Abstract
Plant extracts are gaining more attention as therapeutic agents against inflammation. In this study, four different widely used herbals were selected, such as holy basil leaf, sesame seed, long pepper, and cubeb pepper. We have evaluated the anti-inflammatory action of an aqueous extract from these herbs and tested their effects on monocyte-derived macrophages (MDMs). MDMs were pre-treated with these extracts individually for 2 h, followed by lipopolysaccharide (LPS) stimulation for 24 h and pro-inflammatory gene expression was analyzed. Also, we studied the effect of these extracts on the oxidation of low-density lipoprotein (LDL) by enzymatic (Myeloperoxidase) and non-enzymatic (copper) reactions. All extracts attenuated LPS-induced inflammation and also were able to inhibit the oxidation of LDL. These beneficial actions of extracts led us to identify molecules present in the extracts. A liquid chromatography–high resolution mass spectrometric analysis was performed to identify the chemical composition of extracts. Wide range of molecules were identified across all the extracts, short-chain organic acids, phenolic acids and derivatives, piperine and its structural homologues, eugenol, rosmarinic acid, flavonoids and their glucosides, and others. This study opens a door for future studies on non-pharmacological natural therapeutics that will be useful for consumers and producers, as well as industries utilizing bioactive compounds.
Keywords: anti-inflammation, cubeb pepper, herbal extract, holy basil, long pepper, mass spectrometry, sesame seed
Introduction
Over the decades, botanicals and herbal-derived products have made significant contribution to the overall human health and well-being. Many articles have demonstrated that plant-derived materials contain biological active molecules and are shown to have anti-inflammatory, antihypertensive, anti-atherosclerotic, antidepressant, hepatoprotective, neuroprotective, immunomodulatory, hypoglycemic, anti-microbial, analgesic, antipyretic, anti-hepatotoxic, and antioxidant properties.1–22 These actions of botanicals are attributed to their phytoconstituents, such as flavonoids, carotenoids, poly phenols, methylenedioxy phenol derivatives, methoxy phenols, stilbenes, sterols, etc.19–20,22–33 Most of these molecules are nonpolar in nature, and, hence, there have been many studies on organic solvents extracts of herbal products and their health beneficial properties.8,14,16,18,21,24,27,34–36 On the other hand, a few articles have demonstrated that aqueous extracts of plant materials have also shown several health benefits.2,13,37–44 By considering these therapeutic effects, aqueous extracts of herbs are becoming more popular13,17,37,43,44 as these can be easy to prepare for regular household consumption. In this study, four different kinds of widespread herbal products were selected, (1) Holy basil leaf (Ocimum basilicum L.), (2) Sesame seed (Sesamum indicum L.), (3) Long pepper (Piper longum L.), and (4) Cubeb pepper (Piper cubeba L. f.). However, there are studies in the literature on the health beneficial properties and chemical composition of these selected herbs by using various extraction procedures, and many of them were based on organic solvent extracts.10,14,16,21,45 In this study, we have aimed at evaluating chemical compounds present in the simple aqueous extracts (household way of preparation) of herbs and also compared molecules between different classes of herbs that are attributed to their anti-inflammatory and antioxidant properties. Liquid chromatography–high resolution tandem mass spectrometric (LC-HRMS/MS) method was employed to identify chemical constituents present in herbal aqueous extracts. Anti-inflammatory properties of individual herbal aqueous extracts were evaluated by measuring lipopolysaccharide (LPS)-induced inflammatory gene expression (tumor necrosis factor [TNF]-α and interleukin [IL]-1β) in macrophages. Anti-oxidant properties of the extracts were measured by inhibiting the low-density lipoprotein (LDL) oxidation both enzymatically (Myeloperoxidase—MPO induced) and non-enzymatically (copper-Cu induced). Also, we have tried to establish the chemical networks and their action of anti-inflammatory and anti-oxidant properties. Thus, this study is noteworthy, because the extraction procedure we have followed is a common household way of preparation; having knowledge of beneficial actions of different herbal extracts will attract the attention of consumers and shed light on the non-pharmacologic therapeutic approach.
Materials and Methods
Materials and reagents
Herbal products holy basil leaf, sesame seed, long pepper, and cubeb seeds were purchased from Amazon online store. Analytical-grade reference standard chemicals, LPS (Escherichia coli), Phorbol 12-myristate 13-acetate (PMA), buffers, and mobile phase solvents (liquid chromatography-mass spectrometry [LC-MS] grade) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-purity water (≤18 MΩ) was obtained from Barnstead MegaPure Glass Stills (MP-3A; Thermo Scientific, Waltham, MA, USA). Polymerase chain reaction (PCR) primers and Trizol™ reagent were purchased from Invitrogen (Carlsbad, CA, USA).
Sample preparation
Herbs were finely pulverized by using a mixer grinder (Mr. Coffee Electric Coffee Grinder, IDS77-RB; Amazon). Each individual powder, about 50 mg, was placed in a coffee maker (Better Chef, 91580119M; BestBuy) and extracted with 50 mL of warm water. Extracts were collected in 50-mL falcon tubes, the tubes were left to cool down at room temperature, and the samples were lyophilized to complete dryness. One milligram of each dried extract was dissolved in 50% methanol containing 0.1% formic acid. Samples were vortexed for 2 min and centrifuged at 8,000 g for 10 min. Supernatant was collected and filtered through a 0.45-μm Nylon syringe filter (Alltech Associates, Deerfield, IL, USA). Five microliters of each individual sample solutions were used for LC-MS analysis for characterization of chemical constituents of the extracts. Each extract was analyzed in triplicate. For cell culture experiments, samples were reconstituted in pyrogen-free water under sterile conditions.
Isolation and oxidation of lipoproteins
After obtaining Institutional Review Board approval, blood was collected in heparinized tubes from consented healthy donors and stored on ice. Blood was centrifuged at 3000 rpm for 20 min, and plasma was separated. Lipoproteins were isolated from normal plasma by sequential ultracentrifugation using a Beckman TL-100 tabletop ultracentrifuge (Beckman, Palo Alto, CA, USA).46–48 The isolated lipoproteins were dialyzed against 0.3 mM EDTA in 1 × phosphate-buffered saline (PBS) of pH 7.4 overnight and subsequently filter sterilized. The amount of protein was estimated by using the Bio-Rad DC protein assay (Hercules, CA, USA). The LDL sample was subjected to oxidation immediately after dialysis. Oxidation of LDL (100 μg/mL) was performed with 5 μM copper in both the presence and absence of different concentrations of aqueous extracts (5–50 μg). The formation of conjugated dienes was monitored at an optical density of 234 nm for about 16 h by using Jenway DB-6500 spectrophotometer equipped with an eight-chamber cuvette changer. The degree of LDL oxidation was assessed by determination of peroxide content using leucomethylene blue (LMB) assay48 and thiobarbituric acid reactive substances (TBARS).
In addition to non-enzymatic oxidation, enzymatic oxidation was also performed by using MPO (0.2 U) and 100 μM H2O2 to a 100 μg/mL of LDL in 1 mL of PBS at 37°C in both the presence and absence of 25 μg of extracts, further confirmed by LMB and TBARS assays.
