Abstract
Background
Adverse cardiac remodeling after ST-segment elevation myocardial infarction (STEMI) is a major cause for poor cardiovascular outcomes such as heart failure. The predisposing factors and underlying mechanisms remain not fully understood. This study investigates the association of insulin resistance and dysglycemia with left ventricular (LV) remodeling after STEMI in non-diabetic patients.
Methods
A total of 485 non-diabetic subjects with STEMI who underwent primary percutaneous coronary intervention were consecutively enrolled and followed up for 12 months. Relation of homeostasis model assessment-estimated insulin resistance (HOMA-IR) and glucose levels to changes in echocardiography parameters was studied.
Results
Left ventricular dilation was detected in 49.1% of subjects at 12-month follow-up after STEMI, and was more severe in subjects with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and high HOMA-IR levels. HOMA-IR remained correlated to changes in LV dimensions after adjusting for confounding risk factors. Multivariate regression analysis demonstrated that higher HOMA-IR was independently associated with greater LV dilation after STEMI. A significant interaction term was present between HOMA-IR and IGT in the model (P = 0.001).
Conclusions
Our study reveals that insulin resistance and dysglycemia are prevalent in non-diabetic patients with STEMI and are predictors of the post-infarction LV dilation.
Trial registration Trials number, NCT02089360; registered on March 17, 2014
Electronic supplementary material
The online version of this article (10.1186/s12933-019-0904-3) contains supplementary material, which is available to authorized users.
Keywords: Insulin resistance, Dysglycemia, Left ventricular dilation, Remodeling, Myocardial infarction
Background
Despite rapid advances in acute treatment and secondary prevention measures in recent years, the incidence of chronic heart failure (CHF) after myocardial infarction remains relatively high, and is associated with late mortality [1]. From the onset of myocardial infarction, left ventricular (LV) remodeling occurs in response to abrupt increase in ventricular loading and acute inflammation [2]. Multiple mechanisms are implicated in this process to compensate for the loss of cardiomyocytes [3, 4]. Maladaptive LV remodeling, especially in conditions of disturbed metabolism and neurohormone overaction, predisposes adverse cardiovascular outcomes [5–7].
Insulin resistance is a well-established composite index of systemic inflammatory and metabolic disorders [8]. Mounting evidence reveals that insulin resistance predicts and, to some extent, mediates the development of atherosclerosis [9], myocardial infarction [10] and in-stent restenosis [11]. The predictive role of insulin resistance for LV remodeling and incident CHF has also been proposed [12, 13]. Especially, a unique concentric LV remodeling pattern was characterized in relatively healthy subjects with insulin resistance [12]. However, the role of insulin resistance in LV remodeling after acute myocardial infarction remains poorly understood.
To investigate the relationship between insulin resistance and post-infarction LV remodeling, we analyzed non-diabetic subjects with acute ST-elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI) and performed echocardiography at baseline and 12-month follow-up. The associations between changes in LV geometric parameters and homeostasis model assessment-estimated insulin resistance (HOMA-IR) as well as glucometabolic disorders were examined.
Methods
Study population
This study complies with the Declaration of Helsinki. The study protocol was approved by the local hospital ethics committee, and written informed consent was obtained from all participants.
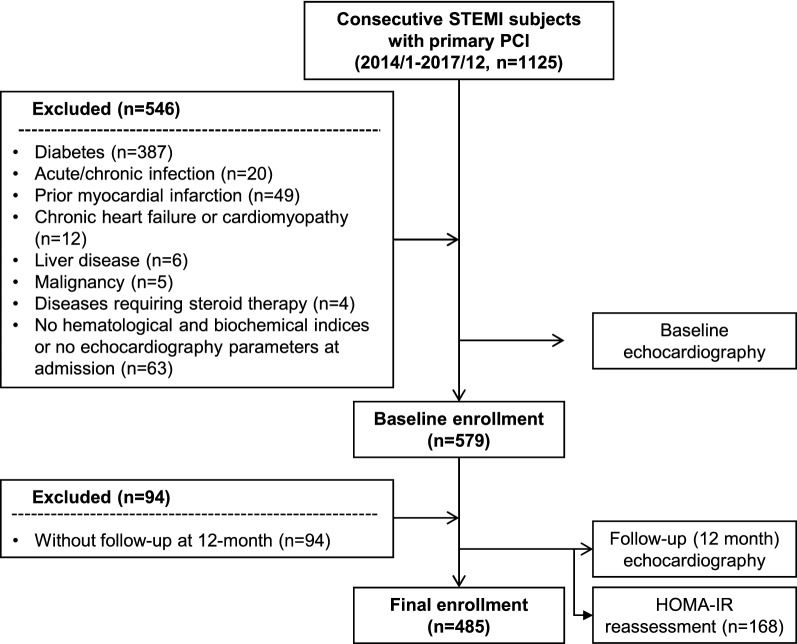
We consecutively enrolled 1125 subjects with the first ever acute STEMI with the onset of symptoms within 12 h preceding hospital admission and received primary PCI from Jan, 2014 to Dec, 2017 in the Department of Cardiology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine (Fig. 1). A total of 483 patients comorbid with diabetes, chronic or acute infection, prior myocardial infarction, chronic heart failure or cardiomyopathy, liver disease, malignancy and diseases requiring steroid therapy, were excluded. Additionally, 63 patients who did not have biochemical indices including fasting glucose and insulin, as well as echocardiography parameters obtained at discharge were not enrolled. Another 94 patients without follow-up echocardiography at 12-month were also excluded. Thus, 485 patients comprised the final enrollment. HOMA-IR levels were reassessed in 168 subjects within the study population at follow-up. The changes in echocardiography parameters were calculated. The association between changes in echocardiograph parameters and basal HOMA-IR as well as glucometabolic disorders was analyzed. The diagnosis of diabetes was made according to the criteria of American Diabetes Association and prediabetes was defined by fasting blood glucose of 100 mg to < 126 mg/dL, 2-h plasma glucose of 140 to < 200 mg/dL, or HbA1C of 5.7 to < 6.5% [14]. Hypertension and dyslipidemia were diagnosed according to seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC 7) and guideline of the National Cholesterol Education Program (ATP III), respectively [15, 16].
