Abstract
Rac1, a small G protein, regulates physiological insulin secretion from the pancreatic β-cell. Interestingly, Rac1 has also been implicated in the onset of metabolic dysfunction of the β-cell under the duress of hyperglycemia (HG). This study is aimed at the identification of interaction partners of Rac1 in β-cells under basal and HG conditions. Using co-immunoprecipitation and UPLC-ESI-MS/MS, we identified 324 Rac1 interaction partners in INS-1832/13cells, which represent the largest Rac1 interactome to date. Furthermore, we identified 27 interaction partners that exhibited increased association with Rac1 in β-cells exposed to HG. Western blotting (INS-1832/13 cells, rat islets and human islets) and co-immunoprecipitation (INS-1832/13 cells) further validated the identity of these Rac1 interaction partners including regulators of GPCR-G protein-effector coupling in the islet. These data form the basis for future investigations on contributory roles of these Rac1-specific signaling pathways in islet β-cell function in health and diabetes.
Keywords: Racl, Pancreatic β-cell, Hyperglycemic conditions, Proteomics, Protein-protein interactions, UPLC-ESI-MS/MS, Insulin secretion, Apoptosis, Diabetes mellitus
1. Introduction
Insulin secretion from the pancreatic β-cell is regulated principally by the extracellular concentration of glucose. However, the molecular and cellular mechanisms underlying the stimulus-secretion coupling of glucose-stimulated insulin secretion (GSIS) remain only partially understood. It is widely accepted that GSIS is mediated largely via the generation of soluble second messengers, such as cyclic nucleotides and hydrolytic products synthesized by phospholipases A2, C and D (Jitrapakdee et al., 2010; Prentki et al., 2013; Berggren and Leibiger, 2006; Regazzi et al., 2016; Wang and Thurmond, 2009). The principal signaling cascade involves the glucose-transporter protein (i.e., Glut-2)-mediated entry of glucose into the β-cell resulting in an increase in the intracellular ATP/ADP ratio that is consequential to glucose metabolism via the glycolytic and the tricarboxylic acid cycle pathways. Such an increase in ATP levels culminates in the closure of membrane-associated ATP-sensitive potassium channels resulting in membrane depolarization followed by influx of the extracellular calcium through the voltage-gated calcium channels on the plasma membrane. A net increase in the intracellular calcium that occurs via the influx of extracellular calcium into the cytosolic fraction of the stimulated β-cell, in addition to the mobilization of calcium from the intracellular storage compartments, has been shown to play critical roles in GSIS (Jitrapakdee et al., 2010; Prentki et al., 2013; Berggren and Leibiger, 2006; Regazzi et al., 2016; Wang and Thurmond, 2009).
Multiple studies have provided convincing evidence to suggest that small G-proteins (e.g., Cdc42 and Rac1) play a significant regulatory role in cytoskeletal remodeling thereby favoring mobilization of secretory granules to the plasma membrane for fusion and release of their cargo into circulation. Published evidence also suggests novel regulatory roles for ADP-ribosylation factor 6 (Arf6) in insulin secretion from the islet β-cell (Kalwat and Thurmond, 2013; Kowluru, 2010, 2017). In this context, specific regulatory proteins/factors for G-proteins, namely guanine nucleotide exchange factors (GEFs; Tiam1, Vav2, β-PIX, Epac and ARNO) and guanine nucleotide dissociation inhibitors (GDIs; Rho GDIα, caveolin-1) have been identified and studied extensively in the islet β-cell (Wang and Thurmond, 2009; Kalwat and Thurmond, 2013; Kowluru, 2010, 2017; Jayaram et al., 2011). In further support of key regulatory roles for Rac1 in physiological insulin secretion in rodent and human islets (Kalwat and Thurmond, 2013; Kowluru, 2010, 2017) are the studies by Asahara et al. (2013) demonstrating impaired glucose tolerance and hypoinsulinemia in Rac1-null (βRac1−/−) mice. Consistent with findings described above, only glucose-induced, but not KCl-induced, insulin secretion was inhibited significantly in islets from βRac1−/− mice. The β-cell mass or islet density remained unchanged in these mice. siRNA-mediated knockdown of Rac1 in INS-1 cells also resulted in a significant defect in glucose-induced, but not KCl-induced, insulin secretion. Based on these findings, it was concluded that Rac1 plays a key regulatory role in insulin secretion primarily by regulating cytoskeletal organization (Asahara et al., 2013). In this context, Greiner et al. (2009) provided evidence to suggest that Rac1-null mice exhibited marked alterations in islet morphogenesis. Taken together, the above-described findings from multiple laboratories involving pharmacological and molecular biological tools as well as knockout animal models provide compelling evidence for novel regulatory roles for Rac1 in islet function, including GSIS (Wang and Thurmond, 2009; Kalwat and Thurmond, 2013; Kowluru, 2010, 2017; Jayaram et al., 2011; Asahara et al., 2013; Greiner et al., 2009).
It is noteworthy that, in addition to its positive modulatory role in insulin secretion, Rac1 has also been implicated in the metabolic dysregulation of the β-cell, specifically at the level of phagocyte-like NADPH oxidase (Nox2)-mediated generation of reactive oxygen species (ROS) thereby creating oxidative stress, mitochondrial dysfunction culminating in the functional abnormalities and eventual demise of the islet β-cell (Kowluru and Kowluru, 2014; Newsholme et al., 2009; Xiang et al., 2010). Data accrued from several recent investigations have implicated sustained activation of Rac1, which is seen under metabolic stress conditions (e.g., chronic hyperglycemia, lipotoxicity and exposure to biologically active sphingolipids like ceramide and proinflammatory cytokines), promotes activation of stress kinases (e.g., p38, JNK1/2 and p53) leading to β-cell dysfunction (Syed et al., 2010, 2011; Sidarala et al., 2015; Sidarala and Kowluru, 2017a, 2017b; Subasinghe et al., 2011; Kowluru and Kowluru, 2018). Together, these findings have led us to propose both “friendly” and “unfriendly” roles of Rac1 in islet β-cell function (Kowluru, 2011). Despite the aforestated evidence for critical regulatory roles of Rac1, potential identity and involvement of interaction between Rac1 and other proteins/factors in islet β-cells under hyperglycemic conditions remain an important, but understudied area of islet biology.
Co-immunoprecipitation (Co-IP) followed by mass spectrometry based proteomics has emerged as a powerful approach to map proteinprotein interactions (Marcilla and Albar, 2013; Wepf et al., 2009). However, most of these studies have utilized protein overexpression and/or epitope tagged bait proteins (Geetha et al., 2011). Recently, we developed a straightforward, label-free approach combining Co-IP with HPLC-ESI-MS/MS, without use of protein overexpression or protein tags, to investigate changes in the abundance of endogenous protein associate with a bait protein (Caruso et al., 2014). Earlier studies from our laboratory have optimized this label-free approach, and discovered novel IRS-1 interaction partners in skeletal muscle from healthy, obese non-diabetic and type 2 diabetic patients (Caruso et al., 2014). We have also used this approach to identify novel interaction partners of protein phosphatase 2A in insulin-secreting pancreatic β-cells (Zhang et al., 2016). In the current study, we used this proteomics approach to identify protein interaction partners of Rac1 in insulin-secreting INS-1832/13 cells exposed to basal or HG conditions. We also validated findings from proteomics approach by immunological approaches (Co-IP and Western blotting) in INS-1832/13 cells, normal rat islets and human islets. Our findings from these studies identified novel interaction partners for Rac1 under normal conditions. We also identified increased association of specific signaling proteins with Rac1 in INS-1832/13 cells exposed to HG conditions that may promote functional dysregulation and apoptosis in these cells.
2. Materials and methods
Antibodies directed against G protein-coupled receptor kinase-interacting protein 1 (GIT1; SC-365084), GIT1/2 (SC-135925), β-Pak–interacting exchange factor (β-PIX; SC-393184), Gαq11/14 (SC-365906) and Karyopherin-α2 (SC-55538) were from Santa Cruz Biotechnology, Inc. Anti-Rac1 GAP (A5298) was from Abclonal. Normal mouse IgG (No. 12–371) and Rac1 antibody (No. 05–389) were from Millipore. Co-Immunoprecipitation kit was obtained from Thermofisher Scientific.
2.1. Rat islets and human islets
Islets from normal male Sprague Dawley rats (~ 6 weeks old; Harlan Laboratories, Oxford, MI, USA) were isolated by the collagenase digestion method (Jayaram et al., 2011; Syed et al., 2011; Sidarala et al., 2015; Sidarala and Kowluru, 2017a). Human islets were obtained from PRODO Laboratories (Irvine, CA, USA). Islets from two human donors were used in these studies. The first donor was a 49-year old Hispanic male (64”, 179 lbs with a BMI of 30.3 and HbA1c of 5.8; Prodo Labs# HP-18196–01). The second donor was a 54-year old Caucasian male (71”, 160 lbs with a BMI of 22.3 and HbA1c of 5.8; Prodo Labs# HP-18164–01). Studies involving human islets were conducted according to the guidelines established by the US Department of Health and Human Services/NIH and were approved by the Research Service Biosafety Committee at the John D. Dingell VA Medical Center. All protocols employed in these studies were reviewed and approved by the Institutional Animal Care and Use Committee at Wayne State University and the Research Service Biosafety Committee at the John D. Dingell VA Medical Center.
2.2. INS-1832/13 cell culture and lysis
INS-1832/13 cells were kindly provided by Prof. Chris Newgard. INS-1832/13 cells were cultured in RPMI-1640 medium containing 10% FBS supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol and 10 mM HEPES (pH 7.4). Prior to the treatment with basal glucose (LG; 2.5 mM) or high glucose (HG; 25 mM), cells were starved overnight in a low glucose/low serum growth medium (2.5 mM glucose and 2.5% FBS). The cells were then treated with growth medium containing 10% FBS with either 2.5 mM or 25 mM glucose for 24–48 h, and were homogenized in RIPA lysis buffer or IP lysis buffer containing protease inhibitors. Protein quantification was done as previously described (Zhang et al., 2016).
2.3. Identification of Rac1 interaction partners by Co-IP and HPLC-ESI-MS/MS
This is carried out according to the methods we published earlier (Caruso et al., 2014; Zhang et al., 2016) with modifications due to a newer mass spectrometry instrument, a Thermo Finnigan LTQ-Orbitrap Lumos, was used to generate UPLC-ESI-MS/MS data (see ESM Methods). In brief, the proteomics’ studies were conducted as follows: immunoprecipitation of the “bait” protein (Rac1), at the endogenous level; followed by 1D-SDS-PAGE to separate co-interaction proteins; ingel trypsin digestion to generate peptide fragments; and UPLC-ESI-MS/ MS analysis to identify co-immunoprecipitating proteins. Multiple biological comparisons and immunoprecipitation of NIgG (as non-specific control) were used to minimize false positives. Extensive bioinformatics and literature search were used to integrate biological and proteomics data and to identify pathways/functional categories, in which identified Rac1 interaction partners were involved, that were impacted by high glucose treatment. Additional experimental details are provided in Supplemental Materials.
2.4. Bioinformatics
Pathway analysis on high glucose-responsive Rac1 interaction partners were performed using Ingenuity Pathway Analysis (Ingenuity Systems, Inc., Redwood City, CA; www.ingenuity.com/), a bioinformatics analysis software package (Zhang et al., 2016) that contains biological and chemical interactions and functional annotations created by manual curation of the scientific literature (Zhang et al., 2016).
Reported Rac1 partners in rat, mouse and/or human cells/tissues were retrieved from 3 large interaction databases, STRING (https://string-db.org/), INTACT (http://www.ebi.ac.uk/intact) and BioGRID (https://thebiogrid.org/) and mapped to the Rac1 partners identified in this study. Default settings were used for the 3 databases except for the String database, for which only the interaction partners with minimum interaction score of 0.90 were considered. In addition, the approach used to identify the interaction and PMIDs for corresponding references in INTACT and BioGRID databases were also retrieved, if such information is available.
