Abstract
Astrocytes protection and functional regulation are important strategies to protect against neuronal damage caused by ischemia. Activation of the delta opioid receptor (DOR) could reduce astrocytes damage, although the mechanism remains unclear. The present study aimed to test the effect of DOR activation on autophagy in astrocytes exposed to oxygen-glucose deprivation (OGD), and to further investigate whether this effect has a protective effect on astrocytes. Primary cultured rat cortical astrocytes were treated with various doses of [d-Ala2, d-Leu5]-Enkephalin (DADLE, a selective DOR agonist) followed by 6 h OGD. Cell viability was evaluated by CCK-8 assay and lactate dehydrogenase release. Autophagic vacuole was analyzed with LC3 immunofluorescent staining. The levels of autophagy and apoptosis-related proteins were analyzed by western blot. Results demonstrated that treatment with 10 nM DADLE was sufficient to increase cell viability and induced autophagy in astrocytes. The DADLE-induced autophagy displayed a cytoprotective effect on astrocytes. Inhibition of autophagy by 3-methyladenine (3-MA, an autophagy inhibitor) reversed the protective effect of DADLE. Naltrindole (a DOR antagonist) only partially antagonized the role of DADLE, which indicated that DADLE might have a cytoprotective mechanism independent of DOR. Further results showed that DADLE significantly enhanced the level of Bcl-2 protein and reduced the level of Bax protein in astrocytes exposed to OGD. Our results suggest a novel mechanism in which DADLE induces autophagy in astrocytes and exerts cytoprotective effects by inhibiting apoptosis.
Keywords: DOR, DADLE, ischemia, autophagy, OGD
Introduction
Stroke is one of the main reasons for death and disability in the world. Approximately 87% of these acute neurological diseases are ischemic and 13% are hemorrhagic1. The brain is very sensitive to ischemia. Only 5 min of blocking blood flow in the brain can lead to neuronal death2. Traditionally, ischemic injury has been viewed from the axiom of neuronal loss. However, more and more evidence indicates that astrocytes may play an important role in ischemic injury3. Astrocytes, which provide structural, trophic, and metabolic support to neurons, are the most abundant type of glial cells in the brain4. In addition to these physiological functions, astrocytes have neuroprotective effects in response to ischemic injury5. Astrocyte damage and dysfunction will jeopardize neuronal survival after ischemia6. Therefore, astrocyte protection and functional regulation are important strategies to protect against neuronal damage caused by ischemia.
Autophagy, which involves the massive degradation of organelles and cytoplasmic macromolecules in cells by the lysosomal system, is an important process for the turnover of intracellular substances7. Autophagy maintains a very low baseline level under normal physiological conditions and plays an important role in maintaining intracellular homeostasis8,9. In recent years, many experiments using ischemic, hypoxic animals, and cell models have confirmed that moderate activation of autophagy rather than overactivation can promote cell survival5,10–13.
In our previous study, activation of the delta opioid receptor (DOR) not only increased neuronal survival but also reduced astrocyte damage in a rat model of global cerebral ischemia14,15. However, the mechanism of activating DOR to protect astrocytes in the ischemic condition is not clear. Autophagy was slightly and continuously activated in primary cultured astrocytes exposed to oxygen-glucose deprivation (OGD). Inhibition of autophagy during OGD resulted in increased apoptosis of astrocytes13. A recent study showed that activation of DOR could protect against lipopolysaccharide (LPS)-induced myocardial damage by promoting autophagy16. In the present study, we aimed to test the effect of DOR activation on autophagy of astrocytes in the OGD model and further investigate whether this effect has a protective effect on astrocytes.
