Abstract
Invasive plants often interact with antagonists that include native parasitic plants and pathogenic soil microbes, which may reduce fitness of the invaders. However, to date, most of the studies on the ecological consequences of antagonistic interactions between invasive plants and the resident biota focused only on pairwise interactions. A full understanding of invasion dynamics requires studies that test the effects of multiple antagonists on fitness of invasive plants and co‐occurring native plants. Here, we used an invasive plant Mikania micrantha, a co‐occurring native plant Coix lacryma‐jobi, and a native holoparasitic plant Cuscuta campestris to test whether parasitism on M. micrantha interacts with soil fungi and bacteria to reduce fitness of the invader and promote growth of the co‐occurring native plant. In a factorial setup, M. micrantha and C. lacryma‐jobi were grown together in pots in the presence versus absence of parasitism on M. micrantha by C. campestris and in the presence versus absence of full complements of soil bacteria and fungi. Fungicide and bactericide were used to suppress soil fungi and bacteria, respectively. Findings show that heavy parasitism by C. campestris caused the greatest reduction in M. micrantha biomass when soil fungi and bacteria were suppressed. In contrast, the co‐occurring native plant C. lacryma‐jobi experienced the greatest increase in biomass when grown with heavily parasitized M. micrantha and in the presence of a full complement of soil fungi and bacteria. Taken together, our results suggest that selective parasitism on susceptible invasive plants by native parasitic plants and soil microorganisms may diminish competitive ability of invasive plants and facilitate native plant coexistence with invasive plants.
Keywords: biotic resistance, coexistence, invasive plants, native plants, parasitic plants, soil microbes
1. INTRODUCTION
Invasion of native communities by exotic plant species is a major element of global environmental change reducing native plant diversity (Kourtev, Ehrenfeld, & Häggblom, 2003; Mack et al., 2000; Vila et al., 2011). Within their introduced ranges, invasive plants often interact with a new suite of antagonists such as native parasitic plants (Li, Jin, & Song, 2012; Miao et al., 2012; Prider, Walting, & Facelli, 2009; Wang, Guan, Li, Yang, & Li, 2012; Yu, Liu, He, Miao, & Dong, 2011; Yu, Yu, Miao, & Dong, 2008) and soil‐borne pathogens (Mitchell et al., 2006). The invasive plants may also interact with soil‐borne microbial mutualists (Kowalski et al., 2015; Richardson, Allsopp, D'antonio, Milton, & Rejmánek, 2000; Simberloff & Von Holle, 1999). The antagonists and mutualists may individually and interactively influence fitness of invasive plants (Hill & Kotanen, 2012; Mitchell et al., 2006). Although the ecological consequences of antagonistic interactions between invasive plants and the resident biota are well documented (Hill & Kotanen, 2012; Levine, Adler, & Yelenik, 2004; Maron & Vilà, 2001; Vila et al., 2011), most of such studies focused only on single interaction types, when in reality, multiple interactions occur simultaneously (van Kleunen, Bossdorf, & Dawson, 2018). A full understanding of invasion dynamics requires studies that test the effects of multiple antagonists on fitness of invasive plants and co‐occurring native plants (van Kleunen et al., 2018; Oduor, 2013; Oduor, Kleunen, & Stift, 2017).
Soil microbial communities may influence individual plant fitness, plant community succession, and invasion by acting as plant pathogens and mutualists (Moora & Zobel, 1996; van der Putten, Klironomos, & Wardle, 2007; Shivega & Aldrich‐Wolfe, 2017). Mycorrhizal fungi and nitrogen‐fixing microbes are the two main groups of plant mutualists (van Kleunen et al., 2018). They can benefit plants by facilitating the availability of major plant nutrients and producing plant growth‐promoting substances (Batten, Scow, Davies, & Harrison, 2006). On the other hand, pathogenic microbes reduce plant fitness (Callaway, Thelen, Rodriguez, & Holben, 2004; Chen et al., 2018; Klironomos, 2002; Maron, Marler, Klironomos, & Cleveland, 2011; van der Putten, Dijk, & Peters, 1993). There is mixed empirical evidence on associations between invasive plants and microbial mutualists. Studies in grasslands and mixed‐grass prairie of North America found that invasive and naturalized alien plants had fewer and weaker associations with arbuscular mycorrhizal (AM) fungi than native plant species (Jordan, Aldrich‐Wolfe, Huerd, Larson, & Muehlbauer, 2012; Pringle et al., 2009; Sigüenza, Crowley, & Allen, 2006; Vogelsang & Bever, 2009). These and other findings that did not find dependency of invasive plants on mycorrhizal fungi led to a suggestion that reduced dependency on microbial mutualists may be an important feature of invasiveness of exotic plants (the degraded mutualism hypothesis; Bunn, Ramsey, & Lekberg, 2015). In contrast, studies in other ecosystems in Europe, New Zealand, and South America (e.g., Dickie, Bolstridge, Cooper, & Peltzer, 2010; Menzel et al., 2017; Nuñez & Dickie, 2014; Štajerová, Šmilauerová, & Šmilauer, 2009) found a majority of exotic plant species to be mycorrhizal. The conflicting results suggest that whether exotic plants benefit from being mycorrhizal may depend upon the plant taxa and ecological context. Associations between invasive plants with nitrogen‐fixing bacteria have also been reported (Le Roux, Hui, Keet, & Ellis, 2017). Invasive plants have also been shown to suffer less from negative effects of pathogenic soil biota than co‐occurring native plant species (Agrawal et al., 2005; Kardol, Cornips, Kempen, Bakx‐Schotman, & Putten, 2007; Klironomos, 2002; Kulmatiski, Beard, Stevens, & Cobbold, 2008). Nevertheless, more recent studies suggest that exotic plants can accumulate soil pathogens over time, which could potentially reduce their impacts on native plants (Diez et al., 2010; Dostál, Müllerová, Pyšek, Pergl, & Klinerová, 2013; Speek et al., 2015; Stricker, Harmon, Goss, Clay, & Luke Flory, 2016). Thus, the net impact of soil microbes (negative, neutral or positive) on fitness of invasive plants and co‐occurring native plants may depend upon the balance of positive effects of mutualists and negative effects of pathogens present in a particular soil (Klironomos, 2002; van der Putten et al., 2013; Westover & Bever, 2001).
As parasitic plants are common in natural communities (Pennings & Callaway, 2002), invasive plants may interact simultaneously with native plants, soil microbes, and native parasitic plants (Li, Jin, Hagedorn, & Li, 2014). Empirical studies have shown that soil microbial communities can mediate competitive interactions between invasive plants and native plants (e.g., Allen, Meyerson, Flick, & Cronin, 2018; Lankau, 2010; Marler, Zabinski, & Callaway, 1999; Shivega & Aldrich‐Wolfe, 2017). For example, rhizospheric soil biota of the invader Phragmites australis increased biomass of a native plant Spartina alterniflora when the two plant species were grown in competition with each other (Allen et al., 2018). In a separate study, microbial taxa inhibited the allelopathic effect of the invader Alliaria petiolata on seedlings of the native plant Platanus occidentalis (Lankau, 2010). In pairwise competition experiments that compared performance of two native prairie plants (Oligoneuron rigidum and Andropogon gerardii) against one invader (Carduus acanthoides), the native plants fared better against the invader in the presence of a native microbial community (Shivega & Aldrich‐Wolfe, 2017). AM fungi increased the negative effects of the invader Centaurea maculosa on a native bunchgrass Festuca idahoensis (Marler et al., 1999). Studies have also shown that native parasitic plants can affect competition between invasive host plants and co‐occurring native plants. For instance, native holoparasitic plants such as Cuscuta campestris (Yu et al., 2008), C. australis (Li et al., 2012; Wang et al., 2012; Yu et al., 2011), and Cassytha pubescens (Prider et al., 2009) caused more damage to their invasive host species than co‐occurring native species. Thus, the holoparasitic plants have been suggested as a potential biological control agent against the plant invaders (Miao et al., 2012). However, previous work only examined the separate effects of soil microbes and native parasitic plants on interactions between invasive plants and native plants. Therefore, whether soil microbial community and native parasitic plants operate independently or interact in ways that exacerbate or ameliorate the effects of each other to influence competitive interactions between invasive plants and native plants remains unexplored.
Here, we used an invasive plant Mikania micrantha, a co‐occurring native plant Coix lacryma‐jobi, and a native holoparasitic plant C. campestris to address the question: Can parasitism on an invasive plant by a native holoparasitic plant interact with soil fungi and bacteria to reduce fitness of the invader and promote growth of a co‐occurring native plant?