Preparation of HPODE and incubation with aqueous extracts
Overall, 13-hydroperoxylinoleic acid (13-HPODE) of 200 nmoles/mL was prepared as previously described,49 and it was used to determine the effect of extracts on free fatty acid peroxides (FFAOOH). HPODE was incubated with increased concentrations of aqueous extracts (0–50 μg) for 1 h at 37°C. The content of lipid peroxides present in the reaction system was analyzed by LMB assay. Similarly, effect of the extracts on hydrogen peroxide was also determined.
Cell culture
THP1 monocytes (ATCC, Manassas, VA, USA) were obtained from ATCC. Cells were grown in flasks and dishes and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma, St Louis, MO, USA), 2 mM l-glutamine, and 1 × penicillin-streptomycin antibiotic solution. Cultures were maintained in a 5% CO2 atmosphere at 37°C. For experiments, THP-1 cells were differentiated with 50 ng/mL PMA for 72 h in complete RPMI 1640. Monocyte-derived macrophages (MDMs) were used for the experiments, and cells were starved in serum-free medium before experiments.
Incubation of MDMs with LPS and aqueous extracts
To monitor the changes in gene expression of TNF-α and IL1β, MDMs (2 × 105cells/well) were pre-incubated in serum-free RPMI 1640 for 3 h. They were pre-treated with aqueous extracts (5 and 25 μg/mL) for 2 h, followed by addition of LPS (100 ng/mL). Cells were incubated for 24 h. At the end of 24 h of incubation, the cells were harvested in Trizol for RNA isolation.
Complementary DNA synthesis and real time-polymerase chain reaction
Total RNA from cells was isolated by using Trizol reagent. One microgram of RNA was reverse-transcribed into complementary DNA (cDNA) by using the Superscript™ III First Strand Synthesis system (Invitrogen). cDNA (50 ng) sample was used to perform quantitative real-time PCR by CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad) with SYBR Green (Invitrogen). PCR was carried out with TNF-α and IL-1β specific primers for human targets (Supplementary Table S1), resulting in 200-bp fragments. As a reference gene we used GAPDH primers, resulting in a 200-bp fragment. PCR was performed with an initial step of denaturation at 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 20 sec and 60°C for 20 sec. Melt curves were established for the reactions. Normalized fold expression was calculated by using the 2−ΔΔCt method.
Liquid chromatography and mass spectrometric conditions
Compounds were separated on Zorbax Eclipse Plus C18 (150 mm L × 4.6 mm inner diameter, 5 μm particle size) column by using an Agilent 1200 series high performance liquid chromatography system coupled with 6520B quadrupole time-of-flight mass spectrometer (Agilent Technologies). Binary mobile phase gradient program was employed to elute the components from the column, pump-A: acetonitrile and pump-B: water, both containing 0.1% HCOOH. The gradient program was as follows: 90% B: 0–12 min; 10% B: 12–35 min; 10% B, 35–50 min; 90% B: 50–50.1; 10% B: 50.1–60 min; at the end of the each run, the column was washed for 5 min with a solvent composition consisting of 50% isopropyl alcohol, 30% methanol, 20% water, and 0.1% HCOOH (v/v). The column was operated at 40°C with a constant mobile phase flow rate at 800 μL/min.
Mass spectral data were acquired in electrospray ionization (ESI) mode over the mass range of 50–1700 m/z and operated in both positive (+) and negative (−) modes separately. The mass spectrometer was tuned and calibrated each day before the analysis to maintain mass accuracy and operated at optimized source conditions as described in our recent article.50 Nitrogen was used as a collision-induced dissociation gas, and studies were performed at different collision energies in the range of 15–35 volts for interpreting the molecules by their tandem mass spectrometry (MS/MS) products. The MS and MS/MS data were collected and processed by using Mass Hunter qualitative analysis software version B.07.00.
Compounds identification
The identification of unknown molecules present in herbal extracts was achieved by considering multiple orthogonal properties of molecules as described in our recent article.50 Briefly, the unknown molecular identification workflow was carried out as follows: (1) Experimental monoisotopic masses obtained from herbal extracts analysis were entered at <10 ppm mass accuracy in publicly available accurate mass spectral libraries such as, Metlin, Massbank, Chemspider, LIPIDMAPS, HMDB, and Pubchem to get tentative molecules; (2) MS/MS fragmentation studies of precursor molecular ions; (3) conformation of a few molecules with authentic reference standards or with its structural analogues, analyzed in identical experimental conditions to compare their chromatographic and mass spectral profiles; and (4) the literature survey on studied herbal species.
Statistical analysis
Values were presented as mean ± standard deviation, and statistical analyses were performed by using Student t test at significance of P < .05.
Results and Discussion
Aqueous extracts of basil leaf, cubeb berries, sesame seeds, and long pepper inhibit the oxidation of lipoproteins
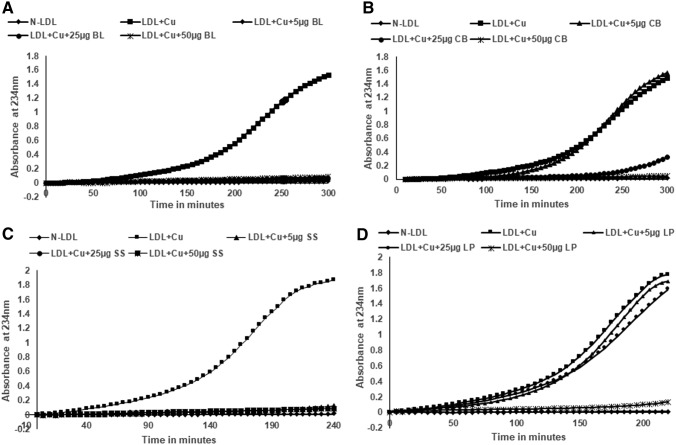
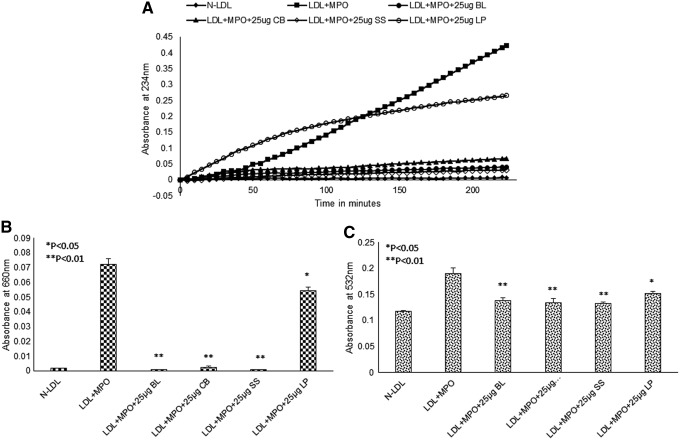
To test the effect of aqueous extracts on the oxidation of LDL, the lipoprotein was incubated with 5 μM copper, and oxidation was performed in the presence and absence of different concentrations of basil leaf, cubeb berries, sesame seeds, and long pepper. The formation of conjugated dienes was measured at 234 nm absorption wavelength. As shown in Figure 1, control incubation generated an oxidized LDL (Ox-LDL) that reached a maximum increase in absorption at about 200 min. The lag phase, which represents the time at which antioxidants are consumed, was 60–80 min. As shown in Figure 1, in the presence of increasing amounts of either basil leaf extract or sesame seeds, there was an increase in lag time, suggesting that even low concentrations are able to delay the oxidation rate. However, cubeb berries and long pepper aqueous extracts were able to delay the oxidation at higher concentrations only. Reduced peroxides and thiobarbituric reactive substances were observed in the presence of aqueous extracts compared with Ox-LDL, as shown in Supplementary Figures S1 and S2. Similarly, all extracts (used 25 μg) were able to inhibit the oxidation of LDL with MPO, as shown in Figure 2.
