Abstract
Study Design:
Prospective pre-clinical and clinical cohort study.
Objectives:
Current spinal navigation systems rely on a dynamic reference frame (DRF) for image-to-patient registration and tool tracking. Working distant to a DRF may generate inaccuracy. Here we quantitate predictors of navigation error as a function of distance from the registered vertebral level, and from intersegmental mobility due to surgical manipulation and patient respiration.
Methods:
Navigation errors from working distant to the registered level, and from surgical manipulation, were quantified in 4 human cadavers. The 3-dimensional (3D) position of a tracked tool tip at 0 to 5 levels from the DRF, and during targeting of pedicle screw tracts, was captured in real-time by an optical navigation system. Respiration-induced vertebral motion was quantified from 10 clinical cases of open posterior instrumentation. The 3D position of a custom spinous-process clamp was tracked over 12 respiratory cycles.
Results:
An increase in mean 3D navigation error of ≥2 mm was observed at ≥2 levels from the DRF in the cervical and lumbar spine. Mean ± SD displacement due to surgical manipulation was 1.55 ± 1.13 mm in 3D across all levels, ≥2 mm in 17.4%, 19.2%, and 38.5% of levels in the cervical, thoracic, and lumbar spine, respectively. Mean ± SD respiration-induced 3D motion was 1.96 ± 1.32 mm, greatest in the lower thoracic spine (P < .001). Tidal volume and positive end-expiratory pressure correlated positively with increased vertebral displacement.
Conclusions:
Vertebral motion is unaccounted for during image-guided surgery when performed at levels distant from the DRF. Navigating instrumentation within 2 levels of the DRF likely minimizes the risk of navigation error.
Keywords: intraoperative navigation, frameless stereotaxy, image guidance, registration, navigation error
Introduction
Intraoperative 3-dimensional (3D) computer-assisted navigation (CAN) in spinal procedures may guide instrumentation placement as well as bony and soft-tissue resection. Contemporary navigation systems register patient anatomy to an imaging dataset, allowing real-time instrument tracking and/or robotic guidance in the virtualized environment. Whether the imaging data is acquired preoperatively, using computed tomography (CT) or magnetic resonance imaging (MRI), or intraoperatively, using 2D/3D-fluoroscopy or CT, current CAN systems rely on a dynamic reference frame (DRF) for maintaining the image-to-patient registration and tool tracking. The accuracy of spinal CAN systems has been studied extensively and varies by registration and imaging technique as well as spinal region.1-4 Concern over registration accuracy is one of several reasons for the relative lack of widespread adoption of CAN among spinal surgeons.5,6
Displacement of vertebral levels distant to the DRF may generate navigation inaccuracy from intersegmental mobility, which is seen to varying extents across the cervical, thoracic and lumbar spines.7 While intersegmental motion due to patient positioning, for instance, between supine pre-operative CT imaging and intraoperative prone positioning, is accounted for by CAN systems registering to intraoperative imaging, there are multiple sources of intraoperative postimaging intersegmental motion. These include patient respiration-induced vertebral motion, as well as displacement from surgeon manipulation during the placement of instrumentation.8,9 In long-segment deformity corrections and minimally invasive lumbosacral procedures, or in some cases of posterior cervical instrumentation, with DRF fixation to the pelvis or Mayfield clamp, respectively, navigation inaccuracy due to intersegmental mobility can become particularly pronounced. However, the current literature on the extent and significance of navigation inaccuracy due to intersegmental mobility is conflicted.8-14
Here, we perform a prospective cadaveric and in vivo human clinical study to quantify intraoperative vertebral motion from patient respiration and surgical manipulation, using continuous tracking enabled by a novel in-house navigation technology based on optical topographic imaging (OTI).
Methods
Specimen/Patient Selection
Preclinical testing was performed in four formalin-fixed human cadavers. All cadavers underwent pre- and postoperative helical CT imaging at 0.5 mm slice thickness, for registration using an OTI navigation system. Institutional ethics board approval was obtained (REB# 16-0051-E).
In vivo testing was performed in 10 clinical cases of open posterior instrumented fusion, for degenerative, traumatic, or neoplastic etiologies. All patients had no history of prior spinal surgery at the operated levels. All patients underwent preoperative helical CT imaging, reformatted at 0.625 mm slice thickness, for registration using an OTI navigation system as part of an ongoing trial of OTI at Sunnybrook Health Sciences Centre (REB# 309-2014 and 086-2015). While the navigation registration technique is novel to OTI, subsequent instrument tracking is performed using standard infrared techniques using passive-reflective spheres.
