Abstract
Nutrigenomic DNA reprogramming in different chronic diseases and cancer has been assessed through the stimulation of gene expression and mRNA synthesis versus DNA silencing by CpG DNA modification (methylation); histone modification (acetylation, methylation) and expression of small noncoding RNAs, known as microRNAs (miRNAs). With regard to the specific nutrigenomic effects in psoriasis, the influence of specific diets on inflammatory cell signaling transcriptional factors such as nuclear factor (NF)-κB and Wnt signaling pathways, on disease-related specific cytokine expression, pro/antioxidant balance, keratinocyte proliferation/apoptosis and on proliferation/differentiation ratio have been documented; however, the influence of dietary compounds on the balance between ‘good and bad’ miRNA expression has not been considered. This review aims to summarize knowledge about aberrant microRNAs expression in psoriasis and to emphasize the potential impact of some dietary compounds on endogenous miRNA synthesis in experimental conditions in vivo and in vitro. Among the aberrantly expressed miRNAs in psoriasis, one of the most prominently upregulated seems to be miR-21. The beneficial effects of phenolic compounds (curcumin and resveratrol), vitamin D, methyl donors, and omega-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid) are discussed. Highly expressed miR-155 has been downregulated by flavonoids (through a quercetin-rich diet) and by vitamin D. Quercetin has been effective in modulating miR-146a. On the other hand, downregulated miR-125b expression was restored by vitamin D, Coenzyme Q10 and by microelement selenium. In conclusion, the miRNA profile, together with other ‘omics’, may constitute a multifaceted approach to explore the impact of diet on psoriasis prevention and treatment.
Keywords: psoriasis, miRNA, nutrition
Introduction
Dietary habits, through specific bioactive food components, may combine with a genetic background and influence the development and progression of diseases; however, nutrients may also counteract or even hinder disease development. Although in the past two decades increasing evidence supports a modulating role of nutrients in several physiological and pathological aspects, the exact mechanisms remain elusive. Among the proposed hypotheses, epigenetic DNA reprogramming has been assessed through the evaluation of: stimulated gene expression and mRNA transcription, DNA silencing through CpG DNA modification (methylation); histone modification (acetylation, methylation); and expression of small noncoding RNAs, known as microRNAs (miRNAs). The altered epigenomic status of psoriatic patients has been documented and discussed in terms of the drug response in regard to histone H3 and H4 acetylation, H3K4 and H3K27 methylation,1 as well as the possible application of histone deacetylase inhibitors (HDACs) to achieve antiproliferative and anti-inflammatory effects.2 As far as miRNA expression is concerned, personalized nutrition may represent the management of dietary habits to serve as possible epigenetic supplements of standard therapy of certain diseases,3 with the aim to improve the balance between ‘good and bad’ miRNA expression patterns.
So far, there have been no data on possible dietary manipulation and related miRNA profiles in psoriasis. This review aims to summarize the knowledge about psoriatic microRNAs modulated by nutrients in clinical studies and experimental conditions in vivo and in vitro.
Current dietary management of psoriasis
Evidence-based clinical reports have highlighted the management of dietary habits in slowing down the clinical course and prognosis of psoriasis.4–6 Recommended diets may also reduce the intake of immunosuppressive drugs and their side effects; from a pharmaco-economic point of view, a healthy diet may reduce the cost of chronic disease treatment and may decrease risk of several comorbidities. Monitored patients have achieved minimal disease activity criteria and improvements in Psoriasis Area and Severity Index scores.7–10
The main nutritional recommendations in psoriasis have been directed towards: (1) reducing body mass index (by hypocaloric dietary regimens,11–13 short-time fasting periods14 or even by gastric bypass for obese patients15); (2) the supplementation of biologically active compounds: hydrosoluble vitamins (B1, B2, B6, B12, C, folic acid, coenzyme Q10), 7,16 liposoluble vitamins (vitamin A/beta-carotene, vitamin D, vitamin E),6,16,17 tryptophan,6 omega-unsaturated fatty acids and oligo elements (selenium, chromium, copper, iron and zinc)17,18; (3) reducing leaky gut-induced immunity and toxicity of gliadorphin by gluten-free diet (in patients with positive serological markers of gluten sensitivity19–24); (4) avoiding cholesterol-rich food25–27; and (5) supplementation of different spices (curcumin).6,8 Recommended foods should contain proteins, mostly from skimmed milk, light cheese, yogurt, fish and livestock; carbohydrates, mostly from rice, green and yellow vegetables, whole cereals, potatoes, beans, nuts and seeds; fruits rich in antioxidants, polyphenols and proanthocyanidins; lipids and omega-3 polyunsaturated fatty acids, mostly from fish and extra virgin olive oil.6–10
Nutritional strategies in psoriasis have been able to suppress local and systemic inflammation, T helper (Th)1 autoimmune reactions, inflammatory cytokines [interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α] and adipocytokine secretion; to impair glucose availability and demand for proliferating keratinocytes; to improve antioxidative cell defense system; to stabilize epidermal cell membrane permeability and to prevent epidermal water loss through the influence on cell structure integrity; and to slow down the progression of atherosclerosis.28,29 Eicosapentaenoic acid (EPA) was documented to be effective in reducing adipocytokine synthesis serving instead of arachidonic acid.30,31 Ceramides, as the main component of the stratum corneum of the epidermis, can stabilize the epidermal cell membrane structure, regulate the epidermal keratinocyte cell proliferation rate and suppress aberrant keratinocyte differentiation, cell senescence, migration and adhesion.32–34 On the other hand, saturated fatty acids can activate the inflammasomes of keratinocytes, activating a Th17 immune response.35 The vitamin D active form (1,25OH D) has been documented to be a key modulator of immune and inflammatory reaction, serving as an immunosuppressive agent and a direct inducer of T regulatory (Treg) cell immune response. The vitamin D receptor (VDR) ligand 1,25-dihydroxycholecalciferol and VDR agonist curcumin and omega-3 fatty acid are able to activate the LCE3 genes (late cornified envelope genes) that are involved in skin repair.36–39
