Abstract
Objective:
To measure the relationship between lactate and mortality in hospital inpatients. Main outcomes of interest were 3-day, 30-day, and 1-year all-cause mortality.
Design:
Retrospective cohort study, October 2011 to September 2013.
Setting:
University-affiliated US Veterans Affairs Hospital.
Patients:
All inpatients with lactate level measured during the study period.
Measurements:
Analysis of peak lactate level (mmol/L) during the most recent admission for patients who died, and peak lactate level during an admission for surviving patients. Covariates including sepsis, ICU admission, code blue and rapid response calls, medical vs surgical ward, liver disease, kidney disease, and hospice status were recorded.
Results:
In total, 3325 inpatients were included; 564 patients had sepsis. Median lactate 1.7 mmol/L (interquartile range [IQR] 1.2-2.6). The 3-day, 30-day, and 1-year mortality were 2.5%, 10%, and 24%, respectively. A lactate level cutoff of ⩾4 mmol/L had best test characteristics (sensitivity 52.4%, specificity 91.4%) to predict increased 3-day mortality. Unadjusted risk ratio of death in 3 days for lactate ⩾4 was 10.3 (95% confidence interval [CI] 6.8-15.7). Patients with sepsis had a consistently higher risk of death compared with patients without sepsis for any given level of lactate. Adjusted odds ratio (OR) of 3-day mortality for lactate ⩾4 was 7.6 (95% CI 4.6-12.5); 30-day mortality was 2.6 (95% CI 1.9-3.6); and 1-year mortality was 1.8 (95% CI 1.4-2.6). Lactates in the normal range (<1.7) were also independently associated with 30-day and 1-year mortality.
Conclusions:
Lactate predicts risk of death in all patients, although patients with sepsis have a higher mortality for any given lactate level. We report the novel finding that serum lactate, including normal values, is associated with long-term mortality.
Keywords: Lactic acidosis, mortality, critical care, sepsis, shock, prognostication
Introduction
Serum lactate level rises with both accelerated glycolysis and tissue hypoxia and is known be a predictor of mortality in certain patient populations.1–4 It has been used extensively to risk-stratify patients with suspected or confirmed sepsis.5–8 The Surviving Sepsis Campaign and the National Quality Forum recommend lactate measurement as a central component in the management of patients with sepsis.9 Previous work has characterized the relationship between lactate clearance and survival after cardiac arrest, subarachnoid hemorrhage, metformin ingestion in diabetics, and cardiogenic shock treated with extracorporeal membrane oxygenation.10 Several studies of trauma patients have shown lactate levels are significantly higher in non-survivors than in survivors.7,9,11–13
In review of the literature, a rigorous analysis of the association between lactate level and mortality risk in unselected patients, independent of level of care, or type of illness, is lacking. We hypothesized serum lactate as an important predictor of all-cause mortality in inpatients, regardless of the presence or absence of sepsis. We also hypothesized medical patients as more likely to carry higher mortality than surgical patients for similar lactate values.
Materials and Methods
Design
A retrospective cohort study at a university-affiliated hospital in the US Veterans Affairs Medical System (VA) was conducted. Study approval was obtained from the VA Research Committee and Stanford University Human Subjects Panel (IRB Protocol #15292).
Participants and measurements
All participants with at least one lactate measurement from October 1, 2011 to September 30, 2013 were eligible for inclusion. Records were reviewed in the VA electronic medical record system. Only inpatients were included, but lactate levels drawn in the Emergency Department prior to admission were also included. Serum lactates were collected from either arterial or venous blood. Typically, samples came from peripheral venous samples when obtained in the Emergency department and wards, while samples from arterial catheters were the most common in the ICU. Sampled blood was transported on ice to the clinical lab, where it was analyzed within 15 min on a Beckman-Coulter AU system (Beckman-Coulter, Brea, CA, USA) following manufacturer’s instructions for sample collection, handling, and calibration intervals.14 The within-run precision of this system has a coefficient of variation of <5%.
The highest of all lactate values recorded during a particular admission was included in the study. For patients with multiple admissions, those separated by 1 year or more were analyzed as separate events. If admissions were separated by less than 1 year, only the most recent admission was analyzed, to prevent over counting of mortality events. At this facility, the cutoff for an elevated lactate was ⩾1.7 mmol/L.