Fig. 1.
Flow chart of patient enrollment. STEMI ST-segment elevation myocardial infarction, PCI percutaneous coronary intervention
Clinical, biochemical and echocardiographic assessments
All the blood samples were drawn and echocardiography was performed at discharge except that the peak cardiac troponin I (cTnI) was recorded after admission. The blood samples were collected in a quiet, air-conditioned room after overnight fasting and after at least 20 min supine rest. Serum glucose, insulin, blood urea nitrogen, creatinine, uric acid, total cholesterol, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, apolipoprotein A-I and apolipoprotein B were assessed (HITACHI 912 Analyzer, Roche Diagnostics, Germany). The estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration equation [17]. Blood HbA1c concentration was measured using ion-exchange high performance liquid chromatography with Bio-rad Variant Hemoglobin Testing System (Bio-Rad Laboratories, USA). Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) was determined using a commercially available electrochemiluminescence immunoassay kit (Roche Diagnostics). Serum levels of high sensitive C-reactive protein (hsCRP) were determined by ELISA (Biocheck Laboratories, Toledo, OH, USA). HOMA-IR was calculated according to the formula: fasting insulin (µU/L) × fasting glucose (mmol/L)/22.5. The detailed information about medical history and lifestyles including smoking status was obtained using a standard questionnaire by trained physicians. Body mass index (BMI) was calculated using the formula of weight/height2 (kilograms per square meter). Body surface area (BSA) was calculated using the formula of 0.0061 × height + 0.0128 × weight − 0.1529. Blood pressure was measured on the non-dominant arm in a seated position after a 10-min rest, using an electronic blood pressure monitor (OMRON Model HEM-752 FUZZY’ Omron Co., Dalian, China). Three measurements were taken at 1-min intervals, and the average was used for analysis.
Transthoracic echocardiography was performed, at least, at the time of enrollment and 12-month follow-up, using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI) with a 1.9- to 3.8-mHz phased-array transducer. Echocardiography was performed by a single sonographer credentialed in cardiac ultrasound. Two-dimensional (2D), pulsed-Doppler imaging was performed from standard parasternal and apical transducer positions with 2D frame rates of 60 to 100 frames/s. All data were stored digitally, and offline data analysis was performed (EchoPac, version 7; GE Healthcare) by two cardiologists at the conclusion of the study, blinded to the study time point.
The LV ejection fraction (LVEF) was calculated using the modified Simpson’s biplane technique. The LV length was measured in the apical 4-chamber view. To facilitate application of clinical normality cut points (20), LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were indexed by BSA calculated at each study time point. LV mass was estimated from M-mode measurements by the formula:
where LVEDD is LV end-diastolic diameter, IVST is interventricular septal thickness, LVPWT is LV posterior wall thickness. LV mass was indexed by BSA. Relative wall thickness (RWT) was determined by the formula:
where IVST is interventricular septal thickness, LVPWT is LV posterior wall thickness, LVEDD is LV end-diastolic diameter.
Statistics
Continuous variables were presented as median (interquartile range) or mean (standard deviation), and categorical data were summarized as frequencies (percentages). For continuous variables, normal distribution was evaluated with Kolmogorov–Smirnov test. Differences among groups were analyzed by Student’s t-test or one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test. Correlation between HOMA-IR and changes in echocardiography parameters was determined by Pearson’s correlation test. Furthermore, multivariate linear regression was implemented to interrogate the association between insulin resistance and LV remodeling parameters. In model 1, changes in LVEDD were employed as dependent variables, and covariates, including male gender, age, history of hypertension, the presence of chronic kidney disease (CKD), levels of lymphocytes, HDL cholesterol, TIMI flow grade after PCI, log-transformed cTnI and baseline LVEDD was adjusted. In model 2, we further adjusted the presence of impaired glucose tolerance (IGT) and BMI (dichotomized by median), as well as interaction terms of each maker with tertiles of HOMA-IR in the model. Regression coefficients of interaction term represent the difference in coefficient of IGT (or BMI) between subgroups stratified by tertiles of HOMA-IR. Coefficients of interaction terms corresponding to intermediate and high tertiles vs. low tertile of HOMA-IR were calculated. All statistical analyses were performed using the SPSS 23.0 for Windows (SPSS, Inc., Chicago, IL, USA). A 2-tailed < 0.05 was considered statistically significant.
Results
Basic characteristics of the studied population
A total of 485 non-diabetic patients with STEMI who underwent PCI were enrolled in this study (Table 1). The level of HOMA-IR (2.36 [1.56–3.92]) was higher than the normal range according to previous reports [10, 18] and the prevalence of impaired fasting glucose (IFG) and IGT was 40.0% and 40.2%, respectively. After dividing the study population into three groups according to HOMA-IR tertiles, we found subjects with high HOMA-IR levels tended to have higher BMI, diastolic blood pressure, liver enzymes, triglyceride and cTnI levels, but were younger and had lower NT-proBNP levels. There was no difference in renal function, smoking history, comorbidities such as hypertension, atrial fibrillation, and CKD between the three groups. On admission, dual antiplatelet agents, statins, ACEI/ARBs and β-blockers were prescribed unless contraindicated. A total of 8.7% of patients were prescribed spirolactone depending on clinical scenario. No significant difference in medication use was detected between different groups.
Table 1.
