Abstract
To analyze the size and location distribution of ruptured intracranial aneurysms (IAs) helps to provide evidence for clinical treatment of unruptured IAs using this feature of aneurysms. In this study, 415 patients who presented with an acute subarachnoid hemorrhage caused by IAs were enrolled from eight tertiary referral centers between June 2016 and March 2018. The size, aspect ratio and anatomic location of ruptured IAs were defined and reported by patient sex. In the study cohort of 415 patients (60.5% women) with saccular ruptured IAs, the three most common locations of ruptured IAs were posterior communicating artery (32.0%), anterior communicating artery (28.7%), and middle cerebral artery (13.5%). The mean size of all ruptured IAs was 5.3±3.1 mm (range 1.1–28.5 mm), but the size varied considerably by location. For example, ruptured IAs of the posterior communicating artery had a mean size of 5.8±3.1 mm, whereas the mean size of ruptured anterior communicating artery aneurysms was 4.6±1.7 mm. The mean AR in all ruptured IAs was 1.66±0.76. Of those aneurysms, 243 (58.6%) had an AR smaller than 1.6 and 318 (76.6%) had an AR smaller than 2.0. Our results suggested that the size of the most ruptured IAs are smaller than 7 mm or even 5 mm. The size and AR varied by sex and location. With the knowledge of size, location and AR, multiplicity should be considered for treatment strategies of unruptured IAs.
Keywords: Intracranial aneurysm, size, location, aspect ratio
Introduction
The annual incidence of aneurysmal subarachnoid hemorrhage (aSAH) is estimated at 7–9 per 100,000 person-years, which results in a high mortality and morbidity1–4. Recent advances in neuroradiological techniques have further increased the detection rate of intracranial aneurysms (IAs), especially in small aneurysms and tiny aneurysms. However, most IAs remain asymptomatic for years or even lifelong. Therefore, it is crucial to identify the risk factors associated with IA rupture as much as possible for clinicians to make treatment decisions.
From previous studies, the most ubiquitous parameter to estimate future aneurysm rupture is size and location. Although the International Study of Unruptured Intracranial Aneurysms demonstrated aneurysms exceeding 7 mm in size have a very low risk of rupture5, a number of large case series have shown that most ruptured IAs presented with sizes <7 mm or even ≤5 mm6,7. Aneurysm location is also an important factor in the risk of rupture. In a consecutive series of 1993 patients with ruptured IAs diagnosed between 1937 and 2013 in Helsinki University Hospital, the rupture rate of the middle cerebral artery aneurysms was the highest, followed by anterior communicating artery aneurysms; however, the rupture risk of ophthalmic artery aneurysms and superior cerebellar artery aneurysms were the lowest8. Although many studies have reported the morphological factors of the ruptured IAs, most of these data are obtained from a single-center, which cannot fully explain the factors affecting the rupture of the aneurysms. In this study, we performed a prospective analysis of the size, aspect ratio (AR), and anatomical location of ruptured IAs in a consecutive series of 415 patients from a multicenter ruptured aneurysm study (AMRAS). We aimed to provide evidence for clinical treatment of unruptured IAs using this feature of aneurysms.
Materials and Methods
Study Cohort
AMRAS was a prospective, multicenter, observational registry of patients who presented with aSAH. Ethical approval to report this case was obtained from Huashan Hospital, Fudan University, Shanghai, China (2017253). All procedures in this study were conducted in accordance with the ethics committee of Huashan Hospital’s approved protocols. Written informed consent was obtained from the patients for their anonymized information to be published in this article. From June 2016 to March 2018, 415 patients who presented with an acute subarachnoid hemorrhage (SAH) caused by IA were enrolled from eight tertiary referral centers. SAH was diagnosed by non-contrast head computed tomography (CT) or cerebrospinal fluid. Once diagnosed as IAs, digital subtraction angiography (DSA) was performed to obtain the morphology parameters of the aneurysms. The size, AR value, and location of ruptured IAs were evaluated by one neurosurgeon and one radiology physician. In patients presenting with multiple aneurysms, the ruptured aneurysm was judged by aneurysm morphology, and/or location, and specific blood distribution on CT scan. The exclusion criteria in this study were as follows: patients presented with non-saccular IAs, such as fusiform aneurysms, dissection aneurysms, and pseudoaneurysms; the interval time between initial SAH and admission to hospital was more than 14 days; patients refused to be enrolled in this study.