MicroRNAs as regulators of cell and tissue function
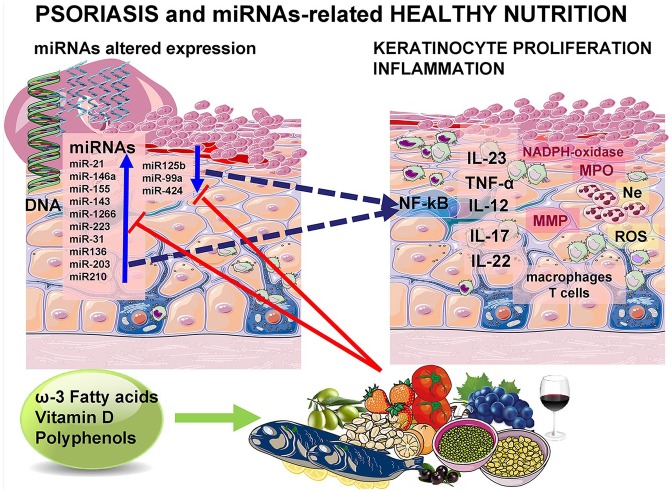
In recent years several studies emphasized a family of small RNAs, known as microRNAs (miRNAs) as powerful regulators of gene expression and transcription, participating in a number of metabolic functions of cells and different disease development and progression. Since dietary factors may have an influence in miRNAs biogenesis, it seems reasonable to assume that some bioactive compounds present in different nutrients may modulate disease development and progression. It has been documented that natural agents, presumably exerting antioxidative and anti-inflammatory properties, such as polyphenolic derivatives present in fruits and wine, were able to modulate miRNA expression in different chronic diseases. Assuming that miRNAs are present in cells from plants to higher organisms, some hypotheses were addressed about possible intestinal absorption of undegraded miRNAs from plant and animal food sources, especially if they are encapsulated in exosomes. A recent report has documented that during the absorption of animal food, such as dairy products, microRNAs may be absorbed in significant amounts, while the absorption of plant-derived microRNAs was insignificant.40 Conversely, it seems more realistic and more convincing to expect that bioactive compounds present in foods may affect endogenous miRNA synthesis (Figure 1).40–47
Figure 1.
Hypothetical influence of dietary compounds on miRNAs expression and modulation of keratinocyte hyperproliferation and inflammation in psoriasis.
IL, interleukin; miRNA, microRNA; MMP, Matrix metalloproteinase; MPO, Mieloperoxidase; NADPH, Nicotinamide adenine dinucleotide phosphate; ROS, Reactive oxigen species; TNF, Tumor necrosis factor.
miRNA synthesis, structure and function
miRNAs are defined as small noncoding RNA molecules, ranging from 21 to 22 nucleotides in length. Their synthesis proceeds through well-defined steps: the hairpin-shaped primary transcripts (pri-miRNAs) are derived from introns of their corresponding transcription parts, from intergenic regions of DNA, catalyzed by RNase II48; the pri-miRNAs undergo catalytic cleavage into smaller transcripts, known as the pre-miRNAs, about 70 nucleotides long, catalyzed by polymerase III Drosha49; the pre-miRNAs as suitable substrate shuttles easily proceed to the cytoplasm through specific nuclear pores, containing the specific carrier Exportin 5; specific helicase, known as Dicer RNase, generates the final, unwound, mature miRNA structure and length; and miRNAs are packaged into exosomes for extracellular transport and may be transported through the bloodstream. So far, hundreds of miRNAs have been identified from plant, animal and human tissues. Their effect is recognized after specific binding to complementary nucleotides of the seed region (about 2–8 nucleotides long) of target mRNA, inducing translational repression, known as a posttranscriptional gene silencing. The other mRNA silencing effect can be related to miR-dependent targeted mRNA degradation.50
The study of the miRNA expression profile was focused after evidence suggested that epigenetic mechanisms may have an influence on DNA outside of promoter and structural DNA genetic regions. About 60% of human mRNAs involved in the coding of cell proteins are regulated by miRNAs and more than 1800 miRNAs were identified, which indicates that miRNAs are capable of regulating almost all living processes. In this way, epigenetic factors can affect the transcriptional regulation of mRNAs involved in cell proliferation, migration, differentiation or inflammation. Since they influence the synthesis of regulatory, catalytic, structural and other important cell and tissue proteins, they may also influence a wide number of diseases in which the dysregulation of proliferation, apoptosis, immune reaction and inflammation may be seen.51
Specific miRNA expression profile in psoriasis and possible influence of nutrients
Psoriasis is a systemic chronic inflammatory disease characterized by a constellation of comorbidities, specifically some of those may also affect the digestive system.52–55 Clinically, it appears as a scaling erythematous and infiltrated plaque, with expression of keratinocyte hyperproliferation and to a rich pabulum of inflammatory cells, comprehending T cells, plasma cells, neutrophils and macrophages.56 The cytokine network involved in the pathogenesis of psoriasis encompasses IL-1, IL-6, IL-17, IL-19, IL-20, IL-22, TNF-α, and interferon (IFN)-stimulated secretion. They exert an influence on keratinocyte proliferation and may serve as potential mitogens for these cells. Although psoriasis is described as being driven by Th1, Th17 and Th22 cells, the exact primum movens remains unknown.57
The etiopathogenesis of psoriasis is complex and is determined by the interaction of genetic and environmental factors. Owing to the high global prevalence of psoriasis worldwide, ranging from 2 to 4.5%,58 and due to the lack of comprehension of the environmental modulation, epigenetic studies have increased, particularly in recent years, to define specific miRNA expression profiles. In this way, the set of specific miRNAs in psoriasis may represent a new diagnostic marker, in addition to established clinical and immune diagnostic markers. The report of Hawkes and colleagues documented aberrant expression of 250 miRNAs, which have been examined in psoriasis-affected keratinocytes, dermal cells, adjacent healthy tissue, compared with the skin tissue of healthy controls and in the peripheral blood of patients with psoriasis.59 Aberrant expression of some miRNA species in psoriasis was cell and tissue-specific and disease-specific, compared with other chronic skin diseases. Increased miRNA levels may be a consequence of stimulated transcription or altered degradation.60
Further research aims to identify the specific biological function of aberrantly expressed miRNA species in psoriasis. Some of them are central mediators of cell proliferation, through the modulation of the downstream intracellular signaling kinases, tumor suppression proteins, apoptotic cascade and inflammatory transcription factors. Current dietary interventions which may be related to miR expression in psoriasis are shown in Table 1.