2. MATERIALS AND METHODS
2.1. Study plant species
Mikania micrantha (Asteraceae) (hereinafter Mikania) is native to Central and South America and was introduced into China in 1919 (Holm, Plucknett, Pancho, & Herberger, 1977). At present, Mikania is distributed widely in Guangdong province in South China where it is invasive (Zhang, Ye, Cao, & Feng, 2004). Cuscuta campestris (hereinafter Cuscuta) is native to China and occurs in the provinces of Fujian, Guangdong, and Xinjiang Uygur Autonomous Region, China (Wang, Wang, & Liao, 2004). As a holoparasitic plant, Cuscuta acquires some or all of its water, carbon, and nutrients via the vascular tissue of the hosts' roots or shoots, which significantly inhibits growth of the host. Previous field observations and greenhouse experiments showed that Cuscuta preferentially parasitized Mikania relative to native plants, which significantly reduced growth and cover of the invader and facilitated native species diversity in invaded patches (Shen, Hong, Ye, Cao, & Wang, 2007; Wang et al., 2004; Yu et al., 2008). The native plant Coix lacryma‐jobi (Poaceae) (hereinafter Coix) was chosen for this experiment because it was the most common native species that co‐occurred with Mikania in the invaded community. Results of a previous field survey suggest that parasitism by Cuscuta may reduce competitive exclusion of Coix by Mikania (Li et al., 2014).
2.2. Location of study
A common garden pot experiment was conducted in Dengshuiling village, in the southeast of Dongguan City (113°31′‐114°15′E; 22°39′‐23°09′N), Guangdong Province, China. The province has a subtropical climate with a mean annual precipitation of 1,819.9 mm, temperature of 23.1°C, and sunshine time of 1,873.7 hr. Mikania first invaded the province in early 1990s where it spread extensively in the shrublands and abandoned agricultural fields.
2.3. Preparation of experimental plant and soil materials
We collected soil from a field near Dengshuiling village. Ten 1 m × 1 m plots were chosen randomly in an abandoned agricultural field site without Mikania. Vegetation and litter were removed from the upper soil surface, and then, soil (red clay) was collected at depths of 0–15 cm from the plots. The soil was mixed with sand (3:1, soil/sand) and homogenized before use. This mixture enabled us to maintain good drainage and accurately harvest roots at the end of the experiment.
We obtained stem cuttings of Mikania from multiple maternal families in a field near Dengshuiling village on 16 July 2006 and then propagated them for use in the experimental setup described below. Sharp pruning shears (sterilized with 70% ethanol) were used to generate the cuttings from upper intact plant parts. Each cutting measured 10 cm in length, and its leaf count was reduced by a half to reduce water loss upon transplant. The cuttings were then inserted into a potted soil (up to a third of the entire length), with the stem maintained in a vertical orientation. Coix was raised from seeds that had been purchased from Shandong Heze Chinese Medicine Institute in March 2006. In order to eliminate any pathogen that might have been present on the Coix seeds, the seeds were surface‐sterilized as follows. The seeds were immersed in 20% CuSO4 for 10 min and later soaked in water for 24 hr, 70% ethanol for 1 min, water again for 5 min, 10% H2O2 for 5 min, and finally rinsed with sterilized water three times (see Li et al., 2014). In June 2006, we sowed similar‐sized seeds in plastic‐plug trays filled with soil of the same source as above. The soil was sterilized before use to prevent any microbes present in the soil from influencing early growth of Coix seedlings and Mikania cuttings.
2.4. Experimental setup
To test whether parasitism by Cuscuta on Mikania interacted with soil fungi and bacteria to influence competitive interactions between Mikania and Coix, we performed a factorial pot experiment. In the experiment, we grew an individual Coix in competition with Mikania (parasitized vs. not parasitized), and when soil fungi and bacteria were suppressed versus not suppressed. In late July 2006, individual Mikania cuttings and Coix seedlings (each measured c. 15 cm in length) that had been raised as described above were carefully removed from the nursery without destroying the roots and transplanted into 3‐L pots (25 cm in diameter) that had been filled with nonsterilized soil from the same source as above. Within the pot, Mikania and Coix were spaced 15 cm apart. Immediately after transplant, the pots were placed under a shade tree to avoid excess evapotranspiration. Then, three days later, the pots were moved to an open‐ field common garden. A week after transplant, bamboo sticks (1 m long) were driven into the soil near Mikania to provide support because Mikania is a climber species. The plants were fertilized with 50% strength Hoagland's nutrient solution once a week. Throughout the experiment, the plants were watered twice a day with tap water.
Three weeks after transplant, Cuscuta stems were collected from a field near the village of Dengshuiling and wound around Mikania stems (Figure S1). We used Cuscuta raised from stem cuttings instead of seeds because there were no mature seeds in the field at the start of the experiment. To represent low‐ and high‐level parasitism, we wound one and three Cuscuta stems (each 15 cm long), respectively, around Mikania stems. As a control, we grew Mikania without Cuscuta infestation. We did not infest Coix with Cuscuta because in the habitat where we sampled experimental plant materials, Cuscuta avoided Coix (although Coix experienced c. 2.5% of parasitism relative to Mikania in other habitats). To suppress fungi that were present in the potted soil, we applied benomyl (purchased from Yida Chemical Inc.). Benomyl had been shown to effectively reduce soil fungi including AM fungi with negligible direct effects on plants (Callaway, Mahall, Wicks, Pankey, & Zabinski, 2003; Hetrick, Wilson, & Hartnett, 1989). The fungicide was applied at a concentration of 50 mg benomyl/kg soil (Callaway et al., 2003; Hetrick et al., 1989). We used streptomycin sulfate (purchased from Linhai Seeds and Vegetation Company) to suppress bacteria in the potted soil. Streptomycin is a commonly used bactericidal antibiotic (El‐Khair & Haggag, 2007) that acts by interfering with normal protein synthesis in bacteria (Bailey, Smith, & Bolton, 2003). We added 40,000 titer units of streptomycin sulfate/kg soil to the soil in the pot every week. The fungicide and bactericide were solubilized in tap water and applied at the rate of 100 ml per pot. As a control against the fungicide and bactericide treatments, we applied 100 ml of tap water. Each of the resulting 12 treatment combinations (i.e., three levels of parasitism on Mikania by Cuscuta [no parasitism, light parasitism, and heavy parasitism] × 2 levels of fungicide [applied vs. not applied] × 2 levels of bactericide [applied vs. not applied]) was replicated five times, resulting in 60 experimental pots. The pots were arranged randomly within the garden and the experiment ran for 7 weeks.
2.5. Measurements
We terminated the experiment at the end of week seven. We then separated Cuscuta from Mikania and harvested individual Mikania and Coix plants separately. We separated roots and shoots of the experimental plants and then dried them to a constant biomass at 80°C for 48 hr. We then determined total biomass (root and shoot) of the dried plant materials.
At harvest, we determined whether fungicide application had suppressed soil fungi by examining root colonization of all the experimental Mikania and Coix plants by AM fungi. We did this before the plant materials were oven‐dried. From each individual plant, we obtained fine roots that were then cut into 1‐cm‐long segments and fixed using formalin/acetic acid/alcohol (FAA) fixative solution. Root samples were cleaned with 10% KOH solution at 90°C for 40 min, acidified in 2% HCl for 5 min, stained with 0.01% acid fuchsin (Kormanik, Bryan, & Schultz, 1980), and then observed under a microscope for presence of AM fungi. We considered a root segment to have AM fungi when it had arbuscules in the cortical cells. For every individual plant, we then determined percentage colonization by AM fungi as follows: AM fungi colonization (%) = 100 × (infected root length/observed root length).
We also determined whether bactericide application had suppressed soil bacteria in the experimental soil material. To do so, we obtained soil samples from individual experimental pots after the plants had been harvested. The soil samples were then stored at 4°C and transported to the laboratory immediately. The soil was then sieved using a sterilized 2‐mm sieve to remove any debris. The number of colony‐forming units (CFUs) in each soil sample was then directly calculated using acridine orange fluorescent staining method under DMLS Fluorescence microscope (Leica Mikrosysteme Vertrieb GmbH Mikroskopie und Histologie; Li & Jin, 2006). To avoid contamination, all the equipments used for processing soil samples were sterilized and cleaned with 70% ethanol before and between uses.