Baseline characteristics
| HOMA-IR tertiles | Low | Intermediate | High | P-value |
|---|---|---|---|---|
| n | 174 | 150 | 161 | |
| Demographic characteristics | ||||
| Age, years, mean (SD) | 65.24 (10.45) | 62.37 (11.23) | 59.93 (11.84) | < 0.001 |
| Male, n (%) | 154 (88.51) | 132 (88.00) | 149 (92.55) | 0.342 |
| Clinical measures, mean (SD) | ||||
| BMI, kg/m2 | 23.09 (3.00) | 24.76 (2.93) | 25.95 (3.78) | < 0.001 |
| SBP, mmHg | 121.07 (17.36) | 124.71 (17.87) | 125.37 (20.30) | 0.078 |
| DBP, mmHg | 71.99 (11.45) | 75.57 (11.98) | 77.76 (12.02) | < 0.001 |
| Current or former smoker, n (%) | 87 (50.00) | 72 (48.00) | 66 (40.99) | 0.228 |
| Diseased vessels, n (%) | ||||
| Single-vessel disease | 59 (33.91) | 46 (30.67) | 52 (32.30) | 0.768 |
| Multi-vessel disease | 115 (66.09) | 104 (69.33) | 109 (67.70) | |
| Medical history, n (%) | ||||
| Hypertension | 90 (51.72) | 86 (57.33) | 101 (62.73) | 0.126 |
| Atrial fibrillation or flutter | 6 (3.45) | 8 (5.33) | 8 (4.97) | 0.682 |
| Chronic kidney disease | 8 (4.60) | 10 (6.67) | 13 (8.07) | 0.424 |
| Cerebrovascular disease | 20 (11.49) | 8 (5.33) | 16 (9.94) | 0.140 |
| Laboratory values, median (IQR) or mean (SD) | ||||
| HbA1c, % | 5.60 (5.40–5.90) | 5.75 (5.40–6.10) | 5.70 (5.40–5.90) | 0.116 |
| Fasting glucose, mmol/L | 5.04 (0.87) | 5.45 (0.76) | 6.17 (0.94) | < 0.001 |
| Fasting insulin, μU/mL | 5.61 (2.12) | 10.32 (1.95) | 21.67 (10.83) | < 0.001 |
| Triglyceride, mmol/L | 1.31 (0.99–1.73) | 1.51 (1.08–2.07) | 1.65 (1.18–2.30) | < 0.001 |
| Total cholesterol, mmol/L | 4.50 (1.44) | 4.37 (0.83) | 4.77 (1.06) | 0.040 |
| HDL cholesterol, mmol/L | 1.04 (0.26) | 1.02 (0.22) | 1.02 (0.23) | 0.855 |
| LDL cholesterol, mmol/L | 2.85 (1.31) | 2.67 (0.68) | 3.02 (0.86) | 0.044 |
| Apolipoprotein A–I, g/L | 1.10 (0.20) | 1.11 (0.19) | 1.14 (0.18) | 0.381 |
| Apolipoprotein B, g/L | 0.89 (0.29) | 0.90 (0.21) | 0.94 (0.19) | 0.084 |
| Alanine aminotransferase, IU/L | 40.96 (23.55) | 37.12 (18.47) | 61.98 (44.55) | < 0.001 |
| Aspatate aminotransferase, IU/L | 140.90 (141.13) | 137.10 (123.68) | 238.58 (213.27) | < 0.001 |
| Blood urea nitrogen, mmol/L | 5.20 (4.10–6.20) | 5.00 (4.20–6.00) | 5.10 (4.20–6.50) | 0.497 |
| Serum creatinine, μmol/L | 78.00 (62.25–87.00) | 77.00 (67.00–87.00) | 80.00 (68.00–92.00) | 0.166 |
| eGFR, mL/min/1.73 m2 | 83.42 (20.70) | 88.11 (21.65) | 85.38 (19.15) | 0.178 |
| hsCRP, mg/L | 5.84 (2.31–13.74) | 4.50 (2.06–11.47) | 3.96 (1.81–12.85) | 0.151 |
| NT-proBNP, pg/mL | 1049.00 (718.10–2668.00) | 859.55 (309.30–1843.00) | 466.60 (278.70–1344.75) | < 0.001 |
| cTnI (ng/mL) | 6.19 (0.35–29.86) | 9.94 (0.32–36.47) | 24.40 (1.78–75.45) | < 0.001 |
| Medication use, n (%) | ||||
| ACEI or ARBs | 125 (71.84) | 114 (76.00) | 133 (82.61) | 0.064 |
| Beta-blockers | 146 (83.91) | 122 (81.33) | 135 (83.85) | 0.787 |
| Statins | 170 (97.70) | 142 (94.67) | 157 (97.52) | 0.243 |
| Spirolactone | 10 (5.75) | 12 (8.00) | 20 (12.42) | 0.089 |
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, cTnI cardiac troponin I, eGFR estimated glomerular filtration rate, BMI body mass index, DBP diastolic blood pressure, HbA1c glycated hemoglobin A1c, HDL high-density lipoprotein, HOMA-IR homeostatic model assessment-insulin resistance, hsCRP high-sensitivity C-reactive protein, IQR interquartile range, LDL low-density lipoprotein, NT-proBNP N-terminal pro-B-type natriuretic peptide, SBP systolic blood pressure, SD standard deviation
HOMA-IR was reassessed in 168 subjects within the study population at the 12-month follow-up. There was no statistic difference in baseline HOMA-IR levels between the selected subjects and the overall population (P = 0.122). Although HOMA-IR levels were lower at follow-up (2.12 [1.61–3.09]) compared to those in the baseline (2.61 [1.77–3.99]; P = 0.022), there was still a stepwise increase in the follow-up HOMA-IR levels across increasing tertiles of the baseline values (P < 0.001; Additional file 1: Figure S1).