Table 1.
Active dietary compounds and their effects on miR expression pattern, local and systemic effects which may be related to psoriasis.
| Active dietary compound | miR type expression | Local and systemic effects |
|---|---|---|
| Resveratrol | miR-21
|
Anti-inflammatory mechanisms: - reduction of proinflammatory transcription factor NF-κB; - decreased expression of TNF-α, IL-1β, IL-6, IL–8. - downregulation of MAP kinase, JNK kinase and transcription factor AP-161–66 |
| Methionine, choline | ||
| Curcumin | ||
| Omega-3 fatty acids (EPA and DHA) Fish oil |
||
| Vitamin D (1,25 OH D3) |
||
| Curcumin | miR-155
|
- downregulation of AKT kinase, mTOR pathway, JNK kinase and transcription factor AP-1. - reduction of proinflammatory transcription factor NF-κB67,68; - reduction of TNF-α and IL-6 synthesis69,70 |
| Resveratrol | ||
| Vitamin D (1,25 OH D3) |
||
| Quercetin | miR-146
|
- reduction of proinflammatory transcription factor NF-κB71; - decreased expression of TNF-α, IL-6, IL-1772 |
| Vitamin D (1,25 OH D3) |
miR-125b
|
- reduction of proinflammatory transcription factor NF-κB; - reduction of TNF-α and IL-6 synthesis - decreased keratinocyte proliferation rate73,74 |
| Quercetin |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IL, interleukin; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor alpha.
miR-21 expression in psoriasis and influence of diet
One of the most prominent miRNAs related to chronic diseases is miR-21, present in different isomiR forms, which differ at the 3′ end. One of them belongs to specific 3′ end-posttranscriptional adenylation, catalyzed by the poly(A) polymerase (PAPD5). Diminished PAPD5 activity may cause an increase in the miR-21 expression level, meaning that the polyA tail may be a switching degradation signal. The literature suggests an increased expression pattern of miR-21 in keratinocytes and inflammatory cells in psoriasis. The miR-21 level has been also documented to rise because of altered degradation via specific PAPD5-mediated polyadenylation, resulting in the upregulated state of keratinocyte proliferation and chronic inflammation in psoriasis. The main target of miRNA-21 contributing to chronic inflammation seems to be the de-repression of TNF-α synthesis.75,76 miR-21 is also a promoter of the inflammatory cascade and an apoptotic suppressor. Experimental results with complementary suppression of miR-21 documented the improvement of psoriatic lesions in experimental animals.71,77
Dietary modulations of miRNA-21 expression were examined in various in vivo and in vitro studies. A very powerful epigenetic modulator of miRNA-21 may be the phenolic compound resveratrol, normally present in red wine, red grape juice, mulberries and peanuts. It is capable of downregulating miRNA-21 in different cell culture models. Experiments using the culture of U251 tumor cells, treated with 10 or 50 µM resveratrol for 12 h, decreased miR-21 expression, and was followed by the reduction in the formation of proinflammatory transcription factor nuclear factor (NF)-κB. Its anti-inflammatory mechanisms are further attributed to the decreased expression of TNF-α, IL-β and IL-6. Regarding the proliferative signaling pathways, resveratrol is able to downregulate mitogen-activated protein kinase (MEK), JNK kinase and transcription factor AP-1.61–63 However, it is not known in patients with psoriasis whether miRNA21 is related to the intake of red wine or other foods containing resveratrol.
