Abstract
Background
The increase in the use of cardiac implantable electronic devices (CIEDs) has been associated with an increase in CIED‐related infections. Transvenous lead extraction is safe and effective for patients with CIED‐related infections; however, the mortality rate in these patients is high. The prognosis after transvenous lead extraction in Japanese patients, especially those with lead‐related infective endocarditis, has not been evaluated. Then, the purpose of this study is to clarify the prognosis after transvenous lead extraction in Japanese patients with CIED‐related infections at a single Japanese center.
Methods
A total of 107 patients who underwent transvenous lead extraction were retrospectively reviewed. The patients were divided into a lead‐related infective endocarditis group (n = 32) and a pocket infection group (n = 75). Procedure success rate and prognosis after lead extraction were evaluated between the two groups.
Results
Procedure success rate was not significantly different between the groups. There were no deaths associated with the procedure or with infection. The survival rate was not significantly different at 1 year or at a median of 816 days (lead‐related infective endocarditis vs pocket infection; 93.7% vs 94.7%, P = 1.000; 78.1% vs 81.3%, P = 0.791) Time to reimplantation and duration of hospital stay and antibiotics therapy were significantly longer for patients with lead‐related infective endocarditis.
Conclusion
In this study, the prognosis for patients with lead‐related infective endocarditis after transvenous lead extraction was favorable. Thus, extraction should be strongly recommended, even if the general condition of the patient is poor.
Keywords: implantable cardioverter defibrillator, lead extraction, lead‐related infective endocarditis, pacemaker, pocket infection
1. INTRODUCTION
The growing evidence of the importance of cardiac implantable electronic devices (CIEDs) in improving both quality of life and survival among specific patients with heart disease has led to a significant growth in the number of these implantations.1, 2 As a result, the number of complications, including CIED‐related infection, has also increased. Although transvenous lead extractions is safe and effective for patients with infections from implantable devices,3, 4 the mortality among these patients has been reported to be very high.5, 6, 7 In patients with lead‐related infective endocarditis, in particular, the mortality at 1‐5 years has been reported to range from 31% to 44%.6, 8, 9, 10, 11, 12, 13, 14 Therefore, it is important to know the prognosis of transvenous lead extractions as well as its influencing factors. Studies of lead extraction in Japanese patients have been reported.15, 16 However, the prognosis after transvenous lead extraction in Japanese patients, especially those with lead‐related infective endocarditis, has not been evaluated. Therefore, we sought to clarify the prognosis after transvenous lead extraction in Japanese patients on the basis of our experience at a single center.
2. METHODS
2.1. Patients
The medical records of all patients with CIED infections who underwent transvenous lead extraction at the Okayama University Hospital from 2010 to 2017 were retrospectively reviewed. All patients were followed and managed by an electrophysiologist and an infectious disease physician. Blood cultures were obtained from all patients before initiating antibiotic therapy at the hospital. Cultures were also obtained from the fibrotic capsule of the device pocket and from the lead tip as well as the attached fibrotic tissue at the time of the device removal. All patients underwent transesophageal echocardiography to confirm the existence or size of vegetation. Survival data were obtained using electronic medical records. The study proposal was approved by the Okayama University Hospital Institutional Review Board.
2.2. Definitions
The patients were divided to two groups: those with lead‐related infective endocarditis and those with infection only of the device pocket. A pocket infection was defined as the presence of local warmth, erythema, swelling, edema, and pain in or discharge from the device pocket or an erosion or impending erosion of the device without lead‐related infective endocarditis. Lead‐related infective endocarditis was diagnosed using the modified Duke criteria for diagnosis of infective endocarditis on the device leads in compliance with the ESC 2015 guidelines.17, 18 The diagnosis of lead‐related infective endocarditis was definite in the presence of two major criteria or one major criterion and three minor criteria. Patients who met one major and one minor criterion, or three minor criteria were also evaluated. Then, pocket infection group consisted of patients with pocket infection and without lead‐related infective endocarditis, and lead‐related infective endocarditis group consisted of patients with lead‐relate infective endocarditis and with or without pocket infection.
2.3. Lead extraction procedure
Laser sheaths were employed in all cases when the leads could not be explanted by traction alone. In brief, the lead was prepared by inserting a locking stylet into the inner coil lumen when possible. A suture was tied onto the insulation and locking stylet at two sites. The laser sheath was advanced over the lead. Laser application was performed at the binding sites and advanced gradually from one binding site to another until the tip of the lead was reached. Once abutting the myocardium, a combination of traction and counter‐traction was performed, and the lead was freed. If laser sheaths were not advanced, mechanical sheaths were used. The femoral and jugular approaches were also attempted with snares.19 In cases with large vegetation (>2 cm), hybrid therapy was used. Initially, lead dissection up to the upper superior vena cava was performed using the laser or mechanical sheaths. Next, open‐heart surgery was performed by a cardiovascular surgeon. The procedural and clinical success definitions in this study were based on the 2009 and 2017 HRS expert consensus statements.20, 21 Complete procedure success was defined as “lead extraction procedure with removal of all targeted leads and all lead material from the vascular space, with the absence of any permanently disabling complication or procedure‐related death.” Clinical success was defined as “lead extraction procedures with removal of all targeted leads and lead material from the vascular space or retention of a small portion of the lead (<4 cm) that does not negatively impact the outcome goals of the procedure.” Failure was defined as “lead extraction procedures in which complete procedural or clinical success cannot be achieved, or the development of any permanently disabling complication or procedure‐related death.”
