Abstract
Background
Monosodium glutamate (MSG) is used as a flavoring and food seasoning. Some studies have reported the oxidative effects of using this substance on various tissues.
Objective
This study has investigated the effects of MSG and the protective effect of vitamin C (vit C) on apoptosis of testicular germ cells and biochemical factors.
Materials and Methods
In this experimental study, 24 adult male Wistar rats were randomly divided into four groups: control (received distilled water), vit C group (150 mg/kg), experimental group 1 (MSG 3 gr/kg), experimental group 2 (MSG 3 gr/kg + vit C 150 mg/kg). The rats were gavaged for 30 days, and then were sacrificed, the right testis was isolated for biochemical examinations for the glutathione, malondialdehyde, and left testis used in histological experiments. Tunnel staining was used to determine the number of apoptotic cells.
Results
The results showed that apoptotic cells in the MSG group had a significant increase compared to the control group (P = 0.001), but the number of these cells in the MSG co-administered with vit C and vit C groups were significantly lower than the MSG group. Germinal epithelial thickness also decreased in MSG group compared to the control group.
Conclusion
MSG can lead to increase apoptotic changes in the germinal epithelial of the testicle, and vit C as an antioxidant can modify the pathological and biochemical changes induced by MSG.
Keywords: Apoptosis, Monosodium glutamate, Rat, Testis, Vitamin C
1. Introduction
Glutamate is known as current amino acids in nature that organize an important part of many peptides and proteins in most tissues. It is produced in the body and acts the main role in the metabolism of the body (1). Monosodium glutamate (MSG) is used as an additive for maintenance of food indices, such as the quality, taste, and durability. MSG is prepared from starch, sugar, beet, sugar cane, or molasses (2). It is used as a seasoning and flavoring in many countries. It may be used in packaged foods without mentioning the label 2. There is a controversy over the use of MSG in foods worldwide (3).
MSG can induce oxidative stress with the production of oxygen radicals and hydrogen peroxide, leading to oxidative DNA damage and peroxidation of cell membranes and cell death. Internal antioxidants including vitamins, minerals may inhibit reactive oxygen species or peroxidation of lipids (4).
Several studies have reported the toxic effects of MSG on human and animal tissues. MSG can cause to change in kidney function, liver, and lipid profile (5–7). Histological findings in the ovaries have demonstrated evidence of cellular hypertrophy, degenerative and atrophic changes that may be the cause of infertility in women (8).
Testicular tissue is one of the most sensitive tissues and can be affected by environmental risk factors (9). Infertility, testicular hemorrhage, morphological changes, and sperm production have been reported after the administration of MSG in male rats (10). Studies have shown the effect of MSG on testicular tissue and the overall structure by conventional staining but have not assessed the amount of apoptotic germinal epithelial cells yet.
The aim of this study was to evaluate the effect of MSG on testicular germ cell apoptosis and biochemical changes in testicular tissue as well as the protective effect of vitamin C (vit C) in concomitant administration with MSG.
2. Materials and Methods
Animals
To this study, we purchased 24 mature male Wistar rats (body weight (BW): 200 250 gr, age: 6–8 wk old) from the animal house of the Faculty of Medicine, Mashhad University, Iran. The rats were kept in a well-ventilated room under controlled conditions (22–25°C, 40–70% relative humidity, 12 hr light/darkness cycle) and away from any stressful conditions. They were allowed free access to food pellets and drinking water.
Chemicals
MSG (C5H8NNaO4 ((Negin Tejarat Payam Co., Iran under the license of Huifenghe, China), Vit C Vials (Osve Co., Iran), and TUNEL kit was purchased from Roche, Germany.
Experimental design
Animals were randomly allocated into four groups (N = 6) as follows:
Control group received distilled water;
Vit C group received vit C 150 mg/kg/BW/Day;
Experimental group 1 received MSG 3gr/kg/BW/Day; and
Experimental group 2 received MSG 3gr/kg/BW/Day and vit C 150 mg/kg/BW/Day (11, 12).