Quantification of Navigation Error From Proximity to DRF
Navigation error due to working at a level distant from that to which the DRF is affixed, was assessed in 4 human cadavers. Cadavers were placed prone on a standard operating table, and a standard midline posterior exposure performed from C1 to S1. Bone screws were implanted into the superolateral edges of the laminae at each level as internal fiducials, to approximate the entry point of typical pedicle screws. The DRF was clamped at various levels in the cervical/thoracic/lumbar spine, and OTI navigation registered. The tip of a tracked awl was then placed into the head of the bone screws at 0 to 5 levels away from that to which the DRF was affixed. The 3D location of the tool tip as seen by the OTI navigation system was recorded at each point, and compared using image-processing software to the actual position of the center of the bone screw head on postoperative CT imaging. All image processing and measurements were performed using a 64-bit OsiriX workstation (version 10.9.5; PIXMEO SARL, Geneva, Switzerland).
Quantification of Navigation Error From Surgical Manipulation
Using the same midline exposures and laminar fiducials in four human cadavers, the tip of a tracked awl (thoracolumbar) or tracked drill guide (cervical) was placed into the heads of the bone screws at each level, and pressure exerted with the appropriate force and trajectory to simulate the creation of pedicle screw tracts. The 3D position of the tracked tool tip prior to and following the exertion of force was recorded by the OTI navigation system, to quantify vertebral movement due to manipulation during pedicle cannulation. All procedures were performed by a single surgeon (DG) at a single center.
Quantification of Navigation Error From Respiration-Induced Motion
Respiration-induced vertebral motion was quantified in vivo in 10 human patients. Patients were positioned prone on a Wilson frame, with Mayfield head clamp for cervical fusions. Following standard midline open posterior exposure, OTI image-to-patient registration was performed. A custom spinous-process clamp with passive-reflective infrared tracking spheres, fabricated in-house, was clamped to the level adjacent to the level to which the DRF was affixed, to 2 to 5 levels distant from the DRF, or to a stationary anatomic target supported by pelvic bolsters or a Mayfield head clamp on the operating table (Figure 1). The 3D position of the spinous-process clamp was tracked at 20 Hz over ∼12 respiratory cycles. A spinous-process clamp was employed to minimize any error from tracking a handheld tool with associated user motion over time. Tracked levels were categorized into cervical, upper thoracic (T1-T6), lower thoracic (T7-T12), and lumbar. Data was collected on multiple parameters that may influence respiration-induced motion, including patient age, gender, body-mass index (BMI), spinal level, respiratory rate (RR), heart rate (HR), mean arterial pressure (MAP), tidal volume (TV), positive end-expiratory pressure (PEEP), and ventilator mode.
Figure 1.
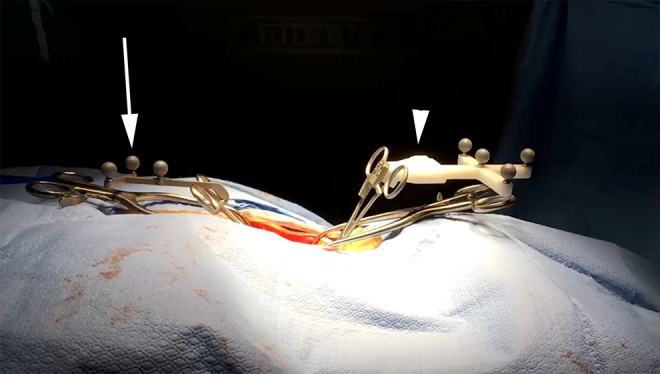
In vivo surgical field with dynamic reference frame (DRF) for in-house optical topographic imaging (OTI) navigation system (arrowhead), and custom spinous-process infrared tracking clamp to quantify vertebral motion (arrow).
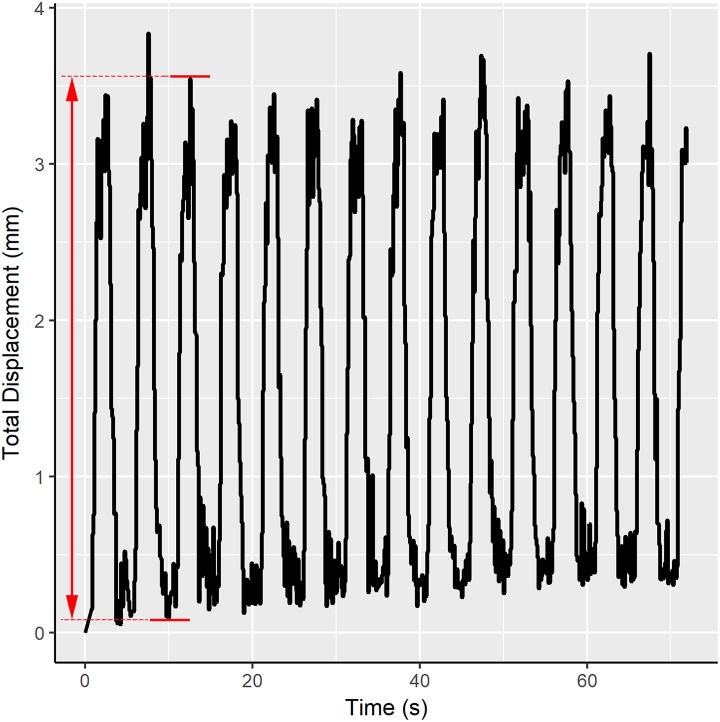
Motion during each respiratory cycle was quantified as the “peak-to-peak” displacement (ie, from end-expiration to end-inspiration) in each of the antero-posterior, cranio-caudal, and medio-lateral axes (Figure 2).