Outcome of interest
For a given admission, the highest recorded lactate level was taken as the independent variable, with mortality at 3, 30, and 365 days taken as the primary outcome. Medical and surgical patients were analyzed separately.
Data collection
Our research group maintains a database of all rapid response (termed “eTeam”) activations, code blue calls, and ICU admissions. Pertinent data for study patients were extracted from these databases. Death information was extracted from the VA electronic records database. If no death date was found in the patient record, patients were considered to be alive. Only episodes of true cardiac arrest were included as code blues. Situations where a code blue was called, but where no cardiopulmonary resuscitation (CPR), defibrillation, or intubation took place, were classified as eTeam calls. A patient was categorized as septic with any of the following criteria: ICD-9 codes for severe sepsis (995.92), septic shock (785.52), or the presence of an infection-related ICD-9 code plus a code for an organ failure or hypotension. History of liver and kidney disease were also determined by ICD-9 codes. The inpatient service at hospital discharge was classified as either medical or surgical.
Statistical analysis
Statistical analysis was performed using Stata 14 (StataCorp LP) and Graph Pad Prism 8.0 (Graph Pad Corp). Continuous variables were reported as means (95% confidence intervals [CIs]) and medians (inter-quartile ranges). Categorical variables were reported as counts and percentages. Categorical data were analyzed using Fisher’s exact test. In some analyses, lactate levels were converted to ordinal intervals of 1.0 mmol/L; in analysis of normal lactate levels, intervals of 0.4 mmol/L were used. Stratified analysis was conducted by sepsis, eTeam (rapid response calls), code blue, ICU admission, and service of discharge. To control for potential confounders (hospice, liver disease, kidney disease, age, and sex), backward stepwise logistic regression was used. For all statistical tests, an alpha of <0.05 was considered statistically significant.
Results
15,104 consecutive serum lactate values drawn from a total of 4038 patients were identified. Table 1 shows the demographic characteristics of patients during the study period. Hospitalized patients with lactate drawn were significantly older, and about twice as likely to have septic shock, ICU admission, or cardiac arrest as the general inpatient population.
Table 1.
Clinical characteristics of all inpatients and lactate study patients.
| Characteristic | All hospital admissions | Lactate analyzed | Lactate, admitted | P valuea |
|---|---|---|---|---|
| Number of patients | 11 428 | 4038 | 3325 | |
| Age (SD) | 63 (15) | 67 (15) | 68 (14) | <.0001 |
| Female (%) | 228 (2) | 223 (5.5) | 131 (3.9) | |
| Median lactate (mmol/L) | — | 1.6 | 1.7 | |
| eTeam (rapid response team) call (%) | 561 (5) | 265 (7) | 277 (8) | <.0001 |
| ICU admissions (%) | 1445 (13) | 565 (17) | ||
| Septic shock (%) | 938 (8) | 581 (14) | 564 (17) | <.0001 |
| Cardiac arrests (%) | 45 (0.4) | 40 (1) | 39 (1.2) | <.0001 |
| Medical patients (%) | 4975 (44) | 2063 (51) | 2063 (62) | <.0001 |
| Surgical patients (%) | 3091 (27) | 627 (15) | 627 (19) | <.0001 |
| Mortality at 3 days (all) | 86 (0.8) | 94 (2.3) | <.0001 | |
| Medical inpatients | 34 (1) | 71 (3.4) | <.0001 | |
| Surgical inpatients | 3 (0) | 10 (1.6) | <.0001 | |
| Mortality at 30 days (all) | 413 (4) | 336 (8.3) | <.0001 | |
| Medical inpatients | 244 (5) | 242 (12) | <.0001 | |
| Surgical inpatients | 31 (1) | 29 (4.6) | <.0001 | |
| Mortality at 1 year (all) | 1266 (11) | 825 (20) | <.0001 | |
| Medical inpatients | 790 (16) | 575 (27) | <.0001 | |
| Surgical inpatients | 151 (6) | 79 (13) | <.0001 |
Comparison of entire inpatient population and subgroup for who lactate was analyzed, and patients with lactates treated as inpatients (admitted). Numbers shown indicate patients in each group, with percentages in parentheses.
Comparisons are made between unselected patients and admitted patients, and between columns containing data for mortality comparisons. Age was compared by Student’s t test; all other variables compared by χ2 using Yates’ method.