2.6. Statistical analysis
We used a three‐way analysis of variance (ANOVA) to test whether parasitism on Mikania by Cuscuta (three levels: no parasitism, light parasitism, and heavy parasitism), soil fungi (suppressed vs. not suppressed), and soil bacteria (suppressed vs. not suppressed) had main and interactive effects on biomass yield of Mikania and Coix. Parasitism, fungicide, and bactericide were specified as independent variables, while total biomass of Mikania and Coix (root and shoot combined) was specified as a dependent variable. We also used ANOVA to test whether colonization of Mikania and Coix roots by AM fungi differed significantly between fungicide treatments, and whether the number of soil bacteria differed between bactericide treatments. In the cases where there were significant main and interactive effects of parasitism, soil fungi, and soil bacteria on the growth of Mikania and Coix, root colonization by AM fungi, and the number of CFUs of soil bacteria, we performed post hoc least‐squares means comparisons between the treatment levels (α = 0.05%). All statistical analyses were performed in SPSS v.16.0. All the figures were generated in Sigma Plot v.11.0.
3. RESULTS
3.1. Biomass of the invasive plant Mikania
Parasitism by Cuscuta on Mikania significantly reduced biomass of the invader (Figure 1a; Table S1). However, heavy and light parasitism caused similar declines in biomass (Figure 1a). Suppression of soil bacteria improved Mikania biomass, although not significantly (Table S1). Mikania produced more biomass when soil fungi were suppressed than when not suppressed (significant main effect of fungicide Figure 1b; Table S1). Soil fungi and bacteria modified the effects of parasitism on Mikania (significant two‐way interactions: parasitism × bactericide; parasitism × fungicide; Figure 1c,d; Table S1). In the presence of a full complement of soil bacteria (bactericide not applied), light and heavy parasitism by Cuscuta reduced Mikania biomass by 62% and 79%, respectively (Figure 1c). However, when bacteria were suppressed (bactericide applied), light and heavy parasitism by Cuscuta reduced Mikania biomass by 31% and 66%, respectively (Figure 1c). Similarly, in the presence of a full complement of soil fungi (fungicide not applied), light and heavy parasitism by Cuscuta reduced Mikania biomass by 68% and 72%, respectively (Figure 1d). On the other hand, when fungi were suppressed (fungicide applied), light and heavy parasitism by Cuscuta reduced Mikania biomass by 35% and 75%, respectively (Figure 1d). Soil bacteria influenced the effect of soil fungi on Mikania biomass (significant interaction between bactericide and fungicide; Figure 1e; Table S1). When bacteria were not suppressed, Mikania produced more biomass when fungi were suppressed than when not suppressed (Figure 1e). However, when bacteria were suppressed, the opposite pattern was observed (Figure 1e). Bacteria and fungi jointly influenced the suppressive effects of Cuscuta on Mikania (significant three‐way interaction: parasitism × bactericide × fungicide; Figure 1f; Table S1). Heavy parasitism by Cuscuta caused the greatest decline in Mikania biomass (−85.3%) when fungi were suppressed while bacteria were not suppressed (Figure 1f).
Figure 1.
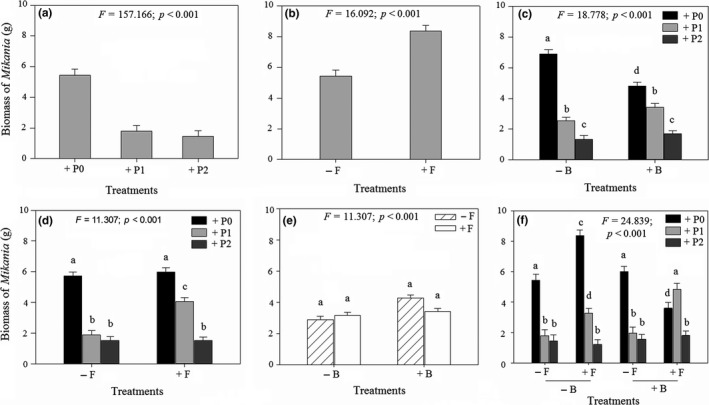
Mean (±1 SE) biomass of Mikania micrantha plants grown in the presence of Coix lacryma‐jobi under different levels of parasitism by Cuscuta campestris and in the presence versus absence of a full complement of soil fungi and bacteria. Fungicide and bactericide were used to suppress soil fungi and bacteria, respectively. (a) Main effect of different levels of parasitism: +P0, +P1, and +P2 indicate no parasitism, light parasitism, and heavy parasitism on C. campestris, respectively; (b) main effect of fungicide; −F indicates without fungicide, +F indicates with fungicide; (c) interactive effects of different level of parasitism and bactericide; (d) interactive effect of different level of parasitism and fungicide; (e) interactive effect of different level of bactericide and fungicide; (f) interactive effect of different level of parasitism, bactericide, and fungicide. Significance of the main and interactive effects was determined by three‐way ANOVA tests (cf. Table S1). Letters above bars indicate the results of post hoc least‐squares mean comparisons (bars that do not share a letter are significantly different)
3.2. Biomass of the native plant Coix
Biomass of the native plant Coix was significantly higher in treatments where Mikania was parasitized (light and heavy) than in the absence of parasitism (Figure 2a and Table S2). Suppression of soil bacteria caused a significant increase in Coix biomass (significant main effect of bactericide; Figure 2b and Table S2). However, suppression of fungi caused a significant decline in Coix biomass (significant main effect of fungicide; Figure 2c and Table S2). Joint suppression of fungi and parasitism on Mikania influenced Coix biomass (significant two‐way interaction: parasitism × fungicide; Figure 2d and Table S2). When the full complement of soil fungi was present, Coix produced similar biomass under light and heavy levels of parasitism (Figure 2d). However, when fungi were suppressed, Coix produced significantly higher biomass when Mikania was heavily parasitized than in the absence of parasitism and under light parasitism (Figure 2d). Coix biomass was also influenced by the joint effects of parasitism on Mikania and soil fungi and bacteria (significant three‐way interaction: parasitism × bactericide × fungicide; Figure 2e and Table S2). Coix experienced the greatest gain in biomass (163.6%) when Mikania was heavily parasitized and in the presence of a full complement of soil fungi and bacteria (Figure 2e). In contrast, Coix experienced a marginal gain in biomass when either fungi or bacteria were suppressed despite heavy parasitism on Mikania (Figure 2e).
Figure 2.
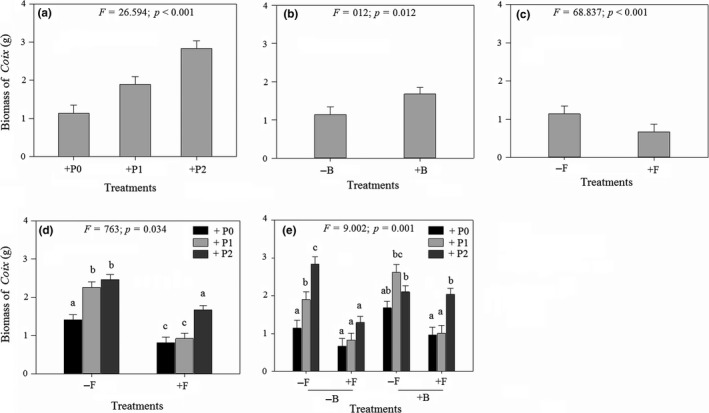
Mean (±1 SE) biomass of Coix lacryma‐jobi plants grown with Mikania micrantha plants that were parasitized by Cuscuta campestris at different intensities and in the presence versus absence of a full complement of soil fungi and bacteria. Fungicide and bactericide were used to suppress soil fungi and bacteria, respectively. (a) Main effect of parasitism by Cuscuta campestris: +P0, +P1, and +P2 indicate no parasitism, light parasitism, and heavy parasitism on C. campestris, respectively; (b) Main effect of bactericide: −B indicates without bactericide, +B indicates with bactericide; (c) main effect of fungicide: −F indicates without fungicide, +F indicates with fungicide; (d) interactive effect of parasitism on C. campestris and fungicide; (e) interactive effect of parasitism on C. campestris, fungicide, and bactericide. Significance of the main and interactive effects was determined by three‐way ANOVAs tests (cf. Table S2). Letters above bars indicate the results of post hoc least‐squares mean comparisons (bars that do not share a letter are significantly different)
3.3. Effects of fungicide and bactericide on AM fungi and soil bacteria
The addition of fungicide significantly reduced colonization of Coix and Mikania roots by AM fungi (Figure 3a,b; Table S3). Fungicide application modified the effect of Cuscuta on colonization of Mikania roots by AM fungi (significant two‐way interaction: parasitism × fungicide; Figure 3c; Table S3). When fungicide was not applied, light and heavy parasitism by Cuscuta had similar effects on colonization by AM fungi, although both parasitism levels caused significant declines in colonization relative to no parasitism (Figure 3c). However, when fungicide was applied, colonization by AM fungi was similar across parasitism levels (Figure 3c). Application of bactericide modified the joint effects of fungicide and parasitism on colonization of Mikania by AM fungi (significant three‐way interaction: parasitism × bactericide × fungicide; Figure 3d and Table S3). Mikania experienced the highest level of colonization (58%) in the absence of parasitism and when fungicide and bactericide were not applied (Figure 3d). In contrast, colonization was lowest when both fungicide and bactericide were applied (Figure 3d). For Coix, parasitism and bactericide did not influence root colonization by AM fungi (Table S4). Similar to the effects of fungicide on colonization by AM fungi, the addition of bactericide significantly reduced the number of CFUs of soil bacteria (Figure 4a; Table S5). Addition of fungicide modified the effect of bactericide on the number of CFUs (significant two‐way interaction: bactericide × fungicide; Figure 4b; Table S5). When bactericide was not added, the number of CFUs was similar between pots where fungicide was applied and in pots without fungicide (Figure 4b). However, when bactericide was applied, pots without fungicide had significantly higher number of CFUs than pots with fungicide (Figure 4b). Parasitism on Mikania by Cuscuta influenced the effects of both fungicide and bactericide on the numbers of CFUs (significant three‐way interaction: parasitism × bactericide × fungicide; Figure 4c and Table S5). The mean number of CFUs was highest (5.37 × 108 CFU/g wet soil) when Mikania was subjected to heavy parasitism by Cuscuta and when fungicide was added but bactericide not added to the pot (Figure 4c). However, the mean number of CFUs was lowest (2.97 × 108 CFU/g wet soil) when neither fungicide nor bactericide was added to the pot and in the presence of light parasitism on Mikania by Cuscuta (Figure 4c).