Aneurysm size was defined as the maximum distance of the dome from the aneurysm neck plane9. The AR was calculated as the ratio of the maximum perpendicular height to the neck width, which has been reported in previous literature10. Locations of ruptured IAs were classified as follows: (1) posterior communicating artery (PcomA); (2) anterior communicating artery (AcomA); (3) middle cerebral artery (MCA); (4) internal carotid artery (ICA), including anterior choroidal artery, ICA bifurcation, supraclinoid ICA, and ophthalmic artery; (5) anterior cerebral artery (ACA); (6) basilar artery (BA); (7) posterior cerebral artery (PCA); (8) posterior inferior cerebellar artery (PICA); and (9) others, including: pericallosal artery, anterior inferior cerebellar artery, vertebral artery.
Patient sex, age, hypertension, and Glasgow Coma Scale (GCS) score and Fisher grades were recorded. Informed written consent was obtained before DSA treatment and included data collection for research purposes. SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) software was used for statistical analysis. Continuous variables (age, aneurysm size, AR value) and categorical variables (hypertension, GCS score, Fisher grades, aneurysm location) were reported as mean±SD and frequency/percentage, respectively.
Result
Patient Characteristics
A total of 415 consecutive patients (251women, 60.5%) aged 56.2±12.2 years identified as ruptured IAs were enrolled in this study. Baseline characteristics of the patients are presented in Table 1. For women, the median age on admission was 58.0 years (mean 58.0±11.9 years, range 17–88 years). For men, the median age was 53.0 years (mean 53.4±12.1 years, range 19–85 years). Of 415 patients, 183 (44.1%) presented with a history of hypertension, 102 (24.6%) presented with a history of drinking, and 78 (18.8%) presented with a history of smoking (Table 1).
Table 1.
Patient Characteristics.
| Characteristics | All Pts | Women | Men |
|---|---|---|---|
| Age (years) | 56.2 ± 12.2 | 58.0 ± 11.9 | 53.4 ± 12.1 |
| Current smoking | 78 (18.8%) | 6 (2.4%) | 72 (43.9%) |
| Current drinking | 102 (24.6%) | 23 (9.2%) | 79 (48.2%) |
| Hypertension | 183 (44.1%) | 118 (47.0%) | 65 (39.6%) |
| Clinical condition | |||
| GCS 3-6 | 35 (8.4%) | 23 (9.2%) | 12 (7.3%) |
| GCS 7-10 | 46 (11.1%) | 28 (11.2%) | 18 (11.0%) |
| GCS 11-15 | 334 (84.5%) | 200 (79.6%) | 134 (81.7%) |
| mFisher grade I | 63 (15.2%) | 45 (17.9%) | 18 (11.0%) |
| mFisher grade II | 201 (48.4%) | 119 (47.4%) | 82 (50.0%) |
| mFisher grade III | 84 (20.2%) | 51 (20.3%) | 33 (20.1%) |
| mFisher grade IV | 67 (16.2%) | 36 (14.4%) | 31 (18.9%) |
| Total | 415 | 251 | 164 |
Pts: patients; GCS: Glasgow Coma Scale
Location of Ruptured IAs
In the anterior circulation, ruptured IAs of the PcomA (32.0%), AcomA (28.7%), MCA (13.5%), ICA (10.9%), and ACA (4.3%) accounted for 89.4% of all saccular ruptured IAs. In the posterior circulation, the BA was the most common location of ruptured IAs, accounting for 3.9% of all IAs, followed by the PCA (2.4%) and PICA (1.9%). Women with ruptured IAs presented most often (39.8%) with ruptured PcomA aneurysms, whereas most (37.2%) men presented with ruptured AcomA aneurysms. The distribution of the MCA aneurysms in women (12.7%) and men (14.6) was similar. Of the ACA aneurysms, 5.6% and 2.5% were found in women and men, respectively (Table 2).
Table 2.
Anatomical Locations of 415 Ruptured Intracranial Aneurysms.