Figure 2.
Representative tracking of the 3-dimensional displacement of a cervical vertebra over ∼14 respiratory cycles, spanning 72 seconds. “Peak-to-peak” displacement is computed as the change in displacement from end-expiration to end-inspiration within one respiratory cycle, indicated by the red arrow.
Statistical Analyses
Differences in absolute navigation errors between spinal levels were quantified with 1-way analysis of variance, with Tukey’s honestly significant difference test for post hoc comparisons. Differences in error dispersion were computed using Levene’s test of homogeneity of variances. Predictors of increased vertebral motion from distance from the DRF, surgical manipulation, or respiratory motion were assessed using multiple linear regression models. Variables were entered simultaneously into a full model, with nonlinearity checked using 3 cubic splines. Models were assessed for collinearity as well as quality of fit. Hierarchical mixed-effects general linear modeling was employed to adjust for second-order differences between cadavers/patients, where required based on univariate analyses.
All statistical analyses were performed in SPSS Statistics (version 21; IBM, Armonk, NY, USA).
Results
For the 4 formalin-fixed specimens used in human cadaveric testing, 2 were female and 2 were male. Mean age at death was 91.4 years (range 83-96 years). There was no radiographic evidence of ankylosing spondylitis (AS), diffuse idiopathic skeletal hyperostosis (DISH), or ossification of the posterior longitudinal ligament (OPLL) in any specimen. In cadaveric testing, 132 laminar fiducials were implanted from C2-S1, 46 cervical, 47 thoracic, and 39 lumbar. In-vivo testing was performed in 10 patients, 5 females and 5 males, with mean age 62.7 years (range 49-76 years). A total of 583 respiratory cycles were tracked in 10 patients in vivo, 136 in the cervical spine, 167 upper thoracic, 74 lower thoracic, and 206 lumbar. All patients were ventilated on a pressure-control mode. Patient and cardiorespiratory characteristics are summarized in Table 1.
Table 1.
Respiration-Induced Vertebral Motion for 10 Clinical Cases, With Patient and Cardiorespiratory Characteristics.
| Patient | Age (Years) | Gender | BMI (kg/m2) | Tracked Levels | RR (/min) | HR (/min) | MAP (mm Hg) | TV (mL) | PEEP (cm H2O) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | F | 29.0 | L4/L5/S1 | 13 | 65 | 82 | 425 | 5 |
| 2 | 52 | F | 27.5 | T6/T8 | 10 | 72 | 65 | 450 | 0 |
| 3 | 58 | F | 24.8 | T9/T10/T12 | 10 | 72 | 70 | 400 | 0 |
| 4 | 74 | M | 26.1 | L2/L3/L5 | 11 | 51 | 68 | 475 | 4 |
| 5 | 52 | F | 38.1 | C4/C5 | 11 | 66 | 103 | 475 | 5 |
| 6 | 76 | M | 24.2 | C5/C6/T1 | 12 | 61 | 93 | 400 | 5 |
| 7 | 69 | M | 23.7 | L5 | 11 | 77 | 97 | 500 | 5 |
| 8 | 68 | M | 20.2 | L2 | 12 | 53 | 65 | 425 | 5 |
| 9 | 69 | F | 21.0 | C4/T1 | 10 | 80 | 74 | 350 | 4 |
| 10 | 60 | M | 25.2 | C3/T1 | 12 | 67 | 81 | 500 | 4 |
Abbreviations: BMI, body mass index; F, female; HR, heart rate; M, male; MAP, mean arterial pressure; PEEP, positive end-expiratory pressure; RR, respiratory rate; TV, tidal volume.
Navigation Error From Proximity to DRF
Quantitative navigation error (Mean ± SD) at the level of the DRF was 1.13 ± 0.27 mm in the anteroposterior (AP) axis, 1.33 ± 0.18 mm in the mediolateral (ML) axis, and 1.62 ± 0.39 mm in the craniocaudal (CC) axis, for an overall 3D error of 2.71 ± 0.43 mm, representing the baseline navigation error.
