Abstract
Stroke is the result of blockage or rupture of blood vessels in the brain and is the leading cause of death and disability in the world. Currently only a very limited number of therapeutic approaches are available for treatment of stroke patients, and the vast majority of neuroprotective agents that tested positively in pre-clinical studies failed in clinical trials. In recent years, the clinical value of the use of exosomes for stroke treatment has received widespread attention due their unique characteristics such as low immunogenicity, low toxicity and biodegradability, ability to cross the blood–brain barrier (BBB), and their important role in communication between cells. More and more evidence suggests that the secretion of exosomes is the mechanism underlying the protection induced by mesenchymal stromal cells (MSCs) after stroke. Exosomes are thought to support brain restoration and induce repairing effects, including neurovascular remodeling, and anti-apoptosis and anti-inflammatory effects. Recent reports have focused on the clinical application of exosomes as a potential drug delivery approach. This review focuses on the ability of exosomes to interrupt the stroke-induced pathologic processes of stroke, and on publications describing how to achieve more effective treatment of stroke with exosomes.
Keywords: stroke, exosome, microRNA
Introduction
Stroke is a devastating neurological disease with high mortality and disability. Strokes can be distinguished as ischemic, which is caused by blockage of blood vessels, or hemorrhagic stroke, induced by the rupture of blood vessels. The main type of stroke is ischemic (in China approximately 73.7% of all strokes are ischemic)1. The main goal of treatment for ischemic stroke is the restoration of blood flow as soon as possible after symptom onset. The two main approaches to achieve recanalization are intravenous thrombolytic therapy and endovascular intracranial thrombectomy, and both methods have a narrow therapeutic time window2,3. For hemorrhagic stroke, specific treatment includes removal of clots or intraventricular blood by minimally invasive surgery, and management of intracranial pressure can effectively reduce mortality4.
Thousands of promising neuroprotective drugs that tested positively in animal models have failed in clinical trials5–7. Therefore, it is urgent to find an alternative therapy for stroke. Exosomes are small (30–150 nm) extracellular vesicles: They contain different types of DNA, RNA, and proteins, and are surrounded by a phospholipid bilayer8. Exosomes are secreted by any cell type, and can be detected in bodily fluids including blood, plasma, serum, etc. Emerging evidence in recent years has shown that exosomes are a key mediator of intercellular communication, which participates in normal physiological processes and plays a role in the development and progression of disease, especially cancer9–13. Increasing evidence indicates that exosomes are closely involved in the modification of the tumor microenvironment, enhancement of tumor cell invasiveness, and formation of premetastatic niches by extracellular matrix remodeling, promoting angiogenesis and expression of integrins contained in the tumor exosomes10,14–17. Furthermore, exosomes can be used for therapeutic approaches such as immunotherapy and delivery of pharmacological agents or genes, and as a potential biomarker for cancer and neurodegenerative diseases17,18.
It is worth noting that exosomes have been extensively researched in stroke19–21. For instance, the secretion of exosomes by mesenchymal stem cells (MSCs) is considered a major mechanism by which MSCs promote neurovascular remodeling and recovery of neurological function after stroke. Upon release by exosomes, RNA, DNA, and proteins target neurovascular cells22 and mediate the neurorestorative effects of MSCs23–25. Here we review the application and possible mechanism of exosomes, especially in ischemic stroke, and discuss the key technologies and advantages of their application and transformation for clinical use.
Biological Characteristics of Exosomes
What are Exosomes?