| Location | No. of ruptured IAs | Mean age in years | ||||
|---|---|---|---|---|---|---|
| All Pts (%) | Women (%) | Men (%) | All Pts | Women | Men | |
| PcomA | 133 (32.0) | 100 (39.8) | 33 (20.1) | 58.3 ± 12.3 | 58.8 ± 12.5 | 56.6 ± 11.6 |
| AcomA | 119 (28.7) | 58 (23.1) | 61 (37.1) | 55.5 ± 11.5 | 59.2 ± 11.4 | 51.9 ± 11.3 |
| MCA | 56 (13.5) | 32 (12.7) | 24 (14.6) | 55.6 ± 11.8 | 57.0 ± 11.0 | 53.7 ± 12.9 |
| ICA | 45 (10.9) | 24 (9.6) | 21 (12.8) | 50.7 ± 12.0 | 52.9 ± 11.3 | 40.8 ± 12.6 |
| ACA | 18 (4.3) | 14 (5.6) | 4 (2.5) | 55.9 ± 9.9 | 54.6 ± 10.5 | 60.3 ± 6.9 |
| BA | 16 (3.9) | 10 (4.0) | 6 (3.6) | 60.2 ± 11.8 | 62.8 ± 9.7 | 55.8 ± 14.6 |
| PCA | 10 (2.4) | 6 (2.4) | 4 (2.5) | 51.6 ± 18.3 | 58.3 ± 19.1 | 41.5 ± 13.1 |
| PICA | 8 (1.9) | 4 (1.6) | 4 (2.5) | 61.4 ± 13.7 | 51.8 ± 7.4 | 71.0 ± 11.5 |
| Others | 10 (2.4) | 3 (1.2) | 7 (4.2) | 58.3 ± 11.4 | 62.3 ± 15.3 | 56.6 ± 10.3 |
| Total | 415 (100) | 251 (100) | 164 (100) | 56.2 ± 12.2 | 57.8 ± 11.9 | 53.4 ± 12.0 |
IA: intracranial aneurysm; Pts: patients; PcomA: posterior communicating artery; AcomA: anterior communicating artery; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; BA: basilar artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar artery
The mean ages of women with ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms were 58.8±12.5, 59.2±11.4, and 57.0±11.0 years, respectively. The mean ages of men with ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms were 56.6±11.6, 51.9±11.3, and 53.7±12.9 years, respectively. It seems possible that patients with ICA ruptured IAs were somewhat younger (mean age of 50.7±12.0 years) than patients with ruptured IAs in other locations. Moreover, the mean age of patients with ruptured PICA aneurysms appeared exceptionally high (mean 61.4±13.7) in our study (Table 2).
Size of Ruptured IAs
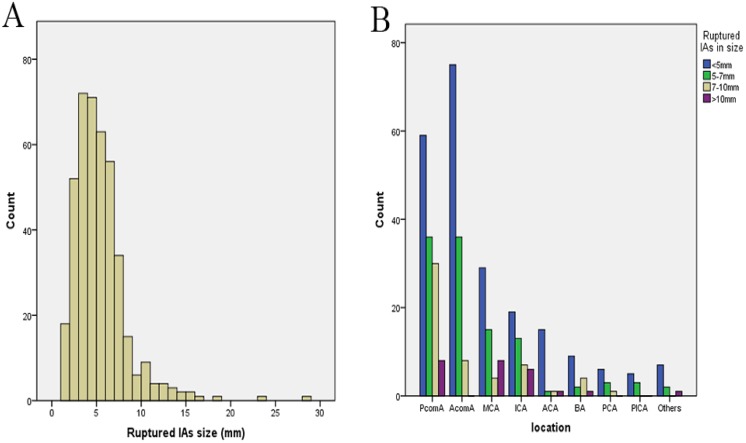
The mean size of all ruptured IAs was 5.3±3.1 mm (range 1.1–28.5 mm) (Fig. 1A). Of those aneurysms, 224 (54.0%) were smaller than 5 mm, 335 (80.7%) were smaller than 7 mm, and 390 (94.0%) were smaller than 10 mm. The mean size of ruptured PcomA aneurysms was 5.8±3.1 mm (range 1.1–23.1 mm). Of those aneurysms, 59 (44.4%) were smaller than 5 mm, 95 (71.4%) were smaller than 7 mm, and 125 (94%) were smaller than 10 mm. The mean size of AcomA aneurysms was 4.6±1.7 mm (range 1.1–10 mm) (Fig. 1B). Of those aneurysms, 75 (63%) were smaller than 5 mm, 111 (93.3%) were smaller than 7 mm, and 117 (98.3%) were smaller than 10 mm. The mean size of ruptured MCA aneurysms was 5.6±3.3 mm (range 1.5–15.3 mm). Of those aneurysms, 29 (51.2%) were smaller than 5 mm, 44 (78.6%) were smaller than 7 mm, and 48 (85.7%) were smaller than 10 mm (Table 3).
Fig. 1.
Size (A) and location distribution (B) of ruptured intracranial aneurysms. IA: intracranial aneurysm; PcomA: posterior communicating artery; AcomA: anterior communicating artery; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; BA: basilar artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar artery.
Table 3.