Treatments were done within 30 days by oral gavage mode. The BW of the rats was recorded weekly at the beginning and end of the experimental duration. The first day, when the animals were treated was considered experimental day 0. At the end of the 30 days of treatment, all animals were anesthetized with chloroform and the abdominal cavity was opened up to expose the testis. The testis tissues after perfusion of heart for full formalin penetration were quickly processed for light microscope investigations and biochemical assessments. The left testes were used for the test of TUNEL and right testes were used for evaluating the level of malondialdehyde (MDA) and glutathione (GSH).
Histological procedures of the testis
We dissected and weighted left testes quickly for doing histological techniques, fixed in 10% formalin. Afterward, the tissues were dehydrated in an ascending grade of alcohol, cleared in xylene, and embedded in paraffin. Using a rotator microtome, serial sections of 5µ thick were taken. Briefly, tissue sections were deparaffinized by xylene and were hydrated in the descending alcohols. Samples were incubated in hydrogen peroxide diluted with methanol (3% solution). They were then incubated with a solution of proteinase K diluted in PBS for 15 min at room temperature in a moist environment. Tissue sections were incubated with TUNEL solvent solution diluted with 50 ml of specific solvent for 2 h at 37°C in a moist environment. Then samples were incubated with a solution convert (Convert POD, HRP) at a rate of 50 ml for 60 min at 37°C in a moist environment. Tissue sections were incubated with a chromogen solution diaminobenzidine tetrachloride (DAB) for 15 min at room temperature. As a final step, sections were counterstained with hematoxylin for 10 sec, dehydrated in ascending alcohols, and were cleared with xylene. Apoptotic cells appeared in brown. Images were captured at 200 and 400 magnifications using Olympus light microscope and were transferred to a computer using a high-resolution camera (BX51, Japan). Morphometric methods were used to evaluate TUNEL-positive cells per unit area in testis by two treatment blinded observers. The number of TUNEL-positive cells was counted using grades unbiased frames. "We calculated the mean number of TUNEL-positive cells per unit area (NA) of testis in different groups of rats using the following formula (13):
In the above formula, “ΣQ” indicates the sum of counted particles appeared in sections, “a/f” indicates the area associated with each frame, and “ΣP” indicates the sum of frame associated points touching space."
Tissues homogenates and estimation of antioxidant parameters
After washing the testicular tissue and removing fatty parts, the tissues were homogeneous in 50 mµ sodium phosphate buffer (pH: 7.4) containing 0.1 mµ ethylenediaminetetraacetic acids (EDTA) on ice-cold. We separated supernatant after centrifugation at 5000 rpm for 20 min at 40°C. To analyze biochemical parameters, supernatant was used.
Reduced glutathione (GSH) assay
To determine the thiol groups, with the aid of Hu, Colorimetric method was used from 5, 5-dithiobis (2-nitrobenzoic acid). DTNB with thiol groups creates a yellow compound that has a maximum optical absorption in 412 nm wavelength and its Optical absorption coefficient is 13.6 Mm cm (14). Enzyme activity was expressed as mmol/gr tissue. For this purpose, 50 μl of homogenates were blended with 1 ml of 10 mM EDTA- Tris buffer and the absorbance was evaluated at 412 nm by using a spectrophotometer (Jenway 6105 UV/vis, UK) (A). To this solution was mixed 20 µl DTNB (10 mM), and after 10 min at the laboratory temperature, sample absorption was evaluated (A). Absorption of DTNB solution was evaluated as Blank alone (B). The total amount of thiol groups was calculated using the following formula:
Total of thiol groups
Measurement of MDA
The amount of MDA was determined in the homogenized tissue samples by the aid of the TBA method. The compound of MDA–TBA can be easily measured in a fluorimetric method (wavelength of irradiation/radiation = 485/535) (15). To prepare a standard curve, 2.39 µl tetraethoxypropane was added to 10 ml of distilled water. This solution was used to prepare dilutions of (3.12, 6.25, 12.5, 25, 50, 100, 200 µmol/ml) in different tubes.
In the start the work, we mixed 0.5 ml homogenated tissue with 0.5 ml of distilled water and 0.5 ml TBA. Then the compound was heated for 60 min in a boiling water bath. As soon as cooling the compound, 25 µl of HCL (PH = 1.6-1.7) and 1.5 ml of n-butanol was added to it and vortex-mixed for 1 min followed by centrifugation at 1000 gr for 10 min. At that point, we separated the upper liquid and transferred to fresh tubes and the absorbance level was read using a fluorimeter (PerkinElmer VICTOR X5 USA). We obtained a calibration curve using MDA tetrabutylammonium. The MDA levels were considered as nmol/gr tissue.