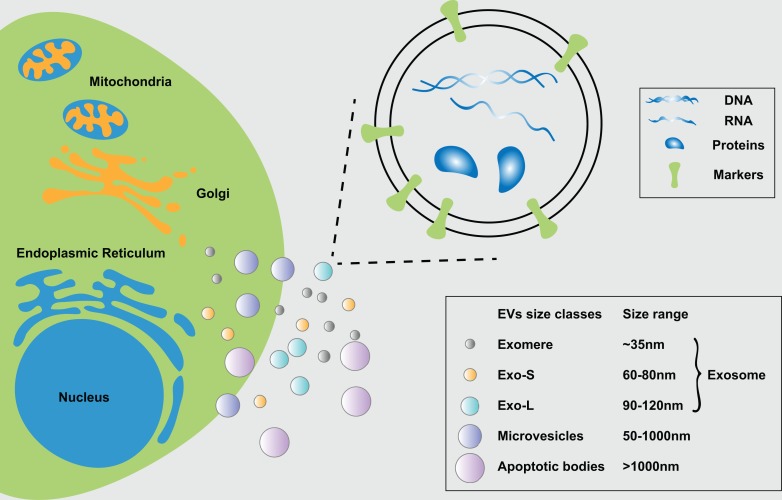
Exosomes are a subspecies of extracellular vesicles (EVs) that originate from multivesicular bodies (MVBs)26. EVs can be divided into three main types based on size, specific proteins, and intracellular sources: exosomes, microvesicles (MVs), and apoptotic bodies. Exosomes are 30–150 nm in diameter and have a cup shape or a uniform spherical shape. The most common markers of exosomes are tetraspanins (CD9, CD63, and CD81), MVB-related endosomal sorting complexes required for transport (ESCRT) proteins (Alix, TSG101), and heat shock proteins (HSPs) (HSP60, HSP70, HSPA5, CCT2, and HSP90)8. MVs are membrane-covered vesicles of various shapes with a diameter of 50–1000 nm or more, and they can be released to the extracellular matrix by budding from the plasma member. The main proteins of MVs are integrins, selectins, and CD40. Apoptotic bodies are larger than other types of EVs, and often have a diameter greater than 1000 nm, and are a product of apoptosis. Due to the lack of a standardized methods for analysis of EVs, some researchers will use the term “EVs” meaning a mixture of these types of vesicles25,27,28.
The technical limitations in efficient separation and detection of exosomal subpopulations have hindered the understanding of their heterogeneous nature and characterization. Recently, Zhang et al. identified two exosome subpopulations (large exosome vesicles, Exo-L, 90–120 nm; small exosome vesicles, Exo-S, 60–80 nm) by using asymmetric-flow field-flow fractionation (AF4). The authors discovered an abundant population of non-membranous nanoparticles termed “exomere” (smaller than 35 nm). They indicated that, since Hsp90-b is highly represented in exomeres, it may be a potential exomere marker, and HSC70/HSPA8 can serve as possible markers for Exo-S/L subpopulations29. Due to technical limitations, the heterogeneous nature of exosomes is not fully understood, and in this review we talk about the exosomes which are 30-150 nm in diameter.
Regulation of Exosome Secretion
Like other types of vesicles, EVs are released into the extracellular matrix by membrane vesicle fission and budding. Exosomes originate from MVBs; when the MVB fuses with the plasma membrane, the contents of the MVB are exocytosed into the extracellular matrix30,31.
Exosomes are specifically initiated within the endosomal system; early endosomes mature into late endosomes or MVBs, and the endosomal membrane is invaginated to produce intraluminal vesicles (ILVs) in the organelle lumen during this process. ILVs eventually mature into exosomes32. Exosomes can enclose protein, RNA, and other signaling molecules, but the mechanism by which signal molecules are sorted into the vesicles is still not clear. Exosomes can be present in all body fluids, such as blood, plasma, serum, cerebrospinal fluid, saliva, urine, and amniotic fluid. They can be isolated from effusions under various disease conditions, including bronchoalveolar lavage fluid, synovial fluid, pleural effusions, and ascites8,33. The contents of exosomes will change under different disease conditions, and can be used as biomarkers for diagnosis2,34–37.
The Role of Exosomes in Intercellular Communication
Almost all cell types secrete exosomes. Tumor cells, especially malignant cells, secrete significantly more exosomes than normal cells. Tumor-derived exosomes are involved in tumor development, angiogenesis, and invasion and metastasis30,38. Metastasis is a complex multi-step process involving invasive changes in tumor cells, angiogenesis, and microenvironmental formation for metastasis. For example, the sentinel lymph nodes induced by melanoma exosomes can increase the expression of a network of interconnected extracellular matrix factors that promote invasion and metastasis of cancer cells14,39. The notch signaling pathway is a relatively conserved signal pathway mediated by cell–cell contact. Delta-like 4 (Notch ligand) expression is up-regulated during angiogenesis, and recent studies have found that Delta-like 4 can be incorporated into exosomes and act on the surface of endothelial cells via exosomes, inhibiting notch signaling40.
It has been demonstrated that injection into normal mice of exosomes isolated from the serum of mice challenged with lipopolysaccharide (LPS) can induce neuroinflammation, suggesting that exosomes may act as a neuroinflammatory mediator in systemic inflammation41. Furthermore, microglia and microglia-derived exosomes, due to their extensive neurotoxicity and neuroprotection, play a critical role in the pathogenesis of Alzheimer’s disease42. Information transfer mediated by exosomes is a current research focus in basic experimental and clinical translational research.