Figure 3.
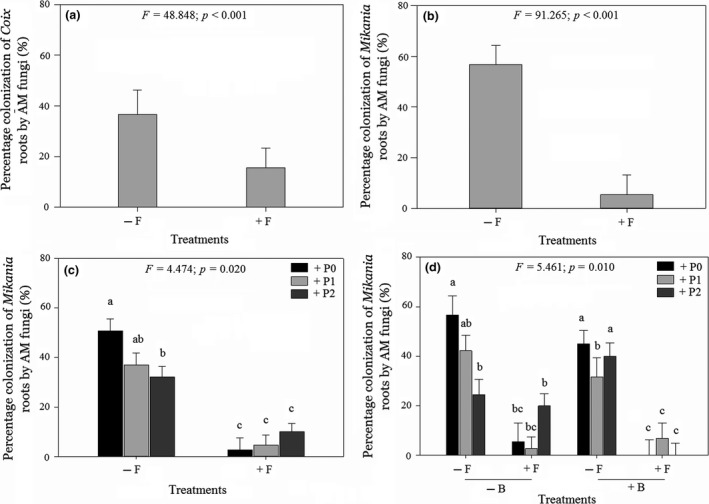
Mean (±1 SE) AM mycorrhizal colonization levels of Coix lacryma‐jobi and Mikania mirantha roots in the presence of different levels of parasitism on M. micrantha by Cuscuta campestris and in the presence versus absence of a full complement of soil fungi and bacteria. Fungicide and bactericide were used to suppress soil fungi and bacteria, respectively. (a) Main effect of fungicide on the mycorrhizal colonization level of Coix root; (b) main effect of fungicide on the mycorrhizal colonization level of Mikania root; (c) interactive effects of different levels of parasitism and fungicide on the AM fungal colonization of Mikania root; (d) interactive effects of different level of parasitism, bactericide, and fungicide on the AM fungal colonization of Mikania root. Significance of the main and interactive effects was determined by three‐way ANOVAs tests (cf. Tables S3 and S4). Letters above bars indicate the results of post hoc least‐squares mean comparisons (bars that do not share a letter are significantly different)
Figure 4.
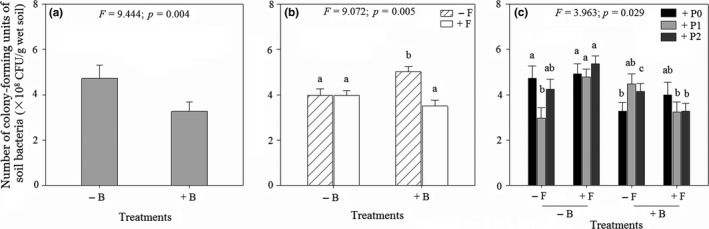
Mean (±1 SE) number of colony‐forming units (CFUs) of soil bacteria in a pot with Coix lacryma‐jobi and Mikania mcirantha in the presence of different levels of parasitism on M. micrantha by Cuscuta campestris and soil fungi and bacteria. Fungicide and bactericide were used to suppress soil fungi and bacteria, respectively. (a) Main effect of bactericide; (b) interactive effect of bactericide and fungicide; (c) interactive effect of parasitism, bactericide, and fungicide. Significance of the main and interactive effects was determined by three‐way ANOVAs tests (cf. Table S5). Letters above bars indicate the results of post hoc least‐squares mean comparisons (bars that do not share a letter are significantly different)
4. DISCUSSION
The factorial manipulation of soil fungi and bacteria and parasitism on the invasive plant Mikania by a native holoparasite Cuscuta permitted us to measure the relative strengths and combined effects of parasitism and soil microbial community on interaction between an invasive plant and a co‐occurring native plant. Parasitism on Mikania by Cuscuta caused a significant decline in biomass of the invader, although the magnitude of impact was modified by the presence of fungi and bacteria in the soil. More specifically, heavy parasitism by C. campestris caused the greatest reduction in M. micrantha biomass when soil fungi and bacteria were suppressed (Figure 1f). In contrast, the co‐occurring native plant Coix experienced the greatest gain in biomass when Mikania was heavily parasitized and in the presence of a full complement of soil bacteria and fungi (Figure 2e). Mikania had the highest level of root colonization by AM fungi in the absence of parasitism and in the presence of a full complement of soil bacteria and fungi (Figure 3d). In contrast, colonization of Coix by AM fungi was not influenced by parasitism on its competitor Mikania or by the presence of soil bacteria (Figure 3d). Heavy parasitism on Mikania by Cuscuta and suppression of soil fungi stimulated bacterial growth in the experimental pots (Figure 4c). Overall, these results suggest that heavy parasitism by Cuscuta and soil bacteria had synergistic negative effects on growth of Mikania, while the co‐occurring Coix benefitted under the same growth conditions. More broadly, the results suggest that native parasitic plants and soil microorganisms can synergistically facilitate coexistence of native plants with invasive plants. Through selective patterns of parasitism by native parasitic plants and in the presence of soil microbes, susceptible invasive hosts may exhibit diminished competitive ability, while co‐occurring nonhost (or less preferred) native species increase in dominance.
4.1. The interactions between parasitism on Mikania by Cuscuta, soil microbes, and the native plant Coix
Heavy parasitism by Cuscuta had the greatest negative effect on Mikania growth when soil fungi were suppressed and in the presence of a full complement of soil bacteria (Figure 1e), which suggests that heavy parasitism weakened defense of Mikania against pathogenic bacteria that were likely present in the soil. The results also suggest that suppressing soil fungi eliminated or reduced beneficial effects of fungal mutualists of Mikania. Parasitic plants can affect growth of their hosts by extracting resources such as water, nutrients, and organic compounds from the host's vascular system (Press, Scholes, & Watling, 1999). Because these same resources are used by plants to make secondary metabolites that have been shown to be toxic to plant pathogens (Bouwmeester, Roux, Lopez‐Raez, & Becard, 2007), it is likely that heavily parasitized Mikania individuals had low concentrations of secondary metabolites and consequently low resistance against pathogenic bacteria that were likely present in the experimental soil. This hypothesis is plausible because species in the genus Cuscuta have been shown to be powerful sinks of host photosynthates and nutrients and can therefore preclude host allocation of resources to growth, stress tolerance, or defense (Jeschke, Bäumel, & Räth, 1994; Shen, Xu, Hong, Wang, & Ye, 2013). The apparent synergistic negative effects of Cuscuta and soil bacteria on Mikania likely released the native plant Coix from strong competition from Mikania as Coix experienced the greatest gain in biomass under similar growth conditions, although when soil fungi were not suppressed (Figure 2e).