Ethical consideration
This study was approved by the Animal Ethics Committee of Mashhad University, according to the recommendations of the national guidelines (IR.MUMS.fm.REC.1395.611).
Statistical analysis
Statistical calculations were performed using SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc, Chicago, Illinois, USA). Statistical significance between groups was evaluated using one-way ANOVA analysis of variance. Differences between groups were determined by the Tukey test. Results were presented as mean SD with p < 0.05 considered to be statistically significant.
3. Results
Effect of MSG and Vit C on BW and testes' weight
Final BWs increased in all of the groups. The comparison of the final BWs with the initial BWs (weight changes) between groups revealed a significant difference in the MSG-treated group with the control group. Likewise, there was a significant difference in the MSG-treated group in comparison with the vit C group (p = 0.021).
A significant difference in the testes' weight of the rats was observed between groups after administration of MSG within a month (Table I).
Effect of MSG and vit C on GSH and MDA level in testis tissue
Evaluation MSG plus vit C group with the control group revealed a significant reduction in MDA level. Likewise, we revealed the MSG plus vit C group in compare with the MSG-treated group had a significant decrease in MDA level. This result indicate effective role of vit C in diminishing MDA levels. There was no significant difference in GSH levels between groups after the administration of MSG within a month (p > 0.05) (Table II).
Histological results
Effect of MSG and vit C on TUNEL-positive cell numbers
We recognized apoptotic cells in the testis of all groups by TUNEL staining. The control group contains only a few TUNEL-positive spermatogonia and primary spermatocyte. However, we saw the number and signal density of TUNEL-positive mentioned cells significantly increased in the MSG-treated group in comparison with the control group (p = 0.001). The reactivity and the number of apoptotic spermatogonia and primary spermatocyte reduced in the vit C-treatment group as compared with the MSG-treated group (p = 0.001). The apoptosis significantly decreased in the group treated with MSG co-administered with vit C as compared with the MSG-treated group (p = 0.001), and these revealed that vit C could prevent from spermatogonia and primary spermatocyte apoptosis (Figures 1 and 2).
Effect of MSG and vit C on germinal epithelial thickness
Germinal epithelial thickness significantly decreased in the MSG-treated group as compared with the control group (p = 0.036). Also, the germinal epithelial thickness in the MSG co-administered with vit C group increased as compared with the MSG group but not significantly (Figures 3 and 4).
Table 1.
Effects of MSG and vit C on initial body weight, final body weight, weight changes, and testes' weight*
|
| ||||
| Groups Parameter | Control | MSG | MSG + Vit C | Vit C |
| Initial Body Weight (gr) | 261.50 5.357 | 229.50 4.593 | 237.83 2.858 | 247.83 3.545 |
| Final Body Weight (gr) | 343.00 10.75 | 282.50 19.31 | 298.67 17.20 | 326.00 18.96 |
| Weight changes (%) | 31.417 | 23.144 | 25.738 | 31.983 |
| Testes' weight (gr) | 1.50 0.14 | 1.40 0.12 | 1.55 0.10 | 1.50 0.06 |
| white<bcol>5</ecol>Note: *Values are the mean standard deviation of the mean; Significantly different from control group (p < 0.05); Significantly different from MSG group (p < 0.05); Significantly different from MSG + vit C group (p < 0.05) | ||||
| white<bcol>5</ecol>MSG: Monosodium glutamate; Vit C: Vitamin C | ||||
Table 2.
Effects of MSG and vit C on biochemical factors (MDA, GSH) *
|
| ||||
| Groups Parameter | Control | MSG | MSG + Vit | Vit C |
| MDA (nmol/gr) | 3.04 0.80 | 3.95 1.24 | 1.37 0.77 | 2.94 1.19 |
| GSH (mmol/gr) | 0.71 0.03 | 0.62 0.04 | 0.69 0.06 | 0.71 0.09 |
| white<bcol>5</ecol>Note: *Values are mean standard deviation of the mean; Significantly different from control group (p < 0.05); Significantly different from MSG group (p < 0.05); | ||||
| white<bcol>5</ecol>MSG: Monosodium glutamate; Vit C: Vitamin C; MDA: malondialdehyde; GSH: glutathione | ||||
Figure 1.
