare A. 2013. Low levels of insulin-like growth factor-1 contribute to alveolar macrophage dysfunction in cystic fibrosis. J. Immunol 191: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, and Hunninghake GW. 2008. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J. Immunol 180: 7485–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kling T, Wenger A, Beck S, and Carén H. 2017. Validation of the MethylationEPIC BeadChip for fresh-frozen and formalin-fixed paraffin-embedded tumours. Clin. Epigenetics 9: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Armstrong DA, Salas LA, Hazlett HF, Nymon AB, Dessaint JA, Aridgides DS, Mellinger DL, Liu X, Christensen BC, and Ashare A. 2018. Genome-wide DNA methylation profiling shows a distinct epigenetic signature associated with lung macrophages in cystic fibrosis. Clin. Epigenetics 10: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, and Irizarry RA. 2014. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houseman EA, Christensen BC, Yeh RF, Marsit CJ, Karagas MR, Wrensch M, Nelson HH, Wiemels J, Zheng S,Wiencke JK, and Kelsey KT. 2008. Model-based clustering of DNA methylation array data: a recursive-partitioning algorithm for high-dimensional data arising as a mixture of beta distributions. BMC Bioinformatics 9: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houseman EA, Kile ML, Christiani DC, Ince TA, Kelsey KT, and Marsit CJ. 2016. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinformatics 17: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West JB 1962. The effect of uneven blood flow in the lung on regional gas exchange. Med. Thorac 19: 392–398. [DOI] [PubMed] [Google Scholar]
- 21.West JB 1963. Distribution of gas and blood in the normal lungs. Br. Med. Bull 19: 53–58. [DOI] [PubMed] [Google Scholar]
- 22.West JB, Holland RA, Dollery CT, and Matthews CM. 1962. Interpretation of radioactive gas clearance rates in the lung. J. Appl. Physiol 17: 14–20. [DOI] [PubMed] [Google Scholar]
- 23.Shi YX, Wang Y, Li X, Zhang W, Zhou HH, Yin JY, and Liu ZQ. 2017. Genome-wide DNA methylation profiling reveals novel epigenetic signatures in squamous cell lung cancer. BMC Genomics 18: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Shang W, Chang Y, and Li X. 2018. Methylation signature genes identification of the lung squamous cell carcinoma occurrence and recognition research. J. Comput. Biol 25: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 25.Um SW, Kim Y, Lee BB, Kim D, Lee KJ, Kim HK, Han J, Kim H, Shim YM, and Kim DH. 2018. Genome-wide analysis of DNA methylation in bronchial washings. Clin. Epigenetics 10: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stueve TR, Li WQ, Shi J, Marconett CN, Zhang T, Yang C, Mullen D, Yan C, Wheeler W, Hua X, et al. 2017. Epigenome-wide analysis of DNA methylation in lung tissue shows concordance with blood studies and identifies tobacco smoke-inducible enhancers. Hum. Mol. Genet 26: 3014–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kettunen E, Hernandez-Vargas H, Cros MP, Durand G, Le Calvez-Kelm F, Stuopelyte K, Jarmalaite S, Salmenkivi K, Anttila S, Wolff H, et al. 2017. Asbestos-associated genome-wide DNA methylation changes in lung cancer. Int. J. Cancer 141: 2014–2029. [DOI] [PubMed] [Google Scholar]
- 28.de Vries M, van der Plaat DA, Nedeljkovic I, Verkaik-Schakel RN, Kooistra W, Amin N, van Duijn CM, Brandsma CA, van Diemen CC, Vonk JM, and Marike Boezen H. 2018. From blood to lung tissue: effect of cigarette smoke on DNA methylation and lung function. Respir. Res 19: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choukrallah MA, Sierro N, Martin F, Baumer K, Thomas J, Ouadi S, Hoeng J, Peitsch MC, and Ivanov NV. 2019. Tobacco heating system 2.2 has a limited impact on DNA methylation of candidate enhancers in mouse lung compared with cigarette smoke. Food Chem. Toxicol 123: 501–510. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Zhao T, Yang X, Sun B, Li Y, Duan J, and Sun Z. 2019. PM2.5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Sci. Total Environ 650: 908–921. [DOI] [PubMed] [Google Scholar]
- 31.Clifford RL, Fishbane N, Patel J, MacIsaac JL, McEwen LM, Fisher AJ, Brandsma CA, Nair P, Kobor MS, Hackett TL, and Knox AJ. 2018. Altered DNA methylation is associated with aberrant gene expression in parenchymal but not airway fibroblasts isolated from individuals with COPD. Clin. Epigenetics 10: 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong DA, Nymon AB, Ringelberg CS, Lesseur C, Hazlett HF, Howard L, Marsit CJ, and Ashare A. 2017. Pulmonary microRNA profiling: implications in upper lobe predominant lung disease. Clin. Epigenetics 9: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ipseiz N, Uderhardt S, Scholtysek C, Steffen M, Schabbauer G, Bozec A, Schett G, and Krönke G. 2014. The nuclear receptor Nr4a1 mediates anti-inflammatory effects of apoptotic cells. J. Immunol 192: 4852–4858. [DOI] [PubMed] [Google Scholar]
- 34.Wohlkoenig C, Leithner K, Olschewski A, Olschewski H, and Hrzenjak A. 2017. TR3 is involved in hypoxia-induced apoptosis resistance in lung cancer cells downstream of HIF-1α. Lung Cancer 111: 15–22. [DOI] [PubMed] [Google Scholar]
- 35.Chao LC, Wroblewski K, Zhang Z, Pei L, Vergnes L, Ilkayeva OR, Ding SY, Reue K, Watt MJ, Newgard CB, et al. 2009. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes 58: 2788–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worby CA, and Dixon JE. 2002. Sorting out the cellular functions of sorting nexins. [Published erratum appears in 2003 Nat. Rev. Mol. Cell Biol. 4: 156.] Nat. Rev. Mol. Cell Biol 3: 919–931. [DOI] [PubMed] [Google Scholar]
- 37.Lou J, Li X, Huang W, Liang J, Zheng M, Xu T, Lyu J, Li D, Xu Q, Jin X, et al. 2017. SNX10 promotes phagosome maturation in macrophages and protects mice against Listeria monocytogenes infection. Oncotarget 8: 53935–53947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamacher J, Hadizamani Y, Borgmann M, Mohaupt M,Männel DN, Moehrlen U, Lucas R, and Stammberger U. 2018. Cytokine-ion channel interactions in pulmonary inflammation. Front. Immunol 8: 1644–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimeloe S, Burgener AV, Grählert J, and Hess C. 2017. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 150: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochocki JD, and Simon MC. 2013. Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol 203: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RA, Hartley RC, Cochemé HM, and Murphy MP. 2012. Mitochondrial pharmacology. Trends Pharmacol. Sci 33: 341–352. [DOI] [PubMed] [Google Scholar]
- 42.Barghouthi S, Everett KD, and Speert DP. 1995. Nonopsonic phagocytosis of Pseudomonas aeruginosa requires facilitated transport of D-glucose by macrophages. J. Immunol 154: 3420–3428. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.