Mikania had the highest level of root colonization by AM fungi in the absence of parasitism by Cuscuta and in the presence of a full complement of soil fungi and bacteria (Figure 3d). This result supports findings on other study systems that infection by parasitic plants can reduce root colonization by AM fungi (Davies & Graves, 1998; Gehring & Whitham, 1992; McKibben & Henning, 2018). The causal mechanism might be a reduced carbon availability (Davies & Graves, 1998). Given that AM fungi and parasitic plants are both carbon sinks (Davies & Graves, 1998), dual infection could lead to the AM fungi and parasitic plants competing for carbon from the host plant. If the parasitic plant is a superior competitor, the reduction in available carbon resources may feedback to disrupt interactions between the host plant and fungal mutualists of the plant (Davies & Graves, 1998; Press & Phoenix, 2005; Stewart & Press, 1990). In support of this, biomass production in Mikania plants parasitized by Cuscuta was significantly reduced relative to nonparasitized Mikania (Figure 1f), suggesting that Cuscuta suppressed the AM fungi through a reduction in the available carbon. Future mechanistic experiments should directly test whether parasitism on Mikania by Cuscuta reduces carbon allocation to AM fungi.
Colonization of Mikania roots by AM fungi was lowest in the presence of parasitism by Cuscuta and when soil fungi and bacteria were suppressed (Figure 3d). In contrast, for the native plant Coix that grew with Mikania in the same pot, only fungicide application reduced root colonization by AM fungi (Figure 3a). These contrasting results could be explained both by the absence of parasitism on Coix by Cuscuta and suppressive effects of the fungicide and bactericide. As Coix was not parasitized, there was no possibility of Cuscuta indirectly reducing colonization of Coix roots by the AM fungi through competition for carbon. On the other hand, suppression of AM fungi in Mikania roots could have been caused by the direct effect of fungicide and indirectly through competition from Cuscuta for carbon. However, whether the bactericide contributed to the decline in AM fungal colonization of Mikania roots indirectly through altered host plant physiology or by acting directly on the fungi remains to be resolved.
In the soil where neither bactericide nor fungicide was applied, Mikania had a higher level of root colonization by AM fungi (58%) (Figure 3d) than Coix (38%) (Figure 3a). These results are counter to the notion that exotic plants are less likely than native plant species to associate with AM fungi (Bunn et al., 2015; Klironomos, 2003; Pringle et al., 2009). Although invasive plants may leave behind coevolved mutualists in the native range (Kowalski et al., 2015), as the density, range, and time‐since‐invasion increase, the plants may acquire novel microbial mutualists (the host‐jumping hypothesis; Shipunov, Newcombe, Raghavendra, & Anderson, 2008; Kowalski et al., 2015). For instance, Cyperus rotundus that invaded the U.S. Gulf coast region harbored a fungal mutualist Balansia cyperi that was native to the region (Stovall & Clay, 1988). The fungus likely jumped from a native Cyperus host to C. rotundus (Kowalski et al., 2015). Invasive plants may also reunite with native‐range mutualists through cointroductions (the cointroduction hypothesis; Shipunov et al., 2008). For instance, communities of endophytic fungi were similar between invaded and native ranges of Centaurea stoebe, suggesting multiple cointroductions of different fungal species (Shipunov et al., 2008). Pinus contorta coinvaded New Zealand with its ectomycorrhizal fungal communities (Dickie et al., 2010). Several Australian ectomycorrhizal fungi were found in plantations of Australian Eucalyptus species in the Iberian Peninsula, further supporting the idea of cointroductions (Díez, 2005). In the Iberian Peninsula, the Australian Acacia longifolia harbored symbiotic nitrogen‐fixing bacteria that are native to Australia (Rodríguez‐Echeverría, 2010). Whether Mikania that has been present in China for close to 100 years (Holm et al., 1977) has acquired new microbial symbionts and/or reunited with those in its native range remains an area of further study.
The number of CFUs of soil bacteria was highest when Mikania was heavily parasitized by Cuscuta and the soil fungi suppressed and in the presence of a full complement of soil bacteria (Figure 4c). These findings support the idea that the impacts of parasitic plants on their hosts can trigger indirect interactions between parasitic plants and other species in the community (Pennings & Callaway, 2002). It is likely that heavy parasitism by Cuscuta caused an increase in Mikania root exudates that in turn promoted bacterial growth in the soil. Root‐derived exudates are a major source of carbon and nutrients for soil bacterial community (Dennis, Miller, & Hirsch, 2010). It is thought that parasitized hosts may increase allocation of resources into the roots, but evidence is scarce and conflicting (Quested, 2008). In a mixed grassland community, infection by a root hemiparasite R. minor stimulated the activity of belowground decomposers, which was attributed to enhanced supply of substrates because the host's root exudation increased (Bardgett et al., 2006). The same study reported a reduced fungal‐to‐bacterial ratio in the presence of the hemiparasite (Bardgett et al., 2006). Soil heterotrophic microbial communities tended to become more abundant and functionally even beneath Pinus nigra trees that were parasitized by mistletoe (Viscum album subsp. austriacum) than beneath nonparasitized trees (Mellado, Morillas, Gallardo, & Zamora, 2016). In contrast, parasitism by C. campestris on Mikania caused a decrease in soil microbial biomass and altered functional diversity of soil microbial communities underneath the invader (Li et al., 2014). Thus, by altering soil microbial biomass and diversity, parasitic plants could influence key soil functions that are driven my microbial communities (e.g., decomposition and nutrient release), which may ultimately influence the growth of native plants around parasitized invasive plants.
It is also likely that the fungicide contributed to an increase in the number of CFUs of soil bacteria (Figure 4c) by suppressing competitive effects of soil fungi on bacteria. Intermicrobial competition occurs in many natural ecosystems and may arise due to limiting nutrients and space, resulting in the reduced growth of some species, and a change in microbial community composition (Bell, Callender, Whyte, & Greer, 2013). This may feedback on plant growth because different components of the microbial community may exert differential effects on plant growth (Bever, Platt, & Morton, 2012). Competitive interactions between fungi and soil bacteria have been observed (Fitter & Garbaye, 1994; Liu, Yu, Xie, & Staehelin, 2016). For instance, suppression of pathogenic fungi (Fusarium oxysporum) by application of fungicides promoted activities of nitrogen‐fixing bacteria in the roots of Ormosia glaberrima seedlings (Liu et al., 2016). Hence, it is likely that in our case, the fungicide suppressed soil fungi, which in turn freed the soil bacteria from fungal competition.
4.2. Conclusion and implication of the findings for the management of Mikania
We found that the native holoparasitic plant Cuscuta and soil microbes had synergistic suppressive effects on growth of the invader Mikania, while the native Coix benefitted from such interactions. Our results suggest that Cuscuta may be used in combination with soil microbes to control Mikania. Practitioners of classical biological are often faced with the challenge of achieving a successful control of invaders at minimal environmental cost (Müller‐Schärer & Schaffner, 2008). Therefore, the native Cuscuta may be a viable alternative to importation of new species to control Mikania. However, as the soil fungi and bacteria modified the effect of Cuscuta, the identity and impact of the soil microbial community should be an important consideration. Thus, we suggest that future studies should identify the lineage‐specific soil‐borne pathogens and mutualists that may be useful in management of Mikania in combination with Cuscusta.
Since parasitic plants selectively depress the biomass of preferred host taxa that may be competitively dominant within a community, plant parasitism can alter the competitive balance between preferred and nonpreferred hosts (Pennings & Callaway, 2002). As a result of this indirect effect, parasitic plants can alter plant community biomass, species composition, and dynamics (Pennings & Callaway, 2002). For instance, field observations and experimental removal of C. salina from a Northern Californian salt marsh found that the parasite reduced the abundance of dominant host species in the community and facilitated plant species evenness, richness, and diversity (Grewell, 2008; Pennings & Callaway, 1996). A perturbation field experiment at two sites in England (Holme and Strumpshaw) found that R. minor structured a grassland community by selectively parasitizing components of the flora and modifying competitive interactions between plants (Gibson & Watkinson, 1992). Empirical studies have shown that the direction and magnitude of effects of parasitic plants may be influenced by environmental contexts like plant community composition, nutrient and moisture availability, and mycorrhizal fungi present (Le, Tennakoon, Metali, Lim, & Bolin, 2015; Matthies & Egli, 1999; Pennings & Callaway, 1996; Stein et al., 2009; Těšitel, Těšitelová, Fisher, Lepš, & Cameron, 2015). Because of the biotic and abiotic complexity inherent in ecological communities, the present results of a pot and mesocosm study should be corroborated by studies that are conducted under more complex ecological conditions in the field.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
AUTHOR CONTRIBUTIONS
JL and MD conceived and designed the experiment; JL conducted the experiment and analyzed the data; JL, AMOO, FHY, and MD wrote, revised, and approved the manuscript.