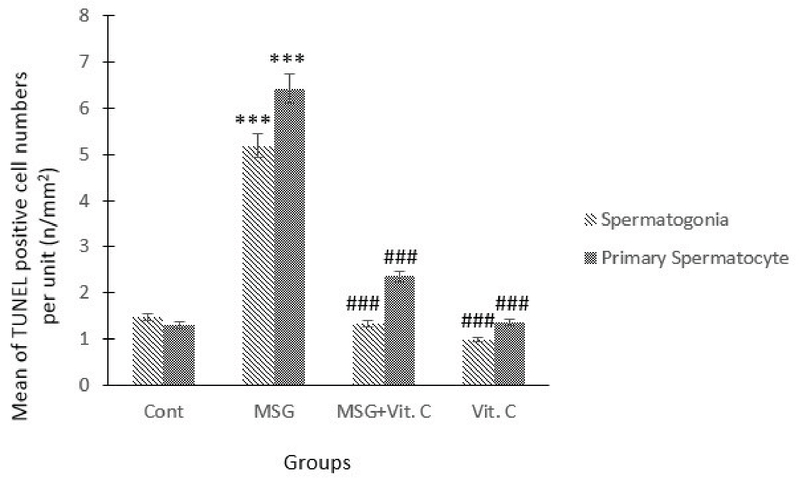
Effects of treatment of MSG, vit C, and their combinations on TUNEL-positive spermatogonia and primary spermatocyte numbers in the testis tissues of rats. Note: ***represents a significant difference between the MSG-treated group and the control group (p 0.001); ###represents a significant difference in vit C and MSG + vit C group with MSG group (p 0.001).
Figure 2.
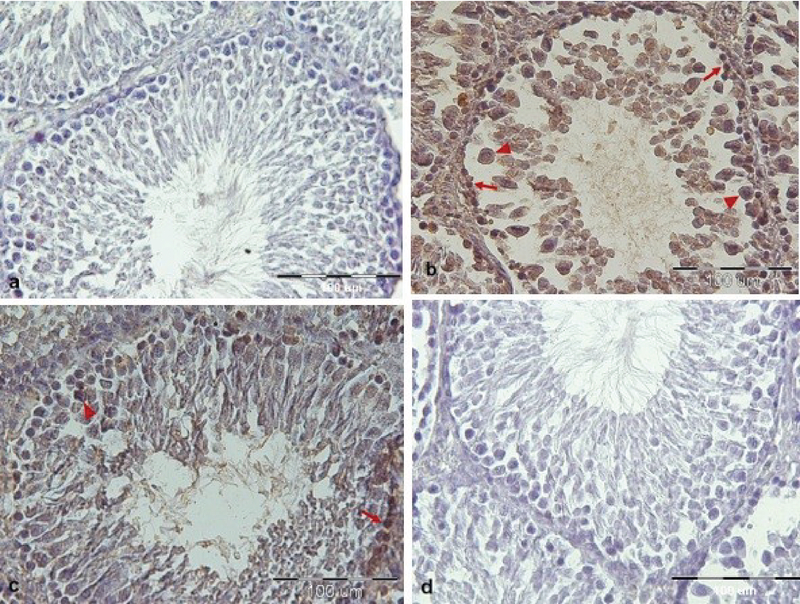
Histological sections of testicular tissues are showing TUNEL-positive spermatogonia and primary spermatocyte in different groups. (a) Control group. (b) MSG-treated group, MSG caused the increase in the apoptotic cells compared to the control group. (c) MSG co-administered with the vit C group, so that the number of apoptotic cells was decreased. (d) vit C group. Arrows show TUNEL-positive spermatogonia, arrows head show TUNEL-positive primary spermatocyte and L (lumen).
Figure 3.