2.4. After total lead extraction
In general, patients with pocket infection were treated with intravenous antibiotics for a minimum of 2 weeks after extraction, and patients with lead‐related infective endocarditis were treated for 4‐6 weeks. Intravenous antibiotic infusion was continued in patients with lead‐related infective endocarditis until the infection was eradicated. After eradication, if necessary, a new device was implanted.
2.5. Statistical analysis
Continuous variables are expressed as mean ± standard deviation if they presented a normal distribution in the Kurtosis and Kolmogorov‐Smirnov tests and as median and interquartile ranges if they did not. Accordingly, the significance of between‐group differences was assessed with a two‐tailed Student's t test, or the equivalent nonparametric test, as appropriate. Discrete variables are expressed as frequencies and percentages, and the significance of a different distribution was determined by the chi‐squared or Fisher's exact test (as appropriate) for binary variables and the Mann‐Whitney test for ordinal variables. Survival and cumulative hazards were calculated using the Kaplan–Meier Method. Log‐rank tests were adopted to assess between‐group survival, and Cox proportional hazards regression analysis was performed to determine characteristics that were related to the outcome. Hazards ratios are reported with 95% confidence intervals (95% CI). All statistical analyses were performed using SPSS version 24.0 software (SPSS, Chicago, IL). Values of P < 0.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
Of the 107 patients included in this study, 32 were in the lead‐related infective endocarditis group and 75 were in the pocket infection group. There were significantly more patients with implantable cardioverter defibrillators in the lead‐related infective endocarditis group. Fever C‐reactive protein, and procalcitonin levels were higher; and hemoglobin, albumin levels were lower in the lead‐related infective endocarditis. There were no significant differences in other parameters between the two groups (Table 1).
Table 1.
Patient characteristics
| Total | Lead‐related infective endocarditis | Pocket infection | P | |
|---|---|---|---|---|
| N | 107 | 32 | 75 | |
| Male, n (%) | 84 (78.5) | 26 (81.3) | 58 (77.3) | 0.799 |
| Age, y | 72.8 ± 13.3 | 69.7 ± 14.3 | 74.1 ± 12.8 | 0.116 |
| Height, m | 1.61 ± 0.10 | 1.63 ± 0.10 | 1.61 ± 0.11 | 0.312 |
| Weight, kg | 58.4 ± 13.8 | 60.8 ± 15.8 | 57.3 ± 12.8 | 0.228 |
| Body mass index, kg/m2 | 22.2 ± 3.8 | 22.7 ± 4.3 | 22.0 ± 3.6 | 0.399 |
| NYHA class, n (%) | 0.322 | |||
| I | 88 (82.2) | 26 (81.3) | 62 (82.7) | |
| II | 8 (7.5) | 4 (12.5) | 4 (5.3) | |
| III | 11 (10.3) | 2 (6.3) | 9 (12.0) | |
| IV | 0 (0) | 0 (0) | 0 (0) | |
| Ejection fraction (%) | 56.8 ± 14.9 | 54.8 ± 16.5 | 56.8 ± 14.9 | 0.407 |
| Log BNP | 2.0 ± 0.6 | 2.0 ± 0.7 | 2.0 ± 0.5 | 0.610 |
| Serum BUN, mg/dL | 21.4 ± 11.8 | 20.9 ± 11.0 | 21.7 ± 12.1 | 0.766 |
| Serum creatinine, mg/dL | 1.10 ± 0.73 | 1.14 ± 0.60 | 1.09 ± 0.78 | 0.740 |
| eGFR, mL/min | 58.2 ± 21.0 | 56.8 ± 21.5 | 58.9 ± 20.9 | 0.643 |