DATA AVAILABILITY
The data have been deposited in Dryad with https://doi.org/10.5061/dryad.92kr452.
Supporting information
ACKNOWLEDGMENTS
The authors thank Hezai Zhong and Renjie Liang for practical assistance with the setup of the experiment and all the members of Jennifer Lau laboratory for useful comments on a previous version of the manuscript. This research received financial support from the National Key Research and Development Program (2016YFC1201100) and the National Natural Science Foundation of China (No. 30800133).
Li J, Oduor AMO, Yu F, Dong M. A native parasitic plant and soil microorganisms facilitate a native plant co‐occurrence with an invasive plant. Ecol Evol. 2019;9:8652–8663. 10.1002/ece3.5407
Data Availability Statement: The data have been deposited in Dryad with https://doi.org/10.5061/dryad.92kr452.
REFERENCES
- Agrawal, A. A. , Kotanen, P. M. , Mitchell, C. E. , Power, A. G. , Godsoe, W. , & Klironomos, J. (2005). Enemy release? An experiment with congeneric plant pairs and diverse above and belowground enemies. Ecology, 86, 2979–2989. 10.1890/05-0219 [DOI] [Google Scholar]
- Allen, W. J. , Meyerson, L. A. , Flick, A. J. , & Cronin, J. T. (2018). Intraspecific variation in indirect plant–soil feedbacks influences a wetland plant invasion. Ecology, 99, 1430–1440. 10.1002/ecy.2344 [DOI] [PubMed] [Google Scholar]
- Bailey, V. L. , Smith, J. L. , & Bolton, H. (2003). Novel antibiotics as inhibitors for the selective respiratory inhibition method of measuring fungal: Bacterial rations in soil. Biology and Fertility of Soils, 38, 154–160. [Google Scholar]
- Bardgett, R. D. , Smith, R. S. , Shiel, R. S. , Peacock, S. , Simkin, J. M. , Quirk, H. , & Hobbs, P. J. (2006). Parasitic plants indirectly regulate below‐ground properties in grassland ecosystems. Nature, 439, 969–972. 10.1038/nature04197 [DOI] [PubMed] [Google Scholar]
- Batten, K. M. , Scow, K. M. , Davies, K. F. , & Harrison, S. P. (2006). Two invasive plants alter soil microbial community composition in serpentine grasslands. Biological Invasions, 8, 217–230. 10.1007/s10530-004-3856-8 [DOI] [Google Scholar]
- Bell, T. H. , Callender, K. L. , Whyte, L. G. , & Greer, C. W. (2013). Microbial competition in polar soils: A review of an understudied but potentially important control on productivity. Biology (Basel), 2, 533–554. 10.3390/biology2020533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever, J. D. , Platt, T. G. , & Morton, E. R. (2012). Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annual Review of Microbiology, 66, 265–283. 10.1146/annurev-micro-092611-150107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester, H. J. , Roux, C. , Lopez‐Raez, J. A. , & Becard, G. (2007). Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science, 12, 224–230. 10.1016/j.tplants.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Bunn, R. A. , Ramsey, P. W. , & Lekberg, Y. (2015). Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta‐analysis. Journal of Ecology, 103, 1547–1556. 10.1111/1365-2745.12456 [DOI] [Google Scholar]
- Callaway, R. M. , Mahall, B. E. , Wicks, C. , Pankey, J. , & Zabinski, C. (2003). Soil fungi and the effects of an invasive forb on grasses: Neighbor identity matters. Ecology, 84, 129–135. 10.1890/0012-9658(2003)084[0129:SFATEO]2.0.CO;2 [DOI] [Google Scholar]
- Callaway, R. , Thelen, G. , Rodriguez, A. , & Holben, W. (2004). Soil biota and exotic plant invasion. Nature, 427, 731–733. 10.1038/nature02322 [DOI] [PubMed] [Google Scholar]
- Chen, T. , Nan, Z. , Kardol, P. , Duan, T. , Song, H. , Wang, J. , … Hou, F. (2018). Effects of interspecific competition on plant‐soil feedbacks generated by long‐term grazing. Soil Biology and Biochemistry, 126, 133–143. 10.1016/j.soilbio.2018.08.029 [DOI] [Google Scholar]
- Davies, D. , & Graves, J. (1998). Interactions between arbuscular mycorrhizal fungi and the hemiparasitic angiosperm Rhinanthus minor during co‐infection of a host. New Phytologist, 139, 555–563. 10.1046/j.1469-8137.1998.00211.x [DOI] [Google Scholar]
- Dennis, P. G. , Miller, A. J. , & Hirsch, P. R. (2010). Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities. FEMS Microbiology Ecology, 72, 313–327. 10.1111/j.1574-6941.2010.00860.x [DOI] [PubMed] [Google Scholar]
- Dickie, I. A. , Bolstridge, N. , Cooper, J. A. , & Peltzer, D. A. (2010). Co‐invasion by Pinus and its mycorrhizal fungi. New Phytologist, 187, 475–484. 10.1111/j.1469-8137.2010.03277.x [DOI] [PubMed] [Google Scholar]
- Díez, J. (2005). Invasion biology of Australian ectomycorrhizal fungi introduced with eucalypt plantations into the Iberian Peninsula. Biological Invasions, 7, 3–15. 10.1007/s10530-004-9624-y [DOI] [Google Scholar]
- Diez, J. M. , Dickie, I. , Edwards, G. , Hulme, P. E. , Sullivan, J. J. , & Duncan, R. P. (2010). Negative soil feedbacks accumulate over time for non‐native plant species. Ecology Letters, 13, 803–809. 10.1111/j.1461-0248.2010.01474.x [DOI] [PubMed] [Google Scholar]
- Dostál, P. , Müllerová, J. , Pyšek, P. , Pergl, J. , & Klinerová, T. (2013). The impact of an invasive plant changes over time. Ecology Letters, 16, 1277–1284. 10.1111/ele.12166 [DOI] [PubMed] [Google Scholar]
- El‐Khair, A. H. , & Haggag, K. H. E. (2007). Application of some bactericides and bioagents for controlling the soft rot disease in potato. Research Journal of Agriculture and Biological Sciences, 3, 463–473. [Google Scholar]
- Fitter, A. H. , & Garbaye, J. (1994). Interactions between mycorrhizal fungi and other soil organisms. Plant and Soil, 159, 123–132. 10.1007/BF00000101 [DOI] [Google Scholar]
- Gehring, C. , & Whitham, T. (1992). Reduced mycorrhizae on Juniperus monosperma with mistletoe: The influence of environmental stress and tree gender on a plant parasite and a plant fungal mutualism. Oecologia, 89, 298–303. 10.1007/BF00317231 [DOI] [PubMed] [Google Scholar]
- Gibson, C. C. , & Watkinson, A. R. (1992). The role of the hemiparasitic annual Rhinanthus minor in determining grassland community structure. Oecologia, 89, 62–68. 10.1007/BF00319016 [DOI] [PubMed] [Google Scholar]
- Grewell, B. J. (2008). Parasite facilitates plant species coexistence in a coastal wetland. Ecology, 89, 1481–1488. 10.1890/07-0896.1 [DOI] [PubMed] [Google Scholar]
- Hetrick, B. A. D. , Wilson, G. T. , & Hartnett, D. C. (1989). Relationship between mycorrhizal dependence and competitive ability of two tallgrass prairie grasses. Canadian Journal of Botany, 67, 2608–2615. 10.1139/b89-337 [DOI] [Google Scholar]
- Hill, S. B. , & Kotanen, P. M. (2012). Biotic interactions experienced by a new invader: Effects of its close relatives at the community scale. Botany‐Botanique, 90, 35–42. 10.1139/b11-084 [DOI] [Google Scholar]
- Holm, L. G. , Plucknett, D. L. , Pancho, J. V. , & Herberger, J. P. (1977). The world's worst weeds: Distribution and biology. Honolulu, HI: University Press of Hawaii. [Google Scholar]
- Jeschke, W. D. , Bäumel, P. , Räth, N. , et al. (1994). Modelling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L.: II. Flows between host and parasite and within the parasitized host. Journal of Experimental Botany, 45, 801–812. [Google Scholar]
- Jordan, N. R. , Aldrich‐Wolfe, L. , Huerd, S. C. , Larson, D. L. , & Muehlbauer, G. (2012). Soil‐occupancy effects of invasive and native grassland plant species on composition and diversity of mycorrhizal associations. Invasive Plant Science and Management, 5, 494–505. 10.1614/IPSM-D-12-00014.1 [DOI] [Google Scholar]