Exosome Changes during Stroke
Pathophysiology of Ischemic Stroke
Ischemic brain injury is induced by oxygen and glucose deprivation of brain tissue in the infarct core. Shortly after onset the deprivation results in ATP deficiency43. Due to the lack of ATP, energy production breaks down and sodium–potassium pumps cannot maintain cell membrane potential. This results in depolarization and an increase in intracellular calcium ion concentration. The increase of intracellular calcium triggers such pathophysiological processes such as the overproduction of reactive oxygen species, phospholipases, and proteases, which in turn induces cell death, causing inflammation and immune response, and destruction of the blood–brain barrier (BBB)4.
The ischemic stroke lesion area can be divided into two major zones: the infarct core and the penumbra. The penumbra is located between the infarct core and normal tissue. While the brain cells in the infarct core are irreversibly damaged during the first few minutes, the penumbra can be salvaged. The restoration of blood flow can attenuate cell damage and progression of infarct into the penumbra area. The shorter the time to blood supply restoration, the smaller the damage from ischemia and the smaller chance of induction of reperfusion damage. Preservation of the penumbra appears to be a promising goal for novel neuroprotective strategies against stroke4,44.
The Role of Exosomes in Stroke
Compared with the study of exosomes in tumors, the application of exosomes in stroke is insufficiently investigated, and has mainly focused on the neuroprotective and neurorestorative effect of MSC-derived exosomes. However, the study of exosomes has been constrained because of the lack of a strict definition of what an exosome is, and the lack of an efficient and economical approach for isolation of exosomes.
Ischemia-reperfusion injury is aggravated with the prolongation of ischemia time. It is difficult to distinguish between transient ischemic attack (TIA) and ischemic stroke within the time window of thrombolysis. Some studies have found that plasma exosomal rno-miR-122-5p and rno-miR-300-3p differ greatly under different ischemic time conditions, and both can be used as potential biomarkers for TIA25. Some studies divide the stroke process into four periods according to time, namely the hyperacute phase ischemic stroke (HIS, within 6 h); the acute phase ischemic stroke (AIS, days 1–7); the sub-acute phase ischemic stroke (SIS, days 8–14); and the recovery phase ischemic stroke (RIS, days >14). Studies have shown that miRNAs in exosomes vary in different periods of stroke; while the plasma exosomal miR-21-5p levels in SIS and RIS were higher than those in controls, the levels of miR-30a-5p in HIS were significantly higher, and in AIS (days 1–3) were lower than controls. The plasma-derived exosomal miR-21-5p and miRNA-30a-5p in combination are suggested to be promising biomarkers for diagnosing ischemic stroke and distinguishing different periods of ischemic stroke45.
The recovery of stroke is associated with the development of neural stem cells (NSCs) and the reconstruction of neurovascular units46. Some studies have analyzed human NSC-derived exosomes and hypoxic pre-conditioning of human NSC-derived exosomes by second-generation sequencing technology, and compared the different expression profiles of miRNAs. After hypoxic pre-stimulation, the miR-98-3p in the human NSC-derived exosomes decreased significantly; NSC-derived exosomal microRNAs are associated with signaling pathways such as PI3K-Akt, Hippo, MAPK, mTOR, and endocytosis. NSC-derived exosomal miRNAs can be used as a basis for the diagnosis and treatment of stroke patients47. Based on the above studies, we can postulate that the contents of exosomes, especially the miRNA content, will change during the progress of stroke. The regulation of cellular process, molecular function, and changes in exosomal miRNAs can be used as diagnostic biomarkers of stroke.
Exosomes in Stroke Diagnosis and Therapy
Exosomes and Stroke Diagnosis
Exosomes are also used as potential biomarkers for the detection of pathological conditions. They carry nucleic acids and proteins that reflect the origin and pathophysiological condition of the parent cells. Furthermore, compared with miRNA directly in the blood, exosomal miRNAs are protected from degradation due to the presence of vesicular structures, making exosomal miRNA a more useful biomarker. Exosomes with specific cargo are present in all body fluids including blood, plasma, and serum, making them easily isolated and analyzed, and are attractive as biomarkers for diagnostic application48 (as shown in Table 1).
Table 1.