Subcellular localization enrichment analysis: was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8, https://david.ncifcrf.gov/.
2.5. Confirmation of Rac1 interaction partners by Co-IP and Western blotting
INS-1832/12 cells treated with either LG or HG were lysed in IP lysis buffer. Equal amounts of lysates prepared from these cells were precleared by rotation for 30 min at 4 °C with 10 μl of a 50% (w/v) slurry of protein G or A/G-agarose beads. After centrifugation, the precleared lysates were incubated overnight with antibodies directed against various signaling proteins or with normal mouse IgG along with protein G or A/G agarose beads at 4 °C with constant rotation. Precleared cell lysate without antibody was used as a negative control. Following incubation, beads were washed in IP lysis buffer, boiled in SDS sample buffer for 5 min and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membrane. Relative abundance of proteins in the immunoprecipitates was determined by Western blotting.
2.6. Western blotting
Following incubations, the cells were harvested and lysed in RIPA buffer containing 1 mg/ml protease inhibitor cocktail, 1 mM NaF, 1 mM PMSF and 1 mM Na3VO4. Cellular lysate proteins (30–50 μg) were separated by SDS-PAGE and electro-transferred onto the nitrocellulose membrane. The membranes were then blocked with 1% casein in 0.2X PBS or 3% BSA in PBS-T for 1 h at room temperature. Blots were then incubated overnight at 4 °C with appropriate primary antibody (1:3000 dilutions for GIT1, GIT1/2, β-PIX, Gα11/14, and karyopherin-α2 and 1:000 dilution for RacGAP1 in 1.5% BSA in PBS-T). The membranes were washed three times for 5 min each with PBS-T and probed with appropriate HRP-conjugated secondary antibody in 1.5% BSA in PBS-T at room temperature for 1 h. After washing, the target proteins were detected by chemiluminescence.
2.7. Statistical analysis of experimental data
All the data were presented as mean ± SEM from number of experiments as indicated in the text. The statistical significance of the differences between the experimental conditions was determined by t-test or ANOVA where appropriate. The p values < 0.05 was considered as statistically significant.
3. Results
3.1. Identification of Rac1 interaction partners in INS-1832/13 cells
As indicated in Fig. 1, INS-1832/13 cells were exposed to either LG or HG conditions for 48 h. Lysates derived from these cells were precleared with normal IgG, followed by co-immunoprecipitation with Rac1 antiserum. The proteins eluted from both anti-Rac1 and NIgG protein-A beads were resolved on 1D-SDS-PAGE and subjected to in-gel trypsin digestion. The resulting peptides were desalted and analyzed by UPLC-ESI-MS/MS.
Fig. 1. Experimental design of the study.

INS-1832/13 cells were treated with basal (2.5 mM) or HG (25 mM) for 48 h. Rac1 is immunoprecipitated and the proteins associated with Rac1 were identified by HPLC-ESI-MS/MS (see Methods for additional details).
As depicted in Fig. 2, a total of 1588 proteins were identified in anti-Rac1 immuno-precipitates with minimum 2 unique peptides with FDR at 0.01 in at least one Rac1 immunopreciptates after excluding common contaminants. Among those, 435 proteins had an enrichment ratio greater than 10. Out of these, 324 proteins were identified with a peak area (PA) in more than half (e.g., > 4 out of 8) Rac1 immunoprecipitates, which were classified as Rac1 interaction partners (Table 1 and Supplemental Table 1). To the best of our knowledge, this represents the largest Rac1 interactome to date in the pancreatic β-cell. Among the 324 Rac1 interaction partners, 39 were listed as Rac1 interaction partners in rat, mouse and/or human cells/tissues in the 3 large interaction partner databases, while 285 were not, and thus appeared to be novel.
Fig. 2.
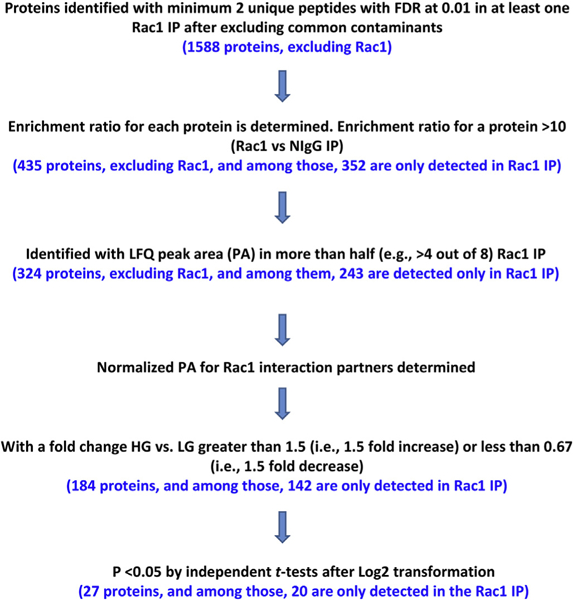
Determination of interaction partners of Rac1 in INS-1832/13 cells exposed to basal or HG conditions.
Table 1.
The 324 proteins that met the criteria for classification as Racl interaction partners.
| Gene Name | Protein Name | Mol. Weight [kD] | Enrichment ratio | Reported as an interaction partner of Racl in 3 large interaction databasesa |
|---|---|---|---|---|
| A1I3 | Alpha-1-inhibitor 3 | 165.76 | Infiniteb | |
| AIM | Alpha-1-macroglobulin | 167.12 | Infinite | |
| ACIN1 | Apoptotic chromatin condensation inducer 1 | 151.04 | Infinite | |
| ACSL1 | Long-chain-fatty-acid-CoA ligase 1 | 78.18 | Infinite | |
| ACTA1 | Actin, alpha | 42.05 | Infinite | Yes |
| ACTG1 | Actin, cytoplasmic 2 | 41.79 | 13000 | Yes |
| ACTN1 | Alpha-actinin-1 | 102.96 | 40 | Yes |
| ACTN3 | Actinin alpha 3 | 103.04 | Infinite | Yes |
| ACTN4 | Alpha-actinin-4 | 104.91 | 13 | Yes |
| ACTR3 | Actin-related protein 3 | 47.58 | 22 | Yes |
| AFDN | Afadin | 207.68 | 51 | |
| AHNAK | AHNAK 1 | 581.12 | Infinite | |
| ALDOA | Fructose-bisphosphate aldolase A | 39.35 | Infinite | |
| AP2M1 | AP-2 complex subunit mu | 49.65 | Infinite | |
| AQR | Aquarius intron-binding spliceosomal factor | 170.76 | Infinite | |
| ARHGAP11A | Rho GTPase-activating protein 11A | 109.72 | Infinite | Yes |
| ARHGEF6 | Rho guanine nucleotide exchange factor 6 | 89.78 | Infinite | Yes |
| ARHGEF7 | Rho guanine nucleotide exchange factor 7 | 79.81 | Infinite | Yes |
| ARPC1A | Actin-related protein 2/3 complex subunit 1A | 41.60 | Infinite | Yes |
| ARPC5 | Actin-related protein 2/3 complex subunit 5 | 16.32 | Infinite | Yes |
| ASCC3L1 | U5 small nuclear ribonucleoprotein 200kDa helicase | 244.87 | 190 | |
| ATP2A1 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 | 109.41 | Infinite | |
| ATP5A1 | ATP synthase subunit alpha, mitochondrial | 59.75 | Infinite | |
| ATXN2 | Ataxin 2 | 117.30 | 36 | |
| BAT1A | Class I histocompatibility antigen, Non-RT1.A alpha-1 chain | 38.95 | Infinite | |
| BCAS2 | BCAS2, pre-mRNA-processing factor | 26.10 | 46 | |
| BCLAF1 | BCL2-associated transcription factor 1 | 184.71 | Infinite | |
| C1QB | Complement C1q subcomponent subunit B | 26.65 | Infinite | |
| C3 | Complement C3 | 186.32 | Infinite | |
| CAD | DNA fragmentation factor subunit beta | 243.37 | Infinite | |
| CAMK2B | Calcium/calmodulin-dependent protein kinase type II subunit beta | 72.83 | Infinite | Yes |
| CAMSAP3 | Calmodulin-regulated spectrin-associated protein family, member 3 | 132.03 | Infinite | |
| CCNA2 | Cyclin A2 variant | 43.86 | Infinite | |
| CDK5RAP3 | CDK5 regulatory subunit-associated protein 3 | 57.13 | Infinite | |
| CELF2 | CUGBP Elav-like family member 2 | 56.96 | Infinite | |
| CHUK | Conserved helix-loop-helix ubiquitous kinase (Predicted) | 84.79 | Infinite | |
| CIRBP | Cold-inducible RNA-binding protein | 18.61 | Infinite | |
| CKM | Creatine kinase M-type | 43.02 | 36 | |
| CLINT1 | Enthoprotin | 52.14 | 230 | |
| CLP1 | Polyribonucleotide 5-hydroxyl-kinase Clp1 | 47.74 | Infinite | |
| CLTB | Clathrin light chain B | 25.12 | Infinite | |
| CLTC | Clathrin heavy chain 1 | 191.56 | 16 | Yes |
| CLU | Clusterin | 51.42 | Infinite | |
| CMAS | N-acylneuraminate cytidylyltransferase | 48.13 | Infinite | |
| CMSS1 | Uncharacterized protein C3orf26 homolog | 31.50 | Infinite | |
| CNOT1 | CCR4-NOT transcription complex, subunit 1 | 266.86 | Infinite | |
| CORO2A | Coronin | 61.99 | Infinite | |
| CORO2B | Coronin | 54.96 | Infinite | |
| CP | Ceruloplasmin | 123.67 | Infinite | |
| CPSF1 | Cleavage and polyadenylation specific factor 1, 160kDa (Predicted), isoform CRA_a | 160.75 | Infinite | |
| CPSF4 | Cleavage and polyadenylation specificity factor subunit 4 | 27.42 | Infinite | |
| CREBBP | CREB-binding protein | 265.63 | Infinite | |
| CSDE1 | Cold shock domain-containing protein E1 | 88.89 | 14 | |
| CSNK1D | Casein kinase I isoform delta | 49.12 | Infinite | |
| CTTN | Cortactin isoform B | 61.08 | 190 | Yes |
| DBN1 | Drebrin | 72.60 | 100 | |
| DCAF7 | DDB1 and CUL4-associated factor 7 | 38.93 | Infinite | |
| DDX1 | ATP-dependent RNA helicase DDX1 | 82.50 | 31 | |
| DDX17 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 17, isoform CRA_a | 72.64 | 22 | |
| DDX23 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 23 (Predicted), isoform CRA_b | 95.50 | Infinite | |
| DDX3Y | DEAD-Box Helicase 3 | 73.15 | Infinite | |
| DDX41 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 41 | 69.80 | Infinite | |
| DDX47 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 47, isoform CRA_a | 50.70 | 12 | |
| DDX6 | DEAD-box helicase 6 | 54.24 | 13 | |
| DHX9 | DEAH (Asp-Glu-Ala-His) box polypeptide 9 (Predicted) | 131.73 | 140 | |
| DIDO1 | Death inducer-obliterator 1 | 243.29 | Infinite | |
| DKC1 | H/ACA ribonucleoprotein complex subunit 4 | 56.58 | Infinite | |
| DNAJC9 | DnaJ (Hsp40) homolog, subfamily C, member 9 (Predicted) | 29.98 | Infinite | |
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 | 182.77 | Infinite | |
| DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific | 166.82 | Infinite | |