| White blood cell, 103 | 6.77 ± 3.68 | 8.13 ± 5.19 | 6.19 ± 2.65 | 0.051 |
| Hemoglobin, g/dL | 12.2 ± 2.1 | 11.6 ± 2.1 | 12.5 ± 2.0 | 0.049 |
| Platelet, 104 | 18.5 ± 8.0 | 17.0 ± 8.6 | 19.1 ± 7.6 | 0.213 |
| Total protein, g/dL | 6.6 ± 1.6 | 6.3 ± 1.4 | 6.7 ± 1.7 | 0.323 |
| Albumin, g/dL | 3.6 ± 0.7 | 3.2 ± 0.9 | 3.8 ± 0.5 | <0.001 |
| C‐reactive protein, mg/dL | 2.8 ± 5.6 | 5.7 ± 7.4 | 1.6 ± 4.0 | 0.005 |
| 0.4 [0.1‐1.8] | 0.4 [0.1‐1.8] | 1.3 [0.3‐7.7] | <0.001 | |
| HbA1c (%) | 6.0 ± 1.3 | 6.1 ± 0.99 | 6.0 ± 1.4 | 0.704 |
| Vegetation, n (%) | 25 (23.4) | 25 (78.1) | — | — |
| Size, mm | 13 [7.8‐28.5] | |||
| Period of antibiotics therapy, d | 28 [21‐36.25] | 29 [22‐40] | 28 [20‐35] | 0.042 |
| Number of antibiotics types | 2 [1‐3] | 2 [3 ‐5] | 1 [1‐2] | < 0.001 |
| Cefazolin | 82 (76.6) | 21 (65.6) | 61 (81.3) | 0.079 |
| Vancomycin | 24 (22.4) | 12 (37.5) | 12 (16.0) | 0.015 |
| Comorbidity, n (%) | ||||
| Hypertension | 56 (52.3) | 16 (50.0) | 40 (53.3) | 0.834 |
| Diabetes mellitus | 30 (28.0) | 8 (25.0) | 22 (29.3) | 0.815 |
| Dyslipidemia | 25 (23.4) | 9 (28.1) | 16 (21.3) | 0.463 |
| Hemodialysis | 1 (0.9) | 0 (0.0) | 1 (1.3) | 1.000 |
| Obstructive pulmonary disease | 19 (17.8) | 2 (6.3) | 17 (22.7) | 0.053 |
| Oral corticosteroid | 5 (4.7) | 1 (3.1) | 4 (5.3) | 1.000 |
| Type of CIED, n (%) | 0.004 | |||
| PM | 81 (75.7) | 19 (59.4) | 62 (82.7) | |
| ICD | 14 (13.1) | 10 (31.3) | 4 (5.3) | |
| CRT‐D | 9 (8.4) | 2 (6.3) | 7 (9.3) | |
| CRT‐P | 3 (2.8) | 1 (3.1) | 2 (2.7) | |
| Number of implanted lead, n (%) | 2.1 ± 0.8 | 2.2 ± 0.8 | 2.1 ± 0.9 | 0.612 |
| Lead dwelling time, y | 8.4 ± 7.0, n = 227 | 8.6 ± 7.1, n = 70 | 7.8 ± 6.8, n = 157 | 0.439 |
Values are mean ± standard deviation, median [interquartile range] or number (%) of patients.
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CIED, cardiac implantable electronic device; CRT‐D, cardiac resynchronization therapy with defibrillator; CRT‐P, cardiac resynchronization therapy without defibrillator; GFR, glomerular filtration rate; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; PM, pacemaker. [Correction added on 01 Aug 2019, after first online publication: the data for “Lead dwelling time, y” has been amended accordingly.]
3.2. Lead extraction
A total of 227 leads were extracted by various lead extraction techniques (Figure 1). Of these, 146 (46.7%) were active fixation leads and 121 (53.3%) were passive fixation leads, and 186 (84.1%) were pacemaker leads, 29 (12.7%) were ICD leads, and 12 (3.2%) were coronary sinus leads. The mean and median dwelling time were 8.4 ± 7.0 and 6.4 (interquartile; 3.1‐11.5) years, respectively. Mean dwelling time of leads was not different between lead‐related infective endocarditis group and pocket infection group (7.8 ± 6.8 years, n = 70 vs 8.6 ± 7.1 years, n = 157, P = 0.439). Overall, 222 (97.8%) of the leads were completely extracted. A small segment (<4 cm) of the lead material was retained in four (1.7%) cases, which did not negatively impact the outcomes of the procedure. A large segment (≥4 cm) of the lead material was retained in one case, which did not negatively impact the outcomes of the procedure.
Figure 1.