FIG. 1.
Herbal aqueous extracts (BL, CB, SS, and LP) inhibit the oxidation of LDL by copper. LDL was isolated from the plasma of consenting subjects and used for oxidation with copper. One hundred micrograms of LDL was oxidized with 5 μM copper in the presence and absence of increased concentrations of extracts (5–50 μg), in 1 mL PBS; formation of conjugated dienes were measured at 234 nm. As concentrations of (A) BL, (B) CB, (C) SS, and (D) LP increased, initiation of LDL oxidation was delayed. BL, basil leaf; CB, cubeb berries/pepper; LDL, low-density lipoprotein; LP, long pepper; PBS, phosphate-buffered saline; SS, sesame seed.
FIG. 2.
Herbal aqueous extracts (BL, CB, SS, and LP) inhibit the oxidation of LDL by MPO. LDL was isolated from the plasma of consenting subjects and used for oxidation with copper. One hundred micrograms of LDL was oxidized with 0.2 U MPO and 100 μM H2O2 in the presence and absence of extracts (25 μg), in 1 mL PBS; formation of conjugated dienes was measured at 234 nm. (A) At the used concentration in the presence of extracts, initiation of LDL oxidation was delayed. (B) LMB assay. (C) TBARS assay. *P < .05; **P < .01; LMB, leucomethylene blue; MPO, myeloperoxidase; TBARS, thiobarbituric acid reactive substances.
All extracts are able to inhibit the oxidation of LDL by copper or MPO in an in vitro system suggests that they could prevent the formation of Ox-LDL in vivo, which has been considered a major pathophysiological step in atherosclerosis. This might be due to the ability of herbal extracts to decrease the rate of initiation of oxidation by reacting with oxygen radicals. They might also act as chain terminators and prevent propagation of oxidation. Pre-treatment of LDL with herbal aqueous extracts renders LDL resistant to oxidation, suggesting that the latter might be able to remove peroxidized lipids (13-HPODE and 15-HPETE) from LDL, which are the major enhancers of the non-enzymatic oxidation of both cholesterol linoleate and 1-palmitoyl-2-arachidonoyl-sn- glycero-3-phosphocholine, which are considered key players in disease progression.51
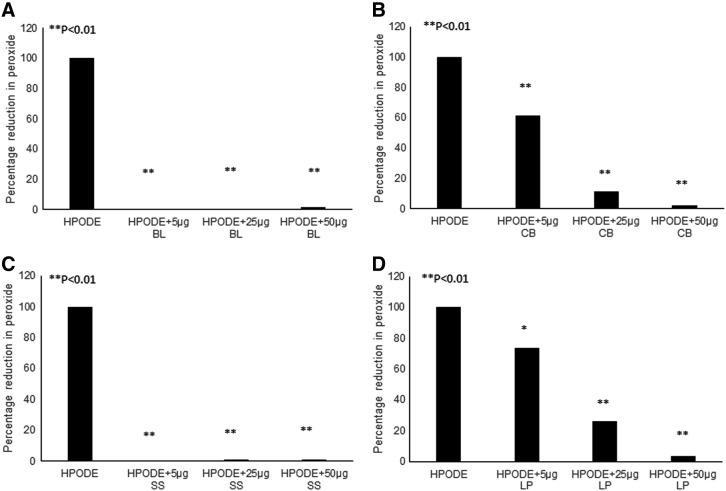
Reduction of peroxides in the presence of extracts
Reduction of peroxides in the presence of bail leaf/cubeb berries/sesame seeds/long pepper is exciting and could have a major impact in preventing propagation of oxidation as well as in reducing the cellular effects of lipid peroxides. FFAOOH (HPODE) was reduced into hydroxides in the presence of increasing concentrations of all the aqueous extracts. As shown in Figure 3, all extracts showed a significant reduction in the peroxide content of FFAOOH, approximately 80–90%, with increasing concentrations. Similarly, all extracts were able to reduce the hydrogen peroxide with increasing concentrations, as shown in Supplementary Figure S3. In our previous studies, we already reported that basil extract52 and associated components contain a methoxy group on phenol that acts as an MPO inhibitor and that might also inhibit the soybean lipoxidase action. This might be due to the presence of similar homology of soybean lipoxidase with human 15 lipoxygenase and MPO.
FIG. 3.
Decomposition of HPODE in the presence of aqueous extracts. Two hundred nmoles per mL of HPODE was prepared by oxidizing linoleic acid with soybean lipoxygenase and used to determine the effect of aqueous extracts on FFAOOHs. HPODE was incubated with increasing concentrations (0–50 μg) of (A) BL (B) CB or (C) SS and (D) LP for 1 h at 37°C. Lipid peroxide present in the reaction system was analyzed by LMB assay. All the extracts showed significant reduction in the peroxide content of FFAOOHs in a concentration-dependent manner. *P < .05; **P < .01. FFAOOHs, free fatty acid peroxides.