| EDC4 | Enhancer of mRNA-decapping protein 4 | 163.25 | 12 | |
| EFHD2 | EF-hand domain-containing protein D2 | 26.76 | Infinite | |
| EHMT1 | Euchromatic histone lysine methyltransferase 1 | 138.91 | Infinite | |
| EHMT2 | Euchromatic histone lysine methyltransferase 2 | 131.83 | Infinite | |
| EIF2C2 | Protein argonaute-2 | 99.54 | Infinite | |
| EIF3D | Eukaryotic translation initiation factor 3 subunit D | 63.99 | Infinite | |
| EIF4G1 | Eukaryotic translation initiation factor 4 gamma, 1 | 175.70 | 18 | |
| ELAVL2 | ELAV-like protein 2 | 42.53 | Infinite | |
| ELAVL4 | ELAV-like protein 4 | 42.37 | Infinite | |
| ELP2 | Elongator complex protein 2 | 91.75 | Infinite | |
| ELP3 | Elongator complex protein 3 | 62.36 | Infinite | |
| ENO3 | Beta-enolase | 47.01 | Infinite | |
| EP300 | E1A-binding protein | 77.34 | Infinite | |
| EPB4.1L3 | Erythrocyte protein band 4.1-like | 107.07 | 14 | |
| ERI1 | 3–5 exoribonuclease 1 | 39.58 | Infinite | |
| ETV6 | Transcription Factor ETV6 | 53.03 | Infinite | |
| EXOSC2 | Exosome component 2 | 32.69 | Infinite | |
| EXOSC5 | Exosome component 5 | 25.22 | Infinite | |
| EXOSC8 | Exosome component 8 | 26.14 | Infinite | |
| FAM120A | Family with sequence similarity 120A | 122.14 | 30 | |
| FAM160A2 | FTS and Hook-interacting protein | 86.27 | Infinite | |
| FAM98A | Protein FAM98A | 55.07 | Infinite | |
| FHOD3 | Formin homology 2 domain-containing 3 | 168.46 | Infinite | |
| FIP1L1 | Pre-mRNA 3-end-processing factor FIP1 | 64.96 | Infinite | |
| FLII | Flightless I homolog | 144.86 | Infinite | |
| FLNB | Filamin, beta (Predicted) | 277.84 | Infinite | Yes |
| FXR2 | FMR1 autosomal homolog 2 | 74.37 | 58 | |
| FYN | Tyrosine-protein kinase Yes | 60.73 | Infinite | Yes |
| G3BP2 | GTPase activating protein (SH3 domain) binding protein 2 | 50.77 | Infinite | |
| GAK | Cyclin-G-associated kinase | 143.71 | Infinite | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 35.83 | Infinite | |
| GAPVD1 | GTPase-activating protein and VPS9 domains 1 | 165.16 | 23 | |
| GEMIN5 | Gem (nuclear organelle)-associated protein 5 | 166.06 | 51 | |
| GIT1 | ARF GTPase-activating protein GIT1 | 85.23 | Infinite | Yes |
| GIT2 | G protein-coupled receptor kinase interacting ArfGAP 2 | 84.52 | Infinite | Yes |
| GKAP1 | G kinase-anchoring protein 1 | 41.93 | Infinite | |
| GNAQ | Guanine nucleotide-binding protein G(q) subunit alpha | 42.16 | Infinite | |
| GPR158 | Probable G-protein-coupled receptor 158 | 134.83 | Infinite | |
| GRAMD1A | GRAM domain-containing protein 1A | 89.52 | Infinite | |
| GRAMD3 | GRAM domain-containing protein 3 | 48.05 | Infinite | |
| GRSF1 | G-rich RNA sequence-binding factor 1 | 52.91 | 16 | |
| GTSE1 | G-2 and S-phase-expressed 1 | 79.98 | Infinite | |
| H1FX | H1 histone family, member X | 20.49 | Infinite | |
| H2AFJ | Histone H2A | 14.15 | Infinite | |
| HIRA | Histone cell cycle regulator | 111.68 | Infinite | |
| HNRNPC | Heterogeneous nuclear ribonucleoprotein C | 33.06 | 24 | |
| HNRNPH3 | Heterogeneous nuclear ribonucleoprotein H3 (2H9) (Predicted), isoform CRA_c | 36.87 | Infinite | |
| HNRNPL | Heterogeneous nuclear ribonucleoprotein L | 67.90 | 62 | |
| HNRNPM | Heterogeneous nuclear ribonucleoprotein M | 77.63 | 13 | |
| HNRNPR | Heterogeneous nuclear ribonucleoprotein R | 70.87 | Infinite | |
| HNRNPUL2 | Heterogeneous nuclear ribonucleoprotein U-like 2 | 84.86 | 15 | |
| HSPA5 | 78 kDa glucose-regulated protein | 72.35 | Infinite | |
| IKBKAP | Elongator complex protein 1 | 149.20 | Infinite | |
| IKBKB | Inhibitor of nuclear factor kappa-B kinase subunit beta | 86.89 | Infinite | |
| IKBKG | NF-kappa-B essential modulator | 48.07 | Infinite | |
| ILF2 | Interleukin enhancer-binding factor 2 | 43.06 | 13 | |
| ILF3 | Interleukin enhancer-binding factor 3 | 98.08 | 120 | |
| INA | Alpha-internexin | 56.12 | Infinite | |
| INPPL1 | Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 | 139.14 | Infinite | |
| ITGB6 | Integrin beta-6 | 85.96 | Infinite | |
| KAB | KARP-1-binding protein 1 | 125.96 | Infinite | |
| KEAP1 | Kelch-like ECH-associated protein 1 | 69.40 | Infinite | |
| KHDRBS1 | KH domain-containing, RNA-binding, signal transduction-associated protein 1 | 48.32 | Infinite | |
| KIDINS220 | Kinase D-interacting substrate of 220 kDa | 196.41 | Infinite | |
| KIF23 | Kinesin-like protein | 107.99 | Infinite | |
| KIF2A | Kinesin-like protein KIF2A | 83.93 | Infinite | |
| KIFC1 | Kinesin-like protein KIFC1 | 76.14 | Infinite | |
| KLHL22 | Kelch-like protein 22 | 78.70 | Infinite | |
| KLHL7 | Kelch-like protein 7 | 65.97 | Infinite | |
| KPNA1 | Importin subunit alpha-1 | 60.14 | Infinite | |
| KPNA4 | Importin subunit alpha | 57.92 | Infinite | Yes |
| KPNA7 | Importin subunit alpha-7 | 59.89 | Infinite | Yes |
| LAD1 | Ladinin-1 | 57.40 | Infinite | |
| LANCL2 | LanC lantibiotic synthetase component C-like 2 | 50.97 | Infinite | |
| LARP1 | La ribonucleoprotein domain family, member 1 | 106.94 | 41 | |
| LARP5 | La ribonucleoprotein domain family, member 4B | 81.08 | 15 | |
| LARS | Leucyl-tRNA synthetase | 134.28 | 11 | |
| LDHA | L-lactate dehydrogenase | 36.45 | Infinite | |
| LIMA1 | Epithelial protein lost in neoplasm | 83.80 | 95 | Yes |
| LIMCH1 | LIM and calponin homology domains 1 | 121.50 | Infinite | |
| LMO7 | LIM domain 7 | 156.76 | 220 | |
| LOC100359980 | Similar to Myosin light chain 1 slow a (Predicted) | 22.81 | Infinite | |
| LOC100360682 | Small nuclear ribonucleoprotein polypeptide E | 10.76 | Infinite | |
| LRRFIP2 | Leucine-rich repeat flightless-interacting protein 2 | 49.77 | Infinite | |
| LYAR | Cell growth-regulating nucleolar protein | 43.68 | Infinite | |
| LZTS2 | Leucine zipper putative tumor suppressor 2 | 72.58 | Infinite | Yes |
| MAGMAS | Mitochondrial import inner membrane translocase subunit TIM16 | 13.77 | Infinite | |
| MATR3 | Matrin-3 | 94.50 | 70 | |
| MISP3 | MISP Family Member 3 | 23.49 | Infinite | |
| MLLT10 | Histone lysine Mmethyltransferase DOT1L cofactor | 103.18 | Infinite | |
| MLLT6 | MLLT6, PHD Finger Containing | 99.17 | Infinite | |
| M0V10 | Mov10 RISC complex RNA helicase | 113.76 | Infinite | |
| MPRIP | Myosin phosphatase Rho-interacting protein | 117.01 | Infinite | |
| MRPL11 | 39S ribosomal protein L11, mitochondrial | 20.75 | Infinite | |
| MRPL16 | 39S ribosomal protein L16, mitochondrial | 28.90 | Infinite | |
| MRPL21 | Mitochondrial ribosomal protein L21 (Predicted), isoform CRA_a | 23.39 | Infinite | |
| MRPS22 | Mitochondrial ribosomal protein S22 | 41.24 | Infinite | |
| MTA2 | Metastasis-associated gene family, member 2 | 74.96 | Infinite | |
| MUC13 | Mucin-13 | 57.54 | Infinite | |
| MYEF2 | Myelin expression factor 2 | 63.38 | 35 | |
| MYH10 | Myosin-10 | 229.00 | 1800 | Yes |
| MYH14 | Myosin heavy chain 14 | 228.91 | 220 | Yes |
| MYH4 | Myosin-4 | 222.69 | 38 | |
| MYH6 | Myosin-6 | 223.38 | Infinite | |
| MYH7 | Myosin-7 | 222.90 | Infinite | |
| MYH9 | Myosin-9 | 226.34 | 39 | Yes |
| MYL1 | Myosin light chain 1/3, skeletal muscle isoform | 20.68 | 250 | |
| MYL12B | Myosin regulatory light chain 12B | 19.78 | Infinite | Yes |
| MYL6 | Myosin light polypeptide 6 | 17.01 | 13 | Yes |
| MYLK | Myosin light chain kinase | 214.91 | Infinite | Yes |
| MYLPF | Myosin regulatory light chain 2, skeletal muscle isoform | 18.97 | Infinite | |
| MYO18A | Myosin XVIIIa | 233.38 | Infinite | |
| MYO1B | Unconventional myosin-Ib | 131.92 | 67 | |
| MYO1C | Unconventional myosin-Ic | 119.81 | 44 | Yes |
| MYO1D | Unconventional myosin-Id | 116.09 | 250 | |
| MY05A | Unconventional myosin-Va | 212.15 | Infinite | |
| MZT2B | Mitotic spindle organizing protein 2B | 16.41 | Infinite | |
| NAB1 | NGFI-A-binding protein 1 | 54.03 | Infinite | |
| NAP1L1 | Nucleosome assembly protein 1-like 1 | 45.37 | Infinite | |
| NCBP1 | Nuclear cap-binding protein subunit 1 | 91.91 | 14 | |
| NCK2 | Non-catalytic region of tyrosine kinase adaptor protein 2 (Predicted) | 42.91 | Infinite | Yes |
| NDUFA8 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 8 | 22.12 | Infinite | |
| NHP2 | H/ACA ribonucleoprotein complex subunit 2 | 17.29 | Infinite | |
| NO66 | Lysine-specific demethylase NO66 | 67.45 | Infinite | |
| N0L5A | Nucleolar protein 5A | 65.40 | 27 | |
| NOL6 | Neuroprotective protein 1 | 128.07 | Infinite | |
| NOP16 | Nucleolar protein 16 | 21.12 | Infinite | |
| N0P58 | Nucleolar protein 58 | 60.07 | 32 | |
| NPAP60 | Nuclear pore complex protein Nup50 | 49.75 | Infinite | |
| NSUN4 | NOL1/NOP2/Sun domain family, member 4 (Predicted) | 42.61 | Infinite | |
| NUFIP2 | NUFIP2, FMR1-interacting protein 2 | 75.66 | Infinite | |
| NUP153 | Nuclear pore complex protein Nup153 | 152.83 | Infinite | |
| OTUD4 | OTU deubiquitinase 4 | 122.72 | Infinite | |
| PABPC1 | Polyadenylate-binding protein 1 | 70.70 | 10 | |
| PABPC4 | Polyadenylate-binding protein 4 | 70.83 | 23 | |
| PABPN1 | Poly(A) binding protein, nuclear 1, isoform CRA_a | 32.31 | Infinite | |