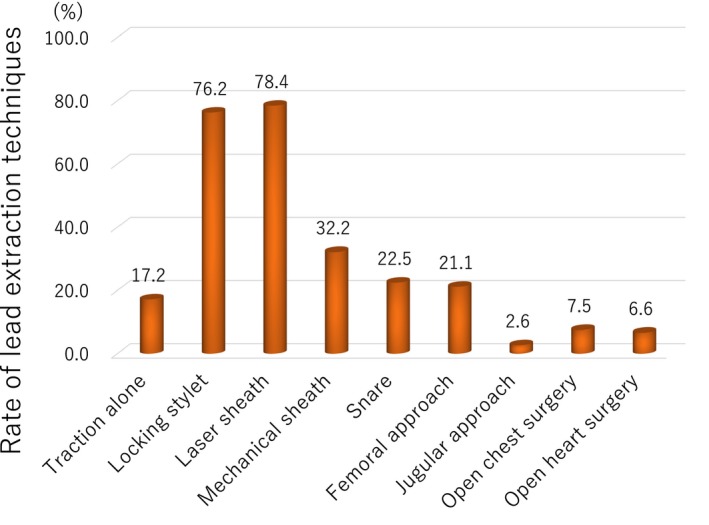
The lead extraction technique. Various procedures were employed, not only locking stylet, laser sheath, but mechanical sheath, snare, femoral approach, jugular approach, and surgical approach
Table 2 presents the results of lead extraction per patient. A total of 109 extraction procedures were performed in 107 patients; two patients had a second procedure because of failure of the first. There were no significant differences between the groups in terms of complete procedure success rate, clinical success rate, or major and minor complications. Neither procedure‐related deaths nor permanently disabling complications were reported.
Table 2.
Results of lead extraction procedure
| Total | Lead‐related infective endocarditis | Pocket infection | P | |
|---|---|---|---|---|
| N | 109 | 32 | 75 | |
| Complete procedural success, n (%) | 102 (93.6) | 32 (100) | 70 (90.9) | 0.211 |
| Clinical success, n (%) | 106 (97.2) | 32 (100) | 74 (96.1) | 0.554 |
| Major complications, n (%) | 3 (2.8) | 2 (6.3) | 1 (1.3) | 0.206 |
| Cardiac tamponade | 3 (2.8) | 2 (6.3) | 1 (1.3) | 0.206 |
| Procedure related death | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Minor complications, n (%) | 9 (8.3) | 1 (3.1) | 8 (10.4) | 0.278 |
| Pericardial effusion not requiring intervention | 2 (1.8) | 0 (0.0) | 2 (2.6) | 1.000 |
| Pocket hematoma at the surgical site | 2 (1.8) | 0 (0.0) | 2 (2.6) | 1.000 |
| Vascular repair at venous entry site | 1 (0.9) | 0 (0.0) | 1 (1.3) | 1.000 |
| Blood transfusion | 3 (2.8) | 0 (0.0) | 3 (3.9) | 0.554 |
| Pulmonary embolism not requiring surgical intervention | 1 (0.9) | 1 (3.1) | 0 (0.0) | 0.294 |
| Femoral vein dissection | 1 (0.9) | 0 (0.0) | 1 (1.3) | 1.000 |
Two patients undertook second lead extraction procedure due to first procedure failure.
3.3. Prognosis after lead extraction
Prognosis after lead extraction on the basis of infection type is shown in Table 3 and Figure 2. Time to reimplantation, duration of hospital stay, and duration of antibiotics therapy were significantly longer in the lead‐related infective endocarditis group than in the pocket infection group. Number of administered antibiotics and patients with catecholamine usage for septic shock were more in the lead‐related infective endocarditis group than in the pocket infection group (Table 3).
Table 3.
Prognosis after lead extraction
| Total | Lead‐related infective endocarditis | Pocket infection | P | |
|---|---|---|---|---|
| N | 107 | 32 | 75 | |
| Reimplantation, n (%) | 81 (75.7) | 22 (68.8) | 63 (84.0) | 0.115 |
| Time to reimplantation, d | 18 [14‐28.5] | 34 [18.75‐126.25] | 15 [14‐22] | <0.001 |
| Duration of hospital stay, d | 38 [31‐50] | 46 [31.25‐84] | 36 [31‐45] | 0.006 |
| Duration of antibiotics therapy, d | 28 [21‐36.25] | 29 [22‐40] | 28 [20‐35] | 0.042 |
| Number of antibiotics types | 2 [1‐3] | 2 [3 ‐5] | 1 [1‐2] | <0.001 |
| Catecholamine for septic shock, n (%) | 6 (5.7) | 6 (18.8) | 0 (0) | <0.001 |
| Follow‐up periods after lead extraction, days | 816 [211‐1311] | 622 [192.25‐1038] | 882 [215‐1372] | 0.118 |
| All cause death, n (%) | 21 (19.6) | 7 (21.9) | 14 (18.7) | 0.791 |
| Hospital death | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Death within 30 d | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Death within 1 y | 6 (5.6) | 2 (6.3) | 4 (5.3) | 1.000 |
| Cause of death, n (%) | ||||
| CIED infection related death | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Cardiac death | 4 (3.7) | 2 (6.3) | 2 (2.7) | 0.581 |
| Non‐cardiac death | 14 (13.1) | 5 (15.6) | 9 (12.0) | 0.755 |
| Unknown | 3 (2.9) | 0 (0) | 3 (4.0) | 0.553 |
Values are mean ± standard deviation, median (interquartile range) or number (%) of patients.
CIED, cardiac implantable electronic device.
Figure 2.