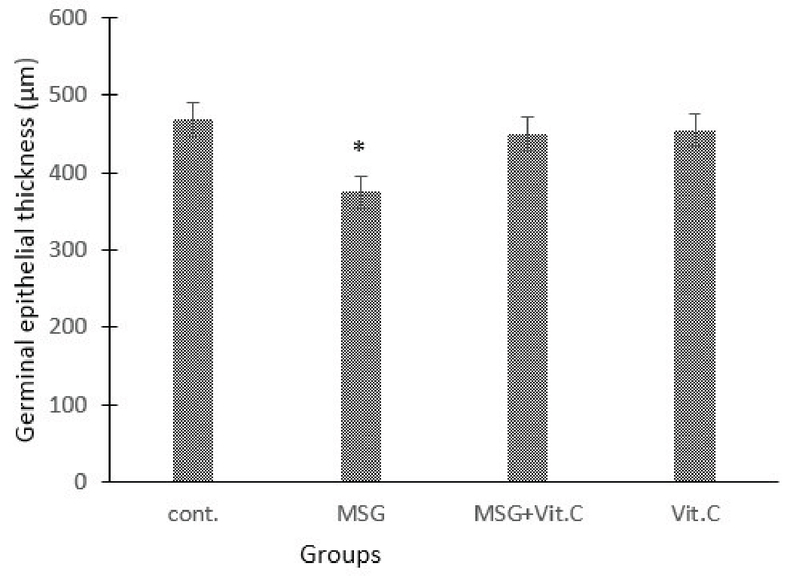
Effects of treatment of MSG, vit C, and their combinations on germinal epithelial thickness in the testis tissues of rats. Note: *represents a significant difference between MSG-treated group and control group (p 0.05).
Figure 4.
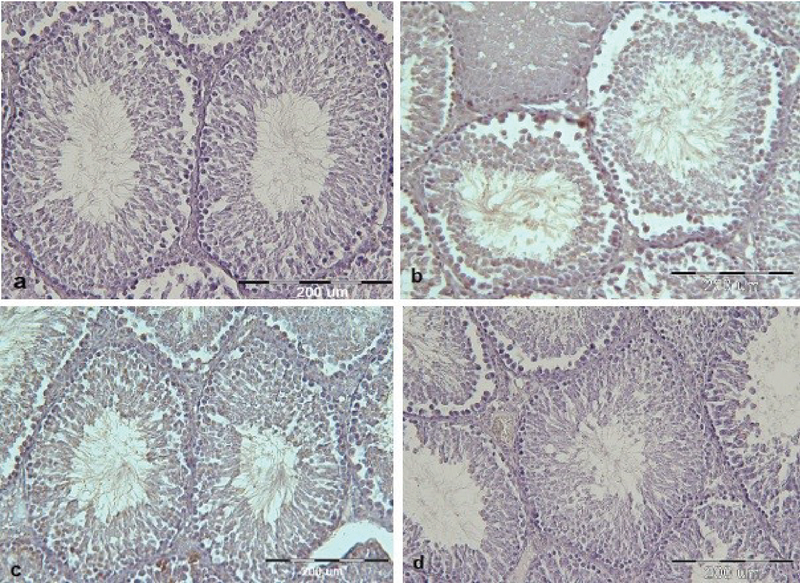
Cross-sections of testicular tissues show germinal epithelial thickness in different groups. (a) Control group. (b) MSG-treated group. (c) MSG co-administered with vit C group. (d) Vit C group. Germinal epithelial thickness shows a decrease in the MSG-treated group in comparison with other groups. Disorganization and distortion epithelial of the base membrane are seen in the MSG-treated group.
4. Discussion
MSG is a common ingredient used in processed foods to enhance favor. In recent decades, MSG has been the target of studies to determine whether it is harmful to the human body. Following the absorption of MSG, glutamate receptors, α-ketoglutarate dehydrogenase and cystine-glutamate antiporter are the main causes of damage in different tissues (1, 6). Studies have found that the metabolism of high levels of glutamate can cause increased lipid peroxidation and decreased levels of major antioxidant enzymes (2, 4).