- Kardol, P. , Cornips, N. J. , van Kempen, M. M. L. , Bakx‐Schotman, J. M. T. , & van der Putten, W. H. (2007). Microbe‐mediated plant‐soil feedback causes historical contingency effects in plan community assembly. Ecological Monographs, 77, 147–162. [Google Scholar]
- Klironomos, J. N. (2002). Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature, 417, 67–70. 10.1038/417067a [DOI] [PubMed] [Google Scholar]
- Klironomos, J. N. (2003). Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology, 84(9), 2292–2301. [Google Scholar]
- Kormanik, P. , Bryan, W. , & Schultz, R. (1980). Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Canadian Journal of Microbiology, 26, 536–538. 10.1139/m80-090 [DOI] [PubMed] [Google Scholar]
- Kourtev, P. , Ehrenfeld, J. , & Häggblom, M. (2003). Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry, 35, 895–905. 10.1016/S0038-0717(03)00120-2 [DOI] [Google Scholar]
- Kowalski, K. P. , Bacon, C. , Bickford, W. , Braun, H. , Clay, K. , Leduc‐Lapierre, M. èlE. , … Wilcox, D. A. (2015). Advancing the science of microbial symbiosis to support invasive species management: A case study on Phragmites in the Great Lakes. Frontiers in Microbiology, 6, 1–14. 10.3389/fmicb.2015.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmatiski, A. , Beard, K. H. , Stevens, J. R. , & Cobbold, S. M. (2008). Plant–soil feedbacks: A meta‐analytical review. Ecology Letters, 11, 980–992. 10.1111/j.1461-0248.2008.01209.x [DOI] [PubMed] [Google Scholar]
- Lankau, R. A. (2010). Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biological Invasions, 12, 2059–2068. 10.1007/s10530-009-9608-z [DOI] [Google Scholar]
- Le, Q.‐V. , Tennakoon, K. U. , Metali, F. , Lim, L. B. L. , & Bolin, J. F. (2015). Impact of Cuscuta australis infection on the photosynthesis of the invasive host, Mikania micrantha, under drought condition. Weed Biology and Management, 15, 138–146. [Google Scholar]
- Le Roux, J. , Hui, C. , Keet, J. , & Ellis, A. (2017). Co‐introduction versus ecological fitting as pathways to the establishment of effective mutualisms during biological invasions. New Phytologist, 215, 1354–1360. [DOI] [PubMed] [Google Scholar]
- Levine, J. M. , Adler, P. B. , & Yelenik, S. G. (2004). A meta‐analysis of biotic resistance to exotic plant invasions. Ecology Letters, 7, 975–989. 10.1111/j.1461-0248.2004.00657.x [DOI] [Google Scholar]
- Li, J.‐M. , & Jin, Z. (2006). Correlation of the soil bacteria count and DNA content and their relationship with environmental factors under Heptacodium miconioides forest. Journal of Zhejiang University, 33, 333–337. [Google Scholar]
- Li, J.‐M. , Jin, Z.‐X. , Hagedorn, F. , & Li, M.‐H. (2014). Short‐term parasite infection alters already the biomass, activity and functional diversity of soil microbial communities. Scientific Reports, 4, 6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Jin, Z. , & Song, W. (2012). Do native parasitic plants cause more damage to exotic invasive hosts than native non‐invasive hosts? An implication for biocontrol. PLoS ONE, 7, e34577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Yu, S. , Xie, Z. , & Staehelin, C. (2016). Distance‐dependent effects of pathogenic fungi on seedlings of a legume tree: Impaired nodule formation and identification of antagonistic rhizosphere bacteria. Journal of Ecology, 104, 1009–1019. 10.1111/1365-2745.12570 [DOI] [Google Scholar]
- Mack, R. N. , Simberloff, D. , Mark Lonsdale, W. , Evans, H. , Clout, M. , & Bazzaz, F. A. (2000). Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications, 10, 689–710. 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2 [DOI] [Google Scholar]
- Marler, M. J. , Zabinski, C. A. , & Callaway, R. M. (1999). Mycorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass. Ecology, 80, 1180–1186. 10.1890/0012-9658(1999)080[1180:MIECEO]2.0.CO;2 [DOI] [Google Scholar]
- Maron, J. L. , Marler, M. , Klironomos, J. N. , & Cleveland, C. C. (2011). Soil fungal pathogens and the relationship between plant diversity and productivity. Ecology Letters, 14, 36–41. 10.1111/j.1461-0248.2010.01547.x [DOI] [PubMed] [Google Scholar]
- Maron, J. L. , & Vilà, M. (2001). Do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos, 95, 363–373. [Google Scholar]
- Matthies, D. , & Egli, P. (1999). Response of a root hemiparasite to elevated CO2 depends on host type and soil nutrients. Oecologia, 120, 156–161. 10.1007/s004420050844 [DOI] [PubMed] [Google Scholar]
- McKibben, M. , & Henning, J. A. (2018). Hemiparasitic plants increase alpine plant richness and evenness but reduce arbuscular mycorrhizal fungal colonization in dominant plant species. PeerJ, 6, e5682 10.7717/peerj.5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado, A. , Morillas, L. , Gallardo, A. , & Zamora, R. (2016). Temporal dynamic of parasite‐mediated linkages between the forest canopy and soil processes and the microbial community. New Phytologist, 211, 1382–1392. 10.1111/nph.13984 [DOI] [PubMed] [Google Scholar]
- Menzel, A. , Hempel, S. , Klotz, S. , Moora, M. , Pyšek, P. , Rillig, M. C. , … Kühn, I. (2017). Mycorrhizal status helps explain invasion success of alien plant species. Ecology, 98, 92–102. 10.1002/ecy.1621 [DOI] [PubMed] [Google Scholar]
- Miao, S. , Li, Y. , Guo, Q. , Yu, H. , Ding, J. Q. , Yu, F. H. , … Dong, M. (2012). Potential alternatives to classical biocontrol: Using native agents in invaded habitats and genetically engineered sterile cultivars for invasive plant management. Tree and Forestry Science and Biotechnology, 6, 17–21. [Google Scholar]
- Mitchell, C. E. , Agrawal, A. A. , Bever, J. D. , Gilbert, G. S. , Hufbauer, R. A. , Klironomos, J. N. , … Vazquez, D. P. (2006). Biotic interactions and plant invasions. Ecology Letters, 9, 726–740. 10.1111/j.1461-0248.2006.00908.x [DOI] [PubMed] [Google Scholar]
- Moora, M. , & Zobel, M. (1996). Effect of arbuscular mycorrhiza on inter‐ and interaspecific competition of two grassland species. Oecologia, 108, 79–84. [DOI] [PubMed] [Google Scholar]
- Müller‐Schärer, H. , & Schaffner, U. (2008). Classical biological control: Exploiting enemy escape to manage plant invasions. Biological Invasions, 10, 859–874. 10.1007/s10530-008-9238-x [DOI] [Google Scholar]
- Nuñez, M. A. , & Dickie, I. A. (2014). Invasive belowground mutualists of woody plants. Biological Invasions, 16, 645–661. 10.1007/s10530-013-0612-y [DOI] [Google Scholar]
- Oduor, A. M. O. (2013). Evolutionary responses of native plant species to invasive plants: A review. New Phytologist, 200, 986–992. 10.1111/nph.12429 [DOI] [PubMed] [Google Scholar]
- Oduor, A. M. O. , van Kleunen, M. , & Stift, M. (2017). In the presence of specialist root and shoot herbivory, invasive‐range Brassica nigra populations have stronger competitive effects than native‐range populations. Journal of Ecology, 105, 1679–1686. 10.1111/1365-2745.12779 [DOI] [Google Scholar]
- Pennings, S. C. , & Callaway, R. M. (1996). Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology, 77, 1410–1419. 10.2307/2265538 [DOI] [Google Scholar]
- Pennings, S. , & Callaway, R. (2002). Parasitic plants: Parallels and contrasts with herbivores. Oecologia, 131, 479–489. 10.1007/s00442-002-0923-7 [DOI] [PubMed] [Google Scholar]
- Press, M. , & Phoenix, K. (2005). Impacts of parasitic plants on natural communities. New Phytologist, 166, 737–751. 10.1111/j.1469-8137.2005.01358.x [DOI] [PubMed] [Google Scholar]