Curcumin, is a yellow polyphenol diferuloylmethane, obtained from curcuma (Curcuma longa) that has been documented to possess antioxidant and anti-inflammatory properties. Clinical studies have documented that miR-21 and miR-155 expression decreased after daily intake of curcumin. This decrease was followed by a subsequent suppression of key proliferation kinases, such as AKT kinase and TOR pathway, JNK kinase and transcription factor AP-1. The inflammation was attenuated by decreased NF-kB activation, TNF-α and IL-6 synthesis. 69,70
The importance of essential amino acids and methyl-compounds for maintaining downregulated miR-21 was documented when using a choline-deficient, or low-methionine and amino acid-defined diet.62
The expression of miR-21 was significantly decreased in experimental models of breast tumors of mice receiving fish oil. Fish oil can be derived from particularly fatty, cold-water fish, such as mackerel, herring, tuna, sardines and salmon. It is rich in omega-3 fatty acids, such as EPA and docosahexaenoic acid (DHA).78 On the other hand, the exposure of hepatocyte culture to oleic acid upregulated miR-21, consequently upregulating the NF-κB p65 inflammatory transcription factor. Oleic acid represents a monounsaturated fat found in vegetable oils, such as a nut oil, animal fat, meat, poultry, cheese, and in dishes prepared with oleic acid-rich vegetable oils. In recent years, omega-3 fish oil supplements have exerted beneficial effects for cardiovascular diseases.79
The beneficial effects of vitamin D in psoriasis was confirmed many years ago, but there is no article related to the effect of vitamin D on the miRNA expression profile in psoriasis. In recent years, the vitamin D effects have been recognized and explained in terms of its influence on the miRNA expression profile, which contributes to immune suppression. The importance of vitamin D in balancing normal miR-21 expression was documented in atherosclerosis and in osteoporosis. Vitamin D is essential for the function of almost every tissue in addition to the cardiovascular system and bone, including the immune system and the skin. As a fat-soluble steroid pro-vitamin, it is localized in the skin layer of epidermal basal and suprabasal keratinocytes in the form of 7-dehydrocholesterol, where under ultraviolet light of about 295 nm, it can be converted into pre-vitamin D3-cholecalciferol. The active form is a 1,25 hydroxylated form. A deficiency may occur due to a diet with insufficient amounts of vitamin D, impaired absorption and limited exposure to sunlight.64,65 Vitamin D exerts an anti-inflammatory effect, downregulating the production of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8, usually upregulated in psoriasis. Regarding the relationship between vitamin D and miRNA expression in psoriasis, before supplementation, the evaluation of its plasma vitamin D level is recommended, afterwards, the adjuvant treatment in deficient states should be recommended as very important.66
Some studies reported that folate supplementation increased the expression of miR-21 in cultures of colon cancer cells.80 It is known that the folate antagonist, methotrexate, can be used to treat patients with psoriasis. It will be intriguing to study whether excessive folate intake (legumes, green vegetables, nuts, seeds) acts as a risk factor for psoriasis by enhancing miR-21 function or expression.
miR-155 expression in psoriasis and influence of diet
Experiments using healthy mononuclear cells (THP-1) incubated with resveratrol in a concentration of 30 and 50 µM for 14 h, downregulated miR-155, which was also known to be highly expressed in psoriasis.81 Among the flavonoids documented to have a potential benefit on miR-155 expression is quercetin. This is naturally present in brightly colored plant-based foods and has antioxidant and anti-inflammatory properties. Experimental protocols in animals involved their treatment with a quercetin-rich diet in a dose of 2 mg/g bodyweight of mice. The downregulation of the expression of miR-155 was followed by the inhibition of NF-κB activation and the consequent anti-inflammatory effect.82 miR-155 was downregulated after vitamin D treatment in both, in vitro and in vivo experiments. The anti-inflammatory effect proceeds through decreased NF-κB signaling and p65 subunit reduction.67,68
miR-146a expression in psoriasis and influence of diet
Among contradictory reports are those considering the functional expression of the miR146 family, miR146a and miR146b, in psoriasis. One one side, miR-146a represents a critical negative regulator of inflammation and the autoimmune response. It acts as a suppressor of mRNAs transcripts of proinflammatory transcription factors and their downstream signaling molecules. miR-146a reflects the sensitivity of keratinocytes to IL-17A, acting as a suppressor of psoriasis-induced skin inflammation. Several mechanisms explain these findings. Since IL-17 represents one of the main cytokines involved in specific immune responses in psoriasis, the obtained results may characterize miR-146a as a very specific miRNA marker for psoriasis.71,83–85 miR-146a-deficient mice showed prominent psoriasis-like skin inflammation, associated with skin thickness, keratinocyte hyperproliferation, production of chemokines and leukocyte skin infiltration. In vivo delivery of miR-146a reduced the experimental psoriatic skin disease in mice.86
Complementary target mRNAs for miR-146a have been reported to be the TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1), followed by indirect suppression of a NF-κB-dependent inflammatory response, IL-6 and TNF-α synthesis and secretion and Epidermal growth factor (EGF) receptor expression.71 The second anti-inflammatory mechanism of miR-146 refers to the downregulation of atypical chemokine receptors (ACKR2) in primary human keratinocytes. The ACKR2 represents a high-affinity receptor for inflammatory chemokine expression. It is elevated in inflammatory conditions such as psoriasis. miR-146b reduces the expression of ACKR2 mRNA in primary human keratinocytes.87 In relation to innate immunity, miR-146b acts as a negative regulator of Toll-like receptor (TLR)-4. The TLR-4 downstream signaling pathway involves myeloid differentiation factor (MyD88), IRAK1 and TRAF6 signaling , what was documented in culture of human monocytes.88,89 Its low expression pattern seems to contribute to an early and prognostically unfavorable disease onset in genetically susceptible patients. Taking into account these facts, the results regarding decreased levels of miR-146a in psoriasis, compared with healthy people, sound logical.90 Besides the findings related to the immune cascade, the other protective mechanism of miR146 concerns the apolipoprotein E (apoE)–atherosclerosis relationship. It has been documented that apoE is important anti-inflammatory apoprotein, capable of protecting against atherosclerosis and other inflammatory diseases. This effect is mediated through the transcriptional control of miR-146a, which may further attenuate monocyte/macrophage activation and atherosclerosis.91 On the other hand, among the upregulated miRNAs referred in psoriatic skin lesions, dermal cells and peripheral blood is referred to be miR-146a.86 This complexity of the interrelations between the proinflammatory cytokine network have an unexpected consequence, related to the upregulation of IL-17 synthesis and secretion, because high levels of IL-17A induce miR-146a expression. This may suggest that miR-146a would not be powerful enough to completely suppress psoriasis-induced IL-17 secretion and subsequent inflammation.92