Size of Ruptured Intracranial Aneurysms by Location.
| Location | Mean size in mm | No. of ruptured IAs by size (women/men) | ||||
|---|---|---|---|---|---|---|
| All Pts | Women | Men | <5mm | <7mm | <10mm | |
| PcomA | 5.8 ± 3.1 | 5.9 ± 3.3 | 5.6 ± 2.6 | 59 (43/16) | 95 (73/22) | 125 (94/31) |
| AcomA | 4.6 ± 1.7 | 4.4 ± 1.5 | 4.7 ± 1.8 | 75 (35/40) | 111 (57/54) | 117 (58/61) |
| MCA | 5.6 ± 3.3 | 5.6 ± 3.2 | 5.6 ± 3.5 | 29 (16/13) | 44 (25/19) | 48 (28/20) |
| ICA | 6.3 ± 4.8 | 6.4 ± 5.3 | 6.2 ± 4.3 | 19 (9/10) | 32 (18/14) | 39 (22/17) |
| ACA | 4.1 ± 2.0 | 4.4 ± 2.2 | 3.0 ± 1.2 | 15 (11/4) | 16 (12/4) | 17 (13/4) |
| BA | 5.5 ± 2.9 | 5.7 ± 3.2 | 5.3 ± 2.6 | 9 (6/3) | 11 (7/4) | 15 (9/6) |
| PCA | 4.6 ± 1.9 | 4.4 ± 1.9 | 4.9 ± 2.1 | 6 (4/2) | 9 (5/4) | 10 (6/4) |
| PICA | 3.9 ± 1.6 | 3.9 ± 2.2 | 4.0 ± 1.1 | 5 (2/3) | 8 (4/4) | 8 (4/4) |
| Others | 5.1 ± 2.9 | 7.9 ± 5.7 | 3.8 ± 1.8 | 7 (2/5) | 9 (2/7) | 9 (2/7) |
| Total | 5.3 ± 3.1 | 5.4 ± 3.2 | 5.1 ± 2.7 | 224 (128/96) | 335 (203/132) | 390 (236/154) |
IA: intracranial aneurysm; Pts: patients; PcomA: posterior communicating artery; AcomA: anterior communicating artery; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; BA: basilar artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar artery
Classified by patient sex, the mean size of ruptured IAs was 5.4±3.2 mm in women and 5.1±2.7 mm in men. In women, the mean size of ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms was 5.9 ±3.3 mm (range 1.1–23.1 mm), 4.4 ±1.5 mm (range 1.1–7.3 mm), and 5.6 ±3.2 mm (range 1.5–15.3 mm), respectively. In particular, the mean size of ruptured IAs was 23.23±7.40 and 5.32±3.02 in patients younger than 18 years old and older than 18 years old, respectively. In men, the mean size of ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms was 5.6±2.6 mm (range 1.1–10.8 mm), 4.7 ±1.8 mm (range 1.3–10 mm), and 5.6 ±3.5 mm (range 2–13.1 mm), respectively (Table 3).
AR of Ruptured IAs
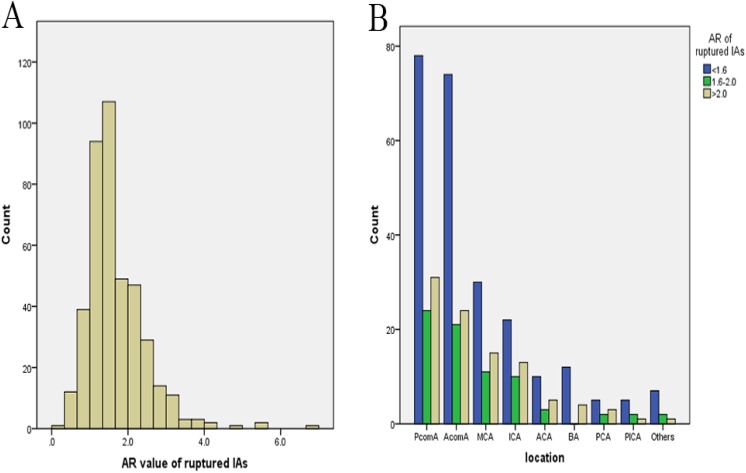
The mean AR was 1.66±0.76 in 415 ruptured IAs (Fig. 2A). Of those aneurysms, 243 (58.6%) had an AR smaller than 1.6 and 318 (76.6%) had an AR smaller than 2.0. The mean AR was 1.63±0.64 in 133 ruptured PcomA aneurysms. Of those aneurysms, 78 (58.6%) had an AR smaller than 1.6 and 102 (76.7%) had an AR smaller than 2.0. The mean AR was 1.59±0.61 in AcomA aneurysms (Fig. 2B). Of those aneurysms, 74 (62.2%) had an AR smaller than 1.6 and 95 (79.8%) had an AR smaller than 2.0. The mean AR was 1.68±0.76 in ruptured MCA aneurysms. Of those aneurysms, 30 (53.6%) had an AR smaller than 1.6 and 41 (73.2%) had an AR smaller than 2.0 (Table 4).