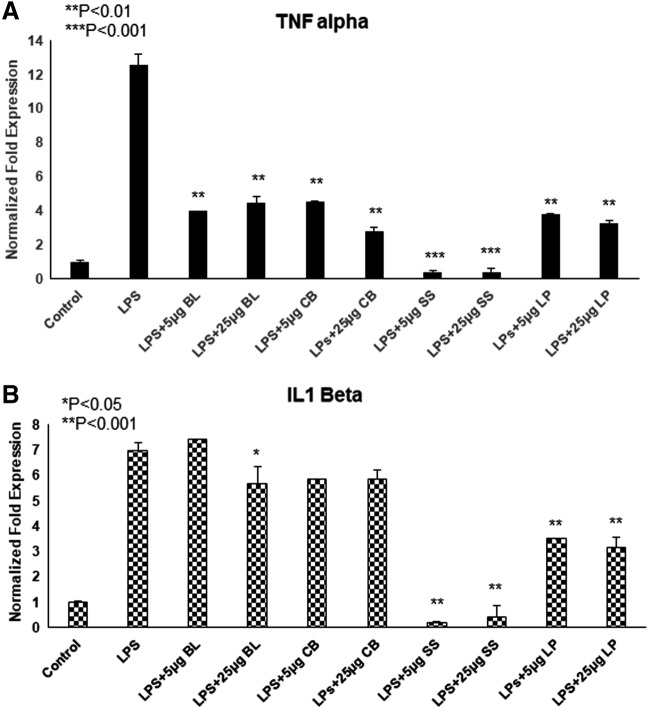
Extracts attenuate LPS-induced inflammatory cytokines in MDMs
MDMs were treated with LPS (100 ng/mL) ± extracts (5 and 25 μg/mL) for 24 h. LPS strongly induced messenger RNA (mRNA) levels of TNF-α and IL-1β in MDMs, whereas all extracts were able to attenuate the LPS-induced inflammatory markers in MDMs in a dose-dependent manner. As shown in Figure 4, a significant reduction in pro-inflammatory cytokine TNF-α was observed in the presence of extracts as compared with the control. All the extracts alone did not induce any of the inflammatory cytokines by itself. LPS has been considered a potent pro-inflammatory stimulant to induce TNF-α in macrophages, which is a key player in several inflammatory diseases. In our studies, we observed the reduction of TNF-α by all herbal extracts, which suggests their efficacy against LPS-induced inflammation. Although proinflammatory cytokine generation is essential for the development of the localized inflammatory response, an imbalance and sustained overproduction of these cytokines might lead to septic shock characterized by coagulopathy, endothelial damage, loss of vascular tone, and multiple system organ failure, often resulting in death.53 Further, their anti-inflammatory nature would shed light on potential non-pharmacological therapy for several inflammation-associated diseases.
FIG. 4.
Herbal aqueous extracts attenuate LPS-induced proinflammatory gene expression in MDMs. MDMs were incubated with BL, CB, SS, and LP (5 and 25 μg) for 2 h, followed by LPS (100 ng/mL) for 24 h. RNA was isolated, and real time-PCR analysis was performed for (A) TNF-α and (B) IL-1β gene expression by using appropriate primers. All are effective in attenuating LPS-induced inflammatory markers. Results are represented as mean ± standard deviation. *P < .05; **P < .01; ***P < .001. IL, interleukin; LPS, lipopolysaccharide; MDMs, monocyte derived macrophages; PCR, polymerase chain reaction; TNF, tumor necrosis factor.
LC-MS experiments
The method development of LC-MS in terms of selection of a column and mobile phase was achieved as described in our recent article.50 The identified compound's polarities have fallen in a broad range from polar to non-polar. Though water is a polar solvent, hot water could extract many non-polar compounds to some extent, due to temperature effect on solubility of molecules in various solvents.54 Small-chain carboxylic acids were commonly identified in all the herbal extracts.
Compound identification by mass spec libraries and fragmentation (MS/MS) studies
In this study, a total of 65 molecules were identified and confirmed in four different types of herbal species. Most of the molecules were confidently confirmed based on their MS, MS/MS spectra, and chromatographic profiles and a few were confirmed with their corresponding reference standards analyzed in identical experimental conditions. The mass spectral libraries and MS/MS studies have played a huge role in the identification and confirmation of molecules present in the extracts. Several monoisotopic masses were shown numerous possible molecules on their entry into mass spectral libraries. Later, appropriate molecules were confirmed based on their MS/MS fragment ions, LC retention time and comparing their corresponding fragments with MS/MS spectra with databases.
Basil leaf
In the basil, 26 molecules were identified and confirmed; these include short-chain organic acids, medium-chain dicarboxylic acids, benzoic acid and cinnamic acid derivatives, flavonoids and its glucosides, and eugenol. The complete list of molecules is shown in Table 1.
Table 1.
Complete List of Molecules Identified in Basil Aqueous Extract
| S. No. | Compound | RT | Detected ion |
|---|---|---|---|
| 1 | Tartaric acid | 1.79 | 149.0109 |
| 2 | Malic acid | 1.88 | 133.0159 |
| 3 | Citric acid | 2.41 | 191.0193 |
| 4 | Glutaconic acid | 2.87 | 129.02 |
| 5 | Quinic acid | 3.33 | 191.0565 |
| 6 | Glutaric acid | 3.86 | 131.0354 |
| 7 | Gentisic acid | 4.63 | 153.0203 |
| 8 | Cis-Caffeoyl tartaric acid | 5.09 | 311.0414 |
| 9 | Phloracetopenone | 8.98 | 167.0338 |
| 10 | Caffeic acid | 10.53 | 179.0356 |
| 11 | 4-Hydroxybenzaldehyde | 12.35 | 121.0301 |
| 12 | 7-Epi-12-hydroxyjasmonic acid glucoside | 12.95 | 387.1671 |
| 13 | Vanillin | 16.65 | 151.0404 |
| 14 | Suberic acid | 18.27 | 173.0818 |
| 15 | Ferulic acid | 18.88 | 193.0494 |
| 16 | Chicoric acid | 19.05 | 473.066 |
| 17 | Luteolin-7-O-glucuronide | 19.15 | 461.0718 |
| 18 | Apigenin | 20.01 | 269.0451 |
| 19 | 2-(2-Oxopropyl)benzoic acid | 20.55 | 177.0563 |
| 20 | Rosmarinic acid | 20.62 | 359.0744 |
| 21 | Azelaic acid | 20.66 | 187.0949 |
| 22 | Salicylic acid | 21.03 | 137.0217 |
| 23 | Eugenol | 22.02 | 163.0741 |
| 24 | Luteolin | 22.02 | 285.0376 |
| 25 | Sebacic acid | 22.41 | 201.1154 |
| 26 | Eugenitol | 24.49 | 205.0507 |
| 27 | Eupatorin | 26.69 | 343.0799 |
RT, retention time.
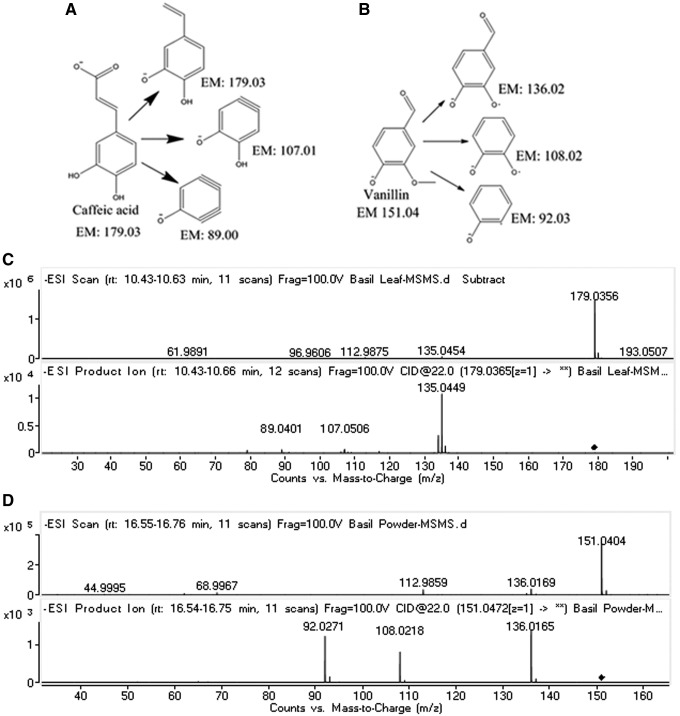
The accurate mass ion at m/z: 179.0341 resulted in various possible molecules on database search, 4-Hydroxyphenylpyruvic acid, 3-(3,5-Dihydroxyphenyl)-2-propeonic acid, and caffeic acid. MS/MS studies have been performed based on the fragments that the caffeic acid molecule established. Glutaric acid, glutaconic acid, 4 hydroxy benzaldehyde, rosmarinic acid, luteolin, and eugenitol were also confirmed by their MS/MS studies and matched with database MS/MS spectra. The parent mass ion at m/z: 151.0404 resulted in many possible molecules, up on MS/MS studies; produced daughter ions at m/z: 136.0165, 108.0218, and 92.0271; based on these fragments, vanillin molecules were confirmed. The fragmentation mechanisms of a few molecules are shown in Figure 5.
