Abstract
Scar formation is a common consequence of skin injuries that lead to a wide range of adverse effects including physical deformities and psychological disorders. Studies demonstrate that mammalian fetal skin in early gestation is capable of regenerating itself after injury without any scar formation. A number of potential therapies have been developed to reduce scar formation in cutaneous wounds based on differences between the process of adult and fetal wound healing. The ideal approach to eliminate scar formation after skin injury is to use a pro-regenerative matrix along with growth factors and cell types that induce regeneration rather than repair. This work provides a comprehensive review of engineering approaches to scar-free wound healing with emphasis on the use of pro-regenerative biomaterials to minimize scar formation in skin injuries.
Impact Statement
Millions of people every year develop scars in response to skin injuries after surgery, trauma, or burns with significant undesired physical and psychological effects. This review provides an update on engineering strategies for scar-free wound healing and discusses the role of different cell types, growth factors, cytokines, and extracellular components in regenerative wound healing. The use of pro-regenerative matrices combined with engineered cells with less intrinsic potential for fibrogenesis is a promising strategy for achieving scar-free skin tissue regeneration.
Keywords: skin wound healing, scarring, fetal and adult wound healing, fibroblasts, macrophages, regenerative biomaterials
Introduction
Millions of people annually develop scars in response to skin injuries after surgery, trauma, or skin burns. These skin injuries result in significant undesired physiological and psychological effects. According to the World Health Organization, more than 11 million burn injuries are reported worldwide annually that require medical attention.1 According to a fact sheet released by the American Burn Association, more than 480,000 burn injuries occur in the United States each year that require treatment.2 It is common for a burn injury to form scar tissue, although the incidence of scar formation is not known.3 In some studies, the prevalence of hypertrophic scar (HTS) formation was estimated between 32% and 72% after burn injuries.4 The market for anti-scarring medication is in excess of $12 billion annually.5 Based on the results of a cohort study, the cost of anti-scar treatment in postburn patients is approximately $6000 per patient.6 Previous studies indicate that scarring dramatically affects the quality of life of patients with skin injuries.7
Excessive deposition of connective tissue (mainly collagen) during wound healing leads to scar formation (Figure 1).8 It was shown for the first time in 1971 that fetal lamb wounds, before day 120 of gestation, heal without scar formation.9 In a similar manner, it was shown that skin injuries in the human fetus in early pregnancy heal to form normal skin tissue without scar formation.10 Engineering approaches that mimic the process of fetal wound healing can lead to enabling technologies that minimize or eliminate scar formation after injury. Many factors including cellular elements, soluble growth factors, and insoluble extracellular matrix (ECM) proteins as well as mechanics of the tissue are involved in fetal wound healing. Recent studies indicate that the application of a single factor, such as transforming growth factor (TGF)-β3 or interleukin-10 (IL-10), is not sufficient for scar-free wound healing.11 Studies centered on the process of fetal regeneration indicate that scar-free wound healing can be realized by combining multiple cellular and morphogenetic factors within a fetal-mimetic matrix or scaffold. In this review, we provide an update on engineering strategies for scar-free wound healing and discuss the role of different cell types, growth factors, cytokines, and extracellular components in regenerative wound healing with emphasis on the potential of pro-regenerative biomaterials in developing skin equivalents. Finally, this review ends with the future of scar-free skin tissue engineering.
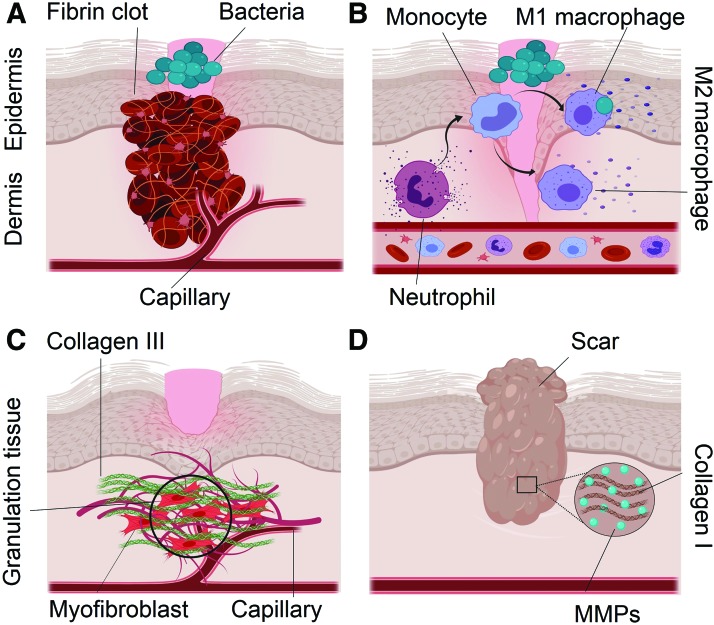
FIG. 1.