In the present study, the study group (MSG group) was compared to the control group. The biochemical parameters (MDA and GSH) in the study group changed after the use of MSG, although it was not significant, However, many studies have shown that MSG leads to the production of free radicals and related reactive oxygen species. Cellular lesions are induced by reactive oxygen species reaction with proteins and lipids, which is harmful to the human body. Furthermore, the use of MSG has been linked to metabolic disturbance and oxidative damage of tissues, which could explain the pathophysiology of many disorders or illnesses (5–7). In the present study, a low dose of 3 g/kg of MSG did not cause oxidative stress, and subsequently did not affect the activity of enzymatic antioxidants and lipid peroxidation. However, more studies should be done on antioxidant enzymes. It should be noted that MSG affects tissues through various mechanisms and oxidative stress is one of its harmful effects.
Our observation has shown that the BW of the rats increased in all groups, although the increase was lower in the MSG group than in the control group. Kondoh and his colleague found that the animals that consumed MSG were reported to have significantly less weight gain and reduced fat deposition (fat mass) and plasma leptin levels compared to the rats that consumed only water, and they linked it to GLU-sensing mechanisms (16). Also, the weight of the testicles in the MSG group was not significant compared to the control group, Ochiogu and colleagues reported that the absence of change in testis weight was probably because mature animals (adult rats) were used in the study (17).
In agreement with this result, Iamsaard and colleagues showed that the oral administration of MSG did not affect testicular weight (12). Ekaluo and colleagues and Sakr and colleagues reported that MSG-treatment caused reduction of testes' weight, epididymis weight, sperm count, and germ cell height (18, 19).
In our study also, the height of epithelial germinal decreased in the MSG group. The degeneration of seminiferous tubules and their germinal epithelium detected in this study may be due to apoptosis caused by MSG. This agrees with the results of studies that imputed pathologies on other tissue to apoptosis after MSG consumption (20, 21).
Many studies have shown the existence of glutamate system including metabotropic (mGlu) and ionotropic glutamate receptors and transporters in different tissues (22, 23). Storto and colleagues showed that mGlu5 and mGlu1 receptors are expressed in rat and human testes or human sperm (22). Activation of mGlu5 receptors has the possibility to produce intracellular Ca waves in cells, which activates many of the reactions that play a basic role in permanence, differentiation, and cell growth (23–25). Therefore, the presence of excess glutamate because of the consumption of monosodium causes severe activation of the glutamate receptors. On the other hand, when Ca in the cell increases or high amounts of calcium enter the organelles such as the endoplasmic reticulum, nucleus, and mitochondria, calcium-dependent enzymes, such as proteases and endonucleases (caspases) become active and provide preliminaries for apoptosis (26, 27). Hence, apoptosis occurs, which is another mechanism of MSG in testicular tissues.
MSG absorption has mostly neurotoxic effects in the brain and it affects the function of the hypothalamic-pituitary-gonadal axis. Therefore, following monosodium consumption, the testis is directly affected by glutamate receptors and can degenerate through the hypothalamus-pituitary-gonadal axis (25). Because of the effect of monosodium on the central nervous system, this axis and subsequently the levels of testosterone, follicle–stimulating hormone and luteinizing hormone, also change, causing a change in spermatogenesis.
When damage occurs, by using enzymatic and non-enzymatic antioxidants that are present in the cells, the damage is repaired, although it may not be fully completed. This study showed that ascorbic acid (vit C) was effective in ameliorating the effect of MSG in apoptotic cells. Pavlovic and colleagues showed that vit C decreased apoptosis in rat thymocytes by increasing the quantity of viable cells and up-regulating the expression of Bcl-2 protein. Hence, the presence of Bcl-2 is essential in the antioxidant defense against apoptosis. Vit C has an important role in the free radical scavengers and protection of cells as an antioxidant and participates in the synthesis of leukotrienes and collagen (28). Reducing the level of vit C in the testicular tissue and subsequently causing cell damage can be considered as another mechanism of MSG effect (29).
5. Conclusion
Considering the importance of the issue of reproduction and its dependence on the lifestyle and according to our study results, vit C as an antioxidant can improve pathological and biochemical changes in testis, but not always. Hence, it may be better to reduce the use of monosodium in the foods or to remove it completely.
Conflict of Interest
There is no conflict of interests
Acknowledgments
This research project was funded by a grant from the Research Council of Mashhad University of Medical Sciences. It was conducted under Contract No. 950996. The authors would like to thank Mr. Jalili-nik for his technical assistance in the Biochemistry Lab.