| PAIP1 | Polyadenylate binding protein-interacting protein 1 (Predicted) | 46.09 | Infinite | |
| PAK1 | Serine/threonine-protein kinase PAK 1 | 60.58 | Infinite | Yes |
| PAK3 | Serine/threonine-protein kinase PAK 3 | 60.71 | Infinite | Yes |
| PCBP3 | Poly (RC) binding protein 3 | 33.78 | Infinite | |
| PCF11 | PCF11 cleavage and polyadenylation factor subunit | 172.66 | Infinite | |
| PDS5B | Sister chromatid cohesion protein PDS5 homolog B | 164.52 | 15 | |
| PLRG1 | Pleiotropic regulator 1 | 57.19 | Infinite | |
| PLS3 | Plastin-3 | 70.75 | Infinite | |
| POLR2A | DNA-directed RNA polymerase | 217.20 | Infinite | |
| PPIE | Peptidyl-prolyl cis-trans isomerase | 33.37 | Infinite | |
| PPP1R12A | Protein phosphatase 1 regulatory subunit 12A | 115.27 | Infinite | Yes |
| PPP1R12B | Protein phosphatase 1 regulatory subunit | 111.39 | Infinite | Yes |
| PPP1R9A | Neurabin-1 | 122.73 | Infinite | |
| PPP1R9B | Neurabin-2 | 89.66 | Infinite | |
| PRMT5 | Protein arginine N-methyltransferase 5 | 72.69 | Infinite | |
| PRPF19 | Pre-mRNA-processing factor 19 | 55.24 | 13 | |
| PRPF8 | Pre-mRNA processing factor 8, isoform CRA_a | 273.61 | Infinite | |
| PRPH | Peripherin | 53.55 | 25 | |
| PRPS1 | Ribose-phosphate pyrophosphokinase 1 | 34.81 | Infinite | |
| PRRC2A | Protein PRRC2A | 229.17 | 21 | |
| PRRC2B | Protein Prrc2b | 243.10 | Infinite | |
| PTBP3 | Polypyrimidine tract-binding protein 3 | 59.81 | Infinite | |
| PTGFRN | Prostaglandin F2 receptor negative regulator | 98.67 | 27 | |
| PTMA | Prothymosin alpha | 12.38 | Infinite | |
| PTOV1 | Prostate tumor-overexpressed gene 1 protein homolog | 46.85 | Infinite | |
| PVALB | Parvalbumin alpha | 11.93 | 24 | |
| PXN | Paxillin | 64.06 | Infinite | Yes |
| PYGM | Glycogen phosphorylase, muscle form | 97.32 | Infinite | |
| QN1 | Protein QN1 homolog | 161.00 | Infinite | |
| RAB11A | Ras-related protein Rab-11B | 24.49 | Infinite | |
| RAB8A | Ras-related protein Rab-8A | 23.67 | Infinite | |
| RAI14 | Ankycorbin | 109.13 | Infinite | |
| RALY | RALY heterogeneous nuclear ribonucleoprotein | 33.05 | 13 | |
| RBFOX2 | RNA binding protein fox-1 homolog 2 | 45.55 | Infinite | |
| RBM15 | RNA binding motif protein 15 | 105.76 | Infinite | |
| RBM47 | RNA-binding motif protein 47 | 64.09 | Infinite | |
| RBMX | RNA-binding motif protein, X chromosome | 42.26 | Infinite | Yes |
| RECQL | ATP-dependent DNA helicase Q1 | 69.64 | 25 | |
| RGD1304704 | LRRGT00192 | 28.17 | 11 | |
| RHOA | Transforming protein RhoA | 21.78 | Infinite | Yes |
| RPL12 | 60S ribosomal protein L12 | 17.85 | Infinite | |
| RPL14 | 60S ribosomal protein L14 | 23.78 | Infinite | |
| RPL19 | 60S ribosomal protein L19 | 23.47 | Infinite | |
| RPL21 | 60S ribosomal protein L21 | 18.68 | Infinite | |
| RPL26 | 60S ribosomal protein L26 | 17.26 | Infinite | |
| RPL27A | 60S ribosomal protein L27a | 16.59 | Infinite | |
| RPL31 | 60S ribosomal protein L31 | 16.32 | Infinite | |
| RPL36 | 60S ribosomal protein L36 | 12.41 | Infinite | |
| RPS27L | 40S ribosomal protein S27-like | 9.48 | Infinite | |
| RQCD1 | Cell differentiation protein RCD1 homolog | 33.60 | Infinite | |
| RRP7A | Ribosomal RNA-processing 7 homolog A | 32.34 | Infinite | |
| SAFB | Scaffold attachment factor B1 | 105.52 | 190 | |
| SCRIB | Scribbled planar cell polarity protein | 179.85 | Infinite | Yes |
| SEC16A | Protein transport protein sec16 | 253.29 | Infinite | |
| SEC61A1 | Protein transport protein Sec61 subunit alpha isoform 1 | 52.26 | Infinite | |
| SF3B1 | Splicing factor 3 b, subunit 1 | 145.83 | 12 | |
| SGPL1 | Sphingosine-1-phosphate lyase 1 | 63.76 | 65 | |
| SHMT1 | Serine hydroxymethyltransferase | 75.37 | Infinite | |
| SHPRH | SNF2 histone linker PHD RING helicase | 194.70 | Infinite | |
| SIPA1L3 | Signal Induced Proliferation Associated 1 Like 3 | 194.93 | Infinite | |
| SMARCA4 | Transcription activator BRG1 | 181.40 | Infinite | |
| SMARCB1 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1 | 43.16 | Infinite | |
| SMC1A | Structural maintenance of chromosomes protein 1A | 143.20 | 16 | |
| SMU1 | WD40 repeat-containing protein SMU1 | 57.54 | Infinite | |
| SND1 | Staphylococcal nuclease domain-containing protein 1 | 101.95 | Infinite | |
| SORBS1 | Sorbin and SH3 domain-containing protein 1 | 143.37 | Infinite | |
| SPECC1L | Cytospin-A | 124.34 | Infinite | |
| SPNA2 | Spectrin alpha chain | 286.71 | 13 | |
| SPOUT1 | SPOUT Domain Containing Methyltransferase 1 | 41.97 | Infinite | |
| SRP68 | Signal recognition particle subunit SRP68 | 70.49 | 11 | |
| SRPK1 | SFRS protein kinase 1 | 73.93 | Infinite | |
| SRSF1 | Serine/arginine-rich splicing factor 1 | 27.74 | 13 | |
| SRSF5 | Serine/arginine-rich splicing factor 5 | 30.89 | Infinite | |
| STAU1 | Protein Phosphatase 1, Regulatory Subunit 150 | 61.33 | 23 | Yes |
| STAU2 | Double-stranded RNA-binding protein Staufen homolog 2 | 62.68 | 52 | |
| STK38L | Serine/Threonine Kinase 38 Like | 53.76 | Infinite | |
| SURF4 | Surfeit 4, isoform CRA_a | 83.26 | Infinite | |
| SVIL | Supervillin; Membrane-Associated F-Actin Binding Protein P205 | 242.05 | Infinite | |
| SYCP2 | Synaptonemal complex protein 2 | 172.59 | Infinite | |
| SYNPO2 | Synaptopodin-2 | 136.02 | 160 | |
| T | T brachyury transcription factor | 47.43 | Infinite | |
| TAF9 | Transcription initiation factor TFIID subunit 9 | 28.99 | Infinite | |
| TARDBP | TAR DNA Binding Protein | 32.15 | 43 | |
| TBC1D5 | TBC1 domain family, member 5 | 93.06 | Infinite | |
| TFRC | Transferrin receptor protein 1 | 85.88 | Infinite | |
| THBS3 | Thrombospondin 3 | 104.13 | Infinite | |
| THOC2 | THO complex 2 | 185.06 | Infinite | |
| THOC5 | THO complex subunit 5 homolog | 78.66 | Infinite | |
| THOC6 | THO complex subunit 6 homolog | 37.42 | 12 | |
| TMEM189 | Ubiquitin-conjugating enzyme E2 variant 2 | 16.36 | Infinite | |
| TMOD2 | Tropomodulin-2 | 39.49 | 20 | |
| TMOD3 | Tropomodulin-3 | 39.47 | Infinite | |
| TNKS1BP1 | Tankyrase 1-binding protein 1 | 180.01 | 34 | |
| TNNC2 | Troponin C2 | 18.10 | 19 | |
| TNNT3 | Troponin T | 30.75 | Infinite | |
| TPM2 | Tropomyosin beta chain | 32.84 | Infinite | |
| TPM4 | Tropomyosin alpha-4 chain | 28.51 | Infinite | |
| TRA2A | Transformer 2 Alpha Homolog | 32.58 | Infinite | |
| TRIM25 | Tripartite motif-containing 25 | 71.92 | Infinite | |
| TRIM27 | Tripartite motif-containing 27 | 58.55 | Infinite | |
| UBTD1 | Ubiquitin domain-containing protein 1 | 25.96 | Infinite | |
| UFL1 | E3 UFMl-protein ligase 1 | 89.58 | Infinite | |
| UPF1 | UPF1, RNA Helicase And ATPase | 122.64 | 35 | |
| USP10 | Ubiquitin carboxyl-terminal hydrolase 10 | 87.31 | Infinite | |
| VIL1 | Villin 1 | 92.66 | 11 | |
| VPS13A | Vacuolar Protein Sorting 13 Homolog A | 166.62 | 370 | |
| WDR77 | Methylosome protein 50 | 37.73 | Infinite | |
| WIZ | Widely-interspaced zinc finger motifs | 103.64 | Infinite | |
| YLPM1 | YLP motif-containing protein 1 | 238.98 | Infinite | |
| YTHDC1 | YTH domain-containing protein 1 | 85.90 | Infinite | |
| YTHDF1 | YTH N (6)-methyladenosine RNA-binding protein 1 | 60.91 | Infinite | |
| ZFP275 | Zinc finger protein 275 | 55.99 | Infinite | |
| ZFP462 | Zinc finger protein 462 | 283.91 | Infinite | |
| ZFR | Zinc finger RNA-binding protein | 116.91 | Infinite | |
| A0A0G2JTG4 | Uncharacterized protein | 14.89 | Infinite | |
| A0A0G2K3S3 | Uncharacterized protein | 11.19 | Infinite | |
| A0A0G2K7N7 | Uncharacterized protein | 31.06 | Infinite | |
| A0A0G2K8A9 | Uncharacterized protein | 23.79 | Infinite |
STRING, IntACT and BioGRID databases.
Proteins only identified in the Rac1 immunoprecipitates, but not in the NIgG immunoprecipitates.
Data in Fig. 3A represent a graphical depiction of eleven significantly enriched pathways for the 324 Rac1 interaction partners identified using Ingenuity Pathway Analysis (IPA). The number of proteins identified as Rac1 interaction partners in a particular pathway is indicated above the bar. Based on the known biological functions of Rac1, we identified 30 interaction partners of Rac1 involved in the actin cytoskeletal signaling pathways. It is noteworthy that our studies identified Rac1 interaction partners involved in calcium (17 partners), RhoGDI (20 partners), PAK (13 partners) and Rho GTPase (19 partners) signaling pathways. Indeed, published evidence implicates these signaling pathways in islet β-cell function, including GSIS (Kalwat and Thurmond, 2013; Kowluru, 2010, 2017). Emerging evidence in multiple cell types including the islet β-cell appear to indicate localization of Rac1 in various subcellular compartments including cytosol, plasma membrane, mitochondria and nucleus (Kowluru et al., 2003; Abdrabou and Wang, 2018; Payapilly and Malliri, 2018; Baidwan et al., 2017). Fig. 3B is representation of significantly enriched subcellular localizations for the Rac1 interaction partners in INS-1832/13 cells. Pathways of actin cytoskeleton and GPCR signaling generated using IPA are provided as Supplemental Figs. 1 and 2, respectively.