Changes in LV geometric and functional properties
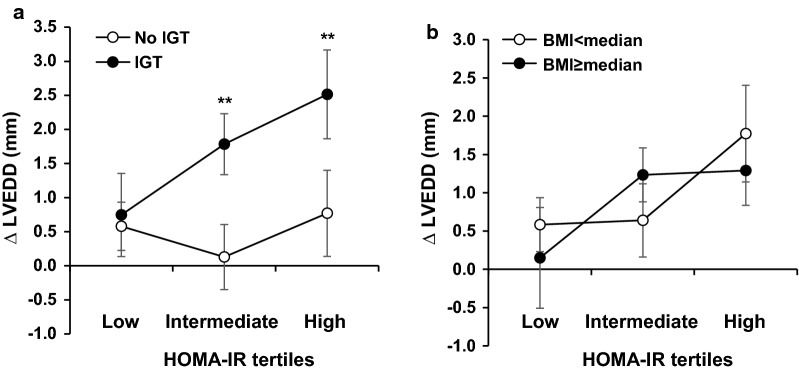
Baseline and 12-month follow-up LV geometric and functional parameters were assessed. Changes in echocardiography parameters were compared in subjects stratified by HOMA-IR tertiles (Table 2). There was an upward trend in post-infarction LV dilation with increasing tertiles of HOMA-IR (LVEDD, LVESD, LVEDV index and LVESV index, all P < 0.001). Especially, subjects with IGT presented greater LV dilation than those without at intermediate and high tertiles of HOMA-IR (Fig. 2; intermediate tertile: 1.78 vs. 0.13, P = 0.001; high tertile: 2.52 vs. 0.77, P = 0.010). When stratified by BMI, however, no difference was detected at any tertile. On the other hand, the post-infarction LV wall thinning appeared to be more prominent (Δ IVST, P = 0.003; Δ LVPWT, P = 0.006; Δ RWT, P = 0.001) in subjects with high HOMA-IR, suggesting a trend toward eccentric remodeling of LV after STEMI in subjects with insulin resistance. No significant difference in changes in LV mass index (P = 0.130) and LVEF recovery (P = 0.089) during follow-up was detected between different tertiles.
Table 2.
Changes in echocardiography parameters during follow-up grouped by HOMA-IR tertiles
| HOMA-IR tertiles | Low | Intermediate | High | P-value |
|---|---|---|---|---|
| LVEDD, mm | ||||
| B | 50.09 (4.35) | 50.77 (5.00) | 51.60 (5.23) | < 0.001 |
| F | 50.60 (4.99) | 51.68 (5.49) | 53.04 (5.33) | |
| Δ | 0.52 (2.47) | 0.91 (3.08) | 1.44 (3.96) | |
| LVESD, mm | ||||
| B | 34.04 (5.09) | 34.93 (5.35) | 35.55 (5.40) | < 0.001 |
| F | 33.89 (5.43) | 34.93 (6.14) | 36.60 (6.37) | |
| Δ | − 0.15 (3.351) | 0.00 (2.94) | 1.05 (4.11) | |
| LVEDVI, mL/m2 | ||||
| B | 70.17 (12.65) | 69.61 (13.88) | 70.28 (16.67) | < 0.001 |
| F | 72.09 (14.60) | 72.66 (16.56) | 75.00 (17.84) | |
| Δ | 1.91 (7.96) | 3.06 (10.63) | 4.66 (13.20) | |
| LVESVI, mL/m2 | ||||
| B | 29.11 (9.97) | 29.46 (10.55) | 30.32 (11.55) | < 0.001 |
| F | 28.65 (11.00) | 29.86 (12.95) | 32.28 (14.21) | |
| Δ | − 0.46 (6.68) | 0.40 (6.38) | 1.96 (9.13) | |
| LVMI, g/m2 | ||||
| B | 93.55 (17.15) | 96.39 (21.13) | 96.21 (21.67) | 0.130 |
| F | 94.47 (18.35) | 95.32 (16.69) | 93.66 (17.95) | |
| Δ | 0.91 (15.84) | − 1.07 (17.75) | − 2.73 (15.24) | |
| IVST, mm | ||||
| B | 9.27 (1.04) | 9.55 (1.15) | 9.60 (1.10) | 0.003 |
| F | 9.14 (1.11) | 9.36 (1.04) | 9.01 (1.16) | |
| Δ | − 0.13 (1.21) | − 0.19 (1.22) | − 0.59 (1.48) | |
| LVPWT, mm | ||||
| B | 8.92 (0.92) | 9.20 (1.07) | 9.19 (0.97) | 0.006 |
| F | 8.93 (0.91) | 8.92 (0.85) | 8.83 (0.75) | |
| Δ | 0.01 (1.03) | − 0.28 (1.09) | − 0.37 (1.25) | |
| RWT | ||||
| B | 0.36 (0.04) | 0.37 (0.05) | 0.37 (0.05) | 0.001 |
| F | 0.36 (0.04) | 0.36 (0.05) | 0.34 (0.05) | |
| Δ | − 0.01 (0.05) | − 0.01 (0.04) | − 0.03 (0.06) | |
| LVEF, % | ||||
| B | 59.04 (8.03) | 58.20 (7.85) | 57.76 (7.35) | 0.089 |
| F | 60.89 (7.63) | 59.99 (7.53) | 58.22 (8.36) | |
| Δ | 1.85 (6.76) | 1.79 (5.40) | 0.47 (6.80) | |
Values are given as mean (standard deviation)
B baseline, Δ changes in corresponding parameters, F follow-up, IVST interventricular septal thickness, LVEDD left ventricular end-diastolic diameter, LVEDVI left ventricular end-diastolic volume indexed to body surface area, LVEF left ventricular ejection fraction, LVESD left ventricular end-systolic diameter, LVESVI left ventricular end-systolic volume indexed to body surface area, LVMI left ventricular mass indexed to body surface area, LVPWT left ventricular posterior wall thickness, RWT relative wall thickness
Fig. 2.
Distribution of changes in LVEDD among HOMA-IR tertiles stratified by IGT and BMI. Shown are distribution of changes in LVEDD according to HOMA-IR tertiles stratified by the presence of IGT (a) or dichotomized BMI (b) levels. Data are expressed as mean ± 95% confidence interval. BMI body mass index, IGT impaired glucose tolerance, HOMA-IR homeostatic model assessment-insulin resistance, LVEDD left ventricular end-diastolic diameter. **P < 0.01 vs. subjects without IGT within the same HOMA-IR tertile
In addition, when grouping the overall study population based on the presence of IFG or IGT, we found subjects with IFG or IGT present more severe LV dilation than those without these glucometabolic abnormalities (Additional file 1: Tables S1, S2). Dividing subjects according to prediabetes status showed a modest but non-significant increase in LVEDD (P = 0.051) and LVEDV index (P = 0.091) in subjects with prediabetes versus those with normoglycemia (Additional file 1: Table S3).