Materials and Methods
Chemicals and Reagents
[d-Ala2, d-Leu5]-Enkephalin (DADLE, a selective DOR agonist), 3-methyladenine (3-MA, an autophagy inhibitor) and antibody against LC3 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Naltrindole (a DOR antagonist) was obtained from Tocris (Ellisville, MO, USA). DMEM, FBS, 0.05% Trypsin-EDTA and normal goat serum were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8) kit, LDH kit, BCA kit, Cyanine 3 (Cy3)-conjugated secondary anti-rabbit antibodies, Alexa Fluor 488 goat anti-mouse IgG and Hoechst were obtained from Beyotime (Nantong, Jiangsu, China). PMSF (a protease inhibitor cocktail and a phosphatase inhibitor cocktail) was obtained from KangChen (Shanghai, China). Antibodies against beclin 1, p62 and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against glial fibrillary acidic protein (GFAP), Bcl-2, Bax and an anti-rabbit IgG antibody linked with HRP were obtained from Bioworld (Shanghai, China). An ECL kit was obtained from Millipore (Billerica, MA, USA).
Primary Culture of Rat Astrocytes
Primary culture of astrocytes was performed according to Juurlink17 and Kasproska’s13 methods. Newborn Wistar rats were purchased from the SLAC Animal Center (Shanghai, China). Briefly, the newborn Wistar rats were decapitated, the meninges were removed and the cerebral cortex was dissected. The cortex was dissociated mechanically in ice-cold DMEM containing 20% FBS and 1% penicillin-streptomycin. The cell suspension was filtered and inoculated in a tissue culture dish. The culture was incubated at 37°C, 95% air and 5% CO2, 95% relative humidity. After 3–4 days, the medium was replaced with medium containing 10% FBS. About 2 weeks later, the culture was shaken at 150 rpm for 3 h in an orbital shaker to remove other types of cells. The cells were further cultured for 2–3 days. Then the cells were trypsinized and reseeded on the plates. Astrocytes purity was assessed by staining with GFAP and Hoechst; ∼95% of cells are GFAP positive. All experimental procedures involving animals were performed in accordance with National Institutes of Health (NIH) guidelines and approved by the Animal Care and Use Committee of the Shanghai Jiaotong University.
OGD
OGD was performed as described previously13. Briefly, when cells reached approximately 80% confluency, the medium was replaced with DMEM free of glucose, FBS, and pyruvate. The astrocytes were then incubated in a hypoxic incubator chamber filled with a mixed composition of 94% N2/5% CO2/1% O2 for 6 h. For drug treatment, various concentrations of DADLE (1 nM, 10 nM, 100 nM, or 1 mM) were added at the beginning of OGD. Naltrindole (10 μM), 3-MA (1 mM) was added 15 min before DADLE, respectively.
Measurement of Cell Viability and Cytotoxicity
Cell viability was assessed by Cell Counting Kit-8 (CCK-8) assay. Astrocytes were cultured in a 96-well plate and underwent 6 h OGD. Then, CCK-8 reagent was added to each well. The culture was incubated at 37°C for 2 h. Optical density (OD) was measured at 450 nm using a Spectra Max M5 reader (Molecular Devices, Sunnyvale, CA, USA).
Cytotoxicity was measured by lactate dehydrogenase (LDH) assay. Samples of medium were prepared following the manufacturer’s protocol for the LDH assay. The LDH reagent was added to each well at room temperature for 30 min in the dark. Absorbance was read at 490 nm and 680 nm. To determine LDH activity, subtract the 680 nm absorbance value from the 490 nm absorbance.
Immunofluorescent Staining
The astrocytic monolayer was fixed for 1 h in 4% paraformaldehyde at room temperature. To detection of autophagy, cells were blocked with 10% normal goat serum with 0.2% Triton X-100 for 1 h. Cells were then incubated with primary antibody for LC-3 (1:200), GFAP (1:100) overnight at 4°C. After washing three times in 0.1 M PBS, sections were incubated with Cyanine 3 (Cy3)-conjugated secondary anti-rabbit antibodies or Alexa Fluor 488 goat anti-mouse IgG (1:500) for 2 h at room temperature. Subsequently, cells were washed three times with PBS and stained with Hoechst for 30 min at 37°C in the dark. Photomicrographs were taken under an epifluorescence microscope (Olympus, Tokyo, Japan).