Fig. 3.

Graphical depiction of significantly enriched pathways and subcellular localizations for the Rac1 interaction partners.
Panel A: Significantly enriched pathways for the Rac1 interaction partners identified using Ingenuity Pathway Analysis. The number of proteins identified as Rac1 interaction partners in a particular pathway is indicated above the bar. The greater the -Log (p value) value (e.g., the smaller p value), the less likely a pathway is significantly enriched just by chance.
Panel B: Significantly enriched subcellular localizations for the Rac1 interaction partners identified using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8, https://davidncifcrf.gov/ . The number of proteins identified as Rac1 interaction partners in a particular subcellular localization is indicated above the bar. The greater the -Log (p value) value (e.g., the smaller p value), the less likely a pathway is significantly enriched just by chance.
3.2. Identification of HG-responsive Rac1 interaction partners in INS-1832/13 cells
As described in Fig. 2 and 142 out of 243 interaction partners, had a fold change greater than 1.5 (i.e., 1.5 fold increase) or less than 0.67 (i.e., 1.5 fold decrease) when comparing lysates from cells treated with LG to HG conditions. Among these, 27 proteins showed a significant difference in Rac1 interaction in response to HG conditions (p < 0.05), and therefore, they were considered as “HG-responsive interaction partners” (Table 2 and Supplemental Table 2). Among these 27 Rac1 interaction partners with a significant difference between LG and HG treatments, 9 were listed as Rac1 interaction partners in rat, mouse and/or human cells/tissues in the 3 large interaction partner databases, while 18 were not, and thus appeared to be novel. Fig. 4 A represents a volcano plot displaying the 324 Rac1 interaction partners in INS-1832/ 13 cells exposed to either basal or HG conditions. Based on their known regulatory roles, these 27 Rac1 interaction partners were grouped into four major categories (Fig. 4 B), namely cytoskeletal proteins (e.g., cortactin, paxillin), nuclear proteins (e.g., importin; also referred to as karyopherin), G-proteins and their regulatory factors (e.g., the α-sub-unit of the trimeric G-protein Gq and the small G-protein Rho) and others (e.g., protein phosphatase 1 regulatory subunit 12A). Our findings also suggested that there were 13 interaction partners with a fold change greater than 5 (also with p < 0.05) in response to HG conditions. Interestingly, 7 out of these 13 proteins exhibited greater than 10 fold increase in the association with Rac1 under HG conditions (Table 2). They are: actin-related protein, LAD1 ladinin-1, leucine-rich repeat flightless-interacting protein 2, protein phosphatase 1 regulatory subunit 12A, peripherin, spectrin alpha chain and supervillin.
Table 2.
The 27 Racl interaction partners with a significant difference between high glucose (HG) and low glucose (LG) treated INS1 cells. All these interaction partners have a enrichment ratio > 10 compared to the NIgG control, detected in > 4 out of the 8 Racl IP samples, and also have a fold change for HG vs. LG greater than 1.5 (i.e., 1.5 fold increase) or less than 0.67 (i.e., 1.5 fold decrease). Data are given as fold changes (means ± SEM). Peak area for each protein identified in a specific IP sample was normalized against the peak area for Rac1 identified in the same sample (See Methods). The normalized peak area for each Rac1 interaction partner was compared between the HG and LG groups to assess effects of HG on protein-protein interactions involving Rac1. Mean of the normalized peak area for each Rac1 interaction partner in the LG sample was set to 1.00, and all the fold changes were relative to LG, n = 4, p < 0.05.
| Gene Name | Protein Name | LG | HG | p-value | Reported as an interaction partner of Rac1 in 3 large interaction databasesa |
|---|---|---|---|---|---|
| ACTR3 | Actin-related protein 3 | 1.0010.35 | 13.74±5.28 | 0.03255 | Yes |
| ARHGEF6 | Rho guanine nucleotide exchange factor 6 | 1.00±0.19 | 1.66±0.1 | 0.03836 | Yes |
| ARPC5 | Actin-related protein 2/3 complex subunit 5 | 1.00±0.23 | 9.114.15 | 0.01267 | Yes |
| CTTN | Cortactin isoform B | 1.00±0.27 | 8.62±2.12 | 0.00729 | Yes |
| DDX3Y | DEAD-Box Helicase 3 | 1.00±0.2 | 1.79±0.14 | 0.03469 | |
| FLII | Flightless 1 homolog | 1.00±0.26 | 3.98±1.02 | 0.01481 | |
| GNAQ | Guanine nucleotide-binding protein G(q) subunit alpha | 1.00+0.20 | 9.82+3.36 | 0.00143 | |
| KPNA1 | Importin subunit alpha-1 | 1.00±0.15 | 2.97±0.78 | 0.02214 | |
| KPNA7 | Importin subunit alpha-7 | 1.00±0.23 | 2.79±0.75 | 0.02914 | Yes |
| LAD1 | Ladinin-1 | 1.00±0.15 | 28.05±11.7 | 0.01261 | |
| NUFIP2 | NUFIP2, FMRl-interacting protein 2 | 1.00±0.16 | 1.94±0.31 | 0.03059 | |
| LRRFIP2 | Leucine-rich repeat flightless-interacting protein 2 | 1.00±0.17 | 12.24±2.77 | 0.00022 | |
| MYOIB | Unconventional myosin-lb | 1.00±0.39 | 8.12±2.97 | 0.03365 | |
| PABPC4 | Polyadenylate-binding protein 4 | 1.00±0.17 | 1.92±0.25 | 0.02448 | |
| PLS3 | Plastin-3 | 1.00±0.01 | 2.34±0.27 | 0.01301 | |
| PPP1R12A | Protein phosphatase 1 regulatory subunit 12A | 1.00±0.13 | 14.12±2.18 | 0.00005 | Yes |
| PRPH | Peripherin | 1.00±0.18 | 17.72±8.66 | 0.01884 | |
| PXN | Paxillin | 1.00±0.24 | 2.95±0.27 | 0.00564 | Yes |
| RAI 14 | Ankycorbin | 1.00±0.01 | 8.73±1.75 | 0.00378 | |
| RALY | RALY heterogeneous nuclear ribonucleoprotein | 1.00±0,17 | 1.66±0.17 | 0.02867 | |
| RPL27A | 60S ribosomal protein L27a | 1.00±0.33 | 2.86±0.51 | 0.02238 | |
| RHOA | Transforming protein RhoA | 1.00±0.34 | 2.38±0.43 | 0.04885 | Yes |
| SCRIB | Scribbled planar cell polarity protein | 1.00±0.18 | 1.83±0.22 | 0.03841 | Yes |
| SPNA2 | Spectrin alpha chain | 1.00±0.38 | 11.34±3.24 | 0.01484 | |
| SRSF5 | Serine/arginine-rich splicing factor 5 | 1.00±0.16 | 2.11±0.45 | 0.04826 | |
| SVIL | Supervillin; Membrane-Associated F-Actin Binding Protein P205 | 1.00±0.27 | 14.69±4.95 | 0.02317 | |
| TRIM27 | Tripartite motif-containing 27 | 1.00±0.25 | 5.07±1.04 | 0.00538 |
Green highlighted proteins are the 7 RAC1 interaction partners with p<0.01 HG vs. LG.
STRING, IntACT and BioGRID databases
Fig. 4.
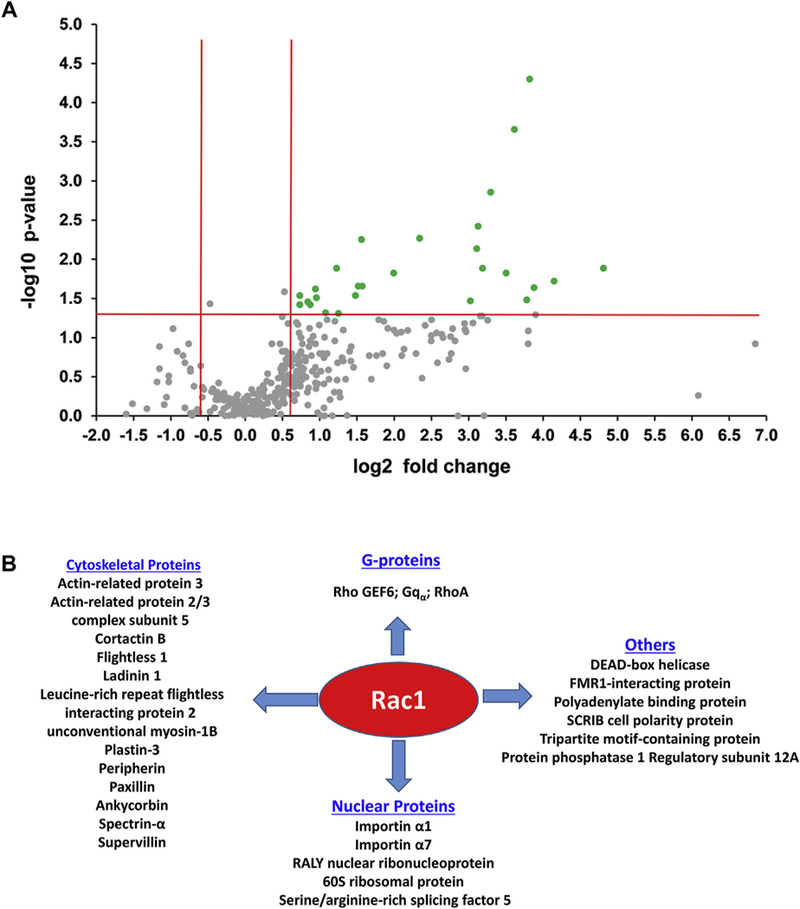
HG-responsive interacting proteins of Rac1 in insulin-secreting INS-1832/13 cells
Panel A: Volcano plot for the 324 Rac1 interaction partners with/out high glucose treatments. The green points indicate Rac1 interaction partners with both large magnitude fold-changes (log 2 of fold change, x axis), and high statistical significance (-log10 of p value, y axis). The horizontal red line shows where p = 0.05 with points above the line having p < 0.05 and points below the line having p > 0.05: log (0.05, 10) = 1.30. The vertical red lines show where a fold change for HG vs. LG greater than 1.5 (i.e., 1.5 fold increase) or less than 0.67 (i.e., 1.5 fold decrease): log (1.5, 2) = 0.585. Those points having a fold-change < 1.5 and/or p > 0.05 are shown in gray and those with a fold-change > 1.5 and p < 0.05 are shown in green.
Panel B: Based on the identity and functions of the HG-responsive proteins identified in the current study, we broadly classified those proteins into four major categories, including G-proteins (subunits and regulatory factors), cytoskeletal proteins, nuclear proteins and others (see Table 2 for additional information on these proteins). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Experimental determination of the protein abundance of Rac1 interaction partners in INS-1832/13 cells, normal rat islets and human islets
In the next series of experiments, we validated our proteomics data (above) by determining the expression/protein abundance of select signaling proteins via Western blotting of lysates derived from INS-1832/13 cells, normal rat islets and human islets. Data in Fig. 5 A suggest that GIT1/2, known GTPase activating proteins for Arf6, a small G-protein that we have implicated in physiological insulin secretion (Jayaram et al., 2011) are expressed in all three cell types studied. We also detected expression of β-PIX, a guanine nucleotide exchange factor for Cdc42 and Rac1 in all the three insulin-secreting cells studied. The expression of α-subunit of the trimeric G-protein Gαq/11/14, which we have shown to be one of the high glucose-responsive proteins in INS-1832/13 cells by proteomics (Fig. 4) was also verified in INS 1832/ 13 cells, normal rat islets and human islets. In addition, we noted the expression of Rac1 GTPase-activating protein (RacGAP1) in all the 3 insulin-secreting cells including human islets. Lastly, we provide the first evidence for the expression of karyopherin, a scaffolding protein, which has been implicated in the nuclear import of Rac1 under conditions of cell dysfunction and apoptosis (see below). Levels of expression of Rac1 in INS-1832/13 cells, normal rat islets and human islets are shown in Fig. 5B. Together, our Western blot data (Fig. 5 A and B) further strengthen our proteomics data. It is noteworthy that our current studies are the first to report expression of GIT1/2, RacGAP1 and karyopherin in clonal β-cells, rodent islets and human islets.