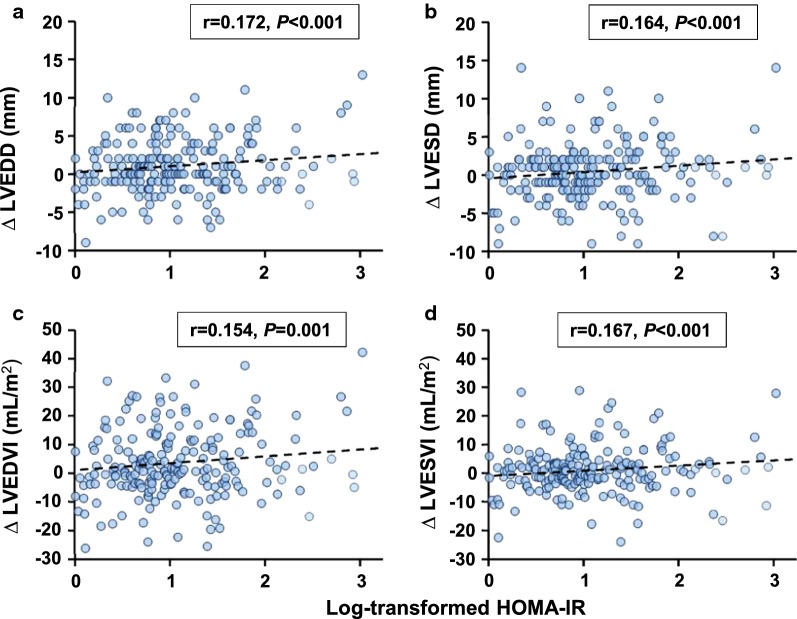
Relation of insulin resistance parameters to LV dilation
Correlation analyses showed that Log-transformed HOMA-IR was correlated positively with changes in LV diameter and volume (LVEDD: r = 0.172, P < 0.001; LVESD: r = 0.164, P < 0.001; LVEDV index: r = 0.154, P = 0.001; LVESV index: r = 0.167, P < 0.001; Table 3 and Fig. 3), and inversely with LV wall thickness (IVST: r = − 0.129, P = 0.004; LVPWT: r = − 0.144, P = 0.001; RWT r = − 0.193, P < 0.001). After adjusting for confounding risk factors, these correlations remained significant. In line with previous reports [19], an inverse correlation was also present between Log-transformed HOMA-IR and changes in LVEF.
Table 3.
Correlation analysis of Log-transformed HOMA-IR and ∆ echocardiography parameters
| ∆ Geometric/functional parameters | Log-transformed HOMA-IR | |||
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| r | P-value | r | P-value | |
| LVEDD | 0.172 | < 0.001 | 0.149 | 0.001 |
| LVESD | 0.164 | < 0.001 | 0.163 | < 0.001 |
| LVEDVI | 0.154 | 0.001 | 0.129 | 0.005 |
| LVESVI | 0.167 | < 0.001 | 0.171 | < 0.001 |
| LVMI | − 0.047 | 0.303 | − 0.106 | 0.022 |
| IVST | − 0.129 | 0.004 | − 0.152 | 0.001 |
| LVPWT | − 0.144 | 0.001 | − 0.203 | < 0.001 |
| RWT | − 0.193 | < 0.001 | − 0.219 | < 0.001 |
| LVEF | − 0.092 | 0.043 | − 0.126 | 0.006 |
IVST interventricular septal thickness, LVEDD left ventricular end-diastolic diameter, LVEDVI left ventricular end-diastolic volume indexed to body surface area, LVEF left ventricular ejection fraction, LVESD left ventricular end-systolic diameter, LVESVI left ventricular end-systolic volume indexed to body surface area, LVMI left ventricular mass indexed to body surface area, LVPWT left ventricular posterior wall thickness, RWT relative wall thickness
aAdjusted for age, sex, history of smoking, hypertension and chronic kidney disease, and LVEF at baseline
Fig. 3.
Correlation between Log-transformed HOMA-IR and changes in LV dimension. HOMA-IR was logarithmically transformed before plotting. HOMA-IR homeostatic model assessment-insulin resistance, LVEDD left ventricular end-diastolic diameter, LVEDVI left ventricular end-diastolic volume indexed to body surface area, LVESD left ventricular end-systolic diameter, LVESVI left ventricular end-systolic volume indexed to body surface area
Multivariate linear regression analysis for HOMA-IR and LV dilation
Finally, we performed multivariate linear regression to interrogate the association between insulin resistance parameters and LV remodeling (Table 4). After adjusting for confounding clinical variables in Model 1, tertiles of HOMA-IR were independently associated with changes in LVEDD (P for trend < 0.001). Compared with the low tertile, intermediate and high tertiles of HOMA-IR corresponded to 1.196-mm and 1.926-mm increases in LVEDD, respectively. By including IGT, BMI (dichotomized by median) and the interaction terms of these variables with tertiles of HOMA-IR in Model 2, we found the presence of IGT (P = 0.007), but not BMI (P = 0.140), was independently associated with changes in LVEDD. Furthermore, IGT modified the association of HOMA-IR with changes in LVEDD (P = 0.001), suggesting that subjects with high HOMA-IR levels perceive greater risk of LV dilation when coinciding with IGT. In contrast, the interaction term between HOMA-IR and BMI was non-significant (P = 0.212), suggesting that HOMA-IR was associated with post-infarction LV dilation irrespective of BMI.
Table 4.