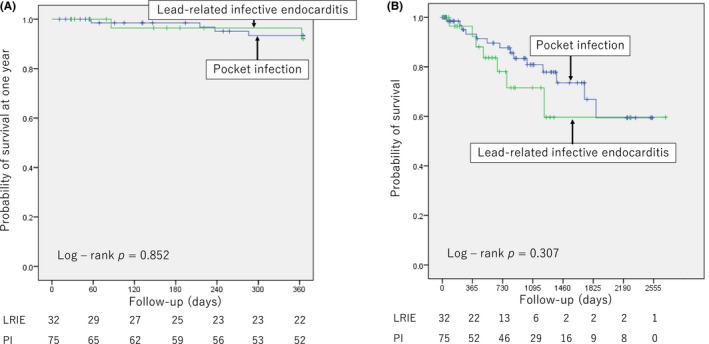
Survival after lead extraction. A, Survival at 1 y after lead extraction between the lead‐related infective endocarditis (LRIE) and pocket infection23 groups. B, Survival at median of 816 d after lead extraction between the LRIE and PI groups. Survival rate was not significantly different at 1 y or at median of 816 d of follow‐up between the two groups (LRIE vs PI; 93.7% vs 94.7%, P = 1.000, at 1 y; 78.1% vs 81.3%, P = 0.791, at median of 816 d)
The survival rate was not significantly different at 1 year or at the median 816 days of follow‐up between the lead‐related infective endocarditis and pocket infection groups: 93.7% vs 94.7%, P = 1.000, at 1 year, and 78.1% vs 81.3%, P = 0.791, at the median of 816 days, respectively (Figure 2). There was no death for 30 days after lead extraction. There was no death associated with implantable device infection during the median of 816 days of follow‐up. In lead‐related infective endocarditis group, only two patients died within 1 year: one due to intestinal bleeding 363 days after lead extraction, which was not related to CIED infection and the other due to aspiration pneumonia 86 days after lead extraction, which was not related to CIED infection. The main cause of other deaths was noncardiac death (Table 3).
On the univariate Cox regression analysis for mortality after lead extraction, age, log of brain natriuretic peptides, serum blood urea nitrogen, estimated glomerular filtration rate, hemoglobin, albumin, platelet, and hypertension were identified as predictors of mortality. (Table 4) Multivariate analysis could not be conducted due to small number of events.
Table 4.
Univariate Cox regression analysis for mortality after lead extraction
| Univariate | |||
|---|---|---|---|
| HR | 95% CI | P | |
| Male, n | 1.033 | [0.378‐2.825] | 0.949 |
| Age, y | 1.080 | [1.016‐1.147] | 0.013 |
| Height, m | 0.968 | [0.927‐1.011] | 0.141 |
| Weight, kg | 0.993 | [0.963‐1.024] | 0.655 |
| Body mass index, kg/m2 | 1.031 | [0.925‐1.150] | 0.579 |
| NYHA class, III/IV, n | 1.845 | [0.675‐5.043] | 0.232 |
| Ejection fraction (%) | 0.977 | [0.952‐1.002] | 0.072 |
| Log BNP | 4.180 | [1.907‐9.176] | < 0.001 |
| Serum BUN, mg/dL | 1.086 | [1.049‐1.125] | < 0.001 |
| Serum creatinine, mg/dL | 1.182 | [1.182‐1.680] | 0.351 |
| eGFR, mL/min | 0.974 | [0.954‐0.955] | 0.015 |
| White blood cell, 103 | 1.099 | [0.986‐1.226] | 0.088 |
| Hemoglobin, g/dL | 0.773 | [0.621‐0.963] | 0.002 |
| Platelet, 104 | 0.993 | [0.986‐1.000] | 0.045 |
| Total protein, g/dL | 0.924 | [0.758‐1.126] | 0.460 |
| Albumin, g/dL | 0.361 | [0.212‐0.616] | < 0.001 |
| HbA1c (%) | 1.034 | [0.770‐1.388] | 0.823 |
| C‐reactive protein, mg/dL | 1.047 | [0.986‐1.112] | 0.133 |
| Procalcitonin, ng/mL | 1.129 | [0.868‐1.467] | 0.412 |
| Fever at administration | 1.430 | [0.862‐2.373] | 0.189 |
| Period of antibiotics therapy, d | 1.006 | [0.999‐1.014] | 0.181 |
| Number of antibiotics types | 1.164 | [0.991‐1.367] | 0.101 |
| Catecholamine for septic shock, n (%) | 2.112 | [0.483‐9.234] | 0.366 |
| Vegetation, n | 0.709 | [0.208‐2.415] | 0.583 |
| Comorbidity, n | |||
| Hypertension | 2.741 | [1.063‐7.071] | 0.037 |
| Diabetes mellitus | 2.221 | [0.942‐5.240] | 0.068 |
| Dyslipidemia | 0.950 | [0.342‐2.557] | 0.895 |
| Obstructive pulmonary disease | 0.993 | [0.334‐2.956] | 0.990 |
| Oral corticosteroid | 1.255 | [0.165‐9.518] | 0.826 |
| CRT, n | 2.240 | [0.818‐6.130] | 0.116 |
| Number of implanted lead, n | 1.033 | [0.593‐1.798] | 0.909 |
| Lead‐related infective endocarditis, n | 1.607 | [0.641‐4.026] | 0.311 |
| Reimplantation, n | 0.705 | [0.232‐2.142] | 0.538 |
Values are mean ± standard deviation, median (interquartile range) or number (%) of patients.