Fig. 5.

Immunological detection of select Rac1 interacting partners in INS-1832/13cells, normal rodent islets and human islets.
Panel A: Lysates derived from INS-1832/13 cells, normal rat islets and human islets were subjected to SDS-PAGE and identified by Western blotting method using specific antibodies directed against each of the proteins indicated (see Methods for additional details). Representative blots from multiple studies are provided in the figure.
Panel B: Rac1 expression in INS-1832/13 cells, normal rat islets and human islets was determined by Western blotting. The membranes were reprobed with anti-actin antibody to serve as loading control. Representative blots from multiple studies are provided in the figure.
3.4. Experimental validation of Gαq/11/14 as a high glucose-responsive Rac1 interaction partner in INS-1832/13 cells
Our proteomics data in Fig. 4 identified Gαq11/14 as one of the high glucose-responsive interaction partners of Rac1 in INS-1832/ 13 cells. Furthermore, our Western blot data (Fig. 5) suggested high degree of expression of this trimeric Ga subunit in INS-1832/13 cells, normal rat islets and human islets. Therefore, we then asked whether hyperglycemic exposure of INS-1832/13 cells leads to increased expression of this protein and/or whether it interacts with Rac1 under those conditions. Data from these studies are included in Fig. 6. First, we noticed no significant effects of hyperglycemic conditions on the expression of Gαq/11/14 (Panel A). Pooled data from 4 independent repeats are represented in Fig. 6 A. Data depicted in Fig. 6 B indicate no significant changes in the expression levels of two HG-responsive proteins (β-PIX and GIT1/2) that we identified in the current study. Interestingly, however, data from Co-IP experiments revealed that HG conditions (24 h) led to increased association of Gαq/11/14 with Rac1 (Panel C). Pooled data from multiple experiments are included in Panel D. Note that data depicted in Panels C and D represent relative abundance of Rac1 in INS 1832/13 lysates immunoprecipitated with an antiserum directed against Gαq/11/14. We next undertook a reverse Co-IP approach in which relative abundance of Gαq/11/14, GIT1/2 and β-PIX in immunoprecipitates derived from LG/HG exposed INS 1832/13 cells using the Rac1 antiserum. Data in Fig. 7 further demonstrate increased association of Rac1 with these three proteins in INS-1832/13 cells exposed to HG conditions. Together, data depicted in Figs. 6 and 7 validate our findings from proteomics experiments.
Fig. 6. Immunological validation of proteomics data Panel.

Panel A: HG conditions do not increase the expression of Gαq/11/14 in INS-1832/13 cells. INS-1832/13 cells were cultured in the presence of basal glucose (2.5 mM; LG) or high glucose (25 mM; HG) for 24 h (see Methods). Cells were lysed and subjected to SDS-PAGE and probed with an antiserum directed against Gaq11/14. GAPDH was used as a loading control. A representative blot from four experiments was shown here. The graph represents the pooled data (mean ± SEM) from four experiments in which relative intensity of Gαq11/14 was normalized to GAPDH.
Panel B: Expression of β-PIX and GIT1/1 under HG conditions in INS-1832/ 13 cells. INS-1832/13 cells were cultured in the presence of basal glucose (2.5 mM; LG) or high glucose (25 mM; HG) for 24 h (see Methods). Cells were lysed in RIPA buffer and subjected to SDS-PAGE and probed with an antiserum directed against β-PIX and GIT1/2. Actin was used as a loading control. A representative blot from four experiments is shown here.
Panel C: High glucose promotes an interaction between Rac1 and Gαq11/14. INS-1 cells were cultured in the presence of either LG (2.5 mM) or HG (25 mM) for 24 h and lysed in IP lysis buffer. Equal volume of precleared cell lysates was immunoprecipitated with anti-Gαq11/14 antibody or normal mouse IgG monoclonal antibody. Precleared cell lysate without antibody was used as negative control. The resulting immunoprecipitates were subjected to SDS-PAGE and analyzed by western blotting with antibody against Rac1. Total cell lysates were probed for Rac1 and Gαq11/14. A representative blot from four experiments is shown.
Panel D: Densitometric quantification of Rac1 abundance in the immunoprecipitates of Gαq11/14 from LG and HG exposed cells. Data are shown mean ± SEM for four independent experiments. * represents p < 0.05.
Fig. 7.
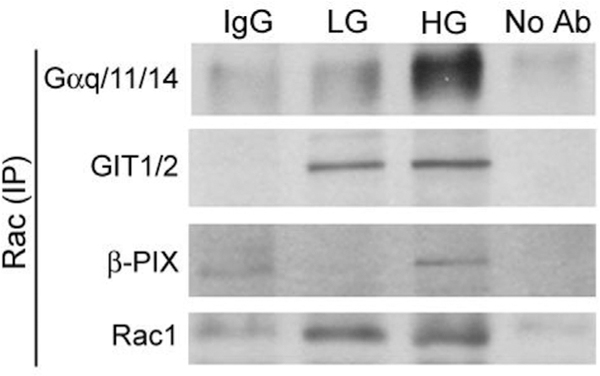
Co-IP experiments to demonstrate increased association between Rac1 and Gαq/11/14, GIT1/2 and β-PIX in INS-1832/13 cells under HG-treatment conditions. INS-1832/13 cells were cultured in the presence of either LG (2.5 mM) or HG (25 mM) for 24 h, and lysed in IP lysis buffer. Equal volume of precleared cell lysates were immunoprecipitated with anti-Rac1 serum or normal mouse IgG monoclonal antibody. Precleared cell lysates without antibody were used as negative controls. The resulting immunoprecipitates were subjected to SDS-PAGE and analyzed by Western blotting with antibody against Gαq/11/14, GIT1/2 and β-PIX. A representative blot from four experiments is shown.
4. Discussion
Using pharmacological, biochemical, and molecular biological approaches, several earlier studies have implicated small G proteins (e.g., Cdc42, Rac1, Arf6) in actin cytoskeletal rearrangements, vesicular transport and fusion of insulin-containing secretory granules to the plasma membrane for fusion and secretion of insulin (Kalwat and Thurmond, 2013; Kowluru, 2010, 2017). In the light of emerging evidence, which indicates regulatory roles of Rac1 in islet β-cell function in health and under metabolic stress (e.g., exposure to hyperglycemia; 8,22), we undertook the current studies to identify interaction partners of Rac1 in insulin-secreting INS-1832/13 cells under normal and HG conditions. It should be noted that, earlier studies from multiple laboratories including our own have documented significant impairment in GSIS in clonal β-cells, rodent islets and human islets following exposure to HG conditions (Sidarala et al., 2015; Sidarala and Kowluru, 2017a; Marshak et al., 1999; Khadija et al., 2014; Kong et al., 2015; Elumalai et al., 2017), Furthermore, we have demonstrated defects in mitochondrial (caspase-3 activation) and nuclear (lamin-B degradation) integrity and function under these conditions culminating in cell demise (Sidarala et al., 2015; Sidarala and Kowluru, 2017a; Khadija et al., 2014).
Using a quantitative proteomic approach (Co-IP and HPLC-ESI-MS/ MS), we identified a total of 324 Rac1 interaction partners in pancreatic β-cells. These Rac1 partners play contributory roles in the regulation of at least 11 signaling pathways, including those involved in actin cytoskeletal rearrangements, calcium- and G protein-dependent signaling. More importantly, we have identified 27 proteins that appear to exhibit increased association with Rac1 under hyperglycemic conditions. Identification of these interacting factors/partners provide fresh insights into delineation of specific signaling pathways that underlie islet β-cell dysregulation and demise under metabolic stress. For brevity, the following discussion is focused on potential roles of five Rac1 partners (i.e., GIT1/2, β-PIX, Gαq/11/14, Rac1GAP and karyopherin) that we have validated via immunological approaches (Figs. 5–7).
4.1. Potential regulatory roles of GIT1/2 in islet function
G protein-coupled receptor kinase-interacting protein-1 (GIT1) is a multidomain protein, which has been shown to interact with a variety of signaling molecules including β-PIX, PAK1, focal adhesion kinase, phospholipase-γ and mitogen-activated protein kinase-1. Functional activation of GITs has been shown to be precisely regulated via tyrosine phosphorylation (Hoefen and Berk, 2006; Zhou et al., 2016). Earlier studies have demonstrated that GITs also subserve the functions as GTPase-activating proteins (GAPs), specifically for Arf6 (Hoefen and Berk, 2006; Zhou et al., 2016). Published evidence indicates that GIT1 exerts both positive as well as negative modulatory roles in actin-driven membrane protrusions by increasing and decreasing activation of Rac1 and/or Cdc42, respectively (Hoefen and Berk, 2006; Zhou et al., 2016). Together, above evidence implicates GITs in the functional activation of Arf6-Cdc42-Rac1 signaling pathways. In this context, earlier studies have investigated roles of Cdc42, Rac1 and Arf6 in GSIS in clonal β-cells, rodent islets and human islets (Kalwat and Thurmond, 2013; Kowluru, 2010, 2017; Jayaram et al., 2011; Jayaram and Kowluru, 2012; Lawrence and Birnbaum, 2003; Ma et al., 2010; Suijun et al., 2014; Guo et al., 2018). Data from these investigations have yielded valuable insights into various regulatory proteins/factors that appear to mediate the activation-deactivation steps of these G proteins. These include GDIs, GEFs, and GAPs. For example, original investigations from our laboratory have reported novel regulatory roles for Arf nucleotide binding site opener (ARNO), a GEF for the small G-protein Arf6, in the functional activation of Arf6 in clonal β-cells and primary rodent and human islets (Jayaram et al., 2011). We reported that ARNO-Arf6 signaling axis controls GSIS by promoting sequential activation of Arf6, Cdc42 and Rac1 with 1, 3 and 15min of exposure to stimulatory glucose concentrations, respectively. Based on these findings and data from complementary studies, we concluded that ARNO-mediates the sequential activation of Arf6, Cdc42 and Rac1 culminating in GSIS (Jayaram et al., 2011). Furthermore, using a selective pharmacological inhibitor of ARNO/Arf6 signaling axis (e.g., secinH3), we reported marked inhibition by secinH3 of glucose-induced phospholipase D (PLD) activation, ERK1/2 phosphorylation and dephosphorylation of cofilin, suggesting that Arf6/ARNO signaling mediates PLD, ERK1/2 and cofilin activation in islet β-cells. In addition, secinH3 blocked glucose-induced Nox2 activation and associated ROS generation, thus placing Nox2 downstream to Arf6/ARNO signaling step (Jayaram and Kowluru, 2012). Together, our previous studies identified signaling steps downstream to ARNO/Arf6 axis leading to insulin secretion. Data from our current (proteomics as well as immunological) investigations provide evidence that GIT1/2 is Racl interacting partner and that its association with Rac1 appears to increase significantly under high glucose exposure conditions. In endothelial cells and non-small-cell lung cancer cells, GIT1-PIX axis has been shown to activate Rac1/Cdc42/cortactin to promote membrane protrusions and cell migration (Majumder et al., 2014). Our current investigations also identified cortactin as one of the interaction partners for Rac1 (Table 1). In endothelial cells, GIT2 has been shown to Vav2-mediated activation of Rac1 induced by C-X-C Motif Chemokine Receptor 2 (Li et al., 2018). Indeed, our recent studies have identified Vav2 as one of the GEFs for Rac1 in pancreatic β-cell (Veluthakal et al., 2015). Therefore, it is likely that GIT1/2 play critical roles in regulation of Arf6, Rac1 and Cdc42 in the islet. Methodical investigations are underway to precisely assess the roles of GIT1/2 in islet β-cell function under normal and metabolic stress conditions.