Fig. 2.
Aspect ratio value (A) and location distribution (B) of ruptured intracranial aneurysms. AR: aspect ratio; PcomA: posterior communicating artery; AcomA: anterior communicating artery; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; BA: basilar artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar arteryPts: patients; GCS: Glasgow Coma Scale.
Table 4.
Aspect Ratio Value of Ruptured Intracranial Aneurysms by Location.
| Location | Mean AR value | No. of ruptured IAs by AR value (women/men) | |||
|---|---|---|---|---|---|
| All Pts | Women | Men | <1.6 | <2.0 | |
| PcomA | 1.63 ± 0.64 | 1.67 ± 0.64 | 1.50 ± 0.65 | 78 (56/22) | 102 (76/26) |
| AcomA | 1.59 ± 0.61 | 1.61 ± 0.63 | 1.57 ± 0.60 | 74 (37/37) | 95 (44/51) |
| MCA | 1.68 ± 0.76 | 1.70 ± 0.84 | 1.65 ± 0.66 | 30 (18/12) | 41 (23/18) |
| ICA | 1.74 ± 0.95 | 1.65 ± 0.72 | 1.84 ± 1.2 | 22 (12/10) | 23 (12/11) |
| ACA | 2.03 ± 1.27 | 2.10 ± 1.24 | 1.76 ± 1.50 | 10 (7/3) | 13 (10/3) |
| BA | 1.63 ± 0.62 | 1.60 ± 0.61 | 1.68 ± 0.67 | 12 (8/4) | 12 (8/4) |
| PCA | 2.17 ± 1.76 | 1.74 ± 0.92 | 2.81 ± 2.65 | 5 (4/1) | 7 (5/2) |
| PICA | 1.61 ± 0.56 | 1.78 ± 0.79 | 1.44 ± 0.20 | 5 (2/3) | 7 (3/4) |
| Others | 1.42 ± 0.32 | 1.67 ± 0.37 | 1.31 ± 0.25 | 7 (2/5) | 9 (2/7) |
| Total | 1.66 ± 0.76 | 1.68 ± 0.72 | 1.63 ± 0.83 | 243 (146/97) | 318 (187/131) |
AR: aspect ratio; IA: intracranial aneurysm; Pts: patients; PcomA: posterior communicating artery; AcomA: anterior communicating artery; MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; BA: basilar artery; PCA: posterior cerebral artery; PICA: posterior inferior cerebellar artery
Classified by patient sex, the mean AR in ruptured IAs was 1.68±0.72 in women and 1.63±0.83 in men. In women, the mean AR in ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms was 1.67±0.64, 1.61±0.63, and 1.70±0.84, respectively. Especially, the mean AR of ruptured IAs was 3.19±1.14 and 1.66±0.76 in patients younger than 18 years old and older than 18 years old, respectively. In men, the mean AR in ruptured PcomA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms was 1.50±0.65, 1.57±0.60, and 1.65±0.66, respectively (Table 4).
Classified by clinical factors, the mean AR was 1.58±0.61 in patients with hypertension history and 1.73±0.86 in patients without hypertension history. The mean AR was 1.64±0.98 in patients with smoking history and 1.66±0.70 in patients without smoking history. The mean AR was 1.60±0.76 in patients with drinking history and 1.68±0.76 in patients without drinking history.
Treatment Strategy
In our study, 249 patients with ruptured IAs underwent endovascular treatment, 156 patients underwent surgical treatment, and 10 patients underwent conservative treatment. Of 249 aneurysms that underwent endovascular treatment, 201 were treated with coiling alone and 48 were treated with stent-assisting coiling. The procedure-related complication rates for all 405 procedures was 5.12% in the surgical treatment group and 4.42% in the endovascular treatment group. Among patients undergoing endovascular treatment, good clinical outcome (Glasgow Outcome Scale 5) was obtained in 226 (90.7%) at discharge. However, good clinical outcome was obtained in 136 patients (87.2%) treated with surgical clipping. Of 10 patients who underwent conservative treatment, eight had clipping or coiling performed one month after SAH, one died of rehemorrhagia, and one referred to continuous conservative treatment without rehemorrhagia at the day of discharge.