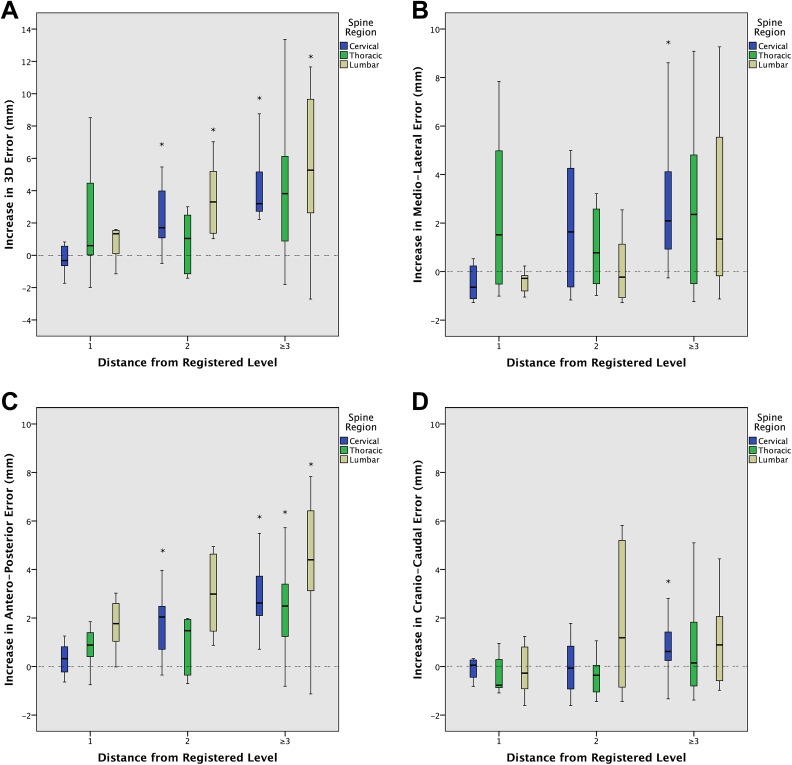
An increase in mean quantitative navigation error of ≥2 mm was seen in 3D in the cervical and lumbar spine at ≥2 levels distant from the DRF, driven largely by an equivalent increase in error in the AP axis at ≥2 levels distant from the DRF, and in the ML axis at ≥3 levels distant (Figure 3). An increase in error of ≥4 mm was seen in 3D at 5 levels from the DRF, driven by an equivalent increase in AP error at ≥4 levels from the DRF. No significant increases in error in the CC axis were seen up to 5 levels from the DRF (Figure 3).
Figure 3.
Standard boxplots demonstrating the increase in translational error from baseline, as a function of the number of levels distant from the dynamic reference frame (DRF), in 3 dimensions (3D) (A) and each of the mediolateral (B), anteroposterior (C), and craniocaudal (D) axes. Boxes represent the first quartile, median, and third quartile. Whiskers represent 1.5× the interquartile range. *Represents significant difference from baseline error, at P < .05.
The variability in navigation error increased significantly in the AP axis at ≥2 levels from the DRF (SD 0.41 vs 0.27 mm, P = .026), and in the ML axis at ≥1 level from the DRF (SD 0.53 vs 0.18 mm, P < .001), by Levene’s test of homogeneity of variances. No significant increases in error dispersion in the CC axis were observed.
Navigation Error From Surgical Manipulation
Displacement (Mean ± SD) due to surgical manipulation was 1.55 ± 1.13 mm in 3D across all levels, nonsignificantly greater in the lumbar spine (1.85 ± 1.48 mm) than in the thoracic (1.51 ± 1.01 mm) and cervical (1.34 ± 0.85 mm) spine. Displacement in the ML axis was significantly greater in the thoracic spine relative to the cervical spine (0.96 ± 0.91 vs 0.45 ± 0.30 mm; P < .001), and in the CC axis in the lumbar spine relative to both the cervical and thoracic spine (1.38 ± 1.12 vs 0.92 ± 0.82 and 0.82 ± 0.72 mm, respectively; P < .001).
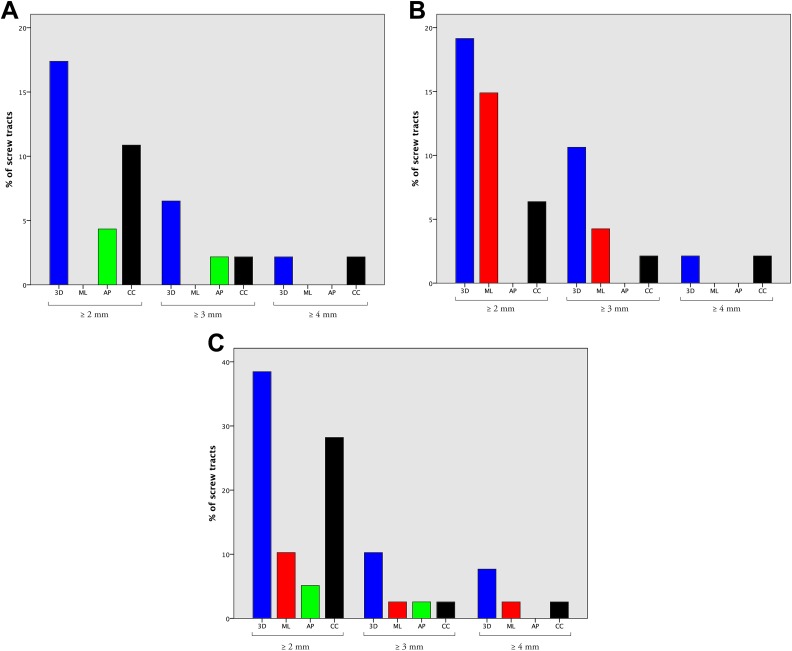
Deviation of ≥2 mm was observed in 3D in 17.4% of cervical levels, 19.2% of thoracic levels, and 38.5% of lumbar levels. Three-dimensional displacement of ≥3 mm was recorded in 6.5% of cervical levels, 10.7% of thoracic levels, and 10.3% of lumbar levels (Figure 4).