A total of 3325 inpatients were included in the final analysis. The flow chart in Figure 1 demonstrates the study screening and inclusion process. The mean age was 68.8 years (SD 14.2). A total of 564 patients (17%) were diagnosed with sepsis; 277 patients (8%) had eTeam activations; 39 patients (1.2%) had cardiac arrest; and 565 patients (17%) were admitted to the ICU. Most patients (67%) were admitted to a medical service, 19% were admitted to a surgical service, with the remainder (14%) unable to be clearly identified as medical or surgical admissions.
Figure 1.
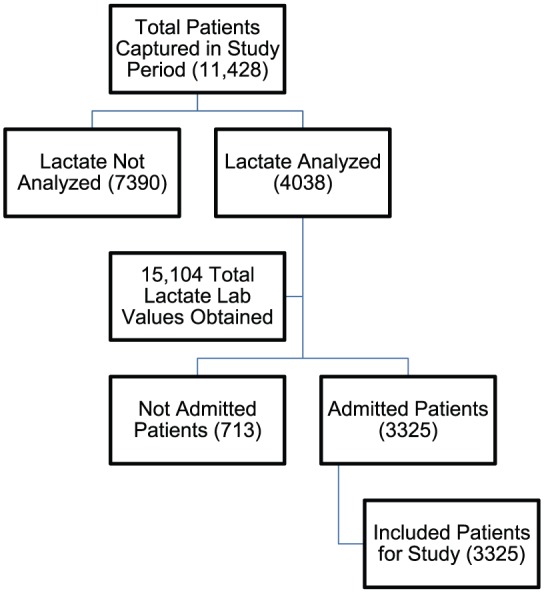
Accounting chart of patients and labs included in the study.
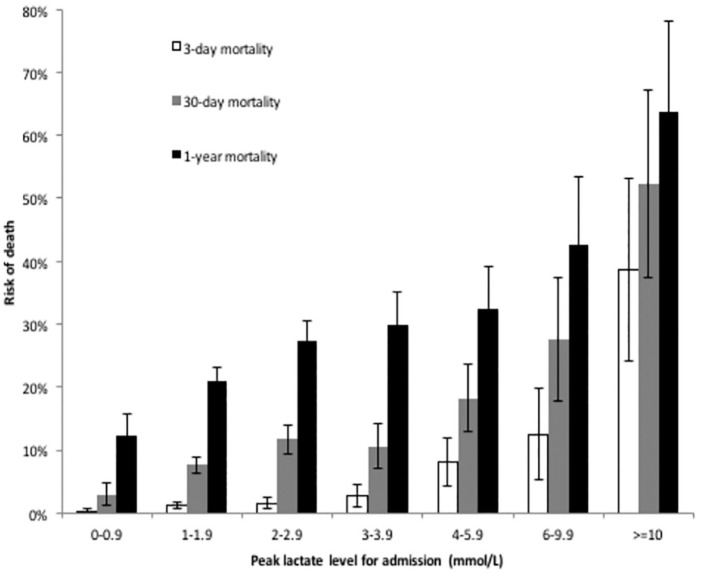
Patients admitted had a median lactate level of 1.7 mmol/L (interquartile range [IQR] 1.2-2.6); 9.7% of which had peak lactates ⩾4.0 mmol/L, and 1.3% had levels ⩾10 mmol/L. The 3-day, 30-day, and 1-year mortalities for inpatients with any lactate level drawn were 2.3%, 8.3%, and 20%, respectively. These were more than double the mortality rate of all inpatients during this time (Table 1). Of the patients with lactate analyzed, the mean lactate of those that died was significantly higher at all mortality intervals. Mean lactate levels among survivors of 3-day, 30-day, and 1-year survival cutoffs were 2.7 (95% CI: 2.7-2.8), 2.7 (95% CI: 2.6-2.7), and 2.6 (95% CI: 2.6-2.7) mmol/L, respectively. Mean lactates among patients who died were 6.9 (95% CI: 5.6-8.1), 4.2 (95% CI: 3.8-4.6), and 3.4 (95% CI: 3.2-3.6) mmol/L, respectively. Survivors and non-survivors at each time interval were compared by Fisher’s exact test, with all three intervals statistically different at P < .0001. Figure 2 shows the risk of death at 3 days, 30 days, and 1 year for all patients by lactate level, demonstrating a rising lactate to be associated with an increased risk of death.