Discussion
Although many studies have demonstrated that the overall size threshold in ruptured IAs was more than 7 mm, the average diameter of ruptured IAs was 5.3±3.1 mm in our study. At the same time, classified by patient sex, the mean size of ruptured IAs was 5.4±3.2 mm in women and 5.1±2.7 mm in men. Additionally, our study suggested that this threshold varied with the site of the aneurysms, which is shown in the Table 3. For ruptured IAs located on distal vessels, such as distal ACA and PICA, the mean size was smaller than at other sites. Carter et al. suggested aneurysms arising from distal vessels have thinner walls, which are prone to rupture under a given pressure11. Therefore, it is not feasible to use a given aneurysm size as an indicator to predict aneurysm rupture. In our study, 54.0% (224) of ruptured IAs were smaller than 5 mm in maximum diameter, 80.7% (335) were smaller than 7 mm in maximum diameter, 94.0% (390) were smaller than 10 mm in maximum diameter. Although ethnic factors were not taken into consideration in our study, unlike many studies from Europe and the United States, our findings showed that the size of ruptured aneurysms was much smaller than that in previous reports. Recently, 1993 ruptured IAs were reported in Korja and colleagues’ study and the median size of all ruptured IAs was 7 mm. They further found that ophthalmic artery ruptured IAs had the largest median size (11 mm). The smallest median size (6 mm) was detected for PICA and pericallosal artery ruptured IAs8. In a study reported by Hernesniemi et al. 15 years ago, they showed the mean maximum diameter of 1007 ruptured IAs was 11 mm, and about 20% were 15 mm or more12. Recent advances in neuroradiological techniques allowed more small or tiny ruptured aneurysms to be detected; in particular, the improvement of living standards was responsible for more people with ruptured tiny IAs going to the hospital. A recent study reported by Bharathi in 2014 demonstrated a mean size of 419 ruptured IAs as 5.7±3.8 mm, which is similar to our study13. Of 1620 consecutive ruptured aneurysms enrolled in a prospective observational cohort study between January 1998 and December 2014, more than half of the aneurysms were smaller than 7 mm14. In summary, we believe that defining 7 mm as a threshold to predict IA rupture cannot meet the precision of current medical requirements. In particular, the risk of IA rupture has certain ethnic and regional characteristics. Therefore, defining the location and size of saccular ruptured IAs in a country or even in a region is of great significance for clinical diagnosis and treatment.
Size ratio (SR) was first reported by Dhar as a parameter associated with the sizes of the aneurysm and parent artery15. For two aneurysms of the same size, a high SR indicates a small-sized parent artery. However, the threshold value of SR to predict aneurysm rupture was unknown, although several previous studies had reported a higher SR value being related to the rupture stature of IAs. Maryam et al. reported the ruptured IAs had significantly larger SR (4.08 ± 0.54 mm) than the unruptured group (2.57 ± 0.24 mm, p <0.01)16. However, Wang et al. reported that the threshold value of SR for predicting ruptured PcomA aneurysms was 1.2117. Therefore, it is still necessary to further accurately divide the SR threshold according to the site of the aneurysm. It is worth noting that vasospasm may affect the parent artery size, and then affect the accuracy of SR18. As vasospasm often occurs five days after SAH and lasts for 7–10 days, the result is different when SR values are measured at different time points after SAH19. Therefore, we believe that it is still very important to assess the size threshold of the ruptured IAs at different sites.