Western Blot Analysis
Western blot analysis was performed as previously described18. Briefly, the cells were washed with cold PBS, harvested, and lysed in RIPA lysis buffer with 1 mM PMSF, then centrifuged at 14,000 g at 4°C for 5 min. Protein concentration was measured using a BCA kit. Equal amounts of protein were loaded and separated on SDS polyacrylamide gels. Then proteins were transferred to PVDF membranes and incubated overnight at 4°C with antibody against LC3 (1:500), Beclin 1 (1:500), p62 (1:500), Bcl-2 (1:200), Bax (1:200), and β-actin (1:1000). The membranes were incubated with secondary antibodies. The proteins were visualized using an ECL kit. The band intensity was detected using a Quantity One Gel Doc XR gel imaging system (Bio-Rad, Hercules, CA, USA). Relative protein levels of each band were normalized to β-actin.
Statistical Analysis
The data were expressed as mean ± SEM. Differences among groups were compared using a one-way ANOVA followed by LSD post hoc test. All results were repeated at least three times from independent experiments. P < 0.05 was considered to be statistically significant. Statistical analysis was performed using the GraphPad Prism 6 software (GraphPad Software Inc, La Jolla, CA, USA).
Results
Effects of DADLE on Cell Viability and LDH Release in Astrocytes
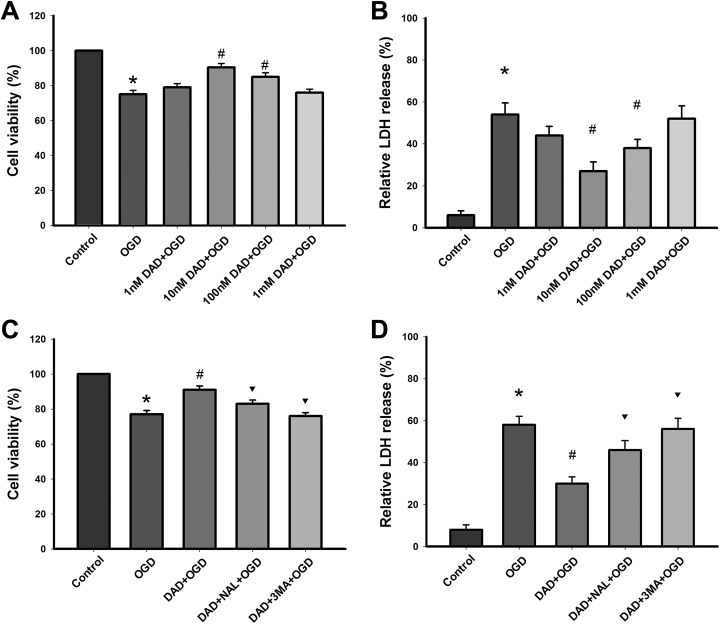
The protective effects of DADLE against OGD exposure are shown in Fig. 1A. It was found that cell viability increased progressively with the DADLE concentrations of added, it reached the highest value at 10 nM and then decreased. These results indicate that treatment with 10 nM DADLE was sufficient to increase cell viability (P < 0.05) (Fig. 1A).
Fig. 1.
DOR activation with DADLE attenuates OGD induced damage in astrocytes. (A) Cell viability was assessed using CCK8 assay. (B) Cytotoxicity was measured by LDH assay. (C) Naltrindole and 3-MA pretreatment reduced the cell viability. (D) Naltrindole and 3-MA pretreatment increased cytotoxicity. *P < 0.05 vs. control; #P < 0.05 vs. OGD group; ▾P < 0.05 vs. DADLE group. DAD, DADLE; NAL, naltrindole; 3-MA, 3-methyladenine.
Compared with the control group, LDH release in the OGD group was significantly elevated (P < 0.05). Compared with the OGD group, OGD-induced LDH release was significantly reduced by DADLE (P < 0.05); 10 nM DADLE most significantly ameliorated the cytotoxicity, as shown in Fig. 1B. Therefore, this dosage (10 nM) was used in the following experiments
Naltrindole partly abolished DADLE-induced protection in astrocytes in the OGD model (P < 0.05) (Fig. 1C). 3-MA completely prevented the protective effects of DADLE (P < 0.05) (Fig. 1D).