Figure 4.
Histograms demonstrating the percentage of screw tracts with ≥2 mm, ≥3 mm, and ≥4 mm deviation with surgeon manipulation, in 3D and in each of the mediolateral (ML), anteroposterior (AP), and craniocaudal (CC) axes. Manipulation-induced displacement is shown in each of the cervical (A), thoracic (B) and lumbar (C) spines.
Navigation Error From Respiration-Induced Motion
Respiration-induced motion was quantified as absolute motion, or motion relative to a DRF clamped 1 to 5 levels adjacent. In general linear modeling, accounting for spinal level, there were no differences in relative vertebral motion in any axis between the DRF at any of 1 to 5 levels distant, hence these are pooled for subsequent analysis as “relative motion.”
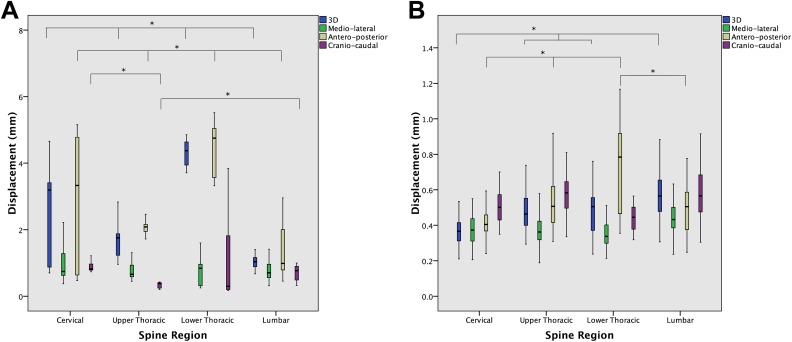
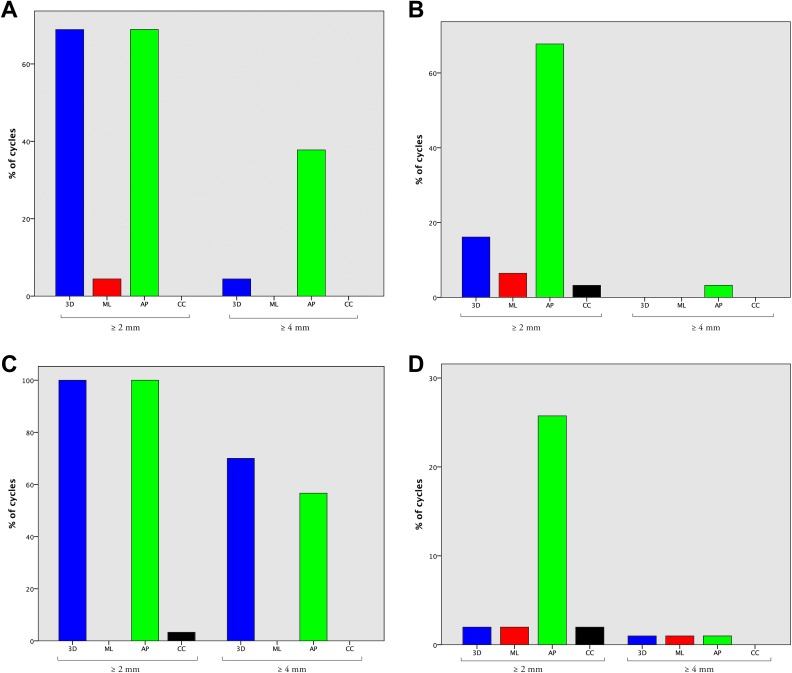
Absolute 3D vertebral motion (mean ± SD) across all levels was 1.96 ± 1.32 mm, significantly greater in the lower thoracic spine than in the cervical, upper thoracic, or lumbar spine (4.30 ± 0.35 vs 2.58 ± 1.24, 1.66 ± 0.47, and 1.09 ± 0.44 mm, respectively; P < .001). Absolute motion was greatest in the AP axis versus the ML and CC axes (2.35 ± 1.75 vs 0.84 ± 0.57 and 0.86 ± 0.61 mm, respectively; P < .001) (Figure 5). Absolute 3D motion was greater than 2 mm in 32.9% of cases and greater than 4 mm in 11.6%, significantly more frequently in the lower thoracic spine (P < .001) (Figure 6). Relative respiration-induced 3D displacement (mean ± SD) across all levels was 0.48 ± 0.14, significantly greater in the lumbar spine and in the AP and CC axes (Figure 5).