Exosomes as Biomarkers in the Diagnosis of Ischemic Stroke.
| Source | Disease model | Contents | potential target | Involved pathways | Ref.(PMID) |
|---|---|---|---|---|---|
| Primary hNSCs | OGD Model | 53 miRNAs were up-regulated, such as miR-214-3p 26 miRNAs were downregulated, such as miR-98-3p |
– | PI3K-Akt, Hippo, MAPK, mTOR, FoxO, Endocytosis, Rap1, and cGMP-PKG | 29843872 |
| MSCs | MCAO in rats | miR-133b | astrocytes | – | 22605481 |
| Plasma | patients with IS | microRNA-21-5p and microRNA-30a-5p | – | – | 29627835 |
| CSF and plasma | MCAO in rats | Rno-miR-122-5p and Rno-miR-300-3p | – | – | 29467645 |
| Plasma | patients with IS | miR-422a and miR-125b-2-3p | – | – | 28982331 |
| Serum | patients with AIS | miR-9 and miR-124 | – | IL-6 | 27661079 |
| Blood samples | AIS patients | miRNA-223 | – | – | 28289400 |
OGD: oxygen glucose deprivation; MSCs: mesenchymal stromal cells; CSF: cerebrospinal fluid; IS: ischemic stroke; AIS: acute ischemic stroke; MCAO: middle cerebral artery occlusion; hNSCs: human neural stem cells; PI3 K: phosphoinositide 3-kinase; AKT: protein kinase B; mTOR: mammalian target of rapamycin; FoxO: forkhead box; Rap1: Ras-related protein 1; cGMP-PKG: cGMP-dependent protein kinase; IL-6: Interleukin 6; “-”: indicates firm conclusions are difficult to draw.
The amount of plasma exosomal rno-miR-122-5p and rno-miR-300-3p differs greatly after different time of ischemia, and both can be used as potential biomarkers for TIA25. Li et al. examined exosomes in 55 ischemic stroke patients and 25 healthy volunteers; plasma exosomal levels of miR-422a and miR-125b-2-3p were examined to explore the potential predictive value in different IS phases (acute and sub-acute phases). The expression levels of plasma exosomal miR-422a and miR-125b-2-3p were significantly decreased in the sub-acute phase group, and the miR-422a levels were increased in the acute phase group as compared with the controls34.
The combination of plasma-derived exosomal miR-21-5p and miRNA-30a-5p is a promising biomarker for diagnosing IS and distinguishing the duration of ischemic stroke45. Exosomal miR-223, miR-9, and miR-124 are significantly higher in acute ischemic stroke patients compared with healthy people, and the expression of exosomal miR-223, miR-9, and miR-124 are positively correlated with National Institutes of Health Stroke Scale (NIHSS) scores. This means exosomal miR-223, miR-9, and miR-124 are promising biomarkers for diagnosing AIS and assessing the extent of ischemic injury49,50.
Exosomes and Stem Cell Therapy for Stroke
MSCs are pluripotent stem cells that have the ability to differentiate into a variety of tissue-specific cells, and can be isolated and extracted from bone marrow, placenta, umbilical cord, cord blood, and adipose tissue51. MSC therapy is very promising for stroke treatment. In addition to replacing damaged cells with MSCs, MSCs can also promote neuroplasticity, angiogenesis, and immunomodulation. Recent studies have found that MSCs achieve neurological recovery through exosomes2,52. Doeppner et al. compared the effects of MSCs with MSC-derived exosomes that were delivered to mice after local cerebral ischemia, and demonstrated that both MSCs and MSC-derived exosomes promote angiogenesis and long-term neuroprotective improvement 28 days after stroke53. The therapeutic effect of the MSC-exosome is consistent with that of MSCs53.
In acute ischemic stroke, inhibition of autophagy can ameliorate the inflammatory effects of hypoxia-ischemia, and exosomes secreted by adipose-derived stem cells (ADSCs) containing MiR-30d-5p inhibit autophagy-mediated oligodendrocyte differentiation to M1 for prevention of cerebral injury54. By co-culturing cortical neurons with glutamate excitotoxicity and adipose-derived MSCs (AMSCs) or conditioned-medium of AMSCs (AMSC-CM), compared with untreated control groups, it was found that the nerve cell damage of the experimental group was reduced, the release of LDH was decreased, and the number of apoptotic cells was decreased; both AMSCs and AMSC-CM similarly reduced nerve cell damage55.
Furthermore, some studies indicate that microRNA 133b (miR-133b) levels are increased after MSC therapy, and MSCs communicate with nerve cells and regulate nerve growth by transferring miR-133b via exosomes. Exosomes secreted by miR-133b-overexpressed MSCs can better promote the recovery of neurological function and increase neural plasticity by stimulating astrocyte secondary secretion of exosomes23,24,56. MSC-derived exosomes enriched with the miR-17-92 cluster increase neurological recovery and neural plasticity by activating the PI3K/Akt/mTOR/GSK-3β signaling pathway57.