BNP, brain natriuretic peptide; BUN, denotes blood urea nitrogen; CI, confidence interval; CRT, cardiac resynchronization therapy with or without defibrillator; GFR, glomerular filtration rate; HR, denotes hazard ratio; NYHA, New York Heart Association.
3.4. A case of lead‐related infective endocarditis
A case of severe lead‐related infective endocarditis is shown in Figure 3. Various infections developed despite intravenous antibiotics therapy. After antibiotics had been continued for 8 months, the infection was no longer evident and a new device was implanted. Infection has not reoccurred.
Figure 3.
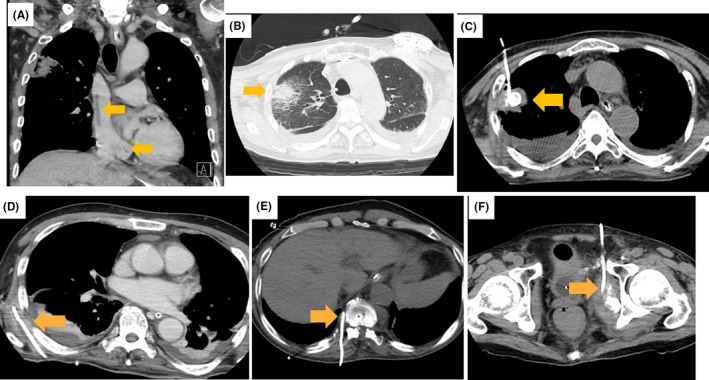
A case of severe lead‐related infective endocarditis. A 68 year old patient with cardiac resynchronization therapy with defibrillator was referred to Okayama University Hospital due to infective endocarditis. He suffered from pulmonary embolism, pulmonary abscess, septic shock, and disseminated intravascular coagulation. On the next day of admission, all leads were extracted without any complication. Methicillin‐sensitive Staphylococcus aureus was found on blood, sputum, urine, lead, and generator cultures. Although intravenous antibiotics have been continued, various infections developed. A, Vegetation in the superior vena cava and coronary sinus after lead extraction. B, Pulmonary abscess. C, Drainage of pulmonary abscess. D, Drainage of abscess beneath the scapula. E, Drainage of abscess around the vertebral body. F, Drainage of abscess around the pelvis. Vertebritis and discitis (not shown in Figure 3) also developed after the above infections disappeared. With continued antibiotics for 8 mo, the infection was no longer evident, and a new device was implanted. Infection has not reoccurred
4. DISCUSSION
The main finding of this study was that the prognosis in Japanese patients with lead‐related infective endocarditis is as favorable as that in patients with only pocket infection.
4.1. Lead extraction techniques
In our institute, various lead extraction techniques are available, including the locking stylet, laser sheath, mechanical sheath, snare, femoral approach, jugular approach, and surgical approach. There were no procedure‐related deaths, in‐hospital deaths, or deaths within 30 days, which is better than the findings previously reported.3, 4 Our staff have been well‐trained by special experts in the field, and we could therefore apply various lead extraction techniques. This strategy no doubt greatly contributed to the good result obtained for the lead extraction procedures. In addition, we were able to communicate with the cardiovascular surgeons and anesthesiologists before or during the extractions, which could also have contributed to the good result. The timing of the extraction depended on the severity of the infection. For more severe infections, the extraction was performed earlier, as early lead extraction in patients with lead‐related infective endocarditis was recommended in a previous study.14
4.2. Prognosis after lead extraction
The prognosis for patients with lead‐related infective endocarditis has been reported to be poor despite lead successful extraction. Survival probability at 1 year has been reported to range from 71% to 85% in these patients.7, 8, 9, 10, 11, 12, 13, 14, 18 However, in this study, survival probability at 1 year was 96.9%. Moreover, the factors associated with severity of heart failure, such as ejection fraction (EF),22 type of device, or New York Heart Association (NYHA) class were not predictors for mortality in the univariate Cox regression analysis. The factors associated with severity of infection, such as C‐reactive protein, vegetation, and lead‐related infective endocarditis, were also not identified as predictors (Table 4). Previously, increased age, low EF, heart failure, chronic renal failure, diabetes mellitus, obstructive pulmonary disease, and high NYHA class were reported to be associated with a poor prognosis in patients with infection from CIEDs.7, 8, 9, 10, 11, 12, 13, 14, 18 In this study, we identified age, chronic renal failure, anemia, and hypertension as predictors for mortality in the univariate Cox regression analysis. The death events were too few in this study to perform the multivariate analysis. Then, it is impossible to demonstrate each variable was truly associated with mortality because confounding factors were not adjusted.