- Press, M. , Scholes, J. , & Watling, J. (1999). Parasitic plants: Physiological and ecological interactions with their hosts In Press M., Scholes J., & Barker M. (Eds.), Physiological plant ecology: The 39th symposium of the British Ecological Society held at the University of York, UK, 7–9 September 1998 (pp. 175–197). Oxford, UK: Blackwell Science. [Google Scholar]
- Prider, J. , Walting, J. , & Facelli, J. (2009). Impacts of a native parasitic plant on an introduced and a native host species: Implications for the control of an invasive weed. Annals of Botany, 103, 107–115. 10.1093/aob/mcn214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, A. , Bever, J. D. , Gardes, M. , Parrent, J. L. , Rillig, M. C. , & Klironomos, J. N. (2009). Mycorrhizal symbioses and plant invasions. Annual Review of Ecology, Evolution, and Systematics, 40, 699–715. 10.1146/annurev.ecolsys.39.110707.173454 [DOI] [Google Scholar]
- Quested, H. M. (2008). Parasitic plants—Impacts on nutrient cycling. Plant and Soil, 311, 269–272. 10.1007/s11104-008-9646-9 [DOI] [Google Scholar]
- Richardson, D. M. , Allsopp, N. , D'antonio, C. M. , Milton, S. J. , & Rejmánek, M. (2000). Plant invasions the role of mutualisms. Biological Reviews of the Cambridge Philosophical Society, 75, 65–93. 10.1017/S0006323199005435 [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Echeverría, S. (2010). Rhizobial hitchhikers from Down Under: Invasional meltdown in a plant–bacteria mutualism? Journal of Biogeography, 37, 1611–1622. 10.1111/j.1365-2699.2010.02284.x [DOI] [Google Scholar]
- Shen, H. , Hong, L. , Ye, W. , Cao, H. , & Wang, Z. (2007). The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha . Journal of Experimental Botany, 58, 2929–2937. 10.1093/jxb/erm168 [DOI] [PubMed] [Google Scholar]
- Shen, H. , Xu, S.‐J. , Hong, L. , Wang, Z.‐M. , & Ye, W.‐H. (2013). Growth but not photosynthesis response of a host plant to infection by a holoparasitic plant depends on nitrogen supply. PLoS ONE, 8, e75555 10.1371/journal.pone.0075555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipunov, A. , Newcombe, G. , Raghavendra, A. K. H. , & Anderson, C. L. (2008). Hidden diversity of endophytic fungi in an invasive plant. American Journal of Botany, 95, 1096–1108. 10.3732/ajb.0800024 [DOI] [PubMed] [Google Scholar]
- Shivega, W. G. , & Aldrich‐Wolfe, L. (2017). Native plants fare better against an introduced competitor with native microbes and lower nitrogen availability. AoB Plants, 9, plx004 10.1093/aobpla/plx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigüenza, C. , Crowley, D. , & Allen, E. (2006). Soil microorganisms of a native shrub and exotic grasses along a nitrogen deposition gradient in southern California. Applied Soil Ecology, 32, 13–26. 10.1016/j.apsoil.2005.02.015 [DOI] [Google Scholar]
- Simberloff, D. , & Von Holle, B. (1999). Positive interactions of nonindigenous species: Invasional meltdown? Biological Invasions, 1, 21–32. [Google Scholar]
- Speek, T. A. A. , Schaminée, J. H. J. , Stam, J. M. , Lotz, L. A. P. , Ozinga, W. A. , & van der Putten, W. H. (2015). Local dominance of exotic plants declines with residence time: A role for plant soil feedback? AoB Plants, 7, plv021 10.1093/aobpla/plv021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štajerová, K. , Šmilauerová, M. , & Šmilauer, P. (2009). Arbuscular mycorrhizal symbiosis of herbaceous invasive neophytes in the Czech Republic. Preslia, 81, 341–355. [Google Scholar]
- Stein, C. , Rißmann, C. , Hempel, S. , Renker, C. , Buscot, F. , Prati, D. , & Auge, H. (2009). Interactive effects of mycorrhizae and a root hemiparasite on plant community productivity and diversity. Oecologia, 159, 191–205. 10.1007/s00442-008-1192-x [DOI] [PubMed] [Google Scholar]
- Stewart, R. , & Press, C. (1990). The physiology and biochemistry of parasitic angiosperms. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 127–151. 10.1146/annurev.pp.41.060190.001015 [DOI] [Google Scholar]
- Stovall, M. , & Clay, K. (1988). The effect of the fungus, Balansia cyperi Edg., on growth and reproduction of purple nutsedge, Cyperus rotundus L. New Phytologist, 109, 351–360. 10.1111/j.1469-8137.1988.tb04205.x [DOI] [Google Scholar]
- Stricker, K. B. , Harmon, P. F. , Goss, E. M. , Clay, K. , & Luke Flory, S. (2016). Emergence and accumulation of novel pathogens suppress an invasive species. Ecology Letters, 19, 469–477. [DOI] [PubMed] [Google Scholar]
- Těšitel, J. , Těšitelová, T. , Fisher, J. P. , Lepš, J. , & Cameron, D. D. (2015). Integrating ecology and physiology of root‐hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytologist, 205, 350–360. 10.1111/nph.13006 [DOI] [PubMed] [Google Scholar]
- van der Putten, W. , Klironomos, J. , & Wardle, D. (2007). Microbial ecology of biological invasions. The ISME Journal, 1, 28–37. 10.1038/ismej.2007.9 [DOI] [PubMed] [Google Scholar]
- van der Putten, W. H. , Van, B. R. D. , Bever, J. D. , Martijn Bezemer, T. , Casper, B. B. , Fukami, T. , … Wardle, D. A. (2013). Plant–soil feedbacks: The past, the present and future challenges. Journal of Ecology, 101, 265–276. 10.1111/1365-2745.12054 [DOI] [Google Scholar]
- van der Putten, W. , van Dijk, C. , & Peters, B. (1993). Plant‐specific soil‐borne diseases contribute to succession in foredune vegetation. Nature, 362, 53–56. 10.1038/362053a0 [DOI] [Google Scholar]
- van Kleunen, M. , Bossdorf, O. , & Dawson, W. (2018). The ecology and evolution of alien plants. Annual Review of Ecology, Evolution, and Systematics, 49, 25–47. 10.1146/annurev-ecolsys-110617-062654 [DOI] [Google Scholar]
- Vilà, M. , Espinar, J. L. , Hejda, M. , Hulme, P. E. , Jarošík, V. , Maron, J. L. , … Pyšek, P. (2011). Ecological impacts of invasive alien plants: A meta‐analysis of their effects on species, communities and ecosystems. Ecology Letters, 14, 702–708. 10.1111/j.1461-0248.2011.01628.x [DOI] [PubMed] [Google Scholar]
- Vogelsang, K. , & Bever, J. (2009). Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology, 90, 399–407. 10.1890/07-2144.1 [DOI] [PubMed] [Google Scholar]
- Wang, B. S. , Wang, Y. J. , Liao, W. B. , et al. (2004). The invasion ecology and management of alien weed Mikania micrantha H.B.K. Beijing, China: Science Press. [Google Scholar]
- Wang, R. , Guan, M. , Li, Y. , Yang, B. , & Li, J. M. (2012). Effect of the parasitic Cuscuta australis on the community diversity and the growth of Alternanthera philoxeroides . Acta Ecologica Sinica, 32, 1917–1923. [Google Scholar]
- Westover, K. M. , & Bever, J. D. (2001). Mechanisms of plant species coexistence: Roles of rhizosphere bacteria and root fungal pathogens. Ecology, 82, 3285–3294. 10.1890/0012-9658(2001)082[3285:MOPSCR]2.0.CO;2 [DOI] [Google Scholar]
- Yu, H. , Liu, J. , He, W.‐M. , Miao, S.‐L. , & Dong, M. (2011). Cuscuta australis restrains three exotic invasive plants and benefits native species. Biological Invasions, 13, 747–756. 10.1007/s10530-010-9865-x [DOI] [Google Scholar]
- Yu, H. , Yu, F. , Miao, S. , & Dong, M. (2008). Holoparasitic Cuscuta campestris suppresses invasive Mikania micrantha and contributes to native community recovery. Biological Conservation, 141, 2653–2661. 10.1016/j.biocon.2008.08.002 [DOI] [Google Scholar]
- Zhang, L. Y. , Ye, W. H. , Cao, H. L. , & Feng, H. L. (2004). Mikania micrantha H.B.K. in China – An overview. Weed Research, 44, 42–49. 10.1111/j.1365-3180.2003.00371.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data have been deposited in Dryad with https://doi.org/10.5061/dryad.92kr452.