Studies have demonstrated that MSCs mainly play a role in neuroprotection and repair by secreting exosomes. However, injection of MSCs may cause vascular occlusion, and there is always a risk of tumor development. As opposed to cell therapy, cell-free exosome therapy has no risk of occluding blood vessels, and exosomes themselves can cross the BBB and can be modified58 (as shown in Table 2). Despite some clear advantages, there are still some problems that need to be solved for the safe and efficient application of exosomes in the clinic, such as how to acquire them on a large scale and which specific mechanisms mediate exosome effects on neurological recovery2.
Table 2.
Exosomes as Therapy Agents in the Treatment of Ischemic Stroke.
| Source | Disease model | Contents | potential target | Assessment standards | Ref.(PMID) |
|---|---|---|---|---|---|
| BM-MSCs | Photothrombosis model in mice | miR-124 | Gli3 and STAT3 in ischemic tissue | Immunohistochemistry of Sox2, Nestin and DCX | 28624203 |
| miR-133b+ MSCs | MCAO in rats and OGD model in Primary Astrocyte | miR-133b | Astrocytes | A foot-fault test and a modified mNSS test. Immunohistochemical staining in the IBZ | 27677799 |
| miR-133b+ MSCs | MCAO in rats | miR-133b | Connective tissue growth factor and ras homolog gene family member A in the IBZ | The adhesive-removal test and foot-fault test for rats | 23630198 |
| miR-17-92+ MSCs | MCAO in rats | miR-17-92 | PTEN Akt, mTOR and GSK-3β | A mNSS and foot-fault tests, histochemistry, immunohistochemistry and Golgi-Cox staining in the IBZ | 28232590 |
| CDCs | Rabbit small-clot embolic stroke model | miR-146a, miR-181b, and miR-126 | Superoxide dismutase-2, | Clinical rating scores and quantal analysis | 29908146 |
| miR-30d-5p+ ADSCs | OGD and murine models of MCAO | miR-30d-5p | Microglial | Immunofluorescence and luciferase reporter assay | 29807362 |
BM-MSCs: bone marrow mesenchymal stem cells; MSCs: mesenchymal stem cells; CDCs: cardiosphere-derived cells; ADSCs: adipose-derived stem cells; MCAO: middle cerebral artery occlusion; OGD: oxygen and glucose deprived; Gli3: glioma-associated oncogene family zinc finger 3; STAT3: signal transducer and activator of transcription 3; IBZ: infarction border zone; PTEN: phosphatase and tensin homolog; AKT: protein kinase B; mTOR: mammalian target of rapamycin; GSK-3β: glycogen synthase kinase 3 beta; DCX: doublecortin; mNSS: modified neurologic severity score.
Compared with clinical trials of tumor-associated exosomes, clinical trials of exosomes in stroke are rare. Currently there is only one clinical trial—Safety and Efficacy of Allogenic Mesenchymal Stem Cells Derived from Exosome Enriched by miR-124 on Disability of Patients With Acute Ischemic—in Phases I and II, investigating the effects of exosome in stoke therapy (https://clinicaltrials.gov/ct2/show/NCT03384433?term=exosome&cond=stroke&rank=1).
Other Functions of Exosomes in Stroke
In the pharmacological treatment of stroke, drug safety and effective drug delivery to the lesion region remain problematic. As a drug vehicle, exosomes have many advantages, such as low immunogenicity, inherent stability, high transmission efficiency, and the ability to cross the BBB59. However, the insufficient targeting ability of exosomes limits their clinical application, and some researchers have modified the surface of the exosomes to increase their targeting ability. Enhanced immunosuppression and inhibition of apoptosis in the lesion region of the ischemic brain has been reported by binding of the c(RGDyK) peptide to the surface of exosomes60.
Exogenous therapy can amplify endogenous nerve repair processes through MSC-derived exosomes or micro-RNAs, thus achieving the purpose of neurorestorative effects after stroke61. In order to more effectively deliver miR-124 to the infarct site, some researchers have modified exosomes to fuse rabies virus glycoprotein (RVG) with exosomal protein lysosome-associated membrane glycoprotein 2b (Lamp2b), finding that RVG-exosomes can be used as a potential treatment for delivering gene drugs to the brain62. Exosomes from human cardiosphere-derived cells (CDCs) have many features that are potentially beneficial for treating acute ischemic stroke. In the small-clot rabbit embolic stroke model, intravenous injection of CDC-exosomes 1 h post-embolization effectively reduced behavioral deficits, and there was no risk of intracranial hemorrhage63.