Stages of wound healing. (A) Coagulation: platelets in the presence of fibrin form a stable clot. Platelets also produce platelet-derived growth factor that along with bacterial products attract inflammatory cells to the site of injury. (B) Inflammation: neutrophils are recruited to the wound bed. Leucocytes secrete proinflammatory cytokines IL-1, IL-6, and TNF-α that exacerbate the inflammation. M2 macrophages are found mostly in the late phase of inflammation and produce anti-inflammatory cytokine IL-10. (C) Proliferation: granulation tissue is the hallmark of this stage, which is composed of myofibroblasts, new capillaries, and Col III. (D) Remodeling: it is the final stage of wound healing in which Col III is replaced by the stiffer bundles of Col I with MMPs as the main effectors. The end result of wound repair is scar formation at the site of injury. IL, interleukin; Col III, collagen type III; TNF, tumor necrosis factor. Color images are available online.
Adult Versus Fetal Wound Healing
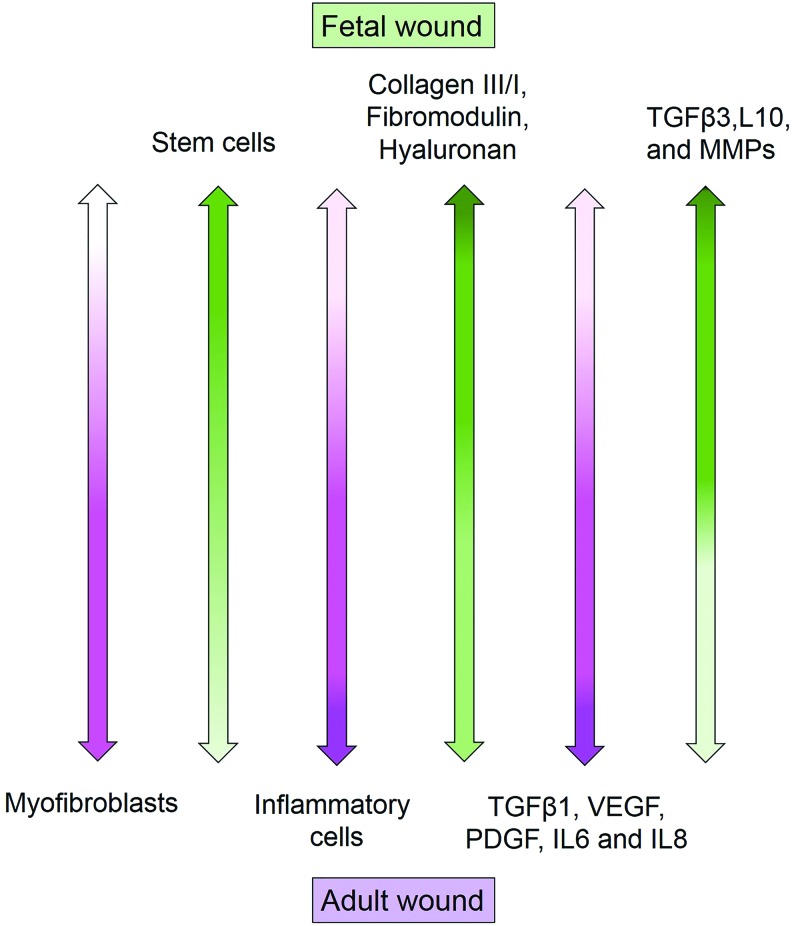
Human fetal skin in early pregnancy can regenerate after injury without forming a scar tissue.10 Understanding the differences between the fetal and adult skin wound healing can lead to scar-free skin regenerative therapies. These are illustrated in Figure 2, which demonstrates the differences in cell types, growth factors, cytokines, and ECM composition.
FIG. 2.
Dissimilarities in cell types, growth factors, and extracellular matrix components between adult and fetal wounds. Color images are available online.