Upregulation of miR-146a among higher consumers of quercetin was documented in patients suffering from lung cancer. Quercetin-rich foods (>0.50 mg/100 g) include apples, grapes, onions, artichoke/fennel/celery, beans, apricots, plums, turnips, peppers, strawberries, tomatoes and broccoli.72 Quercetin and quercetin-rich plants have been documented to be effective in the treatment of experimental psoriasis-like lesions; it decreased the levels of TNF-α, IL-6 and IL-17 in the serum of patients with psoriasis. This effect was mediated by the downregulation of NF-κB-induced inflammation.93,94
miR-125b expression and influence of diet
The downregulated miRNA expression pattern was documented for miR-125b in both, keratinocytes and peripheral blood in psoriasis. This consequently leads to an increased keratinocyte proliferation rate, together with altered differentiation and an upregulated inflammatory cascade by de-repressed mRNA for TNF-α5.80,85,95
In addition to the already documented protective effects of vitamin D supplementation on decreasing miR-21 levels, the other way in which vitamin D may make a balance between ‘good and bad’ miRNAs, is that vitamin D treatment was able to increase the miR-125b level in cultures of prostatic epithelial cells.73 Among the flavonoids documented to have a potential benefit on miR-125b expression is quercetin, whose beneficial effects on miR-155 expression documented its function in the balance between ‘good and bad’ miRNAs.74
Coenzyme Q10 and microelement selenium exert antioxidative properties. By reducing oxidative stress, they have been shown to decrease the risk of a number of chronic diseases, followed by inflammatory reaction and oxidative damage. It has been documented that their effects take place through the modulated expression of more than a hundred miRNAs. They are nonspecific for psoriasis, but the antioxidative properties of coenzyme Q10 and selenium may be recommended as dietary supplements.96
Other aberrantly expressed miRNAs in psoriasis
A number of upregulated miRNAs in psoriasis may exhibit a direct proliferative or inflammatory potential. Others may have an indirect influence by inhibiting a family of anti-inflammatory miRNAs. Aberrant expression in psoriasis has been reported for miR-203, which is predominantly expressed in keratinocytes. It has been related to the following: the upregulation of STAT3 (signal transducer and activator of transcription) protein; the upregulation of protein kinase C, one of the key regulators of keratinocyte differentiation; the upregulation of the transcription of proinflammatory cytokines, such as TNF-α, IL-8, and IL-24; the downregulation of the suppressor of cytokine signaling SOCS3 protein, capable of binding to tyrosine kinase receptor and the JAK/STAT proliferative pathway; the downregulation of transcription factor p63, one of p53-related proteins, required for squamous epithelia differentiation. The role of miR-203 in the above-mentioned processes seems to be essential for aberrant keratinocyte proliferation in psoriasis.97–100 miR-184 was found to be highly upregulated in psoriatic keratinocytes. Its targets for proliferation and inflammation have been shown to be the argonaute proteins, essential components of the RNA-induced silencing complex. Its indirect effect occurs through the suppression of miR-205 synthesis that may result in the constant upregulation of AKT kinase, a fundamental regulatory kinase for cell proliferation.100,101 The upregulation of miR-210 has been documented to be directly related to a stimulated inflammatory response by the alteration of immunosuppressive pathways, through the alteration of Tregs and subsequently upregulated IFN-ϒ and IL-17 synthesis, that occurs via FOXP3. miR-369-3p, miR-143 and miR-223 are similarly expressed.85,102,103 Among other miRNAs responsible for IL-17 secretion in psoriasis is miR-1266, found increased in both, peripheral blood and psoriatic skin.104
The broad spectrum of unwanted effects of some miRNAs are documented in the cases of miR-221 and miR-222, which are capable of stimulating matrix-metalloprotease activity and subsequent skin inflammation and keratinocyte proliferation.105,106 The upregulation of miR-31and miR-135b in psoriasis makes them key elements in a complex network of keratinocyte differentiation, proliferation and migration, followed by the activation of inflammatory transcriptional factor NF-κB upregulation. The inflammatory response is initiated by leukocyte migration to the skin.105 The other downregulated miRNA in keratinocytes is miR-99a that results in the altered expression in epidermal tissue, followed by a subsequent activation of proliferative growth factors receptors, such as the insulin-like growth factor-1 receptor (IGF-1R), leading to a subsequent keratinocyte proliferation response. A similar expression pattern was documented for miR-424, which leads to the depression of cyclin proteins and mitogen-activated protein kinase MEK.105,107
In conclusion, particular nutrients may modulate the miRNA expression pattern. A detailed study of the nutrigenomic profile may be beneficial for patients with psoriasis, and not a superficial supplementation of single nutrients. Nutrigenomics, in line with other ‘omics’, may constitute a similar multifaceted approach that precision medicine is claiming. Future studies should also explore the impact of diet change in the miRNA of patients with psoriasis.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The article preparation and the English language proofreading was supported by the Ministry of Education and Science project: TR31060
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Hristina Kocic  https://orcid.org/0000-0001-9667-5798
https://orcid.org/0000-0001-9667-5798
Contributor Information
Hristina Kocic, Clinic for Dermatology Clinical Center University Nis, Klinicki Centar Nis, Bul Dr Zorana Djindjica 48, Nis, 18000, Serbia.
Giovanni Damiani, Unita Operativa di Dermatologia, IRCCS Fondazione Ca’ Granda, Ospedale Maggiore Policlinico, Milano, Dipartimento di Fisiopatologia Medico-Chirurgica e dei Trapianti, Universita degli Studi di Milano, Milano, Italy.
Bojana Stamenkovic, Department of Rheumatology, Institut za Kardiovaskularne Bolesti Niska Banja University Nis, Nis, Serbia.