Figure 2.
Mortality by lactate level.
Risk of death at 3 days, 30 days, and 1 year, by level of lactate (in mmol/L), grouped as follows: 0 to 0.9, 1 to 1.9, 2 to 2.9, 3 to 3.9, 4 to 5.9, 6 to 9.9, and ⩾10. Values indicate the mean risk of death for all admitted patients during the study period with lactate levels drawn (and 95% confidence intervals).
Logistic regression was used to control for history of acute kidney injury, chronic kidney disease, liver disease, sepsis, ICU admission, code blue, eTeam, hospice, medical service, surgical service, and age ⩾80 years. The adjusted odds ratio (AOR) of 3-day all-cause mortality for lactate ⩾4 was 7.6 (95% CI 4.6-12.5); AOR of 30-day all-cause mortality for lactate ⩾4 was 2.6 (1.9-3.6); AOR of 1-year all-cause mortality for lactate ⩾4 was 1.8 (1.4-2.4).
Medical vs surgical service
A total of 2229 patients (67%) were treated on a medical service, while 632 patients (19%) were treated on a surgical service; 464 patients (14%) did not have a discharge service easily extracted from the electronic records and were excluded from this portion of the analysis. There was no significant difference in 3-day mortality between medical and surgical inpatients. The 30-day mortality in these groups was 12% and 5%, respectively (P < .0001), while 1-year medical and surgical mortality was 27% and 13%, respectively (P < .0001). For comparison, the overall whole-hospital mortality (calculated as all deaths divided by all discharges) during the 24-month period was 11%, and the 30-day and 1-year ICU mortality during the same period was 20% and 33%, respectively, for medical patients, and 2% and 7% for surgical patients, respectively. Multivariate analysis revealed overall worse survival among medical inpatients vs surgical inpatients.
Normal lactate levels
Lactates in the normal range were stratified into 0.4 mmol/L intervals and evaluated in both univariate and multivariate analysis. Very few patients with normal lactates (16 out of 2187, 0.7%) died within 3 days. All of the latter patients were admitted to a medicine service and were significantly older than survivors (77 vs 67 years mean age; P < .01). In multivariable analysis, only renal failure was a significant predictor of 3-day mortality in such patients, when controlling for age, sepsis, code or emergency team calls and lactate (OR: 4.7; 95% CI 1.7-13). Table 2 shows that lactate interval is an independent predictor of mortality at 30 days and 1 year, along with decade of age and admission status. Adjusted odds ratio of 30-day and 1-year mortality based on lactate interval were 1.5 (95% CI: 1.1-2.1) and 1.3 (1.1-1.6), respectively. The mortality risk associated with medical patients receiving lactate analysis was strongly associated with admission status, which was a stronger predictor of 30-day and 1-year mortality; thus, medical admission lacked significance in the logistic model of mortality in patients with normal lactate levels.
Table 2.
Multivariable logistic regression of normal lactate levels and mortality.
| OR | SE | P > z | [95% CI] | |
|---|---|---|---|---|
| 30-day mortality | ||||
| Admitted to hospital | 27.3 | 27.7 | .001 | [3.7-200.3] |
| Decade of age | 1.6 | 0.2 | .0001 | [1.3-1.9] |
| Lactate interval | 1.5 | 0.3 | .012 | [1.1-2.1] |
| Medical admission | 0.99 | 0.2 | .967 | [0.6-1.5] |
| 1-year mortality | ||||
| Admitted to hospital | 4.27 | 1.1 | .0001 | [2.6-7.1] |
| Decade of age | 1.6 | 0.1 | .0001 | [1.4-1.7] |
| Lactate interval | 1.3 | 0.1 | .003 | [1.1-1.6] |
| Medical admission | 1.31 | 0.2 | .057 | [1.0-1.7] |
Abbreviations: CI, confidence interval; OR, odds ratio.
Lactate levels between 0 and 1.6 mmol/L were split into intervals of 0-0.4, 0.41-0.8, 0.81-1.2, and 1.12-1.6 mmol/L. Age was divided in to the following intervals: 18-29, 30-39, 40-49, 50-59, 60-68, 70-79, >80. The lowest interval of each series served as the reference value.