Multivariate linear regression analysis for ∆LVEDD after STEMI
| Covariates | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | Sβ | P-value | Coefficient (95% CI) | Sβ | P-value | |
| Male gender | − 0.009 (− 1.044 to 1.025) | − 0.001 | 0.986 | 0.028 (− 1.004 to 1.061) | 0.003 | 0.957 |
| Age | − 0.190 (− 0.453 to 0.073) | − 0.066 | 0.157 | − 0.125 (− 0.385 to 0.135) | − 0.043 | 0.345 |
| Smoking | 0.446 (− 0.138 to 1.030) | 0.067 | 0.134 | 0.366 (− 0.218 to 0.949) | 0.055 | 0.219 |
| Hypertension | 0.351 (− 0.220 to 0.923) | 0.052 | 0.228 | 0.339 (− 0.226 to 0.905) | 0.050 | 0.239 |
| CKD | 0.886 (− 0.220 to 1.991) | 0.066 | 0.116 | 0.855 (− 0.230 to 1.939) | 0.063 | 0.122 |
| Lymphocytes | − 1.028 (− 1.563 to − 0.492) | − 0.166 | < 0.001 | − 1.049 (− 1.581 to − 0.517) | − 0.169 | < 0.001 |
| HDL cholesterol | − 3.118 (− 4.722 to − 1.515) | − 0.163 | < 0.001 | − 3.265 (− 4.878 to − 1.651) | − 0.170 | < 0.001 |
| TIMI flow ≤ 2 | 1.215 (− 0.460 to 2.890) | 0.059 | 0.155 | 1.489 (− 0.182 to 3.161) | 0.072 | 0.081 |
| Log cTnI | 0.109 (0.007 to 0.211) | 0.092 | 0.036 | 0.114 (0.012 to 0.216) | 0.096 | 0.029 |
| Basal LVEDD | − 0.293 (− 0.359 to − 0.227) | − 0.435 | < 0.001 | − 0.317 (− 0.384 to − 0.251) | − 0.471 | < 0.001 |
| Basal LVEF | − 0.145 (− 0.187 to − 0.103) | − 0.344 | < 0.001 | − 0.145 (− 0.186 to − 0.104) | − 0.343 | < 0.001 |
| HOMA-IR | < 0.001* | 0.088* | ||||
| Tertile 2 vs. 1 | 1.196 (0.541 to 1.851) | 0.170 | < 0.001 | − 0.361 (− 1.360 to 0.638) | − 0.051 | 0.478 |
| Tertile 3 vs. 1 | 1.926 (1.237 to 2.614) | 0.272 | < 0.001 | 1.224 (0.095 to 2.353) | 0.173 | 0.034 |
| IGT | – | – | – | − 1.337 (− 2.311 to − 0.363) | − 0.185 | 0.007 |
| IGT × HOMA-IR | – | – | – | 0.001† | ||
| βint (tertile 2 vs. 1) | – | – | – | 2.453 (1.162 to 3.744) | 0.260 | < 0.001 |
| βint (tertile 3 vs. 1) | – | – | – | 1.925 (0.561 to 3.288) | 0.197 | 0.006 |
| BMI | – | – | – | 0.760 (− 0.249 to 1.769) | 0.113 | 0.140 |
| BMI × HOMA-IR | – | – | – | 0.212† | ||
| βint (tertile 2 vs. 1) | – | – | – | 0.775 (− 0.566 to 2.117) | 0.088 | 0.257 |
| βint (tertile 3 vs. 1) | – | – | – | − 0.405 (− 1.796 to 0.985) | − 0.049 | 0.567 |
BMI body mass index, cTnI cardiac troponin I, CKD chronic kidney disease, HOMA-IR homeostasis model assessment-estimated insulin resistance, IGT impaired glucose tolerance, LVEDD left ventricular end-diastolic diameter, LVEF left ventricular ejection fraction, Sβ standardized coefficient, βint regression coefficient (interaction)
*P for trend; †P for interaction
Discussion
The major finding of the present study is that in non-diabetic patients with STEMI, the development of LV dilation is more severe in those presenting with insulin resistance characterized by high HOMA-IR levels and the presence of IFG or IGT.
Insulin resistance predicts LV dilation
Insulin resistance serves as the pathophysiological basis of type 2 diabetes, as well as the primary metabolic disorders in a great number of patients long before progression into overt diabetes [20]. While diabetes is an established risk factor for the development of heart failure after myocardial infarction [21, 22], the role of insulin resistance in post-infarction LV remodeling in non-diabetic patients is less understood. Existing evidence showed that insulin resistance predicted subsequent CHF incidence independently of established risk factors including diabetes in a large community-based cohort [23]. A separate study of the same cohort revealed that insulin resistance was associated with LV concentric remodeling rather than LV hypertrophy [12]. However, the remodeling process triggered by acute myocardial infarction may be different from that occurred in chronic conditions.
In the present study, we demonstrated that LV dilation was more prominent in subjects presenting with insulin resistance and dysglycemia. There was an upward trend in LV dilation with increasing tertiles of baseline HOMA-IR levels in non-diabetic patients with STEMI. HOMA-IR was positively correlated to changes in LV dimensions. By constructing multivariate regression models, we found HOMA-IR was independently associated with LV dilation after STEMI. On the other hand, the post-infarction LV dilation was more severe in subjects with IFG or IGT. These data are consistent with previous reports that basal glucose levels and poor glycemic control are predictors of adverse LV remodeling and cardiovascular events [24–27]. Noteworthy, glycemic variability after the onset of acute coronary events was shown to predict patient prognosis and subsequent LV dilation [28, 29]. Taken together, these findings provide evidence that patients with insulin resistance, dysglycemia and high glycemic variability perceive higher risk of developing adverse LV remodeling and poor cardiovascular outcomes.