References
- 1.Walker Ronald, Lupien John R. The Safety Evaluation of Monosodium Glutamate. The Journal of Nutrition. 2000;130(4):1049S–1052S. doi: 10.1093/jn/130.4.1049s. [DOI] [PubMed] [Google Scholar]
- 2.Hamza Reham Z., Al-Harbi Mohammad S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicology Reports. 2014;1:1037–1045. doi: 10.1016/j.toxrep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry-Unaeze Helen Nonye. Update on food safety of monosodium l -glutamate (MSG) Pathophysiology. 2017;24(4):243–249. doi: 10.1016/j.pathophys.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Ahluwalia P., Tewari K., Choudhary P. Studies on the effects of monosodium glutamate (MSG) on oxidative stress in erythrocytes of adult male mice. Toxicology Letters. 1996;84(3):161–165. doi: 10.1016/0378-4274(95)03612-1. [DOI] [PubMed] [Google Scholar]
- 5. El-Ezaby MM, Abd-El Hamide N-AH, El-Maksoud MAE, Shaheen EM, Embashi MMR. Effect of some food additives on lipid profile, kidney function and liver function of adult male albino rats. J Bas Environ Sci 2018; 5: 52-59.
- 6.Sharma Amod. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: a mini-review. Journal of Biomedical Science. 2015;22(1) doi: 10.1186/s12929-015-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helal Eman G. E., El-Sayed Rasha A. A., El-Gamal Mariam S. Assessment of the Physiological Changes Induced by Sodium Nitrite, Annatto or Mono Sodium Glutamate in Male Albino Rats. The Egyptian Journal of Hospital Medicine. 2017;67(1):330–335. doi: 10.12816/0036644. [DOI] [Google Scholar]
- 8.Eweka Ao, Eweka A, Om’Iniabohs Fae. Histological studies of the effects of monosodium glutamate on the fallopian tubes of adult female wistar rats. Annals of Biomedical Sciences. 2011;9(1) doi: 10.4314/abs.v9i1.66569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdollahzadeh Azad, Kianifard Davoud, Vafaei Saiah Gholamreza. Study of the Long-Term and Dose Dependent Effects of Methylphenidate and Monosodium Glutamate on the Hormonal Alterations of the Pituitary-Testicular Axis and Sperm Analysis in Adolescence Rats. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Veterinary Medicine. 2017;74(1):75. doi: 10.15835/buasvmcn-vm:12607. [DOI] [Google Scholar]
- 10. Oforofuo IAO, Onakewhor JUE, Idaewor PE. The effect of chronic administration of MSG on the histology of the adult Wistar rat testes. Bioscience Research Communications 1997; 9: 30-56.
- 11.Husarova Veronika, Ostatnikova Daniela. Monosodium Glutamate Toxic Effects and Their Implications for Human Intake: A Review. JMED Research. 2013:1–12. doi: 10.5171/2013.608765. [DOI] [Google Scholar]
- 12. Iamsaard S, Sukhorum W, Samrid R, Yimdee J, Kanla P, Chaisiwamongkol K, et al. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Med Acad 2014; 43: 3-9. [DOI] [PubMed]
- 13.Sargazi Z, Nikravesh Mr, Jalali M, Sadeghnia Hr, Rahimi Anbarkeh F. The Protective Effect of Vitamin E on Serum and Erythrocytes Cholinesterase Levels in Poisoning of Diazinon, in adult female rats. Journal of North Khorasan University of Medical Sciences . 2016;7(4):801–812. doi: 10.29252/jnkums.7.4.801. [DOI] [Google Scholar]
- 14.Sargazi Z, Nikravesh Mr, Jalali M, Sadeghnia Hr, Rahimi Anbarkeh F. The Protective Effect of Vitamin E on Serum and Erythrocytes Cholinesterase Levels in Poisoning of Diazinon, in adult female rats. Journal of North Khorasan University of Medical Sciences . 2016;7(4):801–812. doi: 10.29252/jnkums.7.4.801. [DOI] [Google Scholar]