Patients with sepsis vs patients without sepsis
A total of 564 patients (17% of sample) were diagnosed with sepsis. Risk of death in patients with sepsis vs patients without sepsis was 7% (95% CI: 4%-8%) vs 2% (95% CI: 1%-2%), respectively, at 3 days; 20% (95% CI: 17%-24%) vs 8% (95% CI: 7%-9%) at 30 days; and 39% (95% CI: 35%-43%) vs 21% (95% CI: 19%-23%) at 1 year.
The mean lactate level for patients with sepsis was 3.0 mmol/L (95% CI: 2.8-3.2), compared with 2.1 mmol/L (95% CI: 2.0-2.2) in patients without sepsis (P < .0001). Septic patients had a higher risk of death compared with patients without sepsis for any given level of lactate.
Figure 3 shows a Kaplan-Meier survival curve comparing patients with sepsis vs those without, stratified by lactate cutoffs of <4 and ⩾4 mmol/L. Risk of death for patients with sepsis and lactate <4 mmol/L was similar to that of patients without sepsis with lactate elevated to at least 4 mmol/L.
Figure 3.
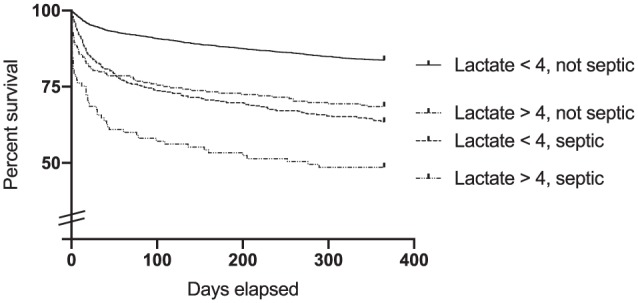
Survival according to interaction between lactate and sepsis.
One-year survival of all admitted patients, into four groups: (1) patients with lactate <4 mmol/L and no diagnosis of sepsis, (2) lactate <4 mmol/L and a diagnosis of sepsis; (3) lactate ⩾4 mmol/L and no diagnosis of sepsis; and, (4) patients with both lactate ⩾4 mmol/L and a diagnosis of sepsis. Identity of curves is indicated by order and position of labels. All intergroup comparisons showed statistically significant differences between groups except 2 vs 3 (P = .35). 1 vs 2 and 4, P < .0001; 4 vs 3, P = .0004.
Discussion
Our investigation supports prior findings of an association between lactate level and mortality.3,4,15–17 Higher levels of lactate had the greatest mortality risk in the short term, while lower elevations of lactate were still associated with mortality at 30 days and 1 year. Further stratification of lactate values within our normal range of <1.7 demonstrated a linear risk of death at 30 days and 1 year. This expands upon the previously published findings Nichol and colleagues demonstrated, in which lactate values within the perceived normal range demonstrated a significant independent relationship between lactate level and hospital mortality.17 Lactate values approaching the upper limit of the normal range had an increasing association with inpatient mortality, similar to our finding in the multivariate analysis for 30-day and 1-year mortality. Our study is unique in demonstrating the risk of death associated with elevated lactate persists up to 1 year after hospitalization in unselected patients.
The analysis is further strengthened by stratification of patients into medical and surgical cohorts, thus defining higher risks at each level associated with medical admission, when controlling for other variables. Multivariate analysis affirmed the risks associated with lactate elevations in the presence of other known risk factors for mortality, including age and sepsis. This association persists after controlling for additional confounding by renal disease, liver disease, and hospice status.
Previous studies of lactate and outcomes have focused on short-term findings, such as ICU, hospital, and 30-day mortalities.3,4,18 The interest in conducting such a study arose from the uncertainty faced by many clinicians when encountering ill patients with an unclear diagnosis, with a lactate level that had already been obtained. Frequently, the clinician encounters mildly elevated lactate values and has little guidance on prognosis, and the need for additional monitoring or diagnostic investigation.
We hypothesize that higher lactate levels may indicate poorer physiologic reserve, and greater autonomic/metabolic stress compensating for an acute illness. Such patients are likely less able to cope with physiologic stressors, are more likely to have prolonged recoveries, making them vulnerable to subsequent insults.