4.3. Reasons of favorable prognosis in patients with lead‐related infective endocarditis after lead extraction
Although there was no clear reason why the prognosis was favorable in this study compared to that previously reported (Table 5), there are some possible explanations. First, the patients could be hospitalized until the infection was completely eradicated, as shown in Figure 3. This is a beneficial aspect of the Japanese National Health Insurance. The maximum duration of hospitalization was 282 days. However, it is difficult to compare this finding to those in previous studies because of a lack of information regarding the duration of hospitalization.7, 8, 9, 10, 11, 12, 13, 14, 18 Second, during hospitalization, extraction team including various specialists, such as cardiovascular surgeons, anesthesiologists, plastic surgeons, respiratory surgeons, and especially infectious disease physicians, could contribute to the management of the infection. Third, most patients in this study were referred to our hospital from other hospitals. Patients with infection who received their implants in our hospital were only 7 (6.5%) out of 107 patients. Therefore, patients who were too sick to be transferred to our hospital may have been excluded. However, some patients with severe sepsis and disseminated intravascular coagulation (Table 3, Figure 3) were included in this study. Finally, although patient characteristics in this study that would influence the prognosis, such as age, EF, renal injury, diabetes mellitus, and obstructive pulmonary disease, were similar to those in previous studies, the rate of heart failure seemed to be lower7, 8, 9, 10, 11, 12, 13, 14, 18 although statistical analysis was not conducted.
Table 5.
Studies reporting long‐term prognosis after lead extraction for CIED infection
| Study | Design | Patients, n | Systemic infection (%) | Local infection (%) | Criteria for systemic infection | 30‐day mortality (%) | 1‐year mortality (%) | 3‐year mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Le et al., 201114 | Retrospective | 416 | N.R. | N.R. | N.R. | 4.5 | 13.2 | N.A. |
| Deharo et al., 20126 | Prospective | 197 | 58.9 | 41.1 | Possible/definite IE (Duke criteria) | 6.9 | 14.3 | N.A. |
| Maytin et al., 201212 | Retrospective | 985 | 18.0 | 32.0 | Bacteremia and/or IE | 5.1 | 20.2 | 49.0 |
| Deckx et al., 20147 | Retrospective | 176 | 17.6 | 34.7 | N.R. | 6.5 | 15.2 | N.A. |
| Tarakji et al., 20148 | Retrospective | 502 | 42.0 | 58.0 | Systemic signs/symptoms + History + Microbiology + echocardiography | 5.8 | 20.0 | N.A. |
| Narducci et al., 201713 | Prospective | 217 | 64.0 | 31.0 | Bacteremia and/or IE (modified Duke criteria) | 5.0 | 10.0 | N.A. |
| Polewczyk et al., 201710 | Retrospective | 500 | 80 | 0 | Possible/definite IE (Duke criteria) | 3.8 | N.R. | 29.3 |
| Diemberger et al., 201811 | Prospective | 121 | 45.5 | 54.5 | Possible/definite IE (Duke criteria) | 0.83 | 14.2 | 28.4 |
| Nishii et al. (present study) | Retrospective | 107 | 30.0 | 70.0 | Possible/definite IE (Duke criteria) | 0.0 | 5.6 | 15.0 |
CIED, cardiac implantable electronic device; IE infective endocarditis; N.A., not available; N.R., not reported.
4.4. Limitations
This study has some limitations. First, this was a single‐center, retrospective study that included a small number of patients in a Japanese population, so the study may not have sufficient power to detect all predictors for mortality. Although in univariate analysis, some risk factors were shown, it is impossible to demonstrate each variable was truly associated with mortality because confounding factors were not adjusted. A prospective, multicenter study with a larger number of Japanese patients could increase the reliability of these results. Second, the number of deaths may be too small to gain enough statistical power. Then, other predictors for mortality may come out, if the number of death increased. Lastly, the background of patients was not same as the previous studies, then, it is difficult to precisely compare the mortality to the previous studies. However, the study was real‐world data in Japanese patients.