Michael Tirant, Psoriasis Eczema Clinic Melbourne, Melbourne, Australia.
Andrija Jovic, Dermatology, Clinic for Dermatology University Clinical Center Nis, Nis, Serbia.
Danica Tiodorovic, Dermatology, Clinic for Dermatology, Medical Faculty University Nis, Nis, Serbia.
Ketty Peris, Dermatology, Institute of Dermatology, Catholic University, Roma, Italy.
References
- 1. Ovejero-Benito MC, Reolid A, Sanchez-Jiménez P, et al. Histone modifications associated with biological drug response in moderate-to-severe psoriasis. Exp Dermatol 2018; 27(12): 1361–1371. [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin F, La Thangue NB. Histone deacetylase inhibitors in psoriasis therapy. Curr Drug Targets Inflamm Allergy 2004; 3: 213–219. [DOI] [PubMed] [Google Scholar]
- 3. Egger G, Liang G, Aparicio A, et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004; 429: 457–463. [DOI] [PubMed] [Google Scholar]
- 4. Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol 2013; 27(Suppl. 3): 36–46. [DOI] [PubMed] [Google Scholar]
- 5. Zeng J, Luo S, Huang Y, et al. Critical role of environmental factors in the pathogenesis of psoriasis. J Dermatol 2017; 44: 863–872. [DOI] [PubMed] [Google Scholar]
- 6. Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J 2019; 25: pii: 13030/qt1p37435s. [PubMed] [Google Scholar]
- 7. Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the national psoriasis foundation: a systematic review. JAMA Dermatol 2018; 154: 934–950. [DOI] [PubMed] [Google Scholar]
- 8. Raut G, Wairkar S. Management of psoriasis with nutraceuticals: an update. Complement Ther Clin Pract 2018; 31: 25–30. [DOI] [PubMed] [Google Scholar]
- 9. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol 2014; 71: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci 2018; 22: 8537–8551. [DOI] [PubMed] [Google Scholar]
- 11. Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus 2018; 10: e3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther 2014; 14: 749–756. [DOI] [PubMed] [Google Scholar]
- 13. Jensen P, Christensen R, Zachariae C, et al. Long-term effect of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: a prospective observational follow-up study. Am J Clin Nutr 2016; 104: 259–265. [DOI] [PubMed] [Google Scholar]
- 14. Damiani G, Watad A, Bridgewood C, et al. The impact of Ramadan fasting on the reduction of PASI score, in moderate-to-severe psoriatic patients: a real-life multicenter study. Nutrients 2019;11: pii: E277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Menezes Ettinger JE, Azaro E, de Souza CA, et al. Remission of psoriasis after open gastric bypass. Obes Surg 2006; 16: 94–97. [DOI] [PubMed] [Google Scholar]
- 16. Kharaeva Z, Gostova E, De Luca C, et al. Clinical and biochemical effects of coenzyme Q(10), vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009; 25: 295–302. [DOI] [PubMed] [Google Scholar]
- 17. Mattozzi C, Paolino G, Salvi M, et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci 2016; 20: 1675–1679. [PubMed] [Google Scholar]
- 18. Ely PH. Is psoriasis a bowel disease? Successful treatment with bile acids and bioflavonoids suggests it is. Clin Dermatol 2018; 36: 376–389. [DOI] [PubMed] [Google Scholar]
- 19. Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients 2018; 10: E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol 2014; 71: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woo WK, McMillan SA, Watson RG, et al. Coeliac disease-associated antibodies correlate with psoriasis activity. Br J Dermatol 2004; 151: 891–894. [DOI] [PubMed] [Google Scholar]
- 22. Michaelsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol 2000; 142: 44–51. [DOI] [PubMed] [Google Scholar]
- 23. Chalmers RJ, Kirby B. Gluten and psoriasis. Br J Dermatol 2000; 142: 5–7. [DOI] [PubMed] [Google Scholar]
- 24. Michaelsson G, Ahs S, Hammarström I, et al. Gluten-free diet in psoriasis patients with antibodies to gliadin results in decreased expression of tissue transglutaminase and fewer Ki67+ cells in the dermis. Acta Derm Venereol 2003; 83: 425–429. [DOI] [PubMed] [Google Scholar]
- 25. Raut G, Wairkar S. Management of psoriasis with nutraceuticals: an update. Complement Ther Clin Pract 2018; 31: 25–30. [DOI] [PubMed] [Google Scholar]
- 26. Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther 2019; 32: e12810. [DOI] [PubMed] [Google Scholar]
- 27. Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol 2018; 154: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guida B, Napoleone A, Trio R, et al. Energy-restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: a randomized control clinical trial. Clin Nutr 2014; 33: 399–405. [DOI] [PubMed] [Google Scholar]
- 29. Herbert D, Franz S, Popkova Y, et al. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J Invest Dermatol 2018; 138: 1999–2009. [DOI] [PubMed] [Google Scholar]
- 30. Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol 1998; 38: 539–547. [DOI] [PubMed] [Google Scholar]
- 31. Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid-based lipid infusion in acute, extended guttate psoriasis. Rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig 1993; 71: 634–643. [DOI] [PubMed] [Google Scholar]
- 32. Hill JR, Wertz PW. Structures of the ceramides from porcine palatal stratum corneum. Lipids 2009; 44: 291–295. [DOI] [PubMed] [Google Scholar]
- 33. Garidel P, Fölting B, Schaller I, et al. The microstructure of the stratum corneum lipid barrier: mid-infrared spectroscopic studies of hydrated ceramide:palmitic acid:cholesterol model systems. Biophys Chem 2010; 150: 144–156. [DOI] [PubMed] [Google Scholar]
- 34. Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 2007; 48: 2531–2546. [DOI] [PubMed] [Google Scholar]
- 35. Eder L, Dey A, Joshi AA, et al. Cardiovascular diseases in psoriasis and psoriatic arthritis. J Rheumatol Suppl 2019; 95: 20–27. [DOI] [PubMed] [Google Scholar]
- 36. Perez A, Raab R, Chen TC, et al. Safety and efficacy of oral calcitriol (1,25- dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol 1996; 134: 1070–1078. [PubMed] [Google Scholar]