Figure 5.
Standard boxplots demonstrating absolute (A) and relative (B) respiration-induced vertebral motion, in 3 dimensions (3D) and in each of the mediolateral (ML), anteroposterior (AP), and craniocaudal (CC) axes, stratified by spinal region. Boxes represent the first quartile, median, and third quartile. Whiskers represent 1.5× the interquartile range. *Represents significant difference, at P < .05.
Figure 6.
Histograms demonstrating the percentage of respiratory cycles with ≥2 mm and ≥4 mm of displacement, in 3 dimensions (3D) and in each of the mediolateral (ML), anteroposterior (AP), and craniocaudal (CC) axes, in the cervical (A), upper thoracic (B), lower thoracic (C), and lumbar (D) spine.
In general linear modeling, TV (r = 0.263, P = .032), PEEP (r = 0.756, P < .001), and MAP (r = 0.150, P < .001) were positively correlated with absolute 3D and AP respiration-induced motion (r is the Pearson correlation coefficient), that is, greater motion is associated with greater TV, PEEP, and MAP Age, gender, and BMI did not significantly correlate with any respiration-induced displacement.
Discussion
While modern 3D CAN has demonstrably increased instrumentation accuracy across all spinal levels, widespread enthusiasm for the technology has been tempered by high costs as well as workflow disruption.1,5,15-19 Initial 3D CAN systems employing registration of patient anatomy to preoperative imaging, using either point- or surface-matching techniques, were highly cumbersome to register. Advances in intraoperative imaging, either 3D fluoroscopy or cone- or fan-beam CT, have allowed for faster automatic patient registration to intraoperatively acquired imaging.20 Intraoperative imaging devices have concurrently eliminated one source of navigation error due to intersegmental mobility, from positional changes from a supine preoperative scan to a prone operating position. However, all contemporary 3D CAN systems, whether registering to pre- or intraoperative imaging, retain dependence on a DRF to maintain patient-to-image registration and allow tool tracking. Navigation errors may therefore arise by virtue of distance from the DRF, a limitation largely of the infrared optical tracking technology used by most current CAN devices, as well as due to intersegmental mobility, from surgical manipulation and patient respiration-induced motion at levels distant to the DRF. Using an OTI navigation system developed in-house, allowing granular control and root-level system access, we quantify and identify predictors of these errors for the first time in the literature.
The impact of working distance from a DRF on navigation error has been studied heterogeneously in the limited literature to date. In the cervical spine, using point-matching registration to preoperative CT, Tauchi et al7 demonstrated a 17% increase in cervical pedicle screw perforation rate when working 1 level distant from the DRF, with greater distance correlated with larger error. The literature in the thoracolumbar spine is more controversial. Using point-matching registration to preoperative CT in the setting of adolescent scoliosis, Uehara et al12 demonstrated significantly increased pedicle screw perforation rates at 3 or more levels distant to the DRF, while Takahashi et al13 showed pedicle violation rates of 1.5% at up to 3 levels distant from the DRF, though without comparison with segmental registration.12,13 Papadopoulos et al14 demonstrated no significant increases in computer-reported registration error, known to correlate poorly with true application error, at up to 4 levels from the DRF.21 Scheufler et al11 claimed safe instrumentation up to 12 levels from the DRF with registration to an intraoperative fan-beam CT, based on radiographic pedicle screw grading, though requiring 3 intraoperative CT spins and with an additional 2.5% rate of K-wire revision. Here, we show quantitatively that an increase in navigation error of ≥2 mm, corresponding to the difference between an “acceptable” and an “unacceptable” pedicle screw in common radiographic grading classifications, occurs at 2 or more levels distant from the DRF in the ML and AP axes.22,23 While AP accuracy, that is, screw depth, may be less important due to typically greater tolerances, inaccuracy in the mediolateral axis may lead to neural or vascular injury. Our study, performed in a cadaveric setting, also eliminates respiration-related motion as a confounder for error due to distance from the DRF.
Intersegmental mobility may also lead to error during surgical manipulation at levels distant from the DRF. This has been studied only once in the literature to our knowledge, with Glossop et al8 demonstrating up to 12 mm of 3D movement in the lumbar spine with paraspinal muscle dissection and pedicle targeting in an in vivo setting. We show absolute 3D movement of only 1.55 mm across all levels, due in part to the rigid formalin-fixed cadaveric setting, as well as because only pedicle targeting was assessed, whereas typically more force may be applied for paraspinal tissue dissection. However, the key point is that while absolute manipulation-related motion is typically small in our study, variability in motion is high particularly in the lumbar spine, and may be expected to be even greater in an in vivo setting with more pliable tissues, as well as a range of degenerative pathologies with greater intersegmental mobility.