DADLE Induced Autophagy in Astrocytes
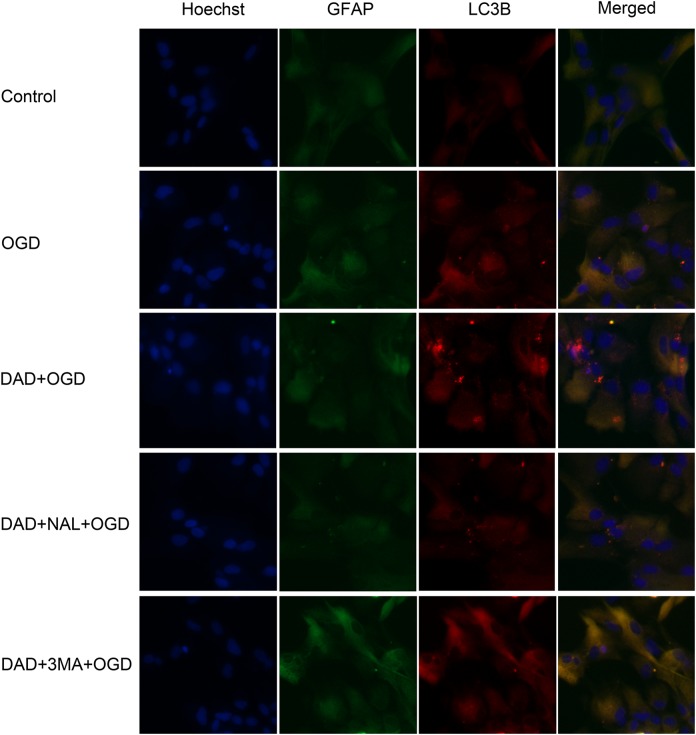
Autophagic vacuole analysis with LC3 staining showed a mildly increasing number of fluorescent particles in astrocytes after OGD. DADLE further increased the number of fluorescent particles in astrocytes. Naltrindole and 3-MA resisted the action of DADLE (Fig. 2).
Fig. 2.
Representative images of LC3 fluorescence staining. Astrocytes were stained using GFAP antibody. Autophagic vacuoles were stained using LC3 antibody, and the cell nuclei were stained with Hoechst. Photomicrographs were taken under an epifluorescence microscope (magnification, x400). DAD, DADLE; NAL, naltrindole; 3-MA, 3-methyladenine.
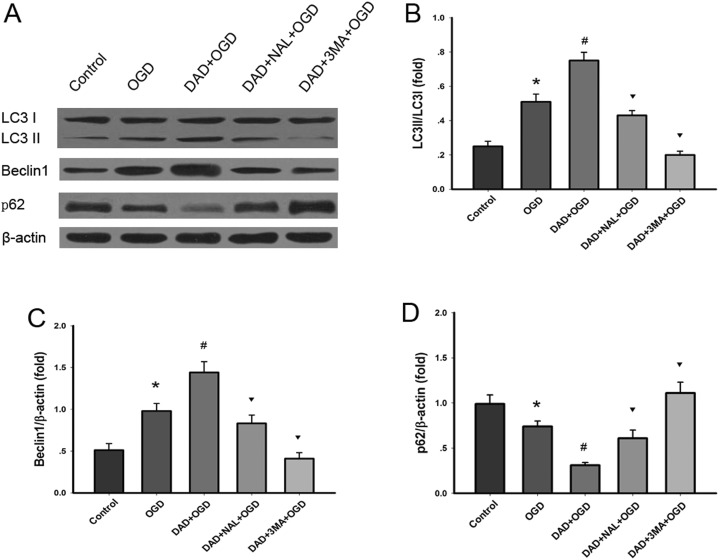
The expression of proteins of autophagy markers was investigated to assess the status of autophagy flux in astrocytes exposed to OGD. As shown in Fig. 3, a statistically significant increase in the level of Beclin 1 and the ratio of LC3-II/ LC3-I was observed in OGD in comparison with control (P < 0.05). A significant reduction in p62 expression was observed in OGD (P < 0.05), which was coincident with the increase in the level of LC3-II.