- 37. Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol 2013; 5: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karrys A, Rady I, Chamcheu RN, et al. Bioactive dietary VDR ligands regulate genes encoding biomarkers of skin repair that are associated with risk for psoriasis. Nutrients 2018; 10: pii: E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mattozzi C, Paolino G, Salvi M, et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci 2016; 20: 1675–1679. [PubMed] [Google Scholar]
- 40. Igaz I, Igaz P. Hypothetic interindividual and interspecies relevance of microRNAs released in body fluids. Exp Suppl 2015; 106: 281–288. [DOI] [PubMed] [Google Scholar]
- 41. Ross SA, Davis CD. The emerging role of microRNAs and nutrition in modulating health and disease. Annu Rev Nutr 2014; 34: 305–336. [DOI] [PubMed] [Google Scholar]
- 42. Lundstrom K. MicroRNA and diet in disease prevention and treatment. J Med Res and Sci 2012; 2: 11–18. [Google Scholar]
- 43. Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res 2007; 61: 24R–29R. [DOI] [PubMed] [Google Scholar]
- 44. Zempleni J, Baier SR, Howard KM, et al. Gene regulation by dietary microRNAs. Can J Physiol Pharmacol 2015; 93: 1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quintanilha BJ, Reis BZ, Duarte GBS, et al. Nutrimiromics: role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients 2017; 9: E1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mico V, Berninches L, Tapia J, et al. NutrimiRAging: micromanaging nutrient sensing pathways through nutrition to promote healthy aging. Int J Mol Sci 2017; 18: E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson RA, Deasy W, Hayes A, et al. High-fat diet and associated changes in the expression of micro-RNAs in tissue: lessons learned from animal studies. Mol Nutr Food Res 2017; 61(6): 1600943. [DOI] [PubMed] [Google Scholar]
- 48. Cui J, Zhou B, Ross SA, et al. Nutrition, microRNAs, and human health. Adv Nutr 2017; 8: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23: 4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228–234. [DOI] [PubMed] [Google Scholar]
- 51. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5: 522–531. [DOI] [PubMed] [Google Scholar]
- 52. Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res 2017; 309: 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fiore M, Leone S, Maraolo AE, et al. Liver illness and psoriatic patients. Biomed Res Int 2018; 2018: 3140983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Damiani G, Franchi C, Pigatto P, et al. Outcomes assessment of hepatitis C virus-positive psoriatic patients treated using pegylated interferon in combination with ribavirin compared to new Direct-Acting Antiviral agents. World J Hepatol 2018; 10: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Della Valle V, Maggioni M, Carrera C, et al. A mysterious abdominal pain during active psoriasis. Intern Emerg Med 2018; 13: 889–892. [DOI] [PubMed] [Google Scholar]
- 56. Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health 2016; 13: pii: E743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rocha-Pereira P, Santos-Silva A, Rebelo I, et al. The inflammatory response in mild and in severe psoriasis. Br J Dermatol 2004; 150: 917–928. [DOI] [PubMed] [Google Scholar]
- 58. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005; 141: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 59. Hawkes JE, Nguyen GH, Fujita M, et al. microRNAs in psoriasis. J Invest Dermatol 2016; 136: 365–371. [DOI] [PubMed] [Google Scholar]
- 60. Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2007; 2: e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li H, Jia Z, Li A, et al. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol Cell Biochem 2013; 382(1–2): 137–143. [DOI] [PubMed] [Google Scholar]
- 62. Latruffe N, Lançon A, Frazzi R, et al. Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann N Y Acad Sci 2015; 1348: 97–106. [DOI] [PubMed] [Google Scholar]
- 63. Tome-Carneiro J, Larrosa M, Yanez-Gascon MJ, et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res 2013; 72: 69–82. [DOI] [PubMed] [Google Scholar]
- 64. Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res 2014; 6: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sheane BJ, Smyth P, Scott K, et al. An association between MicroRNA-21 expression and vitamin D deficiency in coronary artery disease. Microrna 2015; 4: 57–63. [DOI] [PubMed] [Google Scholar]
- 66. Barrea L, Savanelli MC, Di Somma C, et al. Vitamin D and its role in psoriasis: an overview of the dermatologist and nutritionist. Rev Endocr Metab Disord 2017; 18: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, et al. Decreased expression of vitamin D receptor affects an immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway. Int J Mol Sci 2017; 18: pii: E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karkeni E, Bonnet L, Marcotorchino J, et al. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: a new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018; 13: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reuter S, Gupta SC, Park B, et al. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr 2011; 6: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Esatbeyoglu T, Huebbe P, Ernst IM, et al. Curcumin–from molecule to biological function. Angew Chem Int Ed Engl 2012; 51: 5308–5332. [DOI] [PubMed] [Google Scholar]
- 71. Lovendorf MB, Zibert JR, Hagedorn PH, et al. Comparison of microRNA expression using different preservation methods of matched psoriatic skin samples. Exp Dermatol 2012; 21: 299–301. [DOI] [PubMed] [Google Scholar]
- 72. Lam TK, Shao S, Zhao Y, et al. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol Biomarkers Prev 2012; 21: 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Giangreco AA, Vaishnav A, Wagner D, et al. Tumor suppressor microRNAs, miR-100 and –125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev Res (Phila) 2013; 6: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boesch-Saadatmandi C, Wagner AE, Wolffram S, et al. Effect of quercetin on inflammatory gene expression in mice liver in vivo - role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol Res 2012; 65: 523–530. [DOI] [PubMed] [Google Scholar]