Current Challenges in the Therapeutic Application of Exosomes
Due to the heterogeneity of populations and the complexity of stroke mechanisms, only a few pharmacological agents have succeeded in clinical trials and are suitable for the treatment of stroke patients64. Cell therapy in the sub-acute and chronic phases of stroke has demonstrated great potential. It helps to restore neurological function and improve patients’ quality of life65. At the same time, exogenous administration of MSCs always carries the risk of serious side effects, such as malignant transformation, tumor generation, or microvascular obstruction2,58. Recent studies have demonstrated that the therapeutic effects of MSC-based cell therapy can be mediated by the secretion of soluble factors, such as exosomes, etc. The therapeutic effects of MSC-exosomes are consistent with that of MSCs52,53. Webb et al. evaluated NSC EVs (over 90% are less than 200 nm in diameter) in a pig ischemic model and demonstrated for the first time that in a large animal model novel NSC EVs significantly improved neural tissue preservation and functional levels post-middle cerebral artery occlusion (MCAO)66. MSC-exosome may be a potential therapeutic approach in the treatment of stroke. Nevertheless, some problems must be solved before exosomes can be applied in clinical trials.
Firstly, it is essential to develop techniques to obtain exosomes on a large scale at a low cost. Chen et al. generated immortalized MSCs by transformation of the MYC gene; MSCs are the most prolific exosome producer of all cell types known to produce exosomes. Exosomes from MYC-transformed MSCs were able to reduce relative infarct size in a mouse model, and MYC transformation is practical strategy to solve the production of exosomes67,68. Recently, Mendt et al. reported the bioreactor-based, large-scale production of clinical-grade exosomes employing good manufacturing practice (GMP) standards69. Briefly, they collected large amounts of conditioned media through the bioreactor culture of bone marrow-derived MSCs, and exosomes were enriched by filtration and ultracentrifugation (UC)69.
Secondly, standard isolation techniques are required to obtain high-purity exosomes. There are many approaches to isolate exosomes, including UC, precipitation, two-phase isolation, etc. It is essential to highlight the advantages and disadvantages of each method. For example, UC can isolate exosomes from large volumes without additional chemicals. The difficulties of application of UC are associated in part with the expensive equipment and complexity of the procedure, and may be solved with the expansion of production scale, such as the GMP standards27,69.
Thirdly, further studies should look into improving the accuracy of targeted drug therapy by exosome delivery. Due to their physical properties, exosomes can be considered as a promising drug vehicle which allows drugs to cross even an intact BBB and reach the infarct area. Characteristics such as low immunogenicity, low toxicity, biodegradability, and ability to cross the BBB are the advantages of exosomes as an exogenous disease therapy. Modification of exosomes is a further promising option which could increase the accuracy of targeted drug therapy60,62.
Conclusion
Stroke is the one of leading causes of mortality and disability worldwide. Many neuroprotective agents have failed in clinical trials. In recent years the clinical potential of exosomes for stroke diagnosis and therapy has attracted widespread attention due to their unique characteristics. Currently, there is only one clinical trial related to use of exosomes as a stroke treatment, and there is not enough information to allow translation of exosome therapy into clinical practice. Further investigation both of the molecular processes induced by exosome and the generation of clinical-grade exosomes will certainly promote the development of new therapeutic strategies, and decrease mortality and improve the quality of life of stroke patients.
Figure 1.
The biological characteristics of the exosome. (a) According to their approximate diameter, EVs can be divided into three types: exosomes for 35–150 nm, microvesicles for 50–1000 nm, and apoptotic body, which are greater than 1000 nm. Exosomes can be separated exosomal subpopulations as exomere, Exo-L, and Exo-S. (b) Exosomes are small vesicles which contain cargos (DNA, RNA, and protein) surrounded by phospholipid bilayers. Some proteins that are abundant in nanoparticles can be exosome markers, such as tetraspanins (CD9, CD63, and CD81), MVB-related ESCRT proteins (Alix, TSG101) and heat shock proteins (HSP60, HSP70, HSPA5, CCT2, and HSP90)8,29.