4.2. Potential modulatory roles of β-PIX in islet function
The p21-activated kinase-interacting exchange factor (β-PIX), a known GEF for Racl and Cdc42, has been implicated in the regulation of cell function in many cell types (Rathor et al., 2017; Černohorská et al., 2016). Recent investigations by Kepner et al. (2011) have provided novel insights into regulatory roles of β-PIX in physiological insulin secretion. These studies did not investigate putative roles for β-PIX as a GEF for Rac1 in the islet β-cell. However, published evidence clearly identified Cdc42 activation as upstream to Rac1 activation in the cascade of events leading to GSIS (Wang and Thurmond, 2009; Kowluru, 2010, 2017; Jayaram et al., 2011). Therefore, β-PIX might regulate Rac1 functions via the intermediacy of Cdc42. It is noteworthy, however, that recent investigations by Rathor and associates (Rathor et al., 2017) highlighted novel interactions between β-PIX, GIT1 and Rac1 in intestinal epithelial restitution after wound healing. Based on data from complementary studies, these researchers have demonstrated that increased association between β-PIX and GIT1 results in the stimulation of intestinal epithelial restitution by activating Rac1. Along these lines, studies by Cernohorska and coworkers have demonstrated critical roles for GIT1, β-PIX, and p21 protein (Cdc42/Rac1)-activated kinase 1 in microtubule nucleation. Further, they reported that such a signaling step correlated with recruitment of γ-tubulin to the centrosome. The authors proposed that GIT1/β-PIX signaling proteins with PAK1 kinase represent a novel regulatory mechanism of microtubule nucleation in interphase cells (Černohorská et al., 2016).
4.3. Potential regulatory roles of Gαq/11/14 in islet function and dysfunction
We also identified Gαq as one of the interacting partners of Rac1 in INS-1832/13cells. Our findings also suggested that it is expressed in normal rodent and human islets. Using immunocytochemical approaches Skoglund and associates (Skoglund et al., 1999) have reported expression of Gαq in both α-and β-cells of the islet. Using INS-1E β-cells, Shapiro and coworkers have demonstrated regulatory roles of Gαq in fatty acid (palmitate)-induced insulin secretion (Shapiro et al., 2005). Specifically, they implicated novel roles for Gαq-phospholipase C pathway in GPR40-mediated effects of palmitate in promoting intracellular calcium levels and insulin secretion. These studies provided compelling evidence on the identification of Gαq as the putative trimeric G protein that coupled palmitate to its intracellular regulatory signaling pathways. Along these lines, McNelis et al. (2015) have reported regulatory roles for Gαq in (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl) butanamide, a specific agonist for GPR43, induced inositol triphosphate and calcium levels and associated insulin secretion in Min6 cells, murine islets and human islets. Taken together, these studies implicate roles for Gαq in GPR-coupled signaling events leading to insulin secretion. Published evidence in other cell types highlights potential cross-talk between Gαq and Rac1 signaling pathways in transmission of a variety of signaling events. For example. Harmon and Ratner (2008) have implicated Gαq activation in HIV-1 envelope glycoprotein-mediated Rac1 activation and cytoskeletal rearrangement. Sabbatini and associates (Sabbatini et al., 2010) have demonstrated regulatory roles for Gα13 and Gαq in cholecystokinin-mediated activation of Rac1 in mouse pancreatic acini. Despite this evidence in other cells, potential cross-talk between Gαq and Rac1 has not been established in the islet β-cell function and insulin secretion. Furthermore, it is likely that increased association between these two signaling proteins that we observed in pancreatic β-cells under HG conditions could contribute to the genesis of islet dysfunction. Future studies will validate such a postulation.
4.4. Potential roles of karyopherin and RacGAP1 in islet dysfunction under the duress of HG
Two recent studies from our laboratory have provided evidence to indicate that HG conditions promote alterations in subcellular localization of Rac1, a signaling step that we proposed to contribute to cell dysfunction and demise. In the first, we demonstrated that HG conditions promote translocation, phosphorylation and accumulation of p53, a pro-apoptotic gene, in the nuclear fraction in INS-1832/13 cells. Interestingly, EHT1864, a known inhibitor of Rac1 activation (by specifically inhibiting the GDP/GTP exchange), markedly attenuated phosphorylation, but not translocation of p53. These studies implicate potential regulation of p53 phosphorylation by Rac1 in the nuclear fraction under HG conditions (Syed et al., 2011). Second, using pharmacological and molecular biological approaches, we reported mistargeting of biologically-active Rac1 to the nuclear compartment in INS-1832/13 cells, normal rat islets and human islets following exposure to HG conditions (Baidwan et al., 2017). In this context, a growing body of experimental evidence suggests involvement of specific transport proteins that mediate translocation of cytosolic Rac1 to the nuclear compartment. Sandrock et al. (2010) identified the nuclear import receptor karyopherin alpha2 (KPNA2) as a direct interaction partner of Rac1. Using a variety of experimental techniques they demonstrated that the C-terminal polybasic region of Rac1 contains a nuclear localization signal (NLS), whereas Rac2 and Rac3 lack a functional NLS and do not bind to KPNA2. They demonstrated that the presence of the NLS in Rac1 determines the specificity of the interaction and is a prerequisite for the nuclear import. Compatible with our observations (Li et al., 2018), Sandrock et al. (Veluthakal et al., 2015) demonstrated that although this interaction is independent of the Rac1 GDP/GTP loading, the induction of the translocation requires Rac1 activation. Using LC-MS/MS analysis, these investigators have also identified several proteins in the nuclear fraction that interacted with only biologically-active mutant of Rac1. Some of these interaction partners included IQGAP1, IQGAP2, IQGAP3, PIX, GIT1 and KPNA2. Lastly, our studies have also identified RacGAP1 as one of the interacting partners of Rac1. Earlier studies by Kawashima and Kitamura (2008) have demonstrated that Rac1 and RacGAP1 promote nuclear transport of transcriptional factors (e.g., phospho-STAT3). Interestingly, they demonstrated a critical role for importina (KPNA2) in the nuclear import of p-STAT-3 mediated by Rac1/RacGAP1 (Kawashima and Kitamura, 2008). Taken together, the data accrued in the current investigations (via proteomics and immunological approaches) are expected to provide a platform for future investigations to further validate the roles of these Rac1 interaction partners not only in physiological insulin secretion, but also in the pathogenesis of islet dysfunction under conditions of metabolic stress.
4.5. Identification of mitochondrial matrix proteins as potential interaction partners of Rac1
Even though not significantly enriched, our proteomics data captured a number of mitochondrial proteins as interacting partners of Racl in INS-1832/13 cells. Along these lines, original studies from our laboratory have provided the first evidence for localization of Rho G proteins, specifically Cdc42 and Racl in the mitochondrial fraction derived from insulin-secreting βTC-3 cells (Kowluru et al., 2003). Based on complementary physiological and microscopic studies, we proposed that activation of Racl and Cdc42 may be necessary for coupling mitochondrial events to insulin secretion. Velaithan and associates have demonstrated interaction between Racl and BCL2 in BCL-2 overexpressing B-cell lymphoma cells, which, in turn, has been shown to promote BCL-2 mediated generation of superoxide within the mitochondria (Velaithan et al., 2011). Data accrued from studies by Chong et al. (2018) have implicated Rac1 in the generation of mitochondrial superoxides mediated via activation of NADPH oxidase. More recent investigations from our laboratory have demonstrated inhibition of reactive oxygen species, mitochondrial DNA damage and cell demise in bovine retinal endothelial cells chronically exposed to hyperglycemic conditions (Kowluru et al., 2014). Future studies will assess putative regulatory roles of Rac1 in mitochondrial dysregulation of the islet β-cell under the duress of metabolic stress.
4.6. Potential knowledge gaps and future goals
Several lines of experimental evidence suggest that Rac1 undergoes post-translational modifications including prenylation, carboxylmethylation, palmitoylation, phosphorylation and SUMOylation (Olson, 2018; Kowluru and Kowluru, 2015; Castillo-Lluva et al., 2010). At present, we are unsure whether a particular type and/or the degree of these post-translational modification of Rac1 is altered under HG conditions, which could affect binding of various interacting partners with Rac1. This remains a potential caveat in these investigations. Methodical investigations are underway in our laboratory to systematically address potential effects of post-translational modifications of this GTPase on its interaction with islet endogenous proteins under metabolic stress.
In conclusion, data accrued from our current investigations provide the first global analysis of Rac1 interaction partners in β-cells under basal and hyperglycemic conditions. We identify a number of high glucose-responsive Rac1 interaction partners in INS-1832/13 cells. Our immunological findings in rat and human islets validate proteomics data. We envision that these observations form basis for future investigations of these Rac1 interaction partners in furthering our understanding of regulatory roles of specific signaling pathways in islet β-cell function in health and diabetes.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH/NIDDK (R01 DK081750 and R01 DK107666 to ZY and DK94201 and EY22230 to AK). AK is also supported by a MERIT Review Award from the U.S. Department of Veterans Affairs (BX002801) and a Senior Research Career Scientist Award (13S-RCS-006) from the Department of Veterans Affairs. DD and AM were supported by a Pre-Doctoral Fellowship from the Diabetes Obesity Team Science initiative at Wayne State University. The authors thank Prof. Chris Newgard for INS-1832/13 cells.
Abbreviations used
- Arf6
ADP ribosylation factor 6
- ARNO
Arf6 nucleotide binding site opener
- CID
Collision-induced dissociation
- Co-IP
Co-immunoprecipitation
- FDR
False discovery rate
- GAP
GTPase-activating protein
- GDI
Guanine nucleotide dissociation inhibitor
- GEF
Guanine nucleotide exchange factor
- GIT
G protein-coupled receptor kinase-interacting protein-1
- GPCR
G protein-coupled receptor
- GSIS
Glucose-stimulated insulin secretion
- HG
Hyperglycemic
- UPLC-ESI-MS/MS
Ultra-performance liquid chromatography-electrospray-tandem mass spectrometry
- LTQ
Linear ion trap
- NIgG
Normal mouse IgG
- NLS
Nuclear localization signal
- NOX2
Phagocyte-like
- NADPH
oxidase
- PA
Peak area
- PAK
p21-activated kinase
- β-PIX
β-Pak-interacting exchange factor
- ROS
Reactive oxygen species
- Tiam1
T-cell lymphoma invasion and metastasis-inducing protein 1
- Vav2
Vav guanine nucleotide exchange factor 2
Footnotes
Data availability
Individual data points are shown in the Figures. Tabulated data are available upon request from the corresponding author.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mce.2019.110489 .
An abstract highlighting these observations is submitted for presentation at the 55th Annual Meetings of the European Association for the Study of Diabetes to be held in Barcelona in September 2019.
Conflicts of interest
There is no conflict of interest among the authors.