- 75. Boele J, Persson H, Shin JW, et al. PAPD5-mediated 3’ adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proc Natl Acad Sci USA 2014; 111: 11467–11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meisgen F, Xu N, Wei T, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol 2012; 21: 312–324. [DOI] [PubMed] [Google Scholar]
- 77. Guinea-Viniegra J, Jimenez M, Schonthaler HB, et al. Targeting miR-21 to treat psoriasis. Sci Transl Med 2014; 6: 225re1. [DOI] [PubMed] [Google Scholar]
- 78. Mandal CC, Ghosh-Choudhury T, Dey N, et al. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis 2012; 33: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vinciguerra M, Sgroi A, Veyrat-Durebex C, et al. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology 2009; 49: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 80. Beckett EL, Martin C, Choi JH, et al. Folate status, folate-related genes and serum miR-21 expression: implications for miR-21 as a biomarker. BBA Clin 2015; 4: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tili E, Michaille JJ, Adair B, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010; 31: 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boesch-Saadatmandi C, Loboda A, Wagner AE, et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem 2011; 22: 293–299. [DOI] [PubMed] [Google Scholar]
- 83. Zibert JR, Lovendorf MB, Litman T, et al. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci 2010; 58: 177–185. [DOI] [PubMed] [Google Scholar]
- 84. Lovendorf MB, Mitsui H, Zibert JR, et al. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Exp Dermatol 2015; 24: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xia P, Fang X, Zhang ZH, et al. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol Lett 2012; 148: 151–162. [DOI] [PubMed] [Google Scholar]
- 86. Srivastava A, Nikamo P, Lohcharoenkal W, et al. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol 2017; 139: 550–561. [DOI] [PubMed] [Google Scholar]
- 87. Shams K, Kurowska-Stolarska M, Schütte F, et al. MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2. J Biol Chem 2018; 293: 3003–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Curtale G, Mirolo M, Renzi TA, et al. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA 2013; 110: 11499–11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith RL, Hébert HL, Massey J, et al. Association of Toll-like receptor 4 (TLR4) with chronic plaque-type psoriasis and psoriatic arthritis. Arch Dermatol Res 2016; 308: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chatzikyriakidou A, Voulgari PV, Georgiou I, et al. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand J Immunol 2010; 71: 382–385. [DOI] [PubMed] [Google Scholar]
- 91. Li K, Ching D, Luk FS, et al. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res 2015; 117: e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Srivastava A, Nikamo P, Lohcharoenkal W, et al. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol 2017; 139: 550–561. [DOI] [PubMed] [Google Scholar]
- 93. Chen H, Lu C, Liu H, et al. Quercetin amelioratesimiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int Immunopharmacol 2017; 48: 110–117. [DOI] [PubMed] [Google Scholar]
- 94. Bigagli E, Cinci L, Paccosi S, et al. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol 2017; 43: 147–155. [DOI] [PubMed] [Google Scholar]
- 95. Xu N, Brodin P, Wei T, et al. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol 2011; 131: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 96. Alehagen U, Johansson P, Aaseth J, et al. Significant changes in circulating microRNA by dietary supplementation of selenium and coenzyme Q10 in healthy elderly males. A subgroup analysis of a prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS One 2017; 12: e0174880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sonkoly E, Wei T, Pavez Lorie E, et al. Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J Invest Dermatol 2010; 130: 124–134. [DOI] [PubMed] [Google Scholar]
- 98. Primo MN, Bak RO, Schibler B, et al. Regulation of pro-inflammatory cytokines TNF-alpha and IL24 by microRNA-203 in primary keratinocytes. Cytokine 2012; 60: 741–748. [DOI] [PubMed] [Google Scholar]
- 99. Lerman G, Avivi C, Mardoukh C, et al. MiRNA expression in psoriatic skin: reciprocal of regulation of hsa-miR-99a and IGF-1R. PLoS One 2011; 6: e20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yi R, Poy MN, Stoffel M, et al. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008; 452: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Roberts JC, Warren RB, Griffiths CE, et al. Expression of microRNA-184 in keratinocytes represses argonaute 2. J Cell Physiol 2013; 228: 2314–2323. [DOI] [PubMed] [Google Scholar]
- 102. Guo S, Zhang Wei C, et al. Serum and skin levels of miR-369-3p in patients with psoriasis and their correlation with disease severity. Eur J Dermatol 2013; 23: 608–613. [DOI] [PubMed] [Google Scholar]
- 103. Zhao M, Wang LT, Liang GP, et al. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol 2014; 150: 22–30. [DOI] [PubMed] [Google Scholar]
- 104. Ichihara A, Jinnin M, Oyama R, et al. Increased serum levels of miR-1266 in patients with psoriasis vulgaris. Eur J Dermatol 2012; 22: 68–71. [DOI] [PubMed] [Google Scholar]
- 105. Zibert JR, Lovendorf MB, Litman T, et al. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci 2010; 58: 177–185. [DOI] [PubMed] [Google Scholar]
- 106. Joyce CE, Zhou X, Xia J, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet 2011; 20: 4025– 4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lerman G, Avivi C, Mardoukh C, et al. MiRNA expression in psoriatic skin: reciprocal regulation of hsa-miR-99a and IGF-1R. PLoS One 2011; 6: e20916. [DOI] [PMC free article] [PubMed] [Google Scholar]