AR was first proposed by Ujiie et al. and was defined as aneurysm depth to aneurysm neck width. They further proposed that AR in 88% of ruptured aneurysms was higher than 1.6, while 56% of unruptured aneurysms had an AR lower than 1.610. In Weir’s study, the mean AR in 290 ruptured aneurysms was 3.4, while mean AR in 127 unruptured aneurysms was 1.820. In our study, the mean AR in 415 ruptured IAs was 1.66±0.76. Classified by patient sex, the mean AR of ruptured IAs was 1.68±0.72 in women and 1.63±0.83 in men. Furthermore, 58.6% (243) of ruptured IAs were smaller than 1.6 in AR value, 76.6% (318) were smaller than 2.0 in AR value. Although previous studies had shown that a high AR was related to IA rupture, the mean AR value changed according to the location of IAs. Wang et al. reported that the threshold value of AR for predicting ruptured PComA aneurysms was 0.9817. However, there are very few studies reporting a different AR threshold to predict aneurysm rupture for each site. In our study, the mean AR in ruptured PComA aneurysms, ruptured AcomA aneurysms, and ruptured MCA aneurysms was 1.63±0.64, 1.59±0.61, and 1.68±0.76, respectively. In general, the AR value of ruptured IAs in anterior circulation was smaller than those in posterior circulation. Aneurysmal geometry and hemodynamics are mutually causal. Aneurysm morphology determines the specific flow conditions in the aneurysm sac, while blood flow drives aneurysm remodeling/growth, leading to the formation of aneurysm morphology21. Qiu et al. reported that ruptured IAs with a high AR were statistically more likely to have a greater low wall shear stress (WSS) area ratio than unruptured aneurysms. The blood flow in intra-aneurysm was slower as the aneurysm neck was narrow. Furthermore, no blood flow was detected in the dome of dumbbell-shaped aneurysms and daughter aneurysms22. Localized stagnation of blood flow against the wall in the aneurysm dome led to the dysfunction of the endothelium, which is consistent with the low flow theory23. The disruption and dysfunction of endothelial cells caused by non-physiological WSS is the important reason for IA formation and rupture24–27. Therefore, a higher AR means smaller neck and slower intra-aneurysmal blood flow, which reflects a higher risk of rupture.
There are several limitations in our study. First, the IA sizes were measured after IA rupture. Whether IA size changed with rupture was not clear. As vasospasm often occurs after SAH, it is difficult to measure the “real” size of IAs at ruptured status. Second, all cases enrolled in our study were in the hospital, and those who died outside the hospital were not included in the study. Those who suddenly died away from a hospital might have larger aneurysms, as massive bleeding resulting from larger aneurysms could easily lead to sudden death. Excluding people with a larger or giant aneurysm dying outside hospitals may affect the results of size and location distributions of ruptured IAs. Third, apart from size, AR, and location, there are a large number of other factors related to the risk of aneurysm rupture, for example, aneurysm morphology (SR, depth/width ratio, flow angle), previous history of SAH, and use of aspirin, et cetera. This study was not designed to evaluate those risk factors for IA rupture.
Conclusion
In this prospective multicenter study, we reported the size, location, and AR value of ruptured IAs in 415 consecutive patients presenting with aSAH. Our results suggest that the size of most ruptured IAs is smaller than 7 mm, or even 5 mm, and that the size varies by sex and location. Meanwhile, 58.6% of ruptured IAs were smaller than 1.6 in AR value, 76.6% were smaller than 2.0 in AR value. With the knowledge of size, location, and AR value, multiplicity should be considered for treatment strategies of unruptured IAs.
Footnotes
Author Contribution: YZ: conception of the work, data collection, statistical analysis, drafting of manuscript, final approval. BZ, YS: statistical analysis, interpretation and discussion, final approval. HC, XF, PJ, HY: data collection, final approval. QA: interpretation of data, revision for critical and intellectual content, final approval. BL: conception of work, design of study, revision for critical and intellectual content, and final approval. YZ, BZ and XW contributed equally to this study.
Ethical Approval: This study was approved by Huashan Hospital, Fudan University, Shanghai, China (2017253).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the ethics committee of Huashan Hospital’s approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by grants (2016YFC0901003) from Ministry of Science and Technology of China.