Fig. 3.
Effects of treatment with DADLE on the protein expression of autophagy markers. (A) Western blotting was used to measure the protein expression of LC3, Beclin 1, p62, and β-actin. (B) DADLE treatment significantly increased the ratio of LC3-II/LC3-I. (C) DADLE treatment significantly increased Beclin 1 expression. (D) DADLE treatment significantly decreased the protein expression of p62. *P < 0.05 vs. control; #P < 0.05 vs. OGD group; ▾P < 0.05 vs. DADLE group. DAD, DADLE; NAL, naltrindole; 3-MA, 3-methyladenine.
Compared with the OGD group, the level of Beclin 1 and the ratio of LC3-II/ LC3-I in astrocytes were significantly increased after treatment with 10 nM DADLE (P < 0.05). It is notable that the level of LC3-I remained almost unchanged in all experimental groups. Analysis of p62 expression demonstrated that the levels of p62 were decreased after DADLE treatment (P < 0.05). DADLE-induced autophagy in astrocytes was blocked by addition of 3-MA (P < 0.05), and partly by naltrindole (P < 0.05) (Fig. 3).
DADLE-Induced Autophagy Inhibited Apoptosis
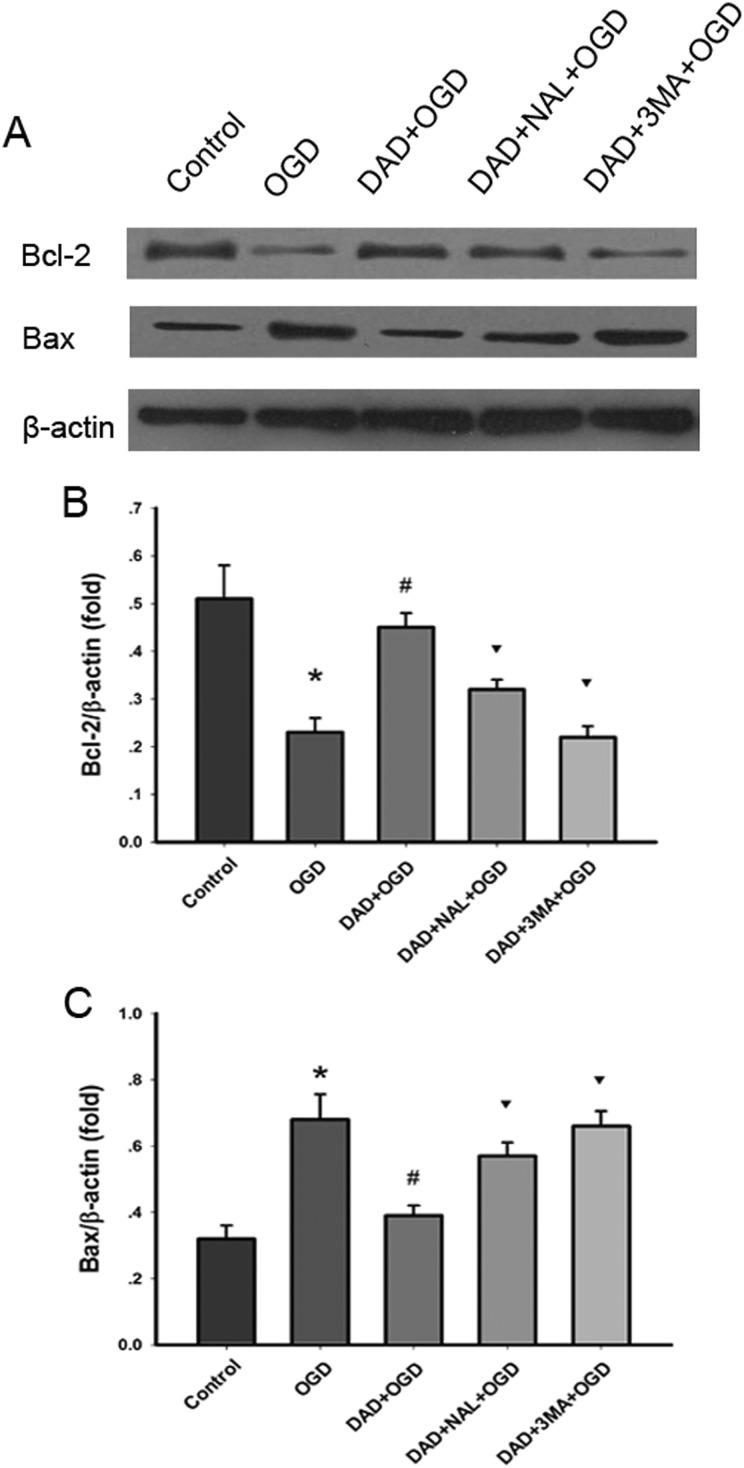
It is well known that Bcl-2 has an anti-apoptotic effect, while Bax has an effect of inducing apoptosis19. In our study, the level of Bcl-2 expression was reduced significantly in OGD and increased in DADLE treated astrocytes (P < 0.05). However, 3-MA and naltrindole inhibited the protein expression of Bcl-2 (P < 0.05).
As expected, the results of western blot demonstrated that the level of Bax expression was obviously increased in the OGD group and decreased in the DADLE group (P < 0.05). 3-MA and naltrindole promoted the protein expression of Bax (P < 0.05) (Fig. 4).
Fig. 4.
DADLE inhibited the expression of apoptosis-related proteins. (A) Western blotting was used to measure the protein expression of Bcl-2 and Bax. (B) DADLE treatment significantly increased the protein expression of Bcl-2. (D) DADLE treatment significantly decreased Bax expression. *p < 0.05 vs. control; #p < 0.05 vs. OGD group; ▾p < 0.05 vs. DADLE group. DAD, DADLE; NAL, naltrindole; 3-MA, 3-methyladenine.
Discussion
In the past few decades, great efforts and impressive advances in neuroprotective agents for the treatment of acute ischemic stroke have been developed in the laboratory, but all of these neuroprotective agents have failed in clinical trials. The central nervous system is a neural network composed of a variety of cells. Protecting neurons alone may not be enough to benefit all cell types, especially astrocytes, in the post-stroke brain.