Cells
Fibroblasts
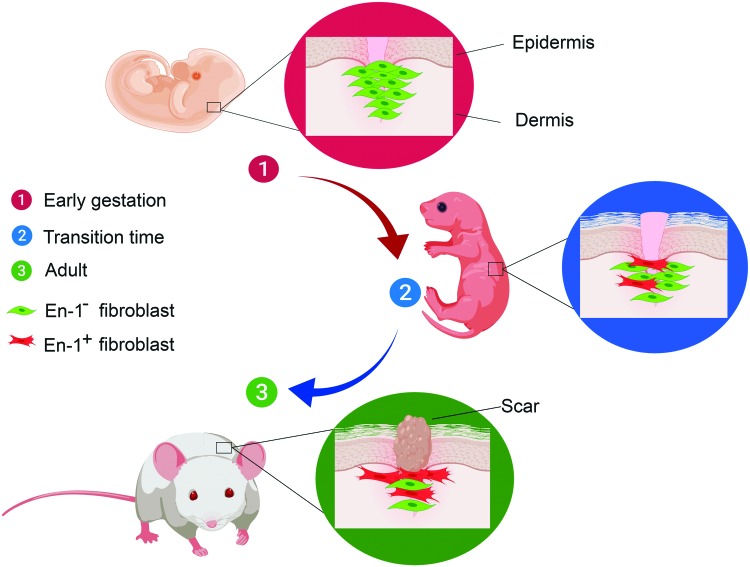
They are the most important cell type in the process of wound healing.12,13 Driskell et al. showed that a unique fibroblast lineage in the lower dermis (reticular layer) is responsible for connective tissue deposition after skin injury.14 Lineage tracing by Rinkevich et al. led to identification of a single fibroblast lineage (Engrailed-1) as the main effector cell in the production of ECM during wound healing as well as embryonic development in mouse dorsal skin.15 This unique fibroblast lineage, which was identified by the cell surface marker adenosine deaminase complexing protein-2 (CD26), was 1% of the dermal fibroblasts at the early stage of gestation but its fraction increased to >75% in postnatal skin.15 Furthermore, it was established that this fibroblast lineage played a major role in the switch from scar-free to scar-forming wound repair in midgestation (Fig. 3).16 Inhibition of CD26 enzymatic activity reduced scar formation in mouse skin injuries.15 CD26, which is also known as dipeptidyl peptidase-IV, is a cell surface exopeptidase that cleaves X-proline dipeptides from the N-terminus of oligopeptides.17 Stromal cell-derived factor-1 (SDF-1), which is also known as C-X-C motif chemokine-12 (CXCL12), is a substrate for CD26. SDF-1 is a strong chemoattractant for the recruitment of CXCR4 (C-X-C chemokine receptor type-4) expressing mesenchymal stem cells (MSCs) to the injury site.18 It has been shown that the overexpression of hypoxia-induced factor-1 (HIF-1) due to ischemia after tissue injury leads to the upregulation of SDF-1 and subsequent recruitment of MSCs to the site of injury to stimulate regeneration.19,20 Ghadge et al. showed in a cardiac ischemia model that the overexpression of HIF-1α by administration of prolyl hydroxylase inhibitor resulted in the upregulation of SDF-1 with subsequent improvement in neovascularization and reduction in the size of myocardial scar.21 They also detected higher expression levels of anti-inflammatory M2 macrophage phenotype at the site of cardiac infarction, which may have played a role in the regeneration of cardiac tissue.21
FIG. 3.
Contribution of Engrailed-1+ (En1) fibroblasts (shown in red color) to matrix deposition and scar formation during skin development in the mouse model of wound healing. The En1+ fibroblast accounts for 1% of the dermal fibroblasts at the early stage of gestation, but its fraction increases to >75% in postnatal skin. Color images are available online.
It has been reported that SDF-1 plays an important role in neovascularization of injured tissue by recruitment of endothelial progenitor cells.22 Rabbany et al. reported that the delivery of SDF-1 in an alginate hydrogel improved wound closure and mitigated scar formation in a pig skin injury model.23 Hu et al. used microarray analysis to show that CXCR4 signaling (SDF-1 receptor) is upregulated in the skin wound of adult mice 12 h postinjury compared with fetal mice.24 Ding et al. reported higher expression of SDF-1/CXCR4 signaling in HTSs caused by burn injuries and the injection of interferon α-2b, which downregulates SDF-1 expression, led to remodeling of the scar tissue.25 These results indicate that more research is needed to understand cell signaling pathways that regulate SDF-1/CXCR4 axis and its interaction with CD26 in scar formation of cutaneous wounds.