Vertebral motion from patient respiration may result in navigation inaccuracy distant to a DRF, due to intersegmental mobility. In the limited literature to date, respiratory motion has been reported up to 1.6 mm in the lumbar spine, with no direct quantification of vertebral motion at other levels.8,9 In our study, we demonstrate via direct measurement that while respiration-induced vertebral motion averages only 0.48 mm at 1 to 5 levels from a DRF, absolute motion with regard to a DRF affixed to a remote fixed anatomical site may be as large as 10 mm in the AP axis and 6 mm in the ML axis. The attenuation of respiration-induced error with an adjacent DRF, even though we demonstrate increased error in optical tracking at 2 or more levels distant from the DRF, is likely due to temporal averaging of errors which does not occur at a single time point. Caution should therefore be exercised when performing navigated procedures with a DRF affixed to the pelvis or Mayfield head clamp, as is commonly done in long-segment lumbosacral or cervical procedures, respectively. Furthermore, greater respiration-induced displacement is correlated with larger TV and PEEP, intuitive given the larger amplitude chest-wall motion expected with greater TV and PEEP; apnea or modification of ventilator parameters may therefore be warranted at critical stages of a navigated procedure where accuracy is paramount.
There are multiple limitations to our analysis. Preclinical testing was conducted in formalin-fixed cadavers, with far more rigid tissues and therefore underestimated vertebral motion than might be expected in clinical application. Respiration-induced motion was assessed up to 5 levels distant to a DRF, due to the limited exposure in most procedures in this series. Further work is therefore required to assess the impact of working at greater distances from a DRF on mitigation of respiration-induced vertebral motion.
Conclusions
Vertebral motion is unaccounted for during image-guided surgery when performed at levels distant from the DRF. Navigating instrumentation within 2 levels of the DRF is likely to minimize the risk of navigation error. While respiration- and manipulation-induced vertebral motion is typically small, there may be significant variability in magnitude, particularly with spinal region and ventilator parameters. Surgeons may mitigate these errors intraoperatively by placing the DRF adjacent to the registered level, rather than on a Mayfield head clamp or pelvis, to minimize respiration-induced error. If performing work distant to a DRF, temporary apnea or adjustment of ventilator parameters may be warranted at critical stages of the procedure, to minimize respiration-induced error. Surgeons should also take care to cease manipulation of bony elements when actively using navigation guidance. Future generations of image-guidance systems should compensate for these errors in real-time to minimize navigation inaccuracy.
Abbreviations
- 3D:
three-dimensional,
- ML:
medio-lateral,
- AP:
antero-posterior,
- CC:
cranio-caudal,
- CAN:
computer-assisted navigation
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VXDY is cofounder and Chief Scientific Officer of 7D Surgical Inc, a company licensing the optical topographic navigation technology developed by our research group. VXDY is a minority shareholder in this company. 7D Surgical Inc. and its technology have not had any influence on the conduction of this study, and all patients included in this analysis have been treated with guidance from commercially available navigation systems.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI). Salary support for DG is provided in part by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship (FRN 142 931).
ORCID iD: Daipayan Guha  https://orcid.org/0000-0002-2052-4819
https://orcid.org/0000-0002-2052-4819
Shaurya Gupta  https://orcid.org/0000-0002-3268-2224
https://orcid.org/0000-0002-3268-2224
References
- 1. Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine. 2014;20:196–203. doi:10.3171/2013.11.spine13413 [DOI] [PubMed] [Google Scholar]
- 2. Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011;20:846–859. doi:10.1007/s00586-010-1577-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laudato PA, Pierzchala K, Schizas C. Pedicle screw insertion accuracy using O-arm, robotic guidance or freehand technique: a comparative study. Spine (Phila Pa 1976). 2018;43:E373–E378. doi:10.1097/BRS.0000000000002449 [DOI] [PubMed] [Google Scholar]
- 4. Du JP, Fan Y, Wu QN, Wang DH, Zhang J, Hao DJ. Accuracy of pedicle screw insertion among 3 image-guided navigation systems: a systematic review and meta-analysis. World Neurosurg. 2018;109:24–30. doi:10.1016/j.wneu.2017.07.154 [DOI] [PubMed] [Google Scholar]