Prior studies of lactate levels have found similar associations with mortality. Initially, this finding was documented in cases of hypovolemic shock,4 with more recent studies examining patients from surgical wards,19,20 the ICU and Emergency Department,9,21–23 and patients with specific conditions such as sepsis,9,22–25 subarachnoid hemorrhage,11 and community acquired pneumonia.22 The use of lactate in stratifying septic patients lacks specificity in some cases;26 however, the overall prognostic value of elevated levels is supported other studies.27 This study further corroborates that the mortality of septic patients increases in proportion lactate levels.
With a new demonstrated association between lactate and mortality in a diverse inpatient population, patients with elevated lactates seem to require greater attention and more aggressive interventions to reverse physiologic derangements. While lactate elevations are not diagnostic for any specific condition, we believe they should be used as a general measure of illness severity. They should factor in to decisions regarding resource allocation. We believe lactate should be incorporated into future clinical prediction tools, given the clear relationship between lactate level and risk of death.
Limitations
The present study is primarily limited by its retrospective nature. Patients were not stratified by disease etiology, except for sepsis. It is possible that other diseases may confer a statistical interaction between lactate and risk of death. Furthermore, only highest lactate level was included. Higher lactates may confer bias toward higher mortality, perhaps with markedly elevated lactate levels factoring into decisions to discontinue life-prolonging therapy. This issue would likely bias short-term mortality, but is unlikely to affect 30-day and 1-year mortality, as well as mortality with normal lactate values.
Lactate clearance was not analyzed, which is known to independently predict short-term clinical outcomes.21–23,28–30 Mortality may be more prominent in patients that are unable to metabolize lactate produced in times of stress.25
Furthermore, no distinction was made between arterial and venous samples, which in some comparative studies have shown to bias toward lower values in the latter.31–33 In our experience, clinicians do not commonly factor these differences into triage or treatment decisions. Similarly, our study does not address the use of point-of-care instruments or capillary blood in lactate analysis.19,34 Despite these limitations, the findings are likely generalizable to the inpatient practice setting. The patients evaluated are similar to many patients at other institutions and present to the hospital with a wide variety of common disease processes. In our experience, lactate levels are obtained more and more as a matter of routine, and in the absence of sepsis, clinicians have questioned the significance of elevated levels.
Conclusions
There is a significant association between lactate level and risk of death that persists many months beyond initial insult. Lactate level should be used to risk-stratify patients’ disease severity, independent of disease process, with elevated lactates indicating higher risk of mortality. This may help identify patients in need of additional scrutiny and monitoring. Future risk stratifying instruments should consider inclusion of lactate level as a component in risk prediction. Prospective studies are needed to validate these results.
Footnotes
Author Contributions: JV contributed as primary manuscript author, data collection and analysis, and project lead. JHS contributed as manuscript revision, research and literature review, formatting, and submission. GL contributed as principle investigator, study design, IRB approval, manuscript revision, and submission.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Jack H Short  https://orcid.org/0000-0001-9606-2772
https://orcid.org/0000-0001-9606-2772
References
- 1. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernandez G, Castro R, Romero C, et al. Persistent sepsis-induced hypotension without hyperlactatemia: is it really septic shock? J Crit Care. 2011;26:435e9–e14. [DOI] [PubMed] [Google Scholar]
- 3. Aduen J, Bernstein WK, Khastgir T, et al. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA. 1994;272:1678–1685. [PubMed] [Google Scholar]
- 4. Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143:1457–1459. [DOI] [PubMed] [Google Scholar]
- 5. Brown JA, Gore DC. In vivo metabolic response of glucose to dichloroacetate in humans. J Surg Res. 1996;61:391–394. [DOI] [PubMed] [Google Scholar]
- 6. Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. [DOI] [PubMed] [Google Scholar]
- 8. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Formica F, Avalli L, Colagrande L, et al. Extracorporeal membrane oxygenation to support adult patients with cardiac failure: predictive factors of 30-day mortality. Interact Cardiovasc Thorac Surg. 2010;10:721–726. [DOI] [PubMed] [Google Scholar]
- 11. Aisiku IP, Chen PR, Truong H, Monsivais DR, Edlow J. Admission serum lactate predicts mortality in aneurysmal subarachnoid hemorrhage. Am J Emerg Med. 2016;34:708–712. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37:96–104. [DOI] [PubMed] [Google Scholar]
- 13. Boucaud-Maitre D, Ropers J, Porokhov B, et al. Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med. 2016;33:1536–1543. [DOI] [PubMed] [Google Scholar]
- 14. Beckman-Coulter. Technical report on lactate assay, 2009. https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/BAOSR6x93/01/EN_LACTATE.pdf.