Acknowledgment
This research was supported by the National Natural Science Foundation of China No. 81671130 to Qin Hu.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL, Investigators NE-C. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Cheng Q, Hu G, Deng T, Wang Q, Zhou J, Su X. Extracellular vesicles in mesenchymal stromal cells: a novel therapeutic strategy for stroke. Exp Ther Med. 2018;15(5):4067–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Z, Lu J, Liu WW, Manaenko A, Hou X, Mei Q, Huang JL, Tang J, Zhang JH, Yao H, Hu Q. Advances in stroke pharmacology. Pharmacol Ther. 2018;191:23–42. [DOI] [PubMed] [Google Scholar]
- 4. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasegawa Y, Nakagawa T, Uekawa K, Ma M, Lin B, Kusaka H, Katayama T, Sueta D, Toyama K, Koibuchi N, Kim-Mitsuyama S. Therapy with the combination of amlodipine and irbesartan has persistent preventative effects on stroke onset associated with BDNF preservation on cerebral vessels in hypertensive rats. Transl Stroke Res. 2016;7(1):79–87. [DOI] [PubMed] [Google Scholar]
- 6. Alhadidi Q, Bin Sayeed MS, Shah ZA. Cofilin as a promising therapeutic target for ischemic and hemorrhagic stroke. Transl Stroke Res. 2016; 7(1):33–41. [DOI] [PubMed] [Google Scholar]
- 7. Boltze J, Wagner DC, Henninger N, Plesnila N, Ayata C. Phase iii preclinical trials in translational stroke research: community response on framework and guidelines. Transl Stroke Res. 2016;7(4):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mincheva-Nilsson L, Baranov V, Nagaeva O, Dehlin E. Isolation and characterization of exosomes from cultures of tissue explants and cell lines. Curr Protoc Immunol. 2016;115:14 42 11–14 42 21. [DOI] [PubMed] [Google Scholar]
- 9. Carretero-González A, Otero I, Carril-Ajuria L, de Velasco G, Manso L. Exosomes: definition, role in tumor development and clinical implications. Cancer Microenviron. 2018;11(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manuel GE, Johnson T, Liu D. Therapeutic angiogenesis of exosomes for ischemic stroke. Int J Physiol Pathophysiol Pharmacol. 2017;9(6):188–191. [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49(5):1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352(1):33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun W, Luo JD, Jiang H, Duan DD. Tumor exosomes: a double-edged sword in cancer therapy. Acta Pharmacol Sin. 2018;39(4):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weidle UH, Birzele F, Kollmorgen G, Ruger R. The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics. 2017;14(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoshino A, Costa-Silva B, Shen TL. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorena U, Sandra B, Krizia S, Giuseppina F, Marco L, Carla E. Exosome-based strategies for diagnosis and therapy. Recent Patents CNS Drug Discovery (Discontinued). 2015;10(1):10–27. [DOI] [PubMed] [Google Scholar]
- 18. Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarmah D, Kaur H, Saraf J, Pravalika K, Goswami A, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P. Getting closer to an effective intervention of ischemic stroke: the big promise of stem cell. Transl Stroke Res. 2018;9(4):356–374. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. Mir-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8(4):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Napoli E, Borlongan CV. Recent advances in stem cell-based therapeutics for stroke. Transl Stroke Res. 2016;7(6):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dykstra-Aiello C, Jickling GC, Ander BP, Zhan X, Liu D, Hull H, Orantia M, Ho C, Stamova B. Intracerebral hemorrhage and ischemic stroke of different etiologies have distinct alternatively spliced mRNA profiles in the blood: a pilot RNA-seq study. Transl Stroke Res. 2015;6(4):284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26(2):243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xin H, Li Y, Buller B, Katakowski M, zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells (Dayton, Ohio). 2012;30(7):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li DB, Liu JL, Wang W, Luo XM, Zhou X, Li JP, Cao XL, Long XH, Chen JG, Qin C. Plasma exosomal miRNA-122-5p and miR-300-3p as potential markers for transient ischaemic attack in rats. Front Aging Neurosci. 2018;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biology. 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res Internat. 2018;2018:8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, Gill J. Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol. 2018;55(12):9280–9293. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Freitas D, Kim HS. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric-flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell. 2016;37(4):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. [DOI] [PubMed] [Google Scholar]
- 32. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplant. 2018;27(3):349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li DB, Liu JL, Wang W, Li RY, Yu DJ, Lan XY, Li JP. Plasma exosomal miR-422a and miR-125b-2-3p serve as biomarkers for ischemic stroke. Curr Neurovasc Res. 2017;14(4):330–337. [DOI] [PubMed] [Google Scholar]
- 35. Cui S, Cheng Z, Qin W, Jiang L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer. 2018;116:46–54. [DOI] [PubMed] [Google Scholar]