References
- Abdrabou A, Wang Z, 2018. Post-translational modification and subcellular distribution of Rac1: an update. Cells 7, E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara S, Shibutani Y, Teruyama K, Inoue HY, Kawada Y, Etoh H, Matsuda T, Kimura-Koyanagi M, Hashimoto N, Sakahara M, Fujimoto W, Takahashi H, Ueda S, Hosooka T, Satoh T, Inoue H, Matsumoto M, Aiba A, Kasuga M, Kido Y, 2013. Ras-related C3 botulinum toxin substrate 1 (Rac1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia 56, 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidwan S, Chekuri A, Hynds DL, Kowluru A, 2017. Glucotoxicity promotes aberrant activation and mislocalization of Ras-related C3 botulinum toxin substrate 1 (Rac1) and metabolic dysfunction in pancreatic islet β-cells: reversal of such metabolic defects by metformin. Apoptosis 22, 1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren PO, Leibiger IB, 2006. Novel aspects on signal transduction in the pancreatic beta cell. Nutr. Metabol. Cardiovasc. Dis. 16 (Suppl. 1), S7–S10. [DOI] [PubMed] [Google Scholar]
- Caruso M, Ma D, Msallaty Z, Lewis M, Seyoum B, Al-janabi W, Diamond M, Abou-Samra AB, H0jlund K, Tagett R, Draghici S, Zhang X, Horowitz JF, Yi Z, 2014. Increased interaction with insulin receptor substrate 1, a novel abnormality in insulin resistance and type 2 diabetes. Diabetes 63, 1933–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, Malliri A, 2010. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat. Cell Biol. 12, 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Černohorská M, Sulimenko V, Hájková Z, Sulimenko T, Slaádková V, Vinopal S, Dráberová E, Dráber P, 2016. GIT1/βPIX signaling proteins and PAK1 kinase regulate microtubule nucleation. Biochim. Biophys. Acta 1863, 1282–1297. [DOI] [PubMed] [Google Scholar]
- Chong SJF, Lai JXH, Eu JQ, Bellot GL, Pervaiz S, 2018. Reactive oxygen species and oncoprotein signaling-A dangerous liaison. Antioxidants Redox Signal. 29, 1553–1588. [DOI] [PubMed] [Google Scholar]
- Elumalai S, Karunakaran U, Lee IK, Moon JS, Won KC, 2017. Rac1-NADPH oxidase signaling promotes CD36 activation under glucotoxic conditions in pancreatic beta cells. Redox Biol 11, 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Langlais P, Luo M, Mapes R, Lefort N, Chen SC, Mandarino LJ, Yi Z, 2011. Label-free proteomic identification of endogenous, insulin-stimulated interaction partners of insulin receptor substrate-1. J. Am. Soc. Mass Spectrom. 22, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner TU, Kesavan G, Ståhlberg A, Semb H, 2009. Rac1 regulates pancreatic islet morphogenesis. BMC Dev. Biol. 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Jiang J, Jing Z, Chen Y, Shi Z, Deng B, 2018. Cysteinyl leukotriene receptor 1 regulates glucose-stimulated insulin secretion (GSIS). Cell. Signal. 46, 129–134. [DOI] [PubMed] [Google Scholar]
- Harmon B, Ratner L, 2008. Induction of the Gαq signaling cascade by the human immunodeficiencyvirus envelope is required forvirus entry. J. Virol. 82, 9191–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefen RJ, Berk BC, 2006. The multifunctional GIT family of proteins. J. Cell Sci. 119, 1469–1475. [DOI] [PubMed] [Google Scholar]
- Jayaram B, Kowluru A, 2012. Phagocytic NADPH oxidase links ARNO-Arf6 signaling pathway in glucose-stimulated insulin secretion from the pancreatic β-cell. Cell. Physiol. Biochem. 30, 1351–1362. [DOI] [PubMed] [Google Scholar]
- Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A, 2011. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 beta-cells and rat islets. Biochem. Pharmacol. 81, 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ, 2010. Regulation of insulin secretion: role of mitochondrial signaling. Diabetologia 53, 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwat MA, Thurmond DC, 2013. Signaling mechanisms of glucose-induced F-actin remodeling in Pancreatic islet β-cells. Exp. Mol. Med. 45, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Kitamura T, 2008. Rac and nuclear translocation of signal transducers and activators of transcription factors. Methods Enzymol. 439, 171–180. [DOI] [PubMed] [Google Scholar]
- Kepner EM, Yoder SM, Oh E, Kalwat MA, Wang Z, Quilliam LA, Thurmond DC, 2011. Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion. Am. J. Physiol. Endocrinol. Metab. 301, E1072–E1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadija S, Veluthakal R, Sidarala V, Kowluru A, 2014. Glucotoxic and diabetic conditions induce caspase 6-mediated degradation of nuclear lamin A in human islets, rodent islets and INS-1 832/13 cells. Apoptosis 19, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Yan D, Wu X, Guan Y, Ma X, 2015. Glucotoxicity inhibits cAMP-protein kinase A-potentiated glucose-stimulated insulin secretion in pancreatic β-cells. J. Diabetes 7, 378–385. [DOI] [PubMed] [Google Scholar]
- Kowluru A, 2010. Small G proteins in islet beta-cell function. Endocr. Rev. 31, 52–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, 2011. Friendly, and not so friendly, roles of Rac1 in islet beta-cell function: lessons learnt from pharmacological and molecular biological approaches. Biochem. Pharmacol. 81, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, 2017. Role of G-proteins in islet function in health and diabetes. Diabetes Obes. Metab. 19 (Suppl. 1), 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Kowluru RA, 2014. Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucotoxicity and diabetes. Biochem. Pharmacol. 88, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Kowluru RA, 2015. Protein prenylation in islet β-cell function in health and diabetes: putting the pieces of the puzzle together. Biochem. Pharmacol. 98, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Kowluru RA, 2018. RACking up ceramide-induced islet β-cell dysfunction. Biochem. Pharmacol. 154, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A, Chen HQ, Tannous M, 2003. Novel roles for the rho subfamily of GTP-binding proteins in succinate-induced insulin secretion brom betaTC3 cells; further evidence in support of the succinate mechanism of insulin release. Endocr. Res. 29, 363–376 2003. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM, Mishra M, 2014. TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 57, 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JT, Birnbaum MJ, 2003. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. U. S. A. 100, 13320–13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yang A, Yin X, Dong S, Luo F, Dou C, Lan X, Xie Z, Hou T, Xu J, 2018. Mesenchymal stem cells promote endothelial progenitor cell migration, vascularization, and bone repair in tissue engineered constructs via activating CXCR2-Src-PKL/Vav2-Rac1. FAESB J 32, 2197–2211. [DOI] [PubMed] [Google Scholar]
- Ma WN, Park SY, Han JS, 2010. Role of phospholipase D1 in glucose-induced insulin secretion in pancreatic Beta cells. Exp. Mol. Med. 42, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Sowden MP, Gerber SA, Thomas T, Christie CK, Mohan A, Yin G, Lord EM, Berk BC, Pang J, 2014. G protein coupled receptor 2 interacting protein-1 is required for endothelial cell directional migration and tumor angiogenesis via cortactin-dependent lamellipodia formation. Arterioscler. Thromb. Vasc. Biol. 34, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla M, Albar JP, 2013. Quantitative proteomics: a strategic ally to map protein interaction networks. IUBMB Life 65, 9–16. [DOI] [PubMed] [Google Scholar]
- Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, Cerasi E, Melloul D, 1999. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes 48, 1230–1236. [DOI] [PubMed] [Google Scholar]
- McNelis JC, Lee YS, Mayorai R, va der Kant R, Johnson AM, Wollam J, Olefsky JM, 2015. GPR43 potentiates β-cell function in obesity. Diabetes 64, 3203–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Morgan D, Rebelato E, Oliveira-Emillo HC, Procopio J, Curi R, et al. , 2009. Insights into the critical role of NADPH oxidases(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52, 2489–2498. [DOI] [PubMed] [Google Scholar]
- Olson MF, 2018. Rho GTPases, their post-translational modifications, disease-associated mutations and pharmacological inhibitors. Small GTPases 9, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payapilly A, Malliri A, 2018. Compartmentalisation of RAC1 signalling. Curr. Opin. Cell Biol. 54, 50–56. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM, Madiraju SR, 2013. Metabolic signaling in fuel-induced insulin secretion. Cell Metabol. 18, 162–185. [DOI] [PubMed] [Google Scholar]
- Rathor N, Chung HK, Wang SR, Qian M, Turner DJ, Wang J-Y, Rao JN, 2017. B-PIX plays an important role in regulation of intestinal epithelial restitution by interacting with GIT1 and Rac1 after wounding. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G399–G407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R, Rodriguez-Trejo A, Jacovetti C, 2016. Insulin secretion in health and disease: nutrients dictate pace. Proc. Nutr. Soc. 75, 19–29. [DOI] [PubMed] [Google Scholar]
- Sabbatini ME, Bi Y, Ji B, Ernst SA, Williams JA, 2010. CCK activates RhoA and Rac1 differentially through Ga13 and Gaq in mouse pancreatic acini. Am. J. Physiol. Cell Physiol. 298, C592–C601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock K, Bielek H, Schradi K, Schmidt G, Klugbauer N, 2010. The nuclear import of the small GTPase Rac1 is mediated by the direct interaction with karyopherin a2. Traffic 11, 198–209. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD, 2005. Role of GPR40 in fatty acid action on beta cell line INS-1E. Biochem. Biophys. Res. Commun. 335, 97–104. [DOI] [PubMed] [Google Scholar]
- Sidarala V, Kowluru A, 2017a. Exposure to chronic hyperglycemic conditions results in Ras-related C3 botulinum toxin substrate 1 (Rac1)-mediated activation of p53 and ATM kinase in pancreatic p-cells. Apoptosis 22, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidarala V, Kowluru A, 2017b. The regulatory roles of mitogen-activated proteinkinase (MAPK) pathways in health and diabetes: lessons learned from the pancreatic beta cell. Recent Pat. Endocr. Metab. Immune Drug Discov. 10, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A, 2015. Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic β-cells under glucotoxic conditions: evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem. Pharmacol. 95, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund G, Basmaciogullari A, Rouot B, Marie JC, Rosselin G, 1999. Cell-specific localization of G protein alpha-subunits in the islets of Langerhans. J. Endocrinol. 162, 31–37. [DOI] [PubMed] [Google Scholar]
- Subasinghe W, Syed I, Kowluru A, 2011. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic β-cells: evidence for regulation by Rac1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R12–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijun W, Zhen Y, Ying G, Yanfang W, 2014. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 444, 451–454. [DOI] [PubMed] [Google Scholar]
- Syed I, Jayaram B, Subasinghe W, Kowluru A, 2010. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem. Pharmacol. 80, 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, Kowluru A, 2011. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 60, 2843–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaithan R, Kang J, Hirpara JL, Loh T, Goh BC, Le Bras M, Brenner C, Clement MV, Pervaiz S, 2011. The small GTPase Rac1 is a novel binding partner of Bcl-2 and stabilizes its antiapoptotic activity. Blood 117, 6214–6226. [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Tunduguru R, Arora DK, Sidarala V, Syeda K, Vlaar CP, Thurmond DC, Kowluru A, 2015. Vav2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cell. Diabetologia 58, 2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Thurmond DC, 2009. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 122, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wepf A, Glatter T, Schmidt A, Aebersold R, Gstaiger M, 2009. Quantitative interaction proteomics using mass spectrometry. Nat. Methods 6, 203–205. [DOI] [PubMed] [Google Scholar]
- Xiang FL, Lu X, Strutt B, Hill DJ, Feng Q, 2010. NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes 59, 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Damacharla D, Ma D, Qi Y, Tagett R, Draghici S, Kowluru A, Yi Z, 2016. Quantitative proteomics reveals novel protein interaction partners of PP2A catalytic subunit in pancreatic β-cells. Mol. Cell. Endocrinol. 424, 1–11 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Li X, Premont RT, 2016. Expanding functions of GT ArfGTPase-activating proteins. PIXRho guanine nucleotide exchange factors and GIT-PIX complexes. J. Cell Sci. 129, 1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