4.5. Conclusions
The prognosis after lead extraction for patients with lead‐related infective endocarditis is favorable. Thus, lead extraction should be strongly recommended, even in patients with lead‐related infective endocarditis in a generally poor condition. To our knowledge, this is the first report of the prognosis of lead extraction in Japanese patients with CIED‐related infection, including lead‐related infective endocarditis.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
ACKNOWLEDGEMENTS
The authors would like to thank Roger Carrillo, MD, PhD, and Maria Grazia Bongiorni, MD, PhD for educating regarding lead extraction; Zenitsu Masuda, MD, PhD, Masami Takagaki, MD, PhD, Yosuke Kroko, MD, PhD, and Hiroki Eto, MD for the surgical backup; Hirotaka Iguchi, Norihiro Nishiyama, and Yuki Takenaka for medical engineering work; and Yuko Kobayashi, Miyuki Fujiwara, and Masayo Ohmori for secretarial work.
Nishii N, Morimoto Y, Miyoshi A, et al. Prognosis after lead extraction in patients with cardiac implantable electronic devices infection: Comparison of lead‐related infective endocarditis with pocket infection in a Japanese single‐center experience. J Arrhythmia. 2019;35:654–663. 10.1002/joa3.12164
REFERENCES
- 1. Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–5. [DOI] [PubMed] [Google Scholar]
- 2. Diemberger I, Biffi M, Martignani C, et al. From lead management to implanted patient management: indications to lead extraction in pacemaker and cardioverter‐defibrillator systems. Expert Rev Med Devices. 2011;8:235–55. [DOI] [PubMed] [Google Scholar]
- 3. Bongiorni MG, Kennergren C, Butter C, et al. The European lead extraction ConTRolled (ELECTRa) study: a European heart rhythm association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J. 2017;38:2995–3005. [DOI] [PubMed] [Google Scholar]
- 4. Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579–86. [DOI] [PubMed] [Google Scholar]
- 5. Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–7. [DOI] [PubMed] [Google Scholar]
- 6. Deharo JC, Quatre A, Mancini J, et al. Long‐term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart. 2012;98:724–31. [DOI] [PubMed] [Google Scholar]
- 7. Deckx S, Marynissen T, Rega F, et al. Predictors of 30‐day and 1‐year mortality after transvenous lead extraction: a single‐centre experience. Europace. 2014;16:1218–25. [DOI] [PubMed] [Google Scholar]
- 8. Tarakji KG, Wazni OM, Harb S, et al. Risk factors for 1‐year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014;16:1490–5. [DOI] [PubMed] [Google Scholar]
- 9. Henrikson CA, Zhang K, Brinker JA. High mid‐term mortality following successful lead extraction for infection. Pacing Clin Electrophysiol. 2011;34:32–6. [DOI] [PubMed] [Google Scholar]
- 10. Polewczyk A, Jachec W, Tomaszewski A, et al. Lead‐related infective endocarditis: Factors influencing early and long‐term survival in patients undergoing transvenous lead extraction. Heart Rhythm. 2017;14:43–9. [DOI] [PubMed] [Google Scholar]
- 11. Diemberger I, Biffi M, Lorenzetti S, et al. Predictors of long‐term survival free from relapses after extraction of infected CIED. Europace. 2018;20:1018–27. [DOI] [PubMed] [Google Scholar]
- 12. Maytin M, Jones SO, Epstein LM. Long‐term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol. 2012;5:252–7. [DOI] [PubMed] [Google Scholar]
- 13. Narducci ML, Di Monaco A, Pelargonio G, et al. Presence of ‘ghosts’ and mortality after transvenous lead extraction. Europace. 2017;19:432–40. [DOI] [PubMed] [Google Scholar]
- 14. Le KY, Sohail MR, Friedman PA, et al. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011;8:1678–85. [DOI] [PubMed] [Google Scholar]
- 15. Goya M, Nagashima M, Hiroshima K, et al. Lead extractions in patients with cardiac implantable electronic device infections: single center experience. J Arrhythm. 2016;32:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okada A, Shoda M, Tabata H, et al. Single‐center experience with percutaneous lead extraction of cardiac implantable electric devices. J Cardiol. 2018;71:192–6. [DOI] [PubMed] [Google Scholar]
- 17. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- 18. Polewczyk A, Jachec W, Janion M, et al. Lead‐dependent infective endocarditis: the role of factors predisposing to its development in an analysis of 414 clinical cases. Pacing Clin Electrophysiol. 2015;38:846–56. [DOI] [PubMed] [Google Scholar]
- 19. Bongiorni MG, Soldati E, Zucchelli G, et al. Transvenous removal of pacing and implantable cardiac defibrillating leads using single sheath mechanical dilatation and multiple venous approaches: high success rate and safety in more than 2000 leads. Eur Heart J. 2008;29:2886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6:1085–104. [DOI] [PubMed] [Google Scholar]
- 21. Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122:985–92. [DOI] [PubMed] [Google Scholar]
- 23. Calo L, Gargaro A, De Ruvo E, et al. Economic impact of remote monitoring on ordinary follow‐up of implantable cardioverter defibrillators as compared with conventional in‐hospital visits. A single‐center prospective and randomized study. J Interv Card Electrophysiol. 2013;37:69–78. [DOI] [PubMed] [Google Scholar]