In ischemic brain, astrocytes exhibited cell hypertrophy and proliferation, which isolated the injured site from viable tissue and prevented uncontrolled tissue damage cascades20. Astrocytes provide important metabolic support for neurons during cerebral ischemia, and destruction of astrocytes function may lead to neuronal death21. It has been demonstrated that astrocytes are more resistant than neurons to ischemia22, and, even within the ischemic core, some astrocytes remained viable and metabolically active23. Enhanced astrocyte survival has been shown to provide neuroprotective effects in a rat model of middle cerebral artery occlusion (MCAO)24. If there are no astrocytes, the neurons are not viable, so neuroprotective therapeutics should consider protecting both neurons and astrocytes.
DOR—a member of the G-protein-coupled receptor family—is distributed widely in various tissues and organs of the human body, including brain, heart, liver, lungs, and gastrointestinal and reproductive tracts25. In recent years, the neuroprotective effects of DOR have received increasing attention. Pharmacological studies found that DOR activation by DADLE significantly promoted neuronal survival in multiple animal models15,26–28. Studies in cultured neurons suggested that DOR-mediated neuroprotection was associated with ionic homeostasis, cellular transcription, antioxidative stress, and antiapoptosis29,30.
DOR is expressed in both neurons and astrocytes31,32. This might mean that DOR has a regulatory effect on both neurons and astrocytes. In our previous study, activation of DOR improved neuronal survival while reducing death of astrocytes in a rat model of global cerebral ischemia14,15. In vitro, the levels of DOR expression increased in astrocytes under hypoxic conditions, but there was no change in neurons. DOR activation effectively protected both the neurons and astrocytes against hypoxic insult. Researchers considered that the protective effects of DOR were related to inhibition of tumor necrosis factor-alpha (TNF-α) levels in astrocytes33. The cited studies demonstrated the effects of DOR on astrocyte function and the mechanism of protecting neurons, but the mechanism by which DOR reduced astrocyte damage remained unclear.