Fetal fibroblasts play a different function during wound healing compared with adults. Fetal fibroblasts proliferate faster and synthesize predominately collagen type III (Col III) during wound repair.26 Myofibroblasts differentiated from fibroblasts and characterized by the expression of α-smooth muscle actin (α-SMA), which play a role in scar formation in adult wounds, are absent in fetal wound healing.26,27 Recently, it has been shown that myofibroblasts have plasticity and they are reprogrammed to fat cells upon exposure to bone morphogenetic proteins.28 These studies indicate that morphogens and cytokines can be used to modify the function of fibroblasts and reverse the scarring process in adult dermal wound injuries. It should be mentioned that there exists a critical wound depth for scar formation. Dunkin et al. investigated the effect of wound depth on scar formation in a clinical trial by creating superficial and deep skin wounds in the lateral hip of 113 volunteers.29 They reported a critical injury depth of 0.56 ± 0.03 mm with deeper injuries leading to fibrosis.
Skin is anatomically composed of three layers, namely epidermis, dermis, and hypodermis with the dermis further divided into superficial (papillary) and deep (reticular) dermis. Wang et al. investigated the properties of fibroblasts isolated from the superficial and deep layers of human normal dermis and HTS tissue in an in vitro tissue culture system.30 They reported that deep dermal fibroblasts were larger, proliferated slower, and expressed higher levels of TGF-β1, connective tissue growth factor (CTGF), α-SMA, and collagen but lower levels of collagenase in comparison with superficial fibroblasts.30 They further reported that deep dermal fibroblasts and fibroblasts isolated from HTS tissue had similar properties.30 Therefore, deep dermal fibroblasts may be responsible for scar formation in relatively deep skin injuries.
Inflammatory cells
Reduced inflammation is the hallmark of fetal wound healing. Neutrophils are the initial inflammatory cells recruited to the site of injury.31 It has been shown that a lower number of neutrophils are recruited to the site of injury in fetal wounds compared with adults. Fetal neutrophils express lower levels of adhesion molecules compared with adults, which in turn decreases their ability to migrate to the wound bed.32,33 Macrophages contribute to cell growth, differentiation, ECM formation, and remodeling in both adult and fetal wound healing by serving as reservoirs for the release of growth factors34; however, their recruitment to the wound bed is reduced in early gestation during fetal development.35 There are fewer mast cells in fetal skin wounds with fewer granules in their cytoplasm.36 Mast cells are believed to play a role in transition from scar-free to scar-forming in fetal wound healing. There was less scar formation during wound healing in a mouse model deficient in skin mast cells compared with the wild type.36 Furthermore, the application of mast cell lysate to a skin injury in a mouse model of scarless wound healing distorted the healing process. Jeong et al. reported that intraperitoneal injection of poly(deoxyribonucleotide), which is known to reduce mast cell degranulation and release of inflammatory cytokines during the inflammatory phase, decreased the number of inflammatory cells and scar size in a rat model of skin wound healing.37 Walraven et al. performed immunohistochemical staining on human adult and second-trimester fetal skin tissues and lymph nodes and reported lower number of macrophages, dendritic and T cells, mast and Langerhans cells in the fetal tissue compared with the adult.38 They showed using the CD45 leukocyte marker that the fetal skin had lower number of leukocytes but higher cell number in the lymph nodes. They further reported a higher ratio of M2 (anti-inflammatory) to M1 (proinflammatory) macrophages in the fetal skin compared with the adult, even though the overall number of macrophages in the fetal skin was lower than that in the adult. They also reported no significant differences in vascular density between the fetal and adult skin. They concluded from the results that the lower number of immune cells in the fetal skin is not due to lower vascular density or the immune system immaturity.
Wounds in the oral mucosa, which heal with less scarring, are also characterized by a lower number of macrophages.39 Wilgus et al. reported that the expression level of inflammatory mediators cyclooxygenase-2 (COX2) and prostaglandin-E2 (PGE2) was significantly lower in scarless wound healing compared with those with scarring.40 Furthermore, they reported that the application of PGE2 to the fetal wound increased the expression of TGF-β1 and scarring.