- 15.Hosseini Mahmoud, Anaeigoudari Akbar, Beheshti Farimah, Soukhtanloo Mohammad, Nosratabadi Reza. Protective effect against brain tissues oxidative damage as a possible mechanism for beneficial effects of L-arginine on lipopolysaccharide induced memory impairment in rats. Drug and Chemical Toxicology. 2017;41(2):175–181. doi: 10.1080/01480545.2017.1336173. [DOI] [PubMed] [Google Scholar]
- 16.Kondoh Takashi, Torii Kunio. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague–Dawley rats. Physiology & Behavior. 2008;95(1-2):135–144. doi: 10.1016/j.physbeh.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Ochiogu Izuchukwu, Ogwu David, Uchendu Chukwuka, Okoye Chidozie, Ihedioha John, Mbegbu Edmund. Effects of monosodium-L-glutamate administration on serum levels of reproductive hormones and cholesterol, epididymal sperm reserves and testicular histomorphology of male albino rats. Acta Veterinaria Hungarica. 2015;63(1):125–139. doi: 10.1556/avet.2015.011. [DOI] [PubMed] [Google Scholar]
- 18.Ekaluo U.B., Ikpeme E.V., Ibiang Y.B., Amaechina O.S. Attenuating Role of Vitamin C on Sperm Toxicity Induced by Monosodium Glutamate in Albino Rats. Journal of Biological Sciences. 2013;13(4):298–301. doi: 10.3923/jbs.2013.298.301. [DOI] [Google Scholar]
- 19. Sakr SA, Badawy GM. Protective effect of curcumin on monosodium glutamate-induced reproductive toxicity in male albino rats. Global J Pharmacol 2013; 7: 416-422.
- 20.Li Han-Min. Effects of Zuogui Wan on neurocyte apoptosis and down-regulation of TGF-β1expression in nuclei of arcuate hypothalamus of monosodium glutamate -liver regeneration rats. World Journal of Gastroenterology. 2004;10(19):2823. doi: 10.3748/wjg.v10.i19.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pavlovic V, Cekic S, Sokolovic D, Djindjic B. Modulatory effect of monosodium glutamate on rat thymocyte proliferation and apoptosis. Bratisl Lek Listy 2006; 107: 185-191. [PubMed]
- 22. Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, Dell'Albani P, et al. Expression of metabotropic glutamate receptors in the rat and human testis. J Endocrinol 2001; 170: 71-78. [DOI] [PubMed]
- 23. Pavlovic V, Cekic S, Kocic G, Sokolovic D, Zivkovic V. Effect of monosodium glutamate on apoptosis and Bcl-2/Bax protein level in rat thymocyte culture. Physiol Res 2007; 56: 619-626. [DOI] [PubMed]
- 24.Miglio Gianluca, Varsaldi Federica, Dianzani Chiara, Fantozzi Roberto, Lombardi Grazia. Stimulation of group I metabotropic glutamate receptors evokes calcium signals and c-jun and c-fos gene expression in human T cells. Biochemical Pharmacology. 2005;70(2):189–199. doi: 10.1016/j.bcp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Alalwani Aisha D. Monosodium glutamate induced testicular lesions in rats (histological study) Middle East Fertility Society Journal. 2014;19(4):274–280. doi: 10.1016/j.mefs.2013.09.003. [DOI] [Google Scholar]
- 26.Meier Pascal, Finch Andrew, Evan Gerard. Apoptosis in development. Nature. 2000;407(6805):796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- 27.Rajaei F, Karja Nwk, Agung B, Wongsrikeao P, Taniguchi M, Murakami M, Sambuu R, Nii M, Otoi T. Analysis of DNA Fragmentation of Porcine Embryos Exposed to Cryoprotectants. Reproduction in Domestic Animals. 2005;40(5):429–432. doi: 10.1111/j.1439-0531.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 28. Pavlovic V, Pavlovic D, Kocic G, Sokolovic D, Sarac M, Jovic Z. Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratisl Lek Listy 2009; 110: 205-209. [PubMed]
- 29. Nayanatara A, Vinodini N, Damodar G, Ahemed B, Ramaswamy C, Shabarinath M, et al. Role of ascorbic acid in monosodium glutamate mediated effect on testicular weight, sperm morphology and sperm count in rat testis. J Chin Clin Med 2008; 3: 1-5.