We found the association between LV dimension and HOMA-IR level was modified by the presence of IGT in the regression model. Subjects in the intermediate and high tertiles of HOMA-IR have greater LV dilation when coinciding with IGT, implying that insulin resistance and dysglycemia may have additive effects on adverse LV remodeling after myocardial infarction. In contrast, no significant interaction term was present between HOMA-IR and BMI with changes in LV dimension. This may be due to the reason that BMI is a crude measure of general adiposity and its impact on LV remodeling is, at least partly, attributable to its association with insulin resistance (Pearson r = 0.344, P < 0.001). In other words, subjects with different BMI but identical HOMA-IR level may develop LV dilation to a similar extent (Fig. 2b). A substantial body of evidence showed that abdominal obesity would be more strongly related to risk of mortality [30], myocardial infarction [31] and type 2 diabetes [32]. Although we did not record waist circumstance or waist-to-hip ratio in this study, they are expected to provide more information than BMI in relation to LV remodeling.
Prevalence of insulin resistance among STEMI patients
The mean HOMA-IR level in this study is beyond the normal range as defined by a substantial number of epidemiological studies [10, 18]. There was also high prevalence of IFG (40.0%), IGT (40.2%) and prediabetes (72.6%) in the study population even though subjects who met the diagnosis of diabetes had already been excluded. These data concur with previous reports demonstrating high prevalence of glucometabolic abnormalities and undiagnosed diabetes in patients with acute myocardial infarction [33–35]. Although the observed hyperglycemia and compromised insulin action might be due to activated production of catecholamines and cortisol in response to infarct extension and myocardial dysfunction, Choi et al. [33] showed that the proportion of abnormal glucose tolerance kept similar at admission and 3 months after discharge when the effects of acute stress and inflammation should have already been lessened. In the present study, HOMA-IR levels were reassessed at 1-year follow-up in 168 subjects within the study population. Although there was a decrease in HOMA-IR at follow-up compared to the baseline, subjects with high HOMA-IR levels in the acute phase were still more likely to exhibit insulin resistance after 1 year.
Possible mechanisms
We showed that subjects with insulin resistance were at higher risk to develop eccentric LV remodeling than those without after STEMI, which was different from previous findings [12] that insulin resistance led to concentric LV remodeling in chronic conditions. Different mechanisms may predominate in distinct pathophysiological conditions that may underlie the paradox. In the chronic phase, insulin resistance leads to altered substrate metabolism, maladaptive immune responses, mitochondrial dysfunction and endoplasmic reticulum stress. These mechanisms promote reactive oxygen species (ROS) production and inflammation, thereby resulting in interstitial collagen deposition, crosslinking, and finally myocardial hypertrophy and relaxation deficiency [36–38]. On the other hand, in the setting of myocardial infarction, insulin resistance is associated with poor myocardial reperfusion, impaired coronary microcirculation [39] and collateralization [40], and reduced collagen deposition in the scar [41]. These factors potentially lead to greater infarct size and post-infarction LV dilation, and finally a higher incidence of heart failure [42]. Nevertheless, the implicated mechanisms await precise characterization in future studies.
Study limitations
We appreciate limitations in our study. First, this study was a retrospective analysis based on prospectively collected data, and all the enrolled patients were from a single center. Second, data were analyzed according to HOMA-IR levels near the acute phase, which may over-estimate insulin resistance status of STEM patients. Third, we evaluated LV remodeling by calculating changes in echocardiography parameters. Performing cardiac magnetic resonance would provide more information. Fourth, anthropometric parameters such as waist circumstance and waist-to-hip ratio were not recorded in this study. They are better measures of central obesity than BMI and should provide more information. Further prospective studies are warranted to analyze the causal link between insulin resistance and LV remodeling, and the prognostic value of HOMA-IR for hard cardiovascular events in subjects with STEMI.
Conclusions
In conclusion, this study reveals high prevalence of insulin resistance and dysglycemia in non-diabetic patients with STEMI and their predictive value for subsequent adverse LV remodeling.
Additional file
Additional file 1: Table S1. Comparison of Δ echocardiography parameters grouped by IFG dichotomy. Table S2. Comparison of Δ echocardiography parameters grouped by IGT dichotomy. Table S3. Comparison of Δ echocardiography parameters grouped by prediabetes status. Figure S1. HOMA-IR levels at follow-up grouped by HOMA-IR tertiles in the baseline.
Acknowledgements
None.
Abbreviations
- CHF
chronic heart failure
- LV
left ventricular
- STEMI
ST-elevation myocardial infarction
- PCI
percutaneous coronary intervention
- HOMA-IR
homeostasis model assessment-estimated insulin resistance
- cTnI
cardiac troponin I
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- eGFR
estimated glomerular filtration rate
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- hsCRP
high sensitive C-reactive protein
- BMI
body mass index
- BSA
body surface area
- 2D
two-dimensional
- LVEF
LV ejection fraction
- LVEDV
LV end-diastolic volume
- LVESV
LV end-systolic volume
- LVEDD
LV end-diastolic diameter
- LVESD
LV end-systolic diameter
- IVST
interventricular septal thickness
- LVPWT
LV posterior wall thickness
- RWT
relative wall thickness
- ANOVA
one-way analysis of variance
- CKD
chronic kidney disease
- IGT
impaired glucose tolerance
- IFG
impaired fasting glucose
- ROS
reactive oxygen species
Authors’ contributions
CY, WJ and XW performed study design, data analysis, data interpretation, and manuscript writing. CY, YS, LL, FD performed data collection. ZY, RZ and WS performed manuscript revision. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 81670451, 81470469, 81770430, 81870179), Shanghai Rising-Star Program (Grant No. 17QA1403000), Shanghai Municipal Commission of Health and Family Planning (Grant No. 2018YQ17).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Hospital Ethics Committee, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Jin, Phone: +86-021-64370045-671503, Email: rj_jinwei@163.com.
Xiao Qun Wang, Phone: +86-021-64370045-663525, Email: Xiaoqun_Wang@hotmail.com.