Platelets
Platelets vary functionally in different stages of gestation. Olutoye et al. reported that there is less platelet aggregation in pig fetal skin than the adult with lower concentrations of TGF-β and platelet-derived growth factor (PDGF). The high amount of hyaluronic acid (HA) in the fetal skin can be attributed to these functional differences.41 Studies on the effect of platelet-rich plasma (PRP) on wound healing have produced controversial results. Kushida et al. reported that PRP induces differentiation of dermal fibroblasts to myofibroblasts with subsequent increase in wound contraction.42 Caceres et al. observed that the expression of myofibroblast differentiation marker α-SMA in gingival fibroblasts increased upon exposure to PRP for 12 h in a dose-dependent manner.43 Chellini et al. reported that the addition of PRP to fibroblasts cultured in a differentiation medium supplemented with TGF-β1 attenuated myofibroblast differentiation.44 This effect was attributed to the inhibition of TGF-β1/SMAD3 signaling pathway upon the addition of PRP, which was mediated by the vascular endothelial growth factor (VEGF) receptor-1. Therefore, the effect of PRP on wound healing remains inconclusive, and more research is needed to understand the role of PRP on scar formation in skin injuries.
Stem cells
Higher number of MSCs is detected in fetal wound healing.45 Exosomes derived from adipose MSCs upregulate the expression of Col III, TGF-β3, and matrix metalloproteinases-3 (MMP3) in skin wounds, which leads to less scarring.46 Kim and Hemmati reported that the co-culture of MSCs and macrophages induced M2 phenotype in the macrophages, which led to the upregulation of IL-10 and IL-6 expressions and downregulation of IL-12 and tumor necrosis factor-α (TNF-α).47 These results indicate that MSCs have an immune-modulatory effect on wound healing. Sabapathy et al. reported that mouse skin wounds treated with Wharton's jelly MSCs cultured on a decellularized amniotic membrane (AM) had less scarring and more tissue regeneration after 10 days.48 Liu et al. reported that intradermal injection of MSCs attenuated scar formation in a rabbit model of skin wound healing.49 They showed increased expression of collagen type I (Col I), α-SMA, TNF-α stimulated gene/protein6, inducible nitric oxide synthase, indoleamine 2,3-dioxygenase, COX2, and IL-10 after injection of MSCs in the wound bed. Subcutaneous injection of adipose-derived stem cells-conditioned medium prevented fibrosis and scar tissue formation by inhibition of the P38/MAPK signaling pathway.50 On the contrary, Doi et al. reported that injection of umbilical cord blood MSCs and Wharton's jelly MSCs to the wound bed of nude mice failed to attenuate scarring.51 However, the failure of MSCs to reduce scarring in Doi's experiments may be related to the absence of immune system in the nude mouse.
Extracellular matrix
The ECM comprising proteins, glycosaminoglycans (GAGs), and proteoglycans (PGs) provides a dynamic scaffold for cell migration, adhesion, proliferation, differentiation, and maturation. The mechanical properties of ECM play a pivotal role in activation of fibroblasts during wound healing. It has been shown that matrix stiffness increases with gestational age as the number of collagen cross-links increases.27,52 The expression of lysyl oxidase that catalyzes collagen cross-linking is downregulated in early-gestation fetal fibroblasts.27,52 Furthermore, fibroblasts cultured in vitro on stiff hydrogels showed higher expression of α-SMA compared with soft hydrogels.53,54 Goffin et al. reported that the granulation tissue in the mouse model of skin injury begins to express α-SMA at 20 kPa tissue stiffness.55 The focal adhesion kinase (FAK) pathway has been implicated as a link between mechanical forces and scar formation. Wong et al. showed that scarring after skin injury in FAK-knockout mice was less than the wild type.56 Furthermore, they reported that the expression of TGF-β1, α-SMA, and monocyte chemoattractant protein-1 (MCP-1) was downregulated and the number of macrophages was lower in FAK-deficient mouse compared with the wild type.56 Their results indicate that mechanical loading activated FAK leading to the expression of MCP-1, which in turn induced the recruitment of inflammatory cells and a fibrotic reaction. Therefore, scar formation can be induced through an inflammatory process by mechanical activation of FAK.
Col I is the most abundant protein in the fetal and adult skin. Fetal skin is characterized by a high ratio of Col III/Col I. Col III is thought to have a critical role in regenerative healing as observed in the mouse blastema during digit tip regeneration.57 Fetal wounds have a high GAG and high-molecular-weight hyaluronic acid (HMW-HA) content compared with adult wounds. HMW-HA reduces angiogenesis and inflammation, whereas low-molecular-weight hyaluronic acid (LMW-HA) is involved in scar formation.58 HA persists for longer times in fetal wound healing. Longaker et al. measured the level of HA in adult and fetal ewe wounds and reported that HA persisted in fetal wounds for 3 weeks, whereas it disappeared from adult wounds after 7 days.59,60 Higher amounts of HA in the blastema of lizard and zebrafish during tail regeneration is evidence of HA importance in regenerative wound healing.61,62
There is also a difference in the concentration of PGs between fetal and adult wounds. Decorin (DCN) expression increases during skin development and reaches its highest amount in adulthood. It is interesting to note that DCN has antifibrotic effects as it is an inhibitor of TGF-β1.13 Higher incidence of scarring in deep wounds is attributed to the lower DCN content of reticular dermis.63 The PG fibromodulin (FMOD) is downregulated in adult wounds, but it is found in high amounts in normal fetal ECM.64 High levels of FMOD was measured in the fetal wound of rodents, which decreased the expression of TGF-β1 and consequently reduced scarring.65 Zheng et al. showed that intradermal injection of human recombinant FMOD in adult rodent or porcine cutaneous wounds decreased the scar size.66 Furthermore, they observed that FMOD had dual effect on TGF-β1 signaling by simultaneously increasing the contraction of fibroblasts to decrease wound size and suppressing fibrosis. Furthermore, they showed in FMOD-deficient mouse model of cutaneous wound that TGF-β3 expression in fibroblasts increased within the first day postinjury, which in turn decreased fibroblast motility, delayed wound closure, increased wound size, and scarring.67 Based on these results, Zheng et al. concluded that the antimotility effect of TGF-β3 promoted scar formation in the early stages of wound healing, whereas its antifibrotic effect impeded scar formation in the later stages.67 In fact, the failure of a single TGF-β3 application or inhibition of TGF-β1 expression in clinical trials for wound healing can be attributed to this dual effect. However, in the failed clinical trials, TGF-β3 was injected in the cutaneous wound bed in the first day after injury.11,66,67 Therefore, the timing of TGF-β3 delivery should be considered in future clinical trials.
Elastin, which is responsible for elasticity and resilience of connective tissue, is expressed 22 weeks after gestation during fetal development.68 Impaired production of elastin fibers in adult cutaneous injuries leads to changes in mechanical properties of the skin.69 The role of elastin in fetal and adult wound healing is not well understood and needs to be investigated in future studies.69
Fibronectin as a major glycoprotein found in various connective tissues including skin plays many roles during wound healing.70 The role of fibronectin in cell–cell and cell–matrix interactions has been thoroughly investigated. The organization of fibronectin, which is connected to the intracellular actin filaments by integrin cell surface receptors, is dependent on the activity of FAK.71 The fetal wound is distinguished from the adult by early deposition of fibronectin, which facilitates cell attachment to the matrix and cell migration within the matrix by association with the adhesion glycoprotein tenascin. As an indication of the role of fibronectin in re-epithelialization, integrin receptors are upregulated on keratinocytes in fetal cutaneous lesions for interaction with fibronectin and tenascin.72,73 Furthermore, the reduced production of fibronectin via the expression of IL-4 in atopic dermatitis delayed re-epithelialization in mouse model of wound healing.74
Growth factors and cytokines
Transforming growth factor-β
TGF-β signaling is required for wound repair and regeneration as the lack of TGF-β expression leads to a chronic nonhealing wound. Inhibition of TGF-β signaling prevents regeneration of limbs in amputated axolotls.75 The three isoforms of TGF-β family of growth factors, namely TGF-β1, TGF-β2, and TGF-β3, are encoded by different genes, but their action is propagated through the SMAD intracellular pathway. Binding of the three TGF-β isoforms to TGF-β receptors I and II initiates a signaling cascade resulting in phosphorylation and activation of SMAD2, SMAD3, and co-SMAD4, their nuclear localization, and the expression of SMAD-specific genes.76,77
The CTGF is known as a downstream mediator of TGF-β.78 A single application of CTGF or TGF-β into mouse subcutaneous tissue resulted in transient formation of granulation tissue, whereas co-injection of CTGF and TGF-β led to long-term formation of fibrotic tissue.79 TGF-β1 and TGF-β2 are involved in scar formation as inhibition of these isoforms by the addition of exogenous antibodies reduced scarring after skin injury. Conversely, TGF-β3 is believed to have an antifibrotic effect80 as the ratio of TGF-β3/TGF-β1 is found to be higher in fetal wounds compared with adults.11,81 However, a phase III clinical trial based on recombinant TGF-β3 therapy to reduce scarring in wound healing failed to meet the primary and secondary endpoints. The failure of TGF-β3 clinical trial is indicative of more complex roles for the TGF-β family of growth factors, which needs to be explored in future studies.11
Wnt/β-catenin
Wingless protein signaling pathway plays a critical role in embryonic development and organogenesis,82 and it is thought to contribute to fibrosis of different organs including skin. Beyer et al. showed that the accumulation of β-catenin in fibroblasts as a result of increased expression of Wnt3 and Wnt10b led to fibrosis.83 Wnt3 induced differentiation of fibroblasts to myofibroblasts in vitro and increased the expression of TGF-β through SMAD2, suggesting a cross talk between the TGF-β signaling and Wnt/β-catenin pathway.84 Carre et al. reported that fetal fibroblasts show higher expression of TGF-β3 in response to Wnt3a, whereas postnatal fibroblasts show higher TGF-β1 expression.85 There is debate on the effect of the Wnt/β-catenin pathway on wound healing. The Wnt/β-catenin pathway is believed to be required for hair follicle formation during prenatal as well as postnatal development.86,87 Collins et al. showed that epidermal activation of β-catenin in vivo in mouse skin reprogrammed adult dermis to a neonatal state with higher fibroblast density and deposition of an immature matrix.86 Topical application of liposomal Wnt3a in adult mouse model of wound healing resulted in faster healing, which was correlated with the activation of dermal Wnt signaling.88 Driskell and colleagues showed that dermal β-catenin is upregulated during wound healing, which resulted in the growth of reticular fibroblasts lacking the ability for hair follicle formation.14,89 However, ablation of β-catenin expression resulted in regeneration of hair follicles in adult mouse model of wound healing after skin injury.14,89 More studies should be performed in the future to understand the precise role of the Wnt/β-catenin signaling pathway in cutaneous wound healing.
Heparin-binding epidermal growth factor
Heparin-binding epidermal growth factor (HB-EGF) has been reported to play a major role in re-epithelialization during wound healing by increasing migration of keratinocytes.90 HB-EGF decreases during gestation in the rat skin. The expression of HB-EGF changes markedly in transition from scar-forming to scar-free wound healing.91
Platelet-derived growth factor
PDGF consists of five isoforms that are produced by platelets, macrophages, endothelial cells, fibroblasts, keratinocytes, and smooth muscle cells.8 PDGF along with TGF-β stabilize the newly formed blood capillaries in the granulation tissue.8 However, PDGF is not necessary for the initiation of angiogenesis as it has been reported that basic fibroblast growth factor and VEGF have a stronger angiogenic effect compared with PDGF.92 PDGF plays a role in re-epithelialization and stimulates proliferation and differentiation of fibroblasts to myofibroblasts.92 PGDF is involved in the remodeling phase of wound healing by increasing the expression of MMPs.8,92 PDGF with two B subunits (PDGF-BB or becaplermin) is the only recombinant growth factor approved by the Food and Drug Administration (FDA) for the treatment of chronic wounds92 and as a profibrotic agent.93 It has been reported that the expression of PDGF-BB gene is increased with gestational age in fetal rat with a sharp increase in expression during the transition from scarless to scar-forming wound healing.91 Furthermore, there is a difference in the expression of PDGF-BB growth factor in fetal and adult wounds after injury as PDGF-BB disappeared after 24 h in fetal wounds, whereas it persisted for up to 72 h in adult wounds.94
Fibroblast growth factor
Fibroblast growth factor (FGF) is a family of growth factors consisting of 23 members.8,92 The most characterized FGFs in wound healing are FGF-2 (basic FGF), FGF-7, FGF-9, and FGF-10.92 FGFs are released by many cell types and interact with a group of receptors that have tyrosine kinase activity.8 It is well established that FGF-2 plays a role in angiogenesis, migration of macrophages and fibroblasts, as well as re-epithelialization.8,92 Ortega et al. showed that wound healing is delayed in FGF-2-knockout mice.95 FGF-7 or keratinocyte growth factor-1 (KGF-