- 5. Hartl R, Lam KS, Wang J, Korge A, Kandziora F, Audige L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013;79:162–172. doi:10.1016/j.wneu.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 6. Choo AD, Regev G, Garfin SR, Kim CW. Surgeons’ perceptions of spinal navigation: analysis of key factors affecting the lack of adoption of spinal navigation technology. SAS J. 2008;2:189–194. doi:10.1016/S1935-9810(08)70038-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tauchi R, Imagama S, Sakai Y, et al. The correlation between cervical range of motion and misplacement of cervical pedicle screws during cervical posterior spinal fixation surgery using a CT-based navigation system. Eur Spine J. 2013;22:1504–1508. doi:10.1007/s00586-013-2719-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glossop N, Hu R. Assessment of vertebral body motion during spine surgery. Spine (Phila Pa 1976). 1997;22:903–909. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Zeng C, Fan M, Hu L, Ma C, Tian W. Assessment of respiration-induced vertebral motion in prone-positioned patients during general anaesthesia. Int J Med Robot. 2016;12:214–218. doi:10.1002/rcs.1676 [DOI] [PubMed] [Google Scholar]
- 10. Scheufler KM, Franke J, Eckardt A, Dohmen H. Accuracy of image-guided pedicle screw placement using intraoperative computed tomography-based navigation with automated referencing, part I: cervicothoracic spine. Neurosurgery. 2011;69:782–795. doi:10.1227/NEU.0b013e318222ae16 [DOI] [PubMed] [Google Scholar]
- 11. Scheufler KM, Franke J, Eckardt A, Dohmen H. Accuracy of image-guided pedicle screw placement using intraoperative computed tomography-based navigation with automated referencing. Part II: thoracolumbar spine. Neurosurgery. 2011;69:1307–1316. doi:10.1227/NEU.0b013e31822ba190 [DOI] [PubMed] [Google Scholar]
- 12. Uehara M, Takahashi J, Ikegami S, et al. Are pedicle screw perforation rates influenced by distance from the reference frame in multilevel registration using a computed tomography-based navigation system in the setting of scoliosis? Spine J. 2017;17:499–504. doi:10.1016/j.spinee.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 13. Takahashi J, Hirabayashi H, Hashidate H, Ogihara N, Kato H. Accuracy of multilevel registration in image-guided pedicle screw insertion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2010;35:347–352. doi:10.1097/BRS.0b013e3181b77f0a [DOI] [PubMed] [Google Scholar]
- 14. Papadopoulos EC, Girardi FP, Sama A, Sandhu HS, Cammisa FP., Jr Accuracy of single-time, multilevel registration in image-guided spinal surgery. Spine J. 2005;5:263–267. doi:10.1016/j.spinee.2004.10.048 [DOI] [PubMed] [Google Scholar]
- 15. Barsa P, Frőhlich R, Šercl M, Buchvald P, Suchomel P. The intraoperative portable CT scanner-based spinal navigation: a viable option for instrumentation in the region of cervico-thoracic junction. Eur Spine J. 2016;25:1643–1650. doi:10.1007/s00586-016-4476-6 [DOI] [PubMed] [Google Scholar]
- 16. Bourgeois AC, Faulkner AR, Bradley YC, et al. Improved accuracy of minimally invasive transpedicular screw placement in the lumbar spine with 3-dimensional stereotactic image guidance: a comparative meta-analysis. J Spinal Disord Tech. 2015;28:324–329. doi:10.1097/BSD.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 17. Hecht N, Kamphuis M, Czabanka M, et al. Intraoperative iso-C C-arm navigation in craniospinal surgery: the first 60 cases. J Neurosurg Spine. 2010;36:E1 doi:10.3171/SPI.2008.9.11.450 [DOI] [PubMed] [Google Scholar]
- 18. Tian W, Weng C, Liu B, et al. Intraoperative 3-dimensional navigation and ultrasonography during posterior decompression with instrumented fusion for ossification of the posterior longitudinal ligament in the thoracic spine. J Spinal Disord Tech. 2013;26:E227–E234. doi:10.1097/BSD.0b013e318286ba39 [DOI] [PubMed] [Google Scholar]
- 19. Wagner SC, Morrissey PB, Kaye ID, Sebastian A, Butler JS, Kepler CK. Intraoperative pedicle screw navigation does not significantly affect complication rates after spine surgery. J Clin Neurosci. 2018;47:198–201. doi:10.1016/j.jocn.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 20. Costa F, Porazzi E, Restelli U, et al. Economic study: a cost-effectiveness analysis of an intraoperative compared with a preoperative image-guided system in lumbar pedicle screw fixation in patients with degenerative spondylolisthesis. Spine J. 2014;14:1790–1796. doi:10.1016/j.spinee.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 21. Guha D, Jakubovic R, Gupta S, et al. Spinal intraoperative three-dimensional navigation: correlation between clinical and absolute engineering accuracy. Spine J. 2017;17:489–498. doi:10.1016/j.spinee.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 22. Neo M, Sakamoto T, Fujibayashi S. The clinical risk of vertebral artery injury from cervical pedicle screws inserted in degenerative vertebrae. Spine (Phila Pa 1976). 2005;30:2800–2805. [DOI] [PubMed] [Google Scholar]
- 23. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976). 1990;15:11–14. [DOI] [PubMed] [Google Scholar]