References
- 1.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 2.Sutton Martin GSJ, Sharpe N. Left ventricular remodeling after myocardial infarction. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.CIR.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, McInnes IB, Dargie HJ, McMurray JJ. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55(3):243–250. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Hung C-L, Verma A, Uno H, Shin S-H, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56(22):1812–1822. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, Wen J, Kubota T, Takeshita A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol. 2003;42(1):165–172. doi: 10.1016/S0735-1097(03)00509-6. [DOI] [PubMed] [Google Scholar]
- 6.Bradham WS, Moe G, Wendt KA, Scott AA, Konig A, Romanova M, Naik G, Spinale FG. TNF-α and myocardial matrix metalloproteinases in heart failure: relationship to LV remodeling. Am J Physiol Heart Circ Physiol. 2002;282(4):H1288–H1295. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 7.van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003;41(11):2084–2092. doi: 10.1016/S0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- 8.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewers M, Zaccaro D, D’Agostino R, Haffner S, Saad MF, Selby JV, Bergman R, Savage P. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27(3):781–787. doi: 10.2337/diacare.27.3.781. [DOI] [PubMed] [Google Scholar]
- 10.Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19(6):470–475. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, Yoshida K. Impact of insulin resistance on neointimal tissue proliferation after coronary stent implantation: intravascular ultrasound studies. J Diabetes Complicat. 2002;16(1):50–55. doi: 10.1016/S1056-8727(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 12.Sundström J, Lind L, Nyström N, Zethelius B, Andrén B, Hales CN, Lithell Hans O. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation. 2000;101(22):2595–2600. doi: 10.1161/01.CIR.101.22.2595. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-K. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes A. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Wang L, Kwong RY, Bluemke DA, Lima JAC, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61(16):1698–1706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasso FC, Carbonara O, Nasti R, Marfella R, Esposito K, Rambaldi P, Mansi L, Salvatore T, Torella R, Cozzolino D. Effects of insulin on left ventricular function during dynamic exercise in overweight and obese subjects. Eur Heart J. 2005;26(12):1205–1212. doi: 10.1093/eurheartj/ehi271. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J. 1984;108(1):31–37. doi: 10.1016/0002-8703(84)90541-6. [DOI] [PubMed] [Google Scholar]
- 22.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women: the Framingham study. JAMA. 1988;260(23):3456–3460. doi: 10.1001/jama.1988.03410230074031. [DOI] [PubMed] [Google Scholar]
- 23.Ingelsson E, Sundström J, Ärnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Nicolau J, Maia L, Vitola J, Mahaffey K, Machado M, Ramires J. Baseline glucose and left ventricular remodeling after acute myocardial infarction. J Diabetes Complicat. 2007;21(5):294–299. doi: 10.1016/j.jdiacomp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Sara J, Taher R, Kolluri N, Vella A, Lerman L, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc Diabetol. 2019;18(1):22. doi: 10.1186/s12933-019-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouchi S, Shimada K, Miyazaki T, Takahashi S, Sugita Y, Shimizu M, Murata A, Kadoguchi T, Kato T, Aikawa T, et al. Low 1,5-anhydroglucitol levels are associated with long-term cardiac mortality in acute coronary syndrome patients with hemoglobin A1c levels less than 7.0. Cardiovasc Diabetol. 2017;16(1):151. doi: 10.1186/s12933-017-0636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee T, Burt M, Heilbronn L, Mangoni A, Wong V, McLean M, Cheung N. Relative hyperglycemia is associated with complications following an acute myocardial infarction: a post hoc analysis of HI-5 data. Cardiovasc Diabetol. 2017;16(1):157. doi: 10.1186/s12933-017-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, Abe T, Okada K, Matsuzawa Y, Konishi M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol. 2018;17(1):116. doi: 10.1186/s12933-018-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gohbara M, Iwahashi N, Kataoka S, Hayakawa Y, Sakamaki K, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, et al. Glycemic variability determined by continuous glucose monitoring system predicts left ventricular remodeling in patients with a first ST-segment elevation myocardial infarction. Circ J. 2015;79(5):1092–1099. doi: 10.1253/circj.CJ-14-1226. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, Zheng W. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167(9):886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 32.Siren R, Eriksson JG, Vanhanen H. Waist circumference a good indicator of future risk for type 2 diabetes and cardiovascular disease. BMC Public Health. 2012;12:631. doi: 10.1186/1471-2458-12-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi DS, Lee KW, Kim NH, Kim SG, Baik SH, Choi KM, Park CG, Oh DJ, Seo HS. Inflammation, insulin resistance, and glucose intolerance in acute myocardial infarction patients without a previous diagnosis of diabetes mellitus. J Clin Endocrinol Metab. 2005;90(1):175–180. doi: 10.1210/jc.2004-1795. [DOI] [PubMed] [Google Scholar]
- 34.Norhammar A, Tenerz Å, Nilsson G, Hamsten A, Efendíc S, Rydén L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359(9324):2140–2144. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic-Radojkovic D, Koracevic G, Stanojevic D, Damjanovic M, Apostolovic S, Pavlovic M. Stress hyperglycemia in acute ST-segment elevation myocardial infarction is a marker of left ventricular remodeling. Acute Card Care. 2013;15(2):38–43. doi: 10.3109/17482941.2013.781190. [DOI] [PubMed] [Google Scholar]
- 36.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 39.Trifunovic D, Stankovic S, Sobic-Saranovic D, Marinkovic J, Petrovic M, Orlic D, Beleslin B, Banovic M, Vujisic-Tesic B, Petrovic M, et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc Diabetol. 2014;13:73. doi: 10.1186/1475-2840-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 41.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291(5):H2504–H2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 42.Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D, et al. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities) JACC Heart Fail. 2013;1(6):531–536. doi: 10.1016/j.jchf.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of Δ echocardiography parameters grouped by IFG dichotomy. Table S2. Comparison of Δ echocardiography parameters grouped by IGT dichotomy. Table S3. Comparison of Δ echocardiography parameters grouped by prediabetes status. Figure S1. HOMA-IR levels at follow-up grouped by HOMA-IR tertiles in the baseline.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.