- 15. Song YH, Shin TG, Kang MJ, et al. Predicting factors associated with clinical deterioration of sepsis patients with intermediate levels of serum lactate. Shock. 2012;38:249–254. [DOI] [PubMed] [Google Scholar]
- 16. Thomas-Rueddel DO, Poidinger B, Weiss M, et al. Hyperlactatemia is an independent predictor of mortality and denotes distinct subtypes of severe sepsis and septic shock. J Crit Care. 2015;30:439.e1–439.e6. [DOI] [PubMed] [Google Scholar]
- 17. Nichol AD, Egi M, Pettila V, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levraut J, Ichai C, Petit I, Ciebiera JP, Perus O, Grimaud D. Low exogenous lactate clearance as an early predictor of mortality in normolactatemic critically ill septic patients. Crit Care Med. 2003;31:705–710. [DOI] [PubMed] [Google Scholar]
- 19. Collange O, Garcia V, Kindo M, et al. Comparison of capillary and arterial lactate levels in patients with shock. Anaesth Crit Care Pain Med. 2017;36:157–162. [DOI] [PubMed] [Google Scholar]
- 20. Venkatesan M, Smith RP, Balasubramanian S, et al. Serum lactate as a marker of mortality in patients with hip fracture: a prospective study. Injury. 2015;46:2201–2205. [DOI] [PubMed] [Google Scholar]
- 21. Haas SA, Lange T, Saugel B, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016;42:202–210. [DOI] [PubMed] [Google Scholar]
- 22. Bhat SR, Swenson KE, Francis MW, Wira CR. Lactate clearance predicts survival among patients in the emergency department with severe sepsis. West J Emerg Med. 2015;16:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holder AL, Gupta N, Lulaj E, et al. Predictors of early progression to severe sepsis or shock among emergency department patients with nonsevere sepsis. Int J Emerg Med. 2016;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aluisio AR, Jain A, Baron BJ, et al. The prognostic role of non-critical lactate levels for in-hospital survival time among ED patients with sepsis. Am J Emerg Med. 2016;34:170–173. [DOI] [PubMed] [Google Scholar]
- 25. Levraut J, Ciebiera JP, Chave S, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than overproduction. Am J Respir Crit Care Med. 1998;157:1021–1026. [DOI] [PubMed] [Google Scholar]
- 26. Dugas AF, Mackenhauer J, Salciccioli JD, Cocchi MN, Gautam S, Donnino MW. Prevalence and characteristics of nonlactate and lactate expressors in septic shock. J Crit Care. 2012;27:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38:4–10. [DOI] [PubMed] [Google Scholar]
- 28. Donnino MW, Miller J, Goyal N, et al. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75:229–234. [DOI] [PubMed] [Google Scholar]
- 29. Shin TG, Jo IJ, Hwang SY, et al. Comprehensive interpretation of central venous oxygen saturation and blood lactate levels during resuscitation of patients with severe sepsis and septic shock in the emergency department. Shock. 2016;45:4–9. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. [DOI] [PubMed] [Google Scholar]
- 31. Bloom B, Pott J, Freund Y, Grundlingh J, Harris T. The agreement between abnormal venous lactate and arterial lactate in the ED: a retrospective chart review. Am J Emerg Med. 2014;32:596–600. [DOI] [PubMed] [Google Scholar]
- 32. Lavery RF, Livingston DH, Tortella BJ, Sambol JT, Slomovitz BM, Siegel JH. The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000;190:656–664. [DOI] [PubMed] [Google Scholar]
- 33. Theerawit P, Na Petvicharn C, Tangsujaritvijit V, Sutherasan Y. The correlation between arterial lactate and venous lactate in patients with sepsis and septic shock. J Intensive Care Med. 2018;33:116–120. [DOI] [PubMed] [Google Scholar]
- 34. Contenti J, Corraze H, Lemoel F, Levraut J. Effectiveness of arterial, venous, and capillary blood lactate as a sepsis triage tool in ED patients. Am J Emerg Med. 2015;33:167–172. [DOI] [PubMed] [Google Scholar]