- 36. Reclusa P, Taverna S, Pucci M, Durendez E, Calabuig S, Manca P, Serrano MJ, Sober L, Pauwels P, Russo A, Rolfo C. Exosomes as diagnostic and predictive biomarkers in lung cancer. J Thorac Dis. 2017;9(suppl 13):S1373–S1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng G, Yuan Y, Wu S, He F, Hu Y, Luo B. MicroRNA let-7e is a potential circulating biomarker of acute stage ischemic stroke. Transl Stroke Res. 2015;6(6):437–445. [DOI] [PubMed] [Google Scholar]
- 38. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. [DOI] [PubMed] [Google Scholar]
- 39. Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. [DOI] [PubMed] [Google Scholar]
- 40. Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, Sargent I, Li JL, Harris AL. New mechanism for notch signaling to endothelium at a distance by delta-like 4 incorporation into exosomes. Blood. 2010;116(13):2385–2394. [DOI] [PubMed] [Google Scholar]
- 41. Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, Lan L, Kumar S, Wang X, Yue J, Liao FF. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation. 2018;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: Focus on microglia. Neuroscience. 2019;405:148–157. [DOI] [PubMed] [Google Scholar]
- 43. Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: a critical review. Stroke. 2012;43(8):2258–2263. [DOI] [PubMed] [Google Scholar]
- 44. Lee TY, Murphy BD, Aviv RI, Fox AJ, Black SE, Sahlas DJ, Symons S, Lee DH, Pelz D, Gulka IB, Chan R, Beletsky V, Hachinski V, Hogan MJ, Goyal M, Demchuk AM, Coutts SB. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37(9):2201; author reply 2203. [DOI] [PubMed] [Google Scholar]
- 45. Wang W, Li DB, Li RY, Zhou X, Yu DJ, Lan XY, Li JP, Liu JL. Diagnosis of hyperacute and acute ischaemic stroke: the potential utility of exosomal microRNA-21-5p and microRNA-30a-5p. Cerebrovasc Dis. 2018;45(5–6):204–212. [DOI] [PubMed] [Google Scholar]
- 46. Hao XZ, Yin LK, Tian JQ, Li CC, Feng XY, Yao ZW, Jiang M, Yang YM. Inhibition of notch1 signaling at the subacute stage of stroke promotes endogenous neurogenesis and motor recovery after stroke. Front Cell Neurosci. 2018;12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang GCL, Guo X, Wang H, Chen W, Wu G, Gu B, Miao W, Kong J, Jin X, Yi G, You Y, Su X, Gu N. Comparative analysis of microrna expression profiles of exosomes derived from normal and hypoxic preconditioning human neural stem cells by next generation sequencing. J Biomed Nanotechnol. 2018;14(6):1075–1089. [DOI] [PubMed] [Google Scholar]
- 48. Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ. Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal. 2015;2015:657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y. Increased brain-specific miR-9 and miR-124 in the serum exosomes of acute ischemic stroke patients. Plos One. 2016;11(9):e0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Y, Song Y, Huang J, Qu M, Zhang Y, Geng J, Zhang Z, Liu J, Yang GY. Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front Neurol. 2017;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181–197. [DOI] [PubMed] [Google Scholar]
- 52. Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig A-K, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translat Med. 2015;4(10):1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from miR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting m2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47(2):864–878. [DOI] [PubMed] [Google Scholar]
- 55. Hao P, Liang Z, Piao H, Ji X, Wang Y, Liu Y, Liu R, Liu J. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and gap-43 expression. Metab Brain Dis. 2014;29(1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells (Dayton, Ohio). 2013;31(12):2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mousavinejad M, Andrews PW, Shoraki EK. Current biosafety considerations in stem cell therapy. Cell J (Yakhteh). 2016;18(2):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos M, Fuentes B, Diekhorst L, Diez-Tejedor E, Gutierrez-Fernandez M. Role of exosomes as a treatment and potential biomarker for stroke. Transl Stroke Res. 2019;10(3):241–249. [DOI] [PubMed] [Google Scholar]
- 60. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. [DOI] [PubMed] [Google Scholar]
- 61. Venkat P, Chen J, Chopp M. Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab. 2018;0(00):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lapchak PA, Boitano PD, de Couto G, Marbán E. Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol. 2018;307:109–117. [DOI] [PubMed] [Google Scholar]
- 64. Neuhaus AA, Rabie T, Sutherland BA, Papadakis M, Hadley G, Cai R, Buchan AM. Importance of preclinical research in the development of neuroprotective strategies for ischemic stroke. JAMA Neurology. 2014;71(5):634–639. [DOI] [PubMed] [Google Scholar]
- 65. Chopp M. Orchestrating recovery: cell-based therapy for stroke. Trans Stroke Res. 2011;2(3):241. [DOI] [PubMed] [Google Scholar]
- 66. Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49(5):1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. [DOI] [PubMed] [Google Scholar]
- 68. Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, Padmanabhan J, Lee CN, de Kleijn DPV, Lim SK. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]