FIG. 5.
Proposed fragmentation pathway and ESI-MS and MS/MS spectra (A, C) for caffeic acid and (B, D) for vanillin. ESI, electrospray ionization; MS/MS, tandem mass spectrometry.
Sesame seed
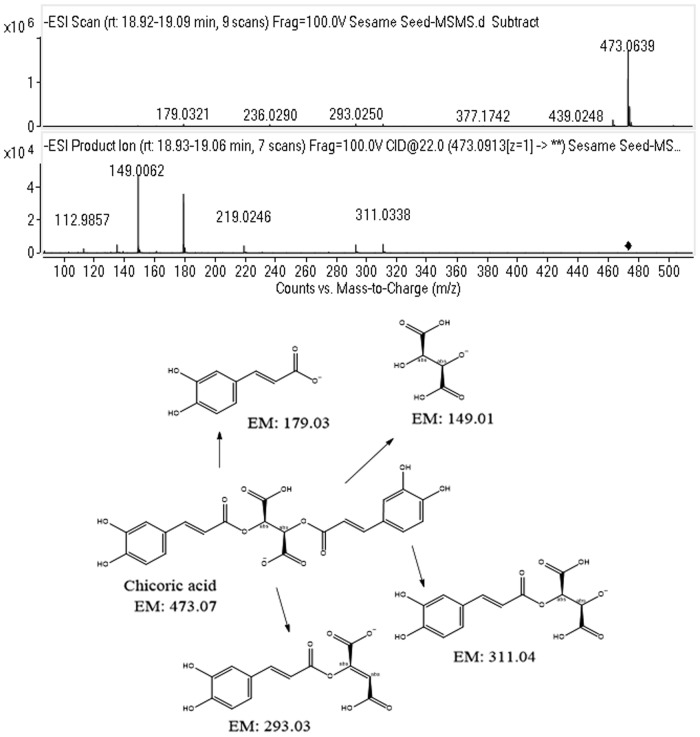
In the sesame seed, 20 molecules were identified and confirmed; these include short-chain organic acids, benzoic acid and its glucosides, cinnamic acid derivatives, and fatty acid degradation products. The complete list of molecules is shown in Table 2. Fertartaric acid, 4-(β-D-Glucosyloxy) benzoate, 2,3 dihydroxy benzoic acid, and Luteolin-7-O-glucuronide were confirmed by their MS and MS/MS fragmentation studies. Chicoric acid MS and MS/MS spectra are shown in Figure 6. Eupatorin was confirmed by its MS and MS/MS spectral match with Metlin database.
Table 2.
Complete List of Molecules Identified in Sesame Seed Aqueous Extract
| S. No. | Compound | RT | Detected ion |
|---|---|---|---|
| 1 | d-Tartaric acid | 1.80 | 149.0114 |
| 2 | Malic acid | 1.86 | 133.0116 |
| 3 | Citric acid | 2.42 | 191.0195 |
| 4 | Glutaric acid | 3.01 | 131.0365 |
| 5 | Quinic acid | 3.28 | 191.0567 |
| 6 | Gentisic acid | 4.67 | 153.0198 |
| 7 | Cis-Caffeoyl tartaric acid | 5.18 | 311.0397 |
| 8 | 4-(β-D-Glucosyloxy)benzoate | 6.24 | 299.0722 |
| 9 | Fertaric acid | 7.95 | 325.0628 |
| 10 | Vanillyl alcohol 4-O-β-glucopyranoside | 9.74 | 315.1069 |
| 11 | Caffeic acid | 10.53 | 179.0341 |
| 12 | 4-Hydroxybenzaldehyde | 12.41 | 121.0290 |
| 13 | 12-Hydroxyjasmonic acid | 17.11 | 225.1141 |
| 14 | Chicoric acid | 19.02 | 473.0639 |
| 15 | Luteolin-7-O-glucuronide | 19.18 | 473.0815 |
| 16 | Rosmarinic acid | 20.6 | 359.0817 |
| 17 | 9S,12S,13S-trihydroxy-10E,15Z-octadecadienoic acid | 23.72 | 327.2217 |
| 18 | Traumatic acid | 24.68 | 227.1289 |
| 19 | Eupatorin | 27.88 | 343.0830 |
| 20 | 9,14-DiHODE | 28.57 | 311.2245 |
FIG. 6.
The ESI-MS and MS/MS spectra of chicoric acid and its proposed fragmentation pathway.
Long pepper
In the long pepper, 21 molecules were identified and confirmed; these include short-chain organic acids, cinnamic acid derivatives, and piperine derivatives. The complete list of molecules is shown in Table 3.
Table 3.
Complete List of Molecules Identified in Long Pepper Aqueous Extract
| S. No. | Compound | RT | Detected ion |
|---|---|---|---|
| 1 | Malic acid | 1.86 | 133.0116 |
| 2 | Citric acid | 2.42 | 191.0195 |
| 3 | Quinic acid | 3.3 | 191.0541 |
| 4 | Gentisic acid | 4.63 | 153.0203 |
| 5 | 1-Acetylpiperidine | 13.27 | 128.1041 |
| 6 | 3,4-Methylenedioxymethamphetamine | 18.27 | 194.112 |
| 7 | Benzoylmalic acid | 19.69 | 237.0375 |
| 8 | Cinnamaldehyde | 23.16 | 133.0612 |
| 9 | Coumaperine | 25.33 | 256.1351 |
| 10 | Piperyline | 26.78 | 272.12 |
| 11 | 4,5-Dihydropiperlonguminine | 27.88 | 276.155 |
| 12 | (E,E)-piperlonguminine | 28.01 | 274.1451 |
| 13 | Piperanine | 28.55 | 288.1523 |
| 14 | Piperine | 28.77 | 286.1441 |
| 15 | Piperittine | 30.58 | 312.1577 |
| 16 | Dehydropipernonaline | 32.59 | 340.1903 |
| 17 | Pipernonaline | 33.28 | 342.2033 |
| 18 | Pipercide | 33.86 | 356.2232 |
| 19 | Piperolein B | 34.18 | 344.2196 |
| 20 | Piperundecalidine | 35.11 | 368.2237 |
| 21 | Guineesine | 36.11 | 384.2489 |
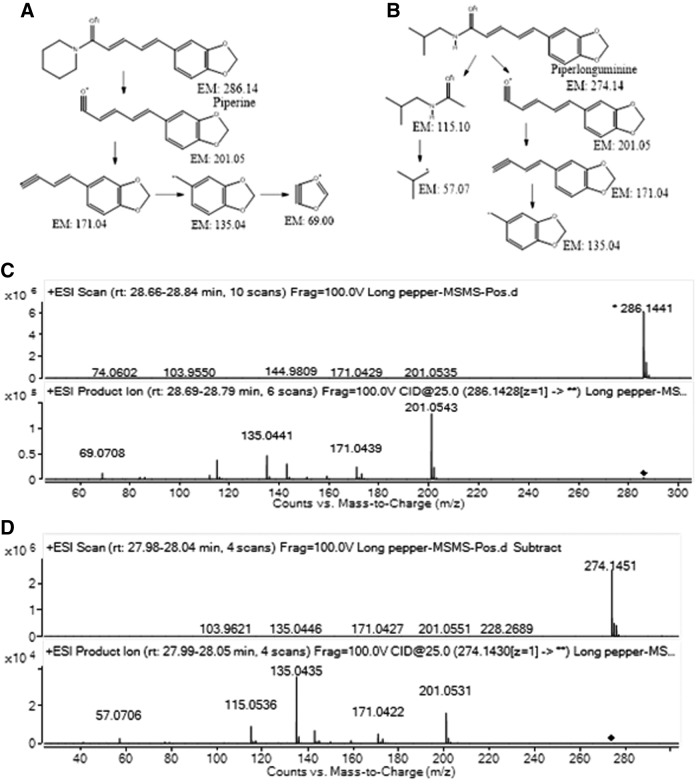
The extract contains a few unique alkaloid molecules containing methylenedioxy phenol moiety attached to them. Piperine and its structural homologues were confirmed by their MS/MS fragmentation studies. The proposed fragmentation pathway of a few molecules is shown in Figure 7.
FIG. 7.
Proposed fragmentation pathway and ESI-MS and MS/MS spectra (A, C) for piperine and (B, D) for piperlonguminine.
Cubeb pepper
In the cubeb pepper, only few molecules were confirmed out of many identified molecules; these include short-chain organic acids and terpenoids. The list of confirmed molecules is shown in Table 4.
Table 4.
Complete List of Molecules Identified in Cubeb Berries Aqueous Extract
| S. No | Compound | RT | Detected ion |
|---|---|---|---|
| 1 | Malic acid | 1.86 | 133.0116 |
| 2 | Citric acid | 2.43 | 191.0195 |
| 3 | Quinic acid | 3.32 | 191.0541 |
| 4 | Gentisic acid | 4.61 | 153.0203 |
| 5 | 5-Acetyl-2,3-dihydro-7-methyl-1H-pyrrolizine | 16.13 | 164.1044 |
Cubeb extract has produced poor ESI-MS spectra due to the presence of planar and nonpolar molecules. Extensive fragmentation was observed in corresponding MS/MS experiments, and, hence, we could not confirm many molecules in cubeb extract.
Identified molecules and inflammatory properties
Among the identified molecules, several molecules such as short-chain carboxylic acids and di-carboxylic acids,55–57 phenolic acids, piperine derivatives, rosmarinic acid, eugenol, etc., have been reported to have anti-inflammatory properties.18,20,49 A few molecules are commonly present in all the extracts and few are specific to types of herbal species. Basil and sesame seed extracts have shown many similar molecules and also shown a similar kind of biological activities. Long pepper and cubeb pepper extracts have shown specific molecules, piperine derivatives, and terpenes (extensive fragmentation in ESI). Basil and sesame seed extracts have acid-containing molecules; whereas long pepper and cubeb pepper extracts have nitrogen (N)-containing basic molecules, mostly heterocyclic compounds. The identified molecules and beneficial properties of herbal extracts are summarized in Table 5. The MS and MS/MS spectra of all herbal extracts are shown as Supplementary Data.
Table 5.
Comparable Parameters Among the Four Herbal Extracts
| Basil | Sesame seed | Long pepper | Cubeb pepper | |
|---|---|---|---|---|
| Anti-inflammatory | TNF-α↓; IL-1β↓ | TNF-α↓; IL-1β↓ | TNF-α↓; IL-1β↓ | TNF-α↓; IL-1β↓ |
| Peroxide reduction | √ | √ | √ | √ |
| Oxidation of LDL | Inhibited | Inhibited | Inhibited | Inhibited |
| Common molecules | Malic acid; citric acid; quinic acid; gentisic acid | |||
| Unique molecules | Eugenol; eugenitol; luteolin | Traumatic acid; 9,14-DiHODE; 9, 12, 15-TriHODE; hydroxy fatty acid derivatives | Piperyline; 4,5-dihydropiperlonguminine; (E,E)-piperlonguminine; piperanine; piperine; piperittine; dehydropipernonaline; pipernonaline; pipercide; piperolein B; piperundecalidine; guineesine | Terpenoids; alkaloids; ESI-non ionizable molecules |
| Poly phenols | √ | √ | ND | ND |
| Medium-chain dicarboxylic acids | √ | √ | ND | ND |
| Small-chain dicarboxylic acids | √ | √ | √ | √ |
| Fatty acid derivatives | ND | √ | ND | ND |
ESI, electrospray ionization; IL, interleukin; LDL, low-density lipoprotein; ND, not detected; TNF, tumor necrosis factor.
TNF-α and IL-1β are two different inflammatory markers. Here, we have investigated how these markers respond to different herbal aqueous extracts based on their chemical constituents. Though sesame seed (SS) and long pepper (LP) extracts have different molecules except a few (Table 5), they attenuate IL-1β more significantly when compared with the other two extracts (basil leaf [BL] and cubeb berries/pepper [CB]), as shown in Figure 4. SS and BL extracts have many similar molecules (Table 5); BL attenuation action on different inflammatory markers, TNF-α and IL-1β is different, whereas SS inhibits both the inflammatory markers significantly. Also, the oxidation mechanism of LDL is different in enzymatic and non-enzymatic conditions. BL and SS extracts inhibitory action on oxidation of LDL is quite similar (5–50 μg) in both the conditions, as shown in Figures 1 and 2; whereas LP and CB extracts are inhibited at higher doses (25–50 μg). Thus, different extracts and their chemical constituents have shown various beneficial properties. Also, all antioxidant molecules may not possess anti-inflammatory properties; further, even some of them might need high doses for their beneficial action.
Oxidative stress and inflammation are closely associated in several diseases, including cardiovascular disease, diabetes, inflammatory bowel disease, cancer, and different types of neurological disorders. At what stage of the disease these two factors play a key role and influence the progression is unknown. The extracts also contain simple carboxylic and dicarboxylic acids; our earlier studies with azelaic acid have suggested that even simple dicarboxylic acids could have anti-inflammatory actions in vivo.58 In this study, the concentrations of individual components could not be determined, hence we have used the microgram concentrations instead of molar concentrations. In addition, our previous studies with sesame oil aqueous extract (SOAE) revealed that the whole extract and SOAE-8 are more beneficial than the corresponding individual components.44,50
In this study, we have generated preliminary results. Based on the observations, we are planning to evaluate the beneficial properties of individuals and groups of molecules in vitro as well as in vivo to understand the molecular mechanisms involved in their anti-inflammatory properties. Thus, herbal extracts can serve as simple non-pharmacological agents, which could prove to be invaluable adjunct therapeutic agents in the treatment of several kinds of oxidative stress as well as in inflammation-associated diseases that will shed light on many future studies.
Supplementary Material
Acknowledgment
This study was supported by National Institutes of Health Grant 5R01AT004106-05.
Author Disclosure Statement
The authors do not have any conflicts of interest.
Supplementary Material
References
- 1. Aslam F, Iqbal S, Nasir M, Anjum AA, Swan P, Sweazea K: Evaluation of white sesame seed oil on glucose control and biomarkers of hepatic, cardiac, and renal functions in male Sprague-Dawley rats with chemically induced diabetes. J Med Food 2017;20:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fathilah AR, Sujata R, Norhanom AW, Adenan MI: Antiproliferative activity of aqueous extract of Piper betle L. and Psidium guajava L. on KB and HeLa cell lines. J Med Plants Res 2010;4:987–990 [Google Scholar]
- 3. Abou El-Soud NH, Deabes M, El-Kassem LA, Khalil M, Abou NH: Chemical composition and antifungal activity of Ocimum basilicum L. essential oil. OA Maced J Med Sci Sep 2012;3:374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsu E, Parthasarathy S: Anti-inflammatory and antioxidant effects of sesame oil on atherosclerosis: A descriptive literature review. Cureus 2017;9:1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nwozo SO, Lewis YT, Oyinloye BE: The effects of Piper guineense versus Sesamum indicum aqueous extracts on lipid metabolism and antioxidants in hypercholesterolemic rats. Iran J Med Sci 2017;4242:449–456 [PMC free article] [PubMed] [Google Scholar]
- 6. Pereira CG, Barreira L, Bijttebier S, Pieters L, Marques C, Santos TF, Rodrigues MJ, Varela J, Custódio L: Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp. maritima: From traditional remedies to prospective products. Sci Rep 2018;8:4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ristic-Medic D, Perunicic-Pekovic G, Rasic-Milutinovic Z, Takic M, Popovic T, Arsic A, Glibetic M. Effects of dietary milled seed mixture on fatty acid status and inflammatory markers in patients on hemodialysis. ScientificWorldJournal 2014;2014:563576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Sayed AM, Ezzat SM, El Naggar MM, EI Hawary SS: In vivo diabetic wound healing effect and HPLC–DAD–ESI–MS/MS profiling of the methanol extracts of eight Aloe species. Rev Bras Farmacogn 2016;26:352–362 [Google Scholar]
- 9. Pandey KB, Rizvi SI: Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vijayakumar RS, Surya D, Nalini N: Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep 2004;9:105–110 [DOI] [PubMed] [Google Scholar]
- 11. Kumar S, Kamboji J, Suman , Sharma S: Overview for various aspects of the health benefits of Piper longum Linn. fruit. J Acupunct Meridian Stud 2011;4:134–140 [DOI] [PubMed] [Google Scholar]
- 12. Damnhouri ZA, Ahmad A: Medicinal & aromatic plants: A review on therapeutic potential of Piper nigrum L. (black pepper): The king of spices. Med Aromat Plants 2014;3:161 [Google Scholar]
- 13. Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA: Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rai S, Ghosh H, Basheer M: Phytochemical characterization and antioxidative property of Ocimum canum: Effect of ethanolic extract of leaves and seeds on basic immunologic and metabolic status of male rats. J Immunol Biol 2016;1:2 [Google Scholar]
- 15. Gorgani L, Mohammadi M, Najafpour D, Nikzad M: Piperine—The bioactive compound of black pepper: From isolation to medicinal formulations. J Chromatogr A 2017;16:124–140 [DOI] [PubMed] [Google Scholar]
- 16. Wang B, Zhang Y, Huang J, Dong L, Li T, Fu X: Anti-inflammatory activity and chemical composition of dichloromethane extract from Piper nigrum and P. longum on permanent focal cerebral ischemia injury in rats. Braz J Pharmacog 2017;27:369–374 [Google Scholar]
- 17. Harnafi H, Aziz M, Amrani S: Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. E Spen Eur E J Clin Nutr Metab 2009;4:e181–e186 [Google Scholar]
- 18. Jin Z, Borjihan G, Zhao R, Sun Z, Hammond GB, Uryu T: Antihyperlipidemic compounds from the fruit of Piper longum L. Phytother Res 2009;23:1194–1196 [DOI] [PubMed] [Google Scholar]
- 19. Ying X, Yu K, Chen X, Chen H, Hong J, Cheng S, Peng L: Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell Immunol 2013;285:49–54 [DOI] [PubMed] [Google Scholar]
- 20. Bae GS, Kim MS: Inhibition of lipopolysaccharide-induced inflammatory responses by piperine, Eur J Pharmacol 2010;642:154–162 [DOI] [PubMed] [Google Scholar]
- 21. Guez CM, De Souza RO, Fischer P, De Moura Leao MF, Duarte JA, Boligon AA, Athayde ML, Zuravski L, Souza de Oliveira LF, Machado MM: Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz J Pharm 2017;53:e15098 [Google Scholar]
- 22. Al-Yafeai A, Bohm V: In vitro bioaccessibility of carotenoids and vitamin E in rosehip products and tomato paste as affected by pectin contents and food processing. J Agric Food Chem 2018;66:3801–3809 [DOI] [PubMed] [Google Scholar]
- 23. Bresciani L, Martini D, Mena P, Tassotti M, Calani L, Brigati G, Brighenti F, Holasek S, Malliga DE, Lamprecht M, Del Rio D: Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ertas A, Bog M, Yılmaz MA, Yesil Y, Hasmil N, Kaya MS, Temel H, Kolak U: Chemical compositions by using LC-MS/MS and GC-MS and biological activities of Sedum sediforme (Jacq.) Pau. J Agric Food Chem 2014;62:4601–4609 [DOI] [PubMed] [Google Scholar]
- 25. Francescato LN, Debenedetti SL, Schwanz TG, Bassani VL, Henriques LT: Identification of phenolic compounds in Equisetum giganteum by ESI-MS/MS and a new approach to total flavonoid quantification. Talenta 2013;105:192–203 [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim RM, El-Halawany AM, Saleh DO, Moataz E, El Naggar B, El-Rahman A, El-Shabrawy O, El-Hawary SS: HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev Bras Farmacogn 2015;25:134–141 [Google Scholar]
- 27. Jayasinghe C, Gotoh N, Aoki T, Wada S: Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J Agric Food Chem 2003;16:4442–4449 [DOI] [PubMed] [Google Scholar]
- 28. Kallenbach M, Baldwin IT, Bonaventure G: A rapid and sensitive method for the simultaneous analysis of aliphatic and polar molecules containing free carboxyl groups in plant extracts by LC-MS/MS. Plant Methods 2009;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li ZH, Guo H, Xu WB, Ge J, Li X, Alimu M, He DJ: Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J Chromatogr Sci 2016; 54:805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu M, Zhang L, Ser S, Cumming J, Ku KM: Comparative phytonutrient analysis of broccoli by-products: The potentials for broccoli by-product utilization. Molecules 2018;23:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G: Therapies for prevention and treatment of Alzheimer's disease. Biomed Res Int 2016;2016:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raju GS, Moghal MR, Dewan SM, Amin MN, Billah M: Characterization of phytoconstituents and evaluation of total phenolic content, anthelmintic, and antimicrobial activities of Solanum violaceum Ortega. Avicenna J Phytomed 2013;3:313–320 [PMC free article] [PubMed] [Google Scholar]
- 33. Okwute SK, Egharevba HO: Piperine-type amides: Review of the chemical and biological characteristics. Int J Chem 2013;5:99–122 [Google Scholar]
- 34. Hossain M, Rai D, Brunton N, Martin-Diana AB, Barry-Ryan C: Characterization of phenolics composition in lamiaceae spices by LC-ESI-MS/MS. J Agric Food Chem 2010;58:10576–10581 [DOI] [PubMed] [Google Scholar]
- 35. Huang N, Rizshsky L, Hauck C, Nikolau BJ, Murphy PA, Birt DF: Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 2011;72:2015–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagesh SV, Muniappan M, Kannan I, Viswanathan S, Simhadri N: Phytochemical analysis and docking study of compounds present in a polyherbal preparation used in the treatment of dermatophytosis. Curr Med Mycol 2017;2017:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abbas WH: Immunomodulatory effect of aqueous extract of Piper nigrum L. in mice model. Int J Sci Technol 2016;11:45–49 [Google Scholar]
- 38. Amran AA, Zakaria Z, Othman F, Das S, Raj S, Nordin NAM: Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis 2010;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Othman SB, Katsuno N, Kanamaru Y, Yabe T: Water-soluble extracts from defatted sesame seed flour show antioxidant activity in vitro. Food Chem 2015;15:306–314 [DOI] [PubMed] [Google Scholar]
- 40. Chaudhary S, Semwal A, Kumar H, Verma HC, Kumar A: In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed Pharmacother 2016;84:2008–2013 [DOI] [PubMed] [Google Scholar]
- 41. Kerkar S, Joshi M, Korgaonkar A, Kerkar S: Phytochemical and pharmacological investigation of hot aqueous extract of Piper betle Linn. leaves and its combination with coffee powder prepared by Bhavana process on the central nervous system of the rats. Int J Sci Eng Res 2015;6:237–262 [Google Scholar]
- 42. Mohd Zainudin M, Zakaria Z, Megat Mohd Nordin NA: The use of Piper sarmentosum leaves aqueous extract (Kadukmy™) as antihypertensive agent in spontaneous hypertensive rats. BMC Complement Altern Med 2015;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tohti I, Tursun M, Umar A, Turdi S, Imin H, Moore N: Aqueous extracts of Ocimum basilicum L. (sweet basil) decrease platelet aggregation induced by ADP and thrombin in vitro and rats arterio–venous shunt thrombosis in vivo. Thromb Res 2016;118:733–739 [DOI] [PubMed] [Google Scholar]
- 44. Narasimhulu CA, Selvarajan K, Burge KY, Litvinov D, Sengupta B, Parthasarathy S: Water-soluble components of sesame oil reduce inflammation and atherosclerosis. J Med Food 2016;19:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pandey R, Chandra P, Srivastava M, Mishra DK, Kumar B: Simultaneous quantitative determination of multiple bioactive markers in Ocimum sanctum obtained from different locations and its marketed herbal formulations using UPLC-ESI- MS/MS combined with principal component analysis. Phytochem Anal 2015;26:383–394 [DOI] [PubMed] [Google Scholar]
- 46. Santanam N, Parthasarathy S: Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest 1995;95:2594–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chandrakala AN, Sukul D, Selvarajan K, Sai-Sudhakar C, Sun B, Parthasarathy S: Induction of brain natriuretic peptide and monocyte chemotactic protein-1 gene expression by oxidized low-density lipoprotein: Relevance to ischemic heart failure. Am J Physiol Cell Physiol 2012;302:C165–C177 [DOI] [PubMed] [Google Scholar]
- 48. Auerbach BJ, Kiely JS, Cornicelli JA: A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem 1992;201:375–380 [DOI] [PubMed] [Google Scholar]
- 49. Khan-Merchant N, Penumetcha M, Meilhac O, Parthasarathy S: Oxidized fatty acids promote atherosclerosis only in the presence of dietarycholesterol in low-density lipoprotein receptor knockout mice. J Nutr 2002;132:3256–3262 [DOI] [PubMed] [Google Scholar]
- 50. Deme P, Narasimhulu CA, Parthasarathy S: Identification and evaluation of anti-inflammatory properties of aqueous components extracted from sesame (Sesamum indicum) oil. J Chromatogr B 2018;1087–1088:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Navab M, Hama SY, Anantharamayya GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman Am: Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J Lipid Res 2000;41:1495–1508 [PubMed] [Google Scholar]
- 52. Chandrakala AN, Vardhan S: Therapeutic potential of Ocimum tenuiflorum in prevention and treatment of atherosclerosis. J Med Food 2015;18:1–9 [DOI] [PubMed] [Google Scholar]
- 53. Cohen J: The immunopathogenesis of sepsis. Nature 2002;420:885–891 [DOI] [PubMed] [Google Scholar]
- 54. Black S, Muller F: On the effect of temperature on aqueous solubility of organic solids. Org Process Res Dev 2010;14:661–665 [Google Scholar]
- 55. Cox MA, Jackson J, Stanton M, Rojas-Triana A, Laverty M, Yang X, Zhu F, Liu J, Maguire M, Cox MA, Bober L, Wang S, Monsma F, Vassileva G, Gustafson E, Jenh CH, Bayne M, Chou CC, Lundell D, Bober L, Jenh CH: Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J Gastroenterol 2009;15:5549–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sieber MA, Hegel JKE: Azelaic acid: Properties and mode of action. Skin Pharmacol Physiol 2014;27:9–17 [DOI] [PubMed] [Google Scholar]
- 57. Schulte BC, Wu W, Rosen T: Azelaic acid: Evidence-based update on mechanism of action and clinical application. J Drugs Dermatol 2015;14:964–968 [PubMed] [Google Scholar]
- 58. Litvinov D, Selvarajan K, Garelnabi M, Brophy L, Parthasarathy S: Anti-atherosclerotic actions of azelaic acid, an end product of linoleic acid peroxidation, in mice. Atherosclerosis 2009;209:449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.