References
- 1. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–636. [DOI] [PubMed] [Google Scholar]
- 2. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360(9342):1267–1274. [DOI] [PubMed] [Google Scholar]
- 3. Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. [DOI] [PubMed] [Google Scholar]
- 4. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93(3):379–387. [DOI] [PubMed] [Google Scholar]
- 5. Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC; International Study of Unruptured Intracranial Aneurysms Investigators. International study of unruptured intracranial aneurysms investigators. unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. [DOI] [PubMed] [Google Scholar]
- 6. Dolati P, Pittman D, Morrish WF, Wong J, Sutherland GR. The frequency of subarachnoid hemorrhage from very small cerebral aneurysms (<5 mm): a population-based study. Cureus. 2015;7(6):e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Froelich JJ, Neilson S, Peters-Wilke J, Dubey A, Thani N, Erasmus A, Carr MW, Hunn AW. Size and location of ruptured intracranial aneurysms: a 5-year clinical survey. World Neurosurg. 2016;91:260–265. [DOI] [PubMed] [Google Scholar]
- 8. Korja M, Kivisaari R, Rezai Jahromi B, Lehto H. Size and location of ruptured intracranial aneurysms: consecutive series of 1993 hospital-admitted patients. J Neurosurg. 2017;127(4):748–753. [DOI] [PubMed] [Google Scholar]
- 9. Raghavan ML, Ma B, Harbaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg. 2005;102(2):355–362. [DOI] [PubMed] [Google Scholar]
- 10. Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 2001;48(3):495–503. [DOI] [PubMed] [Google Scholar]
- 11. Carter BS, Sheth S, Chang E, Sethl M, Ogilvy CS. Epidemiology of the size distribution of intracranial bifurcation aneurysms: smaller size of distal aneurysms and increasing size of unruptured aneurysms with age. Neurosurgery. 2006;58(2):217–223. [DOI] [PubMed] [Google Scholar]
- 12. Hernesniemi J, Vapalahti M, Niskanen M, Tapaninaho A, Kari A, Luukkonen M, Puranen M, Saari T, Rajpar M. One-year outcome in early aneurysm surgery: a 14 years experience. Acta Neurochir (Wien). 1993;122(1-2):1–10. [DOI] [PubMed] [Google Scholar]
- 13. Jagadeesan BD, Delgado Almandoz JE, Kadkhodayan Y, Derdeyn CP, Cross DT, 3rd, Chicoine MR, Rich KM, Zipfel GJ, Dacey RG, Moran CJ. Size and anatomic location of ruptured intracranial aneurysms in patients with single and multiple aneurysms: a retrospective study from a single center. J Neurointerv Surg. 2014;6(3):169–174. [DOI] [PubMed] [Google Scholar]
- 14. van Donkelaar CE, Bakker NA, Veeger NJ, Uyttenboogaart M, Metzemaekers JD, Eshghi O, Mazuri A, Foumani M, Luijckx GJ, Groen RJ, van Dijk JM. Prediction of outcome after subarachnoid hemorrhage: timing of clinical assessment. J Neurosurg. 2017;126(1):52–59. [DOI] [PubMed] [Google Scholar]
- 15. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, Hopkins LN, Meng H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008;63(2):185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A, Levy EI, Meng H, Mocco J. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke. 2010;41(5):916–920. [DOI] [PubMed] [Google Scholar]
- 17. Wang GX, Liu J, Chen YQ, Wen L, Yang MG, Gong MF, Zhang D. Morphological characteristics associated with the rupture risk of mirror posterior communicating artery aneurysms. J Neurointerv Surg. 2018;10(10):995–998. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Jing L, Liu J, Li C, Fan J, Wang S, Li H, Yang X. Clinical, morphological, and hemodynamic independent characteristic factors for rupture of posterior communicating artery aneurysms. J Neurointerv Surg. 2016;8(8):808–812. [DOI] [PubMed] [Google Scholar]
- 19. Beck J, Rohde S, el Beltagy M, Zimmermann M, Berkefeld J, Seifert V, Raabe A. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir (Wien). 2003;145(10):861–865. [DOI] [PubMed] [Google Scholar]
- 20. Weir B, Amidei C, Kongable G, Findlay JM, Kassell NF, Kelly J, Dai L, Karrison TG. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99(3):447–451. [DOI] [PubMed] [Google Scholar]
- 21. Duan Z, Li Y, Guan S, Ma C, Han Y, Ren X, Wei L, Li W, Lou J, Yang Z. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep. 2018;8(1):6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu T, Jin G, Bao W, Lu H. Intercorrelations of morphology with hemodynamics in intracranial aneurysms in computational fluid dynamics. Neurosciences (Riyadh). 2017;22(3):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35(7):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meng H, Wang Z, Kim M, Ecker RD, Hopkins LN. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol. 2006;27(9):1861–1865. [PMC free article] [PubMed] [Google Scholar]
- 25. Xie J, Jones TJ, Feng D, Cook TG, Jester AA, Yi R, Jawed YT, Babbey C, March KL, Murphy MP. Human adipose-derived stem cells suppress elastase-induced murine abdominal aortic inflammation and aneurysm expansion through paracrine factors. Cell Transplant. 2017;26(2):173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaneko N, Mashiko T, Namba K, Tateshima S, Watanabe E, Kawai K. A patient-specific intracranial aneurysm model with endothelial lining: a novel in vitro approach to bridge the gap between biology and flow dynamics. J Neurointerv Surg. 2018;10(3):306–309. [DOI] [PubMed] [Google Scholar]
- 27. Baharoglu MI, Schirmer CM, Hoit DA, Gao BL, Malek AM. Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke. 2010;41(7):1423–1430. [DOI] [PubMed] [Google Scholar]