A recent study found that activation of DOR with DADLE could promote autophagy and protected against LPS-induced myocardial damage. The selective DOR antagonist naltrindole exerted opposite effects16, suggesting that autophagy might be one of the mechanisms by which DOR plays a cytoprotective role. In the present study, DADLE significantly induced autophagy and attenuated OGD-induced injury in primary cultures of rat astrocytes. 3-MA completely blocked the role of DADLE in inducing autophagy and protecting astrocytes. These results showed that the cytoprotective mechanism of DOR was related to autophagy. But, interestingly, naltrindole could only partially antagonize the role of DADLE. Tian’s study showed that DADLE could directly inhibit cellular transcription in neurons, and that DOR was not involved in this process30. These data indicated that DADLE might have a cytoprotective mechanism independent of DOR.
The BCL-2 and Bax protein act as key regulators in the apoptosis pathway. Bcl-2 protein plays a role in inhibiting apoptosis and promoting cell survival, while Bax has the opposite effect34. In the present research, we found that DADLE significantly enhanced the level of Bcl-2 protein, and reduced the level of Bax protein in astrocytes exposed to OGD. These results indicated that DADLE-induced autophagy exerted a cytoprotective effect by inhibiting apoptosis.
In summary, DADLE exerted a cytoprotective effect on astrocytes exposed to OGD, partially through DOR activation. Based on our results, DADLE shows the effect of inducing cell autophagy and inhibiting apoptosis by regulating expression of the proteins Bcl-2 and Bax. These results may suggest a novel mechanism for the cytoprotective effect of DADLE. Of course, this study has its limitations. For example, more methods should be used to prove the conclusion, and there is no further study on the function of astrocytes. Additional research, both in vivo and in vitro, will be required to confirm the effect of DADLE to protect against ischemic injury in preclinical settings.
Footnotes
Author Contributions: SW and XC conducted the experiments. GZ designed the study. YD provided statistical analysis. SW and YD participated in article preparation. All authors approved the final version.
Ethical Approval: This study was approved by the Animal Care and Use Committee of the Shanghai Jiaotong University.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were performed in accordance with National Institutes of Health (NIH) guidelines and approved by the Animal Care and Use Committee of the Shanghai Jiaotong University.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81301122).
ORCID iD: Yale Duan  https://orcid.org/0000-0001-5166-4091
https://orcid.org/0000-0001-5166-4091
References
- 1. Kamalian S, Lev MH. The adult patient with acute neurologic deficit: an update on imaging trends. Neuroimaging Clin N Am. 2018;28(3):319–334. [DOI] [PubMed] [Google Scholar]
- 2. Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106(6):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72(2):111–127. [DOI] [PubMed] [Google Scholar]
- 4. Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18(7):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirayama Y, Koizumi S. Astrocytes and ischemic tolerance. Neurosci Res. 2018;126:53–59. [DOI] [PubMed] [Google Scholar]
- 6. Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11(2):164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. [DOI] [PubMed] [Google Scholar]
- 8. Papackova Z, Cahova M. Important role of autophagy in regulation of metabolic processes in health, disease and aging. Physiol Res. 2014;63(4):409–420. [DOI] [PubMed] [Google Scholar]
- 9. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. [DOI] [PubMed] [Google Scholar]
- 10. Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32(3):329–339. [DOI] [PubMed] [Google Scholar]
- 11. Urbanek T, Kuczmik W, Basta-Kaim A, Gabryel B. Rapamycin induces of protective autophagy in vascular endothelial cells exposed to oxygen-glucose deprivation. Brain Res. 2014;1553:1–11. [DOI] [PubMed] [Google Scholar]
- 12. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasprowska D, Machnik G, Kost A, Gabryel B. Time-Dependent Changes in Apoptosis Upon Autophagy Inhibition in Astrocytes Exposed to Oxygen and Glucose Deprivation. Cell Mol Neurobiol. 2017;37(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan YL, Wang SY, Zeng QW, Su DS, Li W, Wang XR, Zhao Z. Astroglial reaction to delta opioid peptide [D-Ala2, D-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience. 2011;192:81–90. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Duan Y, Su D, Li W, Tan J, Yang D, Wang W, Zhao Z, Wang X. Delta opioid peptide [D-Ala2, D-Leu5] enkephalin (DADLE) triggers postconditioning against transient forebrain ischemia. Eur J Pharmacol. 2011;658(2-3):140–144. [DOI] [PubMed] [Google Scholar]
- 16. Zhao P, Kuai J, Gao J, Sun L, Wang Y, Yao L. Delta opioid receptor agonist attenuates lipopolysaccharide-induced myocardial injury by regulating autophagy. Biochem Biophys Res Commun. 2017;492(1):140–146. [DOI] [PubMed] [Google Scholar]
- 17. Juurlink BH, Hertz L. Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev Neurosci. 1985;7(5-6):263–277. [DOI] [PubMed] [Google Scholar]
- 18. Jia T, Zhang L, Duan Y, Zhang M, Wang G, Zhang J, Zhao Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014;14(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10(11):1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurer G, Gursoy-Ozdemir Y, Erdemli E, Can A, Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol. 2009;19(4):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thoren AE, Helps SC, Nilsson M, Sims NR. Astrocytic function assessed from 1-14C-acetate metabolism after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2005;25(4):440–450. [DOI] [PubMed] [Google Scholar]
- 24. Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Hum Gene Ther. 2004;15(12):1243–1254. [DOI] [PubMed] [Google Scholar]
- 25. Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13(2):230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao CJ, Li JP, Wang W. et al. Effects of intracerebroventricular application of the delta opioid receptor agonist [D-Ala2, D-Leu5] enkephalin on neurological recovery following asphyxial cardiac arrest in rats. Neuroscience. 2010;168(2):531–542. [DOI] [PubMed] [Google Scholar]
- 27. Fu D, Liu H, Li S, Chen L, Yao J. Antioxidative and antiapoptotic effects of Delta-Opioid Peptide [D-Ala(2), D-Leu(5)] Enkephalin on spinal cord ischemia-reperfusion injury in rabbits. Front Neurosci. 2017;11:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lv MR, Li B, Wang MG, Meng FG, Yu JJ, Guo F, Li Y. Activation of the PI3K-Akt pathway promotes neuroprotection of the delta-opioid receptor agonist against cerebral ischemia-reperfusion injury in rat models. Biomed Pharmacother. 2017;93:230–237. [DOI] [PubMed] [Google Scholar]
- 29. He X, Sandhu HK, Yang Y, Hua F, Belser N, Kim DH, Xia Y. Neuroprotection against hypoxia/ischemia: delta-opioid receptor-mediated cellular/molecular events. Cell Mol Life Sci. 2013;70(13):2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian J, Gu Y, Sun K, Wang B, Chen J, Wang X, Su D. [D-Ala2, D-Leu5] encephalin (DADLE) reversibly inhibits cellular transcription in neurons without causing cell injury. Brain Res. 2014;1565:1–7. [DOI] [PubMed] [Google Scholar]
- 31. Persson PA, Thorlin T, Ronnback L, Hansson E, Eriksson PS. Differential expression of delta opioid receptors and mRNA in proliferating astrocytes during the cell cycle. J Neurosci Res. 2000;61(4):371–375. [DOI] [PubMed] [Google Scholar]
- 32. Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220(2):677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Chao D, Chen T, Sandhu H, Xia Y. delta-Opioid receptors and inflammatory cytokines in hypoxia: differential regulation between glial and neuron-like cells. Transl Stroke Res. 2014;5(4):476–483. [DOI] [PubMed] [Google Scholar]
- 34. Leibowitz B, Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther. 2010;9(6):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]