Abstract
Induced pluripotent stem cells (iPS cells) are promising cell source for stem cell replacement strategy applied to brain injury caused by traumatic brain injury (TBI) or stroke. Neural stem cell (NSCs) derived from iPS cells could aid the reconstruction of brain tissue and the restoration of brain function. However, tracing the fate of iPS cells in the host brain is still a challenge. In our study, iPS cells were derived from skin fibroblasts using the four classic factors Oct4, Sox2, Myc, and Klf4. These iPS cells were then induced to differentiate into NSCs, which were incubated with superparamagnetic iron oxides (SPIOs) in vitro. Next, 30 TBI rat models were prepared and divided into three groups (n = 10). One week after brain injury, group A&B rats received implantation of NSCs (labeled with SPIOs), while group C rats received implantation of non-labeled NSCs. After cell implantation, all rats underwent T2*-weighted magnetic resonance imaging (MRI) scan at day 1, and 1 week to 4 weeks, to track the distribution of NSCs in rats’ brains. One month after cell implantation, manganese-enhanced MRI (ME-MRI) scan was performed for all rats. In group B, diltiazem was infused during the ME-MRI scan period. We found that (1) iPS cells were successfully derived from skin fibroblasts using the four classic factors Oct4, Sox2, Myc, and Klf4, expressing typical antigens including SSEA4, Oct4, Sox2, and Nanog; (2) iPS cells were induced to differentiate into NSCs, which could express Nestin and differentiate into neural cells and glial cells; (3) NSCs were incubated with SPIOs overnight, and Prussian blue staining showed intracellular particles; (4) after cell implantation, T2*-weighted MRI scan showed these implanted NSCs could migrate to the injury area in chronological order; (5) the subsequent ME-MRI scan detected NSCs function, which could be blocked by diltiazem. In conclusion, using an in vivo MRI tracking technique to trace the fate of iPS cells-induced NSCs in host brain is feasible.
Keywords: induced pluripotent stem cells, neural stem cells, traumatic brain injury, superparamagnetic iron oxide particles, manganese-enhanced MRI
Introduction
Traumatic brain injury (TBI) is a common emerging situation in the neurosurgical department, and usually causes great economic burden to patients’ families because of long-term hospitalization and therapy in the intensive care unit. Moreover, severe TBI leads to high morbidity and mortality, causing even greater pain to patients and their families1. Thus, improving the life quality for TBI patients is urgent, and effective therapies are key for these patients.
Among different therapeutic strategies, stem cell replacement is undoubtedly very promising. For example, exogenous neural stem cells (NSCs) could be implanted into the brain lesion area for brain protection2–4. NSC transplants not only secrete neurotrophic factors, but also provide cell interactions between exogenous cells and host neural cells, which could aid neural regeneration and restore neural function. However, many questions still remain; for instance, derivation of exogenous NSCs is sometimes not very convenient.
Induced pluripotent stem cells (iPS cells) are the most promising cell sources for NSCs, as demonstrated in our previous studies5,6. iPS cells can be easily obtained from the conversion of skin fibroblasts by the four factors Oct4, Sox2, Myc, and Klf4 (Krüppel-like factors 4). Thus, it is a relatively convenient and safe method to obtain NSCs for animal experiments and pre-clinical trials7–9.
However, tracking these iPS cells-induced NSCs in the host animal brain is still difficult10, especially using non-invasive techniques to achieve in vivo tracking. Currently, magnetic resonance imaging (MRI) is a very important tool used for molecular imaging, and has become the best candidate for tracking stem cells in animal studies and in humans8.
Here, we would like to introduce our studies on MRI tracking of iPS cells-induced NSCs in animal brains. There are two main steps for MRI tracking. The first step is to observe the migration of iPS cells-induced NSCs after transplantation into rats’ brains, and the next step is to detect the functions of NSCs in host animal brains.
Materials and Methods
Derivation of iPS Cells from Skin Fibroblasts
iPS cells were obtained according to the classical method described by the Yamanaka group11,12. Rat skin fibroblasts were acquired from healthy Sprague-Dawley rats, and were cultured at the Department of Laboratory Animal Science, Fudan University. First, rat skin fibroblasts were seeded at 1×105 cells per 100 mm dish covered by feeder cells. On the second day, the fibroblasts were incubated in a cocktail of retroviruses carrying Oct4, Sox2, Myc, and Klf4 for 24 h. At 72 h after virus infection, the infection medium was changed to embryonic stem cells (ES cell) medium. Then, iPS cells selection was performed based on the morphology of ES cell colonies. The characterization of iPS cells included expression of pluripotent markers and differentiation. Detailed methods can be found in the literature11. All animal studies were approved by the Animal Care and Use Committee of Fudan University (20120302-113).
Differentiation of iPS Cells into NSCs
Induction of iPS cells into NSCs was performed using a modified protocol from the literature13. To be specific, iPS cell cultures were dispersed using TrypleTM Express (Invitrogen, MA, USA) for 5 min, washed using ES cell media and pre-plated on gelatin for 1 h at 37°C, adding ROCK inhibitor to remove mouse embryonic fibroblasts. Then, iPS cells were washed and plated on Matrigel with the density of 1×103 cells/cm2 in ES cell medium (containing 10 ng/ml FGF-2 and ROCK inhibitor) (Abcam, Cambridge, UK). When iPS cells were confluent, the ROCK inhibitor was removed.
The initial differentiation media conditions included knockout serum replacement media (containing 10 mM SB431542 and 500 ng/ml Noggin) (Abcam). On day 5, SB431542 was removed and N2 media (25%, 50%, 75%) was added to the knockout serum replacement media every 2 days while maintaining 500 ng/ml Noggin. After 10 days, the cells were digested and plated in NSCs media for sphere culture. After culturing for 10 generations, the cells were used for immunocytochemistry analysis and labeling experiments.
SPIOs Labeling of NSCs
The derived NSCs were digested into single cells with TrypleTM Express (Invitrogen). Then single cells were labeled with superparamagnetic iron oxides (SPIOs) (Sigma-Aldrich, Darmstadt, Germany)7,8. After incubation with SPIOs for 24 h, the Prussian blue method was used to detect iron within the cell plasma. At 72 h after SPIOs labeling, cells were re-plated in NSCs culture medium. About 7 days later, neurospheres formed by SPIOs-labeled NSCs were observed, and were sent for labeling efficiency, cell proliferation, and differentiation analysis14.
In Vitro MRI Scanning of Cells and Solutions
To evaluate the magnetic field difference of iPS cells-derived NSCs (SPIOs-labeled vs. Unlabeled), two Eppendorf tubes (250 µl) of cell suspension (SPIOs-labeled vs. Unlabeled) were prepared. Two control Eppendorf tubes were SPIOs solution and cell medium. The four Eppendorf tubes were then sent for MRI scan using the clinical 3 T MRI scanner (Siemens, Munich, Germany) with an animal coil. T2*-weighted MRI images were acquired with the following parameters: repetition time (TR) = 475 ms, echo time (TE) = 20 ms, field of view (FOV) = 80 mm × 100 mm, matrix = 260 × 320, slice thickness = 2 mm.
Animal Grouping
The TBI model rats were prepared according to the literature15. All animal studies were approved by the Animal Care and Use Committee of Fudan University (20120302-113). For this study, 30 adult normal Sprague-Dawley rats were anesthetized with 10% chloral hydrate intraperitoneally. In general, rat heads were placed in a stereotactic frame after anesthetization, and two holes were drilled in the skull. One hole was drilled 1 mm posterior to the bregma and 2 mm from the midline on the right side, and another hole was made around the first one for cell injection. The dura was kept intact over the cortex. Injury was induced using a small falling ball through a 3 mm diameter tube from a height of 50 cm. Animals were then housed in cages for care and observation after TBI experiments.
One week after TBI, the 30 rats were anesthetized again, and divided randomly into three groups (n = 10). Group A&B rats received 5 μl NSCs bolus injection (labeled with SPIOs, containing about 1×105 cells), while group C rats received non-labeled NSCs implantation.
In Vivo MRI Tracking
After implantation of NSCs, we used MRI to track their migration in TBI rat brains. MRI scan was performed at day 1, and 1 week, 2 weeks, 3 weeks, and 4 weeks post-transplantation. The T2*-weighted MRI images were acquired using a clinical 3 T MRI scanner (Siemens) with an animal coil. The scans were performed with the following parameters: TR = 4651 ms, TE = 96.5 ms, FOV = 60 mm × 48 mm, matrix = 320 × 256, slice thickness = 1.8 mm, band width = 15.6 kHz.
Manganese-enhanced MRI Scan for TBI Rats
As mentioned above, approximately 1 month after transplantation of NSCs into the TBI rat brains, SPIOs-labeled NSCs could migrate to the injured brain areas. Then, manganese-enhanced MRI (ME-MRI) scan was performed to detect the function of these NSCs16.
In brief, 1% MnCl2 (Sigma-Aldrich) was first intravenously infused into group A TBI rats within 1 h. At approximately the halfway stage of MnCl2 infusion, blood–brain barrier (BBB) of the right-side cerebral hemisphere (the injury site) was opened by 20% mannitol. Then, left (contralateral) forepaw electrical stimulation was conducted for 30 min, and the ME-MRI scan was then performed. In group B TBI rats, the same procedures were performed, but the Ca2+ channel inhibitor diltiazem (Sigma-Aldrich) was infused 10 min before electrical stimulation and was continued during the entire stimulus period.
Next, ME-MRI scan was performed using a clinical 3 T MRI scanner (Siemens) with an animal coil and the three-dimensional spoiled gradient recalled acquisition in a steady state (3D-SPGR) pulse sequence was used. The scan was performed using the following parameters: TE = 2.4 ms; TR = 8.8 ms; FOV = 5 cm × 4 cm; flip angle = 45°; repetition = 6 NEX.
Prussian Blue Staining
All rats were sacrificed and transcardially perfused with 4% poly-formaldehyde (PFA) after the final MRI scan. In detail, brain tissues were fixed in 4% PFA overnight, dehydrated in 30% sucrose solution, frozen on dry ice, and cryosected into 30 μm slices for histology according to the MRI images. The sections were doubly stained by hematoxylin-eosin and Prussian blue to detect intracellular iron oxide particles17.
Results
Characterization of iPS Cells
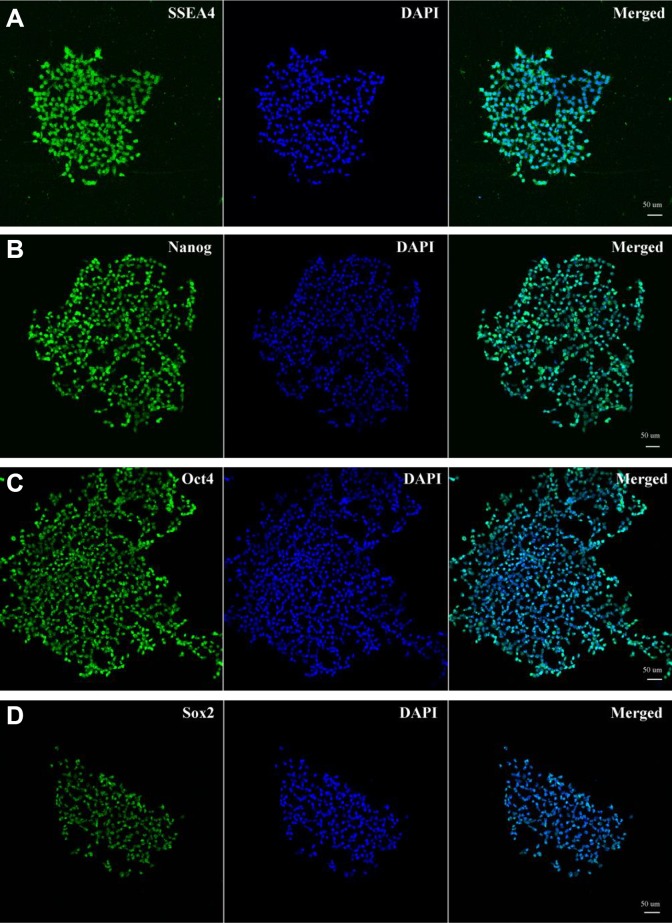
The iPS cells were successfully reprogrammed from rat skin fibroblasts using Yamanaka’s method11. The characteristics of the iPS cells were similar to ES cells in morphology, and these iPS cells were positive for alkaline phosphatase. Moreover, these iPS cells could express ES cell-specific surface antigens, including SSEA-4 (stage-specific embryonic antigen-4), Nanog, Oct4, and Sox2 (Abcam) (Fig. 1A–D).
Figure 1.
Characterization of iPS cells. The characteristics of the iPS cells were similar to embryonic stem (ES) cells in morphology, and these iPS cells were positive for alkaline phosphatase (AKP). Moreover, these iPS cells could express ES cell-specific surface antigens, including SSEA-4, Nanog, Oct4, and Sox2 (Fig. 1A-D).
Preparation of NSCs from iPS Cells
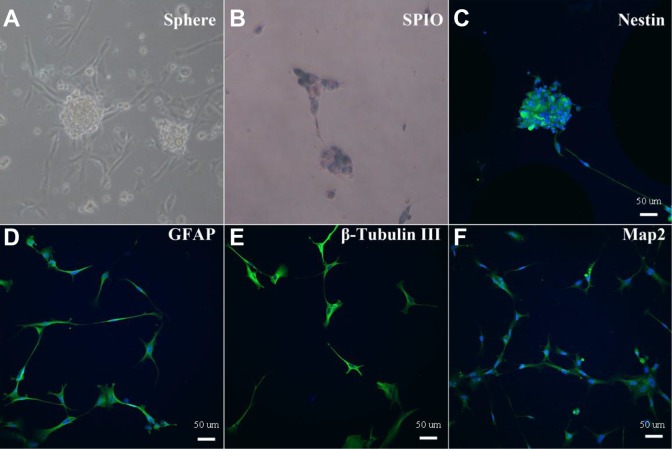
Following our protocol, NSCs were induced from the above iPS cells. We used microscopy to observe the formation of NSCs neurospheres (Fig. 2A). We then incubated SPIO particles with NSCs in medium overnight to allow them to permeate the cells, thereby labeling them. Labeled NSCs then underwent Prussian blue staining to confirm the labeling, which resulted in the presentation of blue particles within the cytoplasm (Fig. 2B).
Figure 2.
iPS cells-induced NSCs, SPIOs labeling and identification. iPS cells-induced NSCs neurospheres were observed under microscope after passage (A); SPIOs-labeled NSCs had blue particles in the cytoplasm revealed by Prussian blue staining (B); SPIOs-labeled NSCs were identified with immunofluorescence staining for Nestin (C), GFAP (D), β-Tubulin III (E), and Map2 (F).
We then determined whether SPIOs labeling would affect the activity of NSCs. These SPIOs-labeled NSCs were identified based on their self-renewal and differentiation abilities, using immunofluorescence staining for Nestin, glial fibrillary acidic protein (GFAP), β-Tubulin III, and microtubule-associated protein 2 (MAP2) (Abcam) (Fig. 2C–F). The results showed that SPIOs labeling does not affect the function of NSCs, as SPIOs-labeled NSCs retain their self-renewal ability (Nestin, Fig. 2C), and could differentiate into neurons (β-Tubulin III and MAP2, Fig. 2E, 2F) and glial cells (GFAP, Fig. 2D).
In Vitro MRI Scanning of NSCs and Solutions
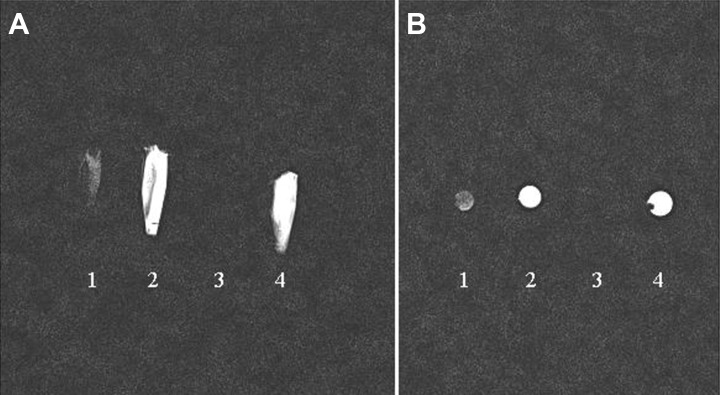
To evaluate the magnetic field difference of iPS cells-derived NSCs (SPIOs-labeled vs. Unlabeled), two Eppendorf tubes (250 µl) of cell suspension (SPIOs-labeled vs. Unlabeled) were prepared. Two control Eppendorf tubes were SPIOs solution and cell medium. The four Eppendorf tubes were then sent for MRI scan using the clinical 3 T MRI scanner (Siemens) with an animal coil. The data showed that SPIOs-labeled NSCs presented with hypointense signals compared with unlabeled cells and cell medium (Fig. 3A, 3B). However, the SPIOs solution could not be visualized due to the effect of the high magnetic field.
Figure 3.
In vitro MRI scanning of iPS cells-derived NSCs and solutions. The data showed that SPIOs-labeled NSCs (1) presented with hypointense signals compared with unlabeled cells (2) and cell medium (4). However, the SPIOs solution could not be visualized due to high magnetic field effect (3). (A: sagittal view; B: axial view).
MRI Tracking of iPS Cell-Induced NSCs Migration
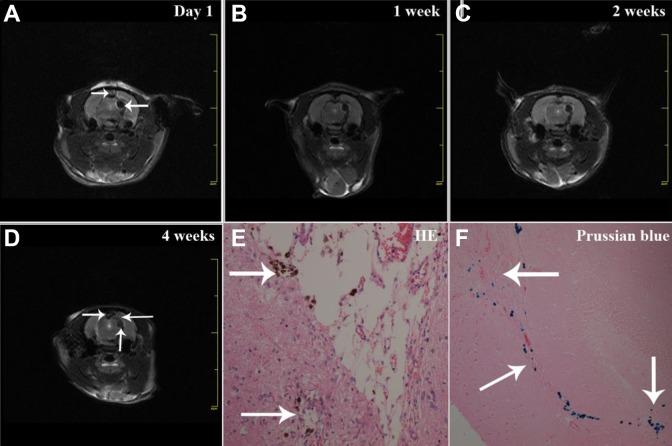
After implantation of NSCs, we used MRI to track the migration of NSCs in TBI rat brains. Remarkably, based on the MRI images, there were pronounced hypointense signals around cell injection sites on day 1 (Fig. 4A). These hypointense signals indicated the existence of transplanted NSCs. As time proceeded, hypointense signals gradually vanished and spread to the edge of the brain injury areas (Fig. 4B–D), which illustrated the migration of transplanted NSCs. These findings were consistent with the presence of SPIOs-labeled NSCs revealed by hematoxylin-eosin staining and Prussian blue staining (Fig. 4E, 4F), exhibiting scattered blue particles within the brain tissues.
Figure 4.
Migration of implanted iPS cells-induced NSCs in TBI rat brains. T2*-weighted MRI scan was performed after iPS cells-induced NSCs (SPIOs-labeled) transplantation, showing pronounced hypointense signals at the cell injection site (A, as indicated by the lower white arrow). The dark signals gradually spread to the border of the damaged brain area from 1 (B) and 2 weeks (C) to 4 weeks (D, the left white arrow indicates the brain lesion, the lower white arrow indicates the cell injection site, the right white arrow indicates “migrated” signals). Hematoxylin-eosin staining (E) and Prussian blue staining of brain sections showed the presence of SPIOs-labeled NSCs with blue particles (F, the upper white arrow indicates NSCs in the brain lesion area, the lower white arrow indicates migrated NSCs, the right white arrow indicates the cell injection site).
Functional MRI Tracking of iPS Cell-Induced NSCs
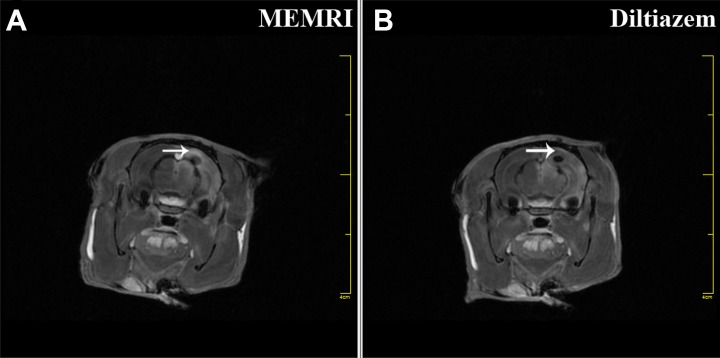
ME-MRI results showed that regional signal increase (Fig. 5A, white arrow) was produced in the brain injury area. However, this enhancement could be blocked (Fig. 5B, white arrow) by diltiazem in another group of TBI rats, suggesting that localized neural activity was induced by implanted NSCs, which could be blocked by a Ca2+ channel antagonist. In the study, we found that TBI rats that received implantation of iPS cell-induced NSCs could produce regional signal increase, based on ME-MRI.
Figure 5.
ME-MRI tracking of iPS cells-induced NSCs function in TBI rats. TBI rats underwent ME-MRI scan, showing that a regional signal increase (A, white arrow) was produced in the lesion area. However, this enhancement could be blocked (B, white arrow) with diltiazem in another group of TBI rats, suggesting that localized neural activity was provided by implanted iPS cells-induced NSCs.
Discussion
iPS Cells are Potential Cell Sources for Stem Cell Replacement
In 1992, Reynolds and Weiss first reported the derivation of NSCs from the embryonic mouse brain18. In 2000, Gage defined NSCs as cells that have the potential of self-renewal and proliferation, and demonstrated that these cells could differentiate into neurons, astrocytes, and oligodendroglia19. The common consensus is that endogenous NSCs are located at the subventricular zone and dentate gyrus areas in rodents. In 2001, Zhu et al. first isolated human adult NSCs from brain trauma patients7. All of these pioneering studies brought great innovation to stem cell research and regenerative medicine, and techniques to obtain NSCs from embryonic and adult rodent brains are now established. Subsequently, revolutionary work by Zhu et al. showed that NSCs could be collected from adult patients with open brain trauma, which expanded the field of neurogenesis7,8. Later, it was found that NSCs could also be differentiated from iPS cells5,6,20, which increased the cell sources for replacement of stem cells.
In our study, iPS cells were successfully derived from rat skin fibroblasts using the four classic factors Oct4, Sox2, Myc, and Klf4. These iPS cells were then induced to differentiate into NSCs, which could still express the typical antigens for NSCs after SPIOs labeling, including Nestin, GFAP, β-Tubulin III, and MAP2.
Before cell transplantation, NSCs should be processed using magnetic nanoparticles. In this way, NSCs can be detected by MRI. Currently, SPIO particles are most commonly used for labeling of NSCs. These particles can decrease the relax time for T2*-weighted MRI or gradient-echo scan by virtue of susceptibility differences to the adjacent environment. We incubated SPIO particles with NSCs in medium overnight to allow them to permeate the cells, thereby labeling them. Labeled NSCs then underwent Prussian blue staining, which resulted in the presentation of blue particles within the cytoplasm, to confirm the labeling.
MRI Tracking of iPS Cell-Induced NSC Migration in Host Animal Brains
Next, we used MRI to track the distribution of these iPS cells-induced NSCs which were transplanted into animal brains. Applications of in vivo MRI tracking of NSCs can be found both in research and clinical settings, due to the generation of MRI contrast from the ex vivo pre-labeling of NSCs with magnetic SPIO nanoparticles. The high spatial resolution of MRI makes it possible to observe transplanted NSCs in their anatomical areas21,22.
For cell transplantation, we first established the TBI animal model according to Feeney et al.’s method15. One week later, these TBI rats could be used for cell transplantation. SPIOs-labeled NSCs (derived from iPS cells) were stereotactically23 transplanted into TBI rat brains around the injury. After implantation of the NSCs, we used MRI to track the migration of NSCs in the TBI rat brains. MRI scanning was performed at day 1, and at 1 week, 2 weeks, 3 weeks, and 4 weeks post-transplantation. On the MRI images, there were pronounced hypointense signals around cell injection sites on day 1. These hypointense signals indicated the existence of transplanted NSCs. As time proceeded, hypointense signals gradually vanished and spread to the edge of the brain injury areas, which illustrated the migration of transplanted NSCs. These findings were consistent with the presence of SPIOs-labeled NSCs revealed by hematoxylin-eosin staining and Prussian blue staining.
As described above, we could successfully track the implanted iPS cells-induced NSCs in animal brains by MRI. However, there were still some outstanding questions. One concern was if these NSCs could survive in the host brain, and the appropriate methodology to assess viability. It is possible that NSC death could occur after transplantation, at which time the SPIO nanoparticles would leach out from cytoplasm. In such situations, the SPIO nanoparticles would still be detectable by MRI as hypointense signals, leading to difficulty in distinguishing live and dead cells.
With the recent advent of new molecular MRI technology such as chemical exchange saturation transfer (CEST), this problem can be resolved24. CEST agents formally belong to the class of negative contrast agents because they indicate a reduction in the signal intensity of water protons in MRI images acquired using specific off-radiation pulses. In contrast to paramagnetic agents, their effect is not due to T2 shortening, but to a saturation transfer mediated by chemical exchange. Properly designed CEST agents can result in significant improvements in imaging strength of engrafted cells, with a large pool of exchangeable protons associated with these carriers, allowing for the detection of nanocarriers at nanomolar concentrations25,26.
Another question is how to evaluate the function of these implanted iPS cell-induced NSCs in animal brains by MRI. We need to determine whether these implanted NSCs can self-renewal, differentiate into their offspring, and functionally participate in the local neural circuits. This is critically important from the neurogenesis point of view. According to our experience, the ME-MRI technique could be an alternative way to resolve the problem, rather than the BOLD (blood oxygenation level-dependent) functional MRI.
Functional Tracking of iPS Cell-Induced NSCs by ME-MRI
In experimental studies, patch clamp is usually used for the detection of electrophysiological function of transplanted cells27. However, the drawback of this approach is its invasiveness, because pathological sections are required. Exploring a method to evaluate cell function in vivo by molecular MRI is thus necessary.
ME-MRI is a classic molecular imaging technique based on the principle that the manganese ion (Mn2 +) is a calcium (Ca2+) analog and also an MRI contrast agent28. Mn2 + enters cells through active voltage-gated calcium channels, but unlike Ca2+, Mn2 + has a long half-life within the cells. This property allows Mn2 + to be used for functional evaluation by ME-MRI. ME-MRI is completely different from BOLD functional MRI evaluation, which is based on increases in blood volume, but not direct cell activity29,30.
As mentioned above, approximately 1 month after transplantation of iPS cells-induced NSCs into TBI rat brains, SPIOs-labeled NSCs migrated to the injured brain areas, which was revealed based on T2*-weighted MRI images. Then, ME-MRI scan was performed to detect the function of these NSCs. The ME-MRI results showed that regional signal increase was produced in the brain injury area. However, this enhancement could be blocked by diltiazem in another group of TBI rats, suggesting that localized neural activity was induced by implanted NSCs, which could be blocked by a Ca2+ channel antagonist.
In the study, we found that TBI rats that received implantation of iPS cells-induced NSCs could produce regional signal increase based on ME-MRI. When Mn2+ is infused into the rat brain, it accumulates in the activated brain area because of calcium influx, and this could be detected by ME-MRI. These increased signals could be blocked by diltiazem, a Ca2+ channel antagonist, proving that ME-MRI could detect localized neuronal activity. ME-MRI has the advantage of in vivo functional tracking, compared with conventional patch clamp methods used after sacrificing the animals31–33. However, the manganese ion has the disadvantage of potential toxicity, which would restrict its application in clinical trials.
Conclusion
TBI contributes to approximately 30% of all brain injury-related deaths in the neurosurgery department, resulting in great economic burden and severe morbidity and mortality34. Determining how to provide better therapy and achieve better life quality for TBI patients is a great task. Currently, iPS cell-induced NSCs could be a promising strategy for stem cells replacement applied for brain injury in animal studies and pre-clinical trials6. However, tracking these iPS cells-induced NSCs in host animal brains with a non-invasive method is still a great challenge35,36. In our study, we introduced research on MRI tracking of iPS cells-induced NSCs in host animal brains by two steps. First, we found that implanted iPS cells-induced NSCs (after SPIOs labeling) could migrate to the injured brain areas from the injection site, as revealed by T2*-weighted MRI. Then, we used ME-MRI to detect neural activity caused by implanted iPS cells-induced NSCs in the local brain area.
Acknowledgments
We are very grateful to Dr. Li Yin (from Siemens Corporation) for her kind assistance in MRI scan. This study was supported by grant (No.2018YFA0107900) from National Key R&D Program of China; grant (No.81200936) from the National Nature Science Foundation, and grant (No.134119a8500) from Shanghai Committee of Science and Technology.
Author Contributions: Lili Jiang, Ronggang Li, Hailiang Tang: These authors contribute equally to the paper.
Ethical Approval: Ethical approval to report this study was obtained from the Animal Care and Use Committee of Fudan University (20120302-113).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the *Animal Care and Use Committee of Fudan University (20120302-113)* approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Esnault P, Cardinale M, Boret H, D’Aranda E, Montcriol A, Bordes J, Prunet B, Joubert C, Dagain A, Goutorbe P, Kaiser E, Meaudre E. Blunt cerebrovascular injuries in severe traumatic brain injury: incidence, risk factors, and evolution. J Neurosurg. 2017;127(1):16–22. [DOI] [PubMed] [Google Scholar]
- 2. Aertker BM, Bedi S, Cox CS., Jr Strategies for CNS repair following TBI. Exp Neurol. 2016;275(Pt 3):411–426. [DOI] [PubMed] [Google Scholar]
- 3. Tso D, McKinnon RD. Cell replacement therapy for central nervous system diseases. Neural Regen Res. 2015;10(9):1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gennai S, Monsel A, Hao Q, Liu J, Gudapati V, Barbier EL, Lee JW. Cell-based therapy for traumatic brain injury. Br J Anaesth. 2015;115(2):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang P, Zhang HL, Li W, Sha H, Xu C, Yao L, Tang Q, Tang H, Chen L, Zhu J. Generation of patient-specific induced neuronal cells using a direct reprogramming strategy. Stem Cells Dev. 2014;23(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang H, Sha H, Sun H, Wu X, Xie L, Wang P, Xu C, Larsen C, Zhang HL, Gong Y, Mao Y, Chen X, Zhou L, Feng X, Zhu J. Tracking induced pluripotent stem cells-derived neural stem cells in the central nervous system of rats and monkeys. Cell Reprogram. 2013;15(5):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu J, Wu X, Zhang HL. Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr Drug Targets. 2005;6(1):97–110. [DOI] [PubMed] [Google Scholar]
- 8. Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355(22):2376–2378. [DOI] [PubMed] [Google Scholar]
- 9. Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J, Zhu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108(2):320–329. [DOI] [PubMed] [Google Scholar]
- 10. Connell JJ, Patrick PS, Yu Y, Lythgoe MF, Kalber TL. Advanced cell therapies: targeting, tracking and actuation of cells with magnetic particles. Regen Med. 2015;10(6):757–772. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 13. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neri M, Maderna C, Cavazzin C, Deidda-Vigoriti V, Politi LS, Scotti G, Marzola P, Sbarbati A, Vescovi AL, Gritti A. Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells. 2008;26(2):505–516. [DOI] [PubMed] [Google Scholar]
- 15. Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211(1):67–77. [DOI] [PubMed] [Google Scholar]
- 16. Tang HL, Sun HP, Wu X, Sha HY, Feng XY, Zhu JH. Detection of neural stem cells function in rats with traumatic brain injury by manganese-enhanced magnetic resonance imaging. Chin Med J (Engl). 2011;124(12):1848–1853. [PubMed] [Google Scholar]
- 17. Shapiro EM, Gonzalez-Perez O, Manuel García-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32(3):1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 19. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. [DOI] [PubMed] [Google Scholar]
- 20. Yao L, Chen R, Wang P, Zhang Q, Tang H, Sun H. Generation of induced pluripotent stem cells with high efficiency from human embryonic renal cortical cells. Am J Transl Res. 2016;8(11):4982–4993. [PMC free article] [PubMed] [Google Scholar]
- 21. Gera A, Steinberg GK, Guzman R. In vivo neural stem cell imaging: current modalities and future directions. Regen Med. 2010;5(1):73–86. [DOI] [PubMed] [Google Scholar]
- 22. Villa C, Erratico S, Razini P, Farini A, Meregalli M, Belicchi M, Torrente Y. In vivo tracking of stem cell by nanotechnologies: future prospects for mouse to human translation. Tissue Eng Part B Rev. 2011;17(1):1–11. [DOI] [PubMed] [Google Scholar]
- 23. De Vloo P, Nuttin B. Stereotaxy in rat models: current state of the art, proposals to improve targeting accuracy and reporting guideline. Behav Brain Res. 2017. pii: S0166-4328(17)31561-31569. [DOI] [PubMed] [Google Scholar]
- 24. Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PC, Bulte JW, McMahon MT. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater. 2013;12(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pumphrey AL, Ye S, Yang Z, Simkin J, Gensel JC, Abdel-Latif A, Vandsburger MH. Cardiac chemical exchange saturation transfer MR imaging tracking of cell survival or rejection in mouse models of cell therapy. Radiology. 2017;282(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Randtke EA, Granados JC, Howison CM, Pagel MD, Cárdenas-Rodríguez J. Multislice CEST MRI improves the spatial assessment of tumor pH. Magn Reson Med. 2017;78(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao H, Steiger A, Nohner M, Ye H. Specific intensity direct current (DC) electric field improves neural stem cell migration and enhances differentiation towards βIII-Tubulin+ neurons. Plos One. 2015;10(6):e0129625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malheiros JM, Paiva FF, Longo BM, Hamani C, Covolan L. Manganese-enhanced MRI: biological applications in neuroscience. Front Neurol. 2015;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koretsky AP. Is there a path beyond BOLD? Molecular imaging of brain function. Neuroimage. 2012;62(2):1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva AC. Using manganese-enhanced MRI to understand BOLD. Neuroimage. 2012;62(2):1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talley Watts L, Shen Q, Deng S, Chemello J, Duong TQ. Manganese-enhanced magnetic resonance imaging of traumatic brain injury. J Neurotrauma. 2015;32(13):1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med. 1997;38(3):378–388. [DOI] [PubMed] [Google Scholar]
- 33. Lu H, Xi ZX, Gitajn L, Rea W, Yang Y, Stein EA. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI). Proc Natl Acad Sci U S A. 2007;104(7):2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reis C, Wang Y, Akyol O, Ho WM, Ii RA, Stier G, Martin R, Zhang JH. What’s new in traumatic brain injury: update on tracking, monitoring and treatment. Int J Mol Sci. 2015;16(6):11903–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomaidou D. Neural stem cell transplantation in an animal model of traumatic brain injury. Methods Mol Biol. 2014;1210:9–21. [DOI] [PubMed] [Google Scholar]
- 36. Shen WB, Plachez C, Tsymbalyuk O, Tsymbalyuk N, Xu S, Smith AM, Michel SL, Yarnell D, Mullins R, Gullapalli RP, Puche A, Simard JM, Fishman PS, Yarowsky P. Cell-based therapy in TBI: Magnetic retention of neural stem cells in vivo. Cell Transplant. 2016;25(6):1085–1099. [DOI] [PubMed] [Google Scholar]