Abstract
Ganglioside GM1 is a member of the ganglioside family which has been used in many countries and is thought of as a promising alternative treatment for preventing several neurological diseases, including cerebral ischemic injury. The therapeutic effects of GM1 have been proved both in neonates and in adults following ischemic brain damage; however, its clinical efficacy in patients with ischemic stroke is still uncertain. This review examines the recent knowledge of the neuroprotective properties of GM1 in ischemic stroke, collected in the past two decades. We conclude that GM1 may have potential for stroke treatment, although we need to be cautious in respect of its complications.
Keywords: Ganglioside GM1, monosialotetrahexosylganglioside, stroke
Introduction
Considerable interest lies in the evaluation of ganglioside GM1, an important member of the ganglioside family. Ganglioside GM1 became of great importance to scientists in the early 1970s after its role was established as a functional tissue receptor for the cholera toxin1. Since then, scientists aimed to identify further functions of gangliosides, which became the subject of numerous global conferences. Furthermore GM1 has been shown to increase the activities of neurotrophic factors, thereby promoting protective effects on the neural system by encouraging neural stem cell survival and proliferation2, facilitating the stability and regeneration of axons, and by further preventing neurodegeneration3–6. GM1 supplementation could also afford a protective intervention in high altitude cerebral edema by suppressing oxidative stress and inflammatory response7. Because treatment with intravenous ganglioside was found to cause an acute inflammatory polyneuropathy also known as Guillain–Barré syndrome (GBS), GM1 was withdrawn from Europe8. However, this complication was not common9 and GM1 continued to be available in Asian countries, including China, where GM1 has long and widely been used in various nervous system diseases, mostly without occurrence of GBS or other severe complications10–13. Recent findings have suggested that GM1 may be related to the outcomes in the stroke progress by regulation of cell death and survival14. Our team has been working on stroke research for many years. We have previously reviewed and explored the role of GM1 in ischemic stroke15. In this paper, we will update the information for a better understanding of this field. The literature search was conducted using Medline. The following search terms were used: “Ganglioside GM1,” “Monosialotetrahexosylganglioside,” “ischemic stroke,” “ischemic brain injury,” and “stroke”. The keywords used in the Medline search were cross-referenced and the literature search was limited to English language publications on the subject. All relevant articles were included after a careful evaluation.
GM1 in Animal Studies
In Neonatal Ischemic Brain Injury
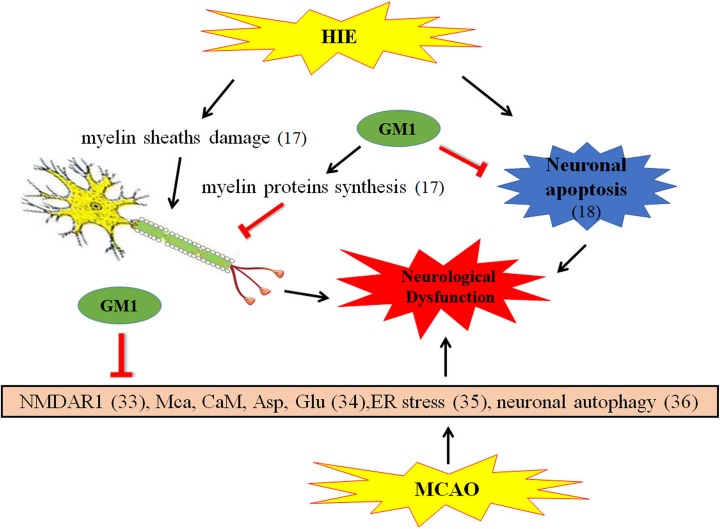
Neonates with hypoxic ischemic encephalopathy (HIE) is susceptible to develop cerebral white matter injury (WMI), a major cause of neonatal death and long-term disability16. The expression of GM1 has been found to exhibit an obvious decline at 48 h after hypoxic ischemia in lipid rafts of p 7 neonatal rats17. Myelin sheath damage, the main feature of WMI, was efficiently improved by GM1 treatment and eventually prevented secondary brain injury. The nerve repair and myelination benefit from GM1 possibly involves helping the connection of paranode proteins with lipid rafts, promoting myelin basic proteins synthesis, thus stabilizing paranode structures, and eventually repairing the damage to the myelin sheath14,17. Recently, monosialotetrahexosylganglioside GM1 administration was found to largely attenuate the neurological impairment manifestations in P 10 rats subjected to hypoxic ischemia by protecting against neuronal apoptosis (Figure 1)18.
Figure 1.
The mechanism of GM1 on ischemic brain injury.
HIE: hypoxic ischemic encephalopathy; MCAO: middle cerebral artery occlusion; NMDAR: N-methyl-D-aspartate receptor; MCa: mitochondrial calcium; CaM: calmodulin; Asp: aspartate; Glu: glutamate; ER: endoplasmic reticulum
In Adult Ischemic Brain Injury
Difference in the decrease of GM1 in neonates following HIE, in a recent study by Whitehead et al.19, which aimed to examine the spatial profile of ganglioside species using matrix-assisted laser desorption/ionization imaging, found that GM1 d18:1 (one of the GM1 moieties, which differs in carbon chain length within the sphingosine base) signal was up-regulated within the ipsilateral cortex, striatum and hippocampus, peaked by seven days post middle cerebral artery occlusion (MCAO), and dropped within the ipsilateral hemisphere within brain areas where tissue viability had been lost in adult mice. GM1 d20:1 was up-regulated at 24 h and peaked by three days post-MCAO within the ipsilateral cortex and in the hippocampi of both sides of the hemisphere. By seven days post-MCAO, GM1 d20:1 was restricted to regions surrounding the infarct core, as determined by Cresyl violet staining. This trend is similar to the results of an endothelin-1 (ET-1) subcortical stroke with the Alzheimer’s disease model, in which Wistar rats were injected with both ET-1 and beta-amyloid (Aβ), and much more serious damage was in the brain of the animal20. Both GM1d18:1 and d20:1 demonstrated a significant increase three days after administration of ET-1 and Aβ compared with a sham group. However, following stroke alone (only ET-1 injection) there was a decrease in GM1 after injury. The differences in these results may be explained by the severity of injury the animals received. These findings suggest that GM1 may play a role in mediating functions of those areas of the brain mentioned above following MCAO in mice. Consistent with the previous studies, both high-performance thin-layer chromatography and immunofluorescence microscopy revealed an increase in GM1 expression in rat cerebral cortex after MCAO, the changes of GM1 expression being thought to be an autologous mechanism against ischemic damage21. One possible explanation for the differences in the GM1 expression between neonates and adults following ischemia is that this protection mechanism may not be sufficiently developed in neonates.
Ischemic stroke is characterized by high mortality and significant neurological deficits in long-term survivors22–27. Its mechanisms of neuronal cell death have only partially been elucidated28–30, which is thought to be the key to this serious condition. Glutamate receptor N-methyl-D-aspartate receptor (NMDAR) is considered to be an important regulator in the cerebral ischemia pathological processes31,32. GM1 was reported to maintain the normal neurological functions by reducing the expression of NMDAR subunit NMDAR1 in a MCAO rat model33. In the present study, the authors did not measure the expression of GM1 after focal cerebral ischemia/reperfusion, but they found that GM1 could significantly reduce the infarct size, and this effect was time-dependent. Specifically, GM1 delivered in a short period of 5 min or 1 h after surgery was effective, while the administration at 2 h following MCAO was not. Therefore, early use of GM1 was recommended for cerebral ischemia. Besides the inhibition of NMDA, GM1 has been shown to decrease the content of mitochondrial calcium and calmodulin and increase the expression of aspartate and glutamate in neurons of the hippocampus CA1 region and frontal cerebral cortex after cerebral ischemia–reperfusion injury34. Su et al. found that GM1 (15 mg/kg) intraperitoneally administered at 20 min prior to reperfusion modulated endoplasmic reticulum stress-related protein levels, by increasing GRP78 and reducing CHOP/GADD153 expression along with activation of caspase-12 in the ischemic brain hemispheres in rats with diabetes35.These results imply that GM1 attenuates diabetes-associated cerebral ischemia–reperfusion injury by suppressing the endoplasmic reticulum stress. GM1 has also been shown to improve neurobehavioral performances, reduce infarction size, and to decrease mortality rate after ischemic brain injury by inhibiting autophagy makers36. The ratio of LC3-I to LC3-II, P62 level, and Beclin-1 expression were all significantly reduced after GM1 treatment, without finding any significant adverse effects (Figure 1).
GM1 in Clinical Trials
Previous studies showed tissue-type plasminogen activator (tPA) improved thrombolytic efficacy and long-term neurological outcomes in stroke patients37–40. However, the narrow therapeutic window and the potential risk of intracerebral hemorrhage of tPA lead to the fact that only few patients benefit from it41. Given its good neuroprotective effects in animal experiments, GM1 successfully attracted the attention of neurologists and began to be used in patients with acute ischemic stroke and other neurological disorders13,42,43. The clinical application of GM1 achieved initial benefits soon after in Parkinson’s and Alzheimer’s patients; however, its clinical efficiency in ischemic stroke disease still needs to be validated11,44,45. Candelise et al. reviewed the Cochrane Stroke Group trials register9, in which 12 clinical trials involving about 2300 patents with definite or presumed ischemic stroke were identified and analyzed. The results did not show any significant differences with respect to the incidence of disability and fatality between groups. GM1 has even been banned because of the risk of GBS in Europe countries8. Interestingly, it is still being widely used in Asian countries for its potential neuroprotective effects by improving behavioral outcomes in stroke patients10–13, without any severe complications (see Table 1).
Table 1.
GM1 in Clinical Trials.
| Patients | Symptoms improved or not | References |
|---|---|---|
| Ischemic stroke PD AD |
Not/improved Improved Improved |
9, 10, 11, 12, 13 11, 44 45 |
PD: Parkinson’s Disease; AD: Alzheimer’s Disease.
Conclusion
Overall, ganglioside GM1 treatment for ischemic stroke needs to be implemented with caution. The related clinical trials were conducted with several limitations. For example, one important noticeable issue is that many severe stroke patients were included in those trials, and more severe injuries, more bad outcomes. Ethnical and regional disparities may also account for part of the differing incidence of GBS. It is worth mentioning that GBS was not found in the Asian area, maybe because GBS was not reported to the local health departments in some Asian countries. It is apparent that additional clinical studies are needed to test the effect of GM1, given the above-mentioned limitations. Overall, GM1 may have potential for stroke treatment.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: National Natural Science Foundation of China No. 81500996 to L. Li; Beijing Jishuitan Hospital Nova Program xkxx201619 to W. Zhang and National Clinical Research for Geriatric Disorders, Xuanwu Hospital of Capital Medical University No.20170026 to T. Wang.
References
- 1. Holmgren J, Lonnroth I, Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973;8(2):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584(9):1694–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta. 2010;1801(8):878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc Natl Acad Sci U S A. 2009;106(52):22405–22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584(9):1748–1759. [DOI] [PubMed] [Google Scholar]
- 6. Caughlin S, Maheshwari S, Agca Y, Agca C, Harris AJ, Jurcic K, Yeung KK, Cechetto DF, Whitehead SN. Membrane-lipid homeostasis in a prodromal rat model of Alzheimer’s disease: characteristic profiles in ganglioside distributions during aging detected using MALDI imaging mass spectrometry. Biochim Biophys Acta. 2018;1862(6):1327–1338. [DOI] [PubMed] [Google Scholar]
- 7. Gong G, Yin L, Yuan L, Sui D, Sun Y, Fu H, Chen L, Wang X. Ganglioside GM1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol Immunol. 2018;95:91–98. [DOI] [PubMed] [Google Scholar]
- 8. Figueras A, Morales-Olivas FJ, Capella D, Palop V, Laporte JR. Bovine gangliosides and acute motor polyneuropathy. BMJ. 1992;305(6865):1330–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Candelise L, Ciccone A. Gangliosides for acute ischaemic stroke. Cochrane Database Syst Rev. 2001;4:CD000094. [DOI] [PubMed] [Google Scholar]
- 10. Zhu Y, Yang J, Jiao S, Ji T. Ganglioside-monosialic acid (GM1) prevents oxaliplatin-induced peripheral neurotoxicity in patients with gastrointestinal tumors. World J Surg Oncol. 2013;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schneider JS, Sendek S, Daskalakis C, Cambi F. GM1 ganglioside in Parkinson’s disease: results of a five year open study. J Neurol Sci. 2010;292(1–2):45–51. [DOI] [PubMed] [Google Scholar]
- 12. McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–425. [DOI] [PubMed] [Google Scholar]
- 13. Ganglioside GM1 in acute ischemic stroke. The SASS Trial. Stroke. 1994;25(6):1141–1148. [DOI] [PubMed] [Google Scholar]
- 14. Rong X, Zhou W, Xiao-Wen C, Tao L, Tang J. Ganglioside GM1 reduces white matter damage in neonatal rats. Acta Neurobiol Exp (Wars). 2013;73(3):379–386. [DOI] [PubMed] [Google Scholar]
- 15. Li L, Wang T. Ganglioside GM1 for ischemic stroke: an update (2005-2015). Pulmonary Critical Care Med. 2016;1(1):23–24. [Google Scholar]
- 16. Al Mamun A, Yu H, Romana S, Liu F. Inflammatory responses are sex specific in chronic hypoxic-ischemic encephalopathy. Cell Transplant. 2018;1:963689718766362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang YP, Huang QL, Zhao CM, Tang JL, Wang YL. GM1 improves neurofascin155 association with lipid rafts and prevents rat brain myelin injury after hypoxia-ischemia. Braz J Med Biol Res. 2011;44(6):553–561. [DOI] [PubMed] [Google Scholar]
- 18. Tang B, Wang D, Li M, Wu Q, Yang Q, Shi W, Chen C. An in vivo study of hypoxia-inducible factor-1alpha signaling in ginsenoside Rg1-mediated brain repair after hypoxia/ischemia brain injury. Pediatr Res. 2017;81(1–1):120–126. [DOI] [PubMed] [Google Scholar]
- 19. Whitehead SN, Chan KH, Gangaraju S, Slinn J, Li J, Hou ST. Imaging mass spectrometry detection of gangliosides species in the mouse brain following transient focal cerebral ischemia and long-term recovery. PLoS One. 2011;6(6):e20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caughlin S, Hepburn JD, Park DH, Jurcic K, Yeung KK, Cechetto DF, Whitehead SN. Increased expression of simple ganglioside species GM2 and GM3 detected by MALDI imaging mass spectrometry in a combined rat model of abeta toxicity and stroke. PLoS One. 2015;10(6):e0130364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwak DH, Kim SM, Lee DH, Kim JS, Lee SU, Jung KY, Seo BB, Choo YK. Differential expression patterns of gangliosides in the ischemic cerebral cortex produced by middle cerebral artery occlusion. Mol Cells. 2005;20(3):354–360. [PubMed] [Google Scholar]
- 22. Yigitkanli K, Zheng Y, Pekcec A, Lo EH, van Leyen K. Increased 12/15-lipoxygenase leads to widespread brain injury following global cerebral ischemia. Transl Stroke Res. 2017;8(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakata M, Nakagomi T, Maeda M, Nakano-Doi A, Momota Y, Matsuyama T. Induction of perivascular neural stem cells and possible contribution to neurogenesis following transient brain ischemia/reperfusion injury. Transl Stroke Res. 2017;8(2):131–143. [DOI] [PubMed] [Google Scholar]
- 24. Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8(2):107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao Z, Wang L, Wu X, Zhao L, Chi C, Guo L, Tong D, Yang X, Dong Z, Deng R, Novakovic VA, Thatte HS, Bi Y, Tian Y, Shi J, Zhou J, Kou J, Hu S. Enhanced procoagulant activity on blood cells after acute ischemic stroke. Transl Stroke Res. 2017;8(1):83–91. [DOI] [PubMed] [Google Scholar]
- 26. Pischiutta F, Sammali E, Parolini O, Carswell HVO, Zanier ER. Placenta-derived cells for acute brain injury. Cell Transplant. 2018;27(1):151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn JH, Chen BH, Shin BN, Cho JH, Kim IH, Park JH, Lee JC, Tae HJ, Lee YL, Lee J, Byun K, Jeong GB, Lee B, Kim SU, Kim YM, Won MH, Choi SY. Intravenously infused F3.olig2 improves memory deficits via restoring myelination in the aged hippocampus following experimental ischemic stroke. Cell Transplant. 2016;25(12):2129–2144. [DOI] [PubMed] [Google Scholar]
- 28. Gautam J, Yao Y. Roles of pericytes in stroke pathogenesis. Cell Transplant. 2018;1:963689718768455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hocum Stone LL, Xiao F, Rotschafer J, Nan Z, Juliano M, Sanberg CD, Sanberg PR, Kuzmin-Nichols N, Grande A, Cheeran MC, Low WC. Amelioration of ischemic brain injury in rats with human umbilical cord blood stem cells: mechanisms of action. Cell Transplant. 2016;25(8):1473–1488. [DOI] [PubMed] [Google Scholar]
- 30. Yamashita T, Abe K. Recent progress in therapeutic strategies for ischemic stroke. Cell Transplant. 2016;25(5):893–898. [DOI] [PubMed] [Google Scholar]
- 31. Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. [DOI] [PubMed] [Google Scholar]
- 32. Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11(7):290–296. [DOI] [PubMed] [Google Scholar]
- 33. Liu JR, Ding MP, Wei EQ, Luo JH, Song Y, Huang JZ, Ge QF, Hu H, Zhu LJ. GM1 stabilizes expression of NMDA receptor subunit 1 in the ischemic hemisphere of MCAo/reperfusion rat. J Zhejiang Univ Sci B. 2005;6(4):254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Fang X, Zhou Y, Deng X, Lu Y, Li J, Li S, Wang B, Xu R. The possible damaged mechanism and the preventive effect of monosialotetrahexosylganglioside in a rat model of cerebral ischemia-reperfusion injury. J Stroke Cerebrovasc Dis. 2015;24(7):1471–1478. [DOI] [PubMed] [Google Scholar]
- 35. Su D, Ma J, Yang J, Kang Y, Lv M, Li Y. Monosialotetrahexosy-1 ganglioside attenuates diabetes-associated cerebral ischemia/reperfusion injury through suppression of the endoplasmic reticulum stress-induced apoptosis. J Clin Neurosci. 2017;41:54–59. [DOI] [PubMed] [Google Scholar]
- 36. Li L, Tian J, Long MK, Chen Y, Lu J, Zhou C, Wang T. Protection against experimental stroke by ganglioside GM1 is associated with the inhibition of autophagy. PLoS One. 2016;11(1):e0144219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan X, Jiang Y, Yu Z, Liu Q, Guo S, Sun X, van Leyen K, Ning M, Gao X, Lo EH, Wang X. Annexin A2 plus low-dose tissue plasminogen activator combination attenuates cerebrovascular dysfunction after focal embolic stroke of rats. Transl Stroke Res. 2017;8(6):549–559. [DOI] [PubMed] [Google Scholar]
- 38. Ji B, Zhou F, Han L, Yang J, Fan H, Li S, Li J, Zhang X, Wang X, Chen X, Yun X. Sodium Tanshinone IIA sulfonate enhances effectiveness Rt-PA treatment in acute ischemic stroke patients associated with ameliorating blood-brain barrier damage. Transl Stroke Res. 2017;8(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lapchak PA, Lara JM, Boitano PD. Cytoprotective drug-tissue plasminogen activator protease interaction assays: screening of two novel cytoprotective chromones. Transl Stroke Res. 2017;8(5):494–506. [DOI] [PubMed] [Google Scholar]
- 40. Jeanneret V, Yepes M. The plasminogen activation system promotes dendritic spine recovery and improvement in neurological function after an ischemic stroke. Transl Stroke Res. 2017;8(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoo J, Seo JJ, Eom JH, Hwang DY. Enhanced recovery from chronic ischemic injury by bone marrow cells in a rat model of ischemic stroke. Cell Transplant. 2015;24(2):167–182. [DOI] [PubMed] [Google Scholar]
- 42. Favaron M, Manev H, Alho H, Bertolino M, Ferret B, Guidotti A, Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci U S A. 1988;85(19):7351–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ledeen RW. Biology of gangliosides: neuritogenic and neuronotrophic properties. J Neurosci Res. 1984;12(2–3):147–159. [DOI] [PubMed] [Google Scholar]
- 44. Schneider JS, Gollomp SM, Sendek S, Colcher A, Cambi F, Du W. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J Neurol Sci. 2013;324(1–2):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Svennerholm L, Brane G, Karlsson I, Lekman A, Ramstrom I, Wikkelso C. Alzheimer disease – effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement Geriatr Cogn Disord. 2002;14(3):128–136. [DOI] [PubMed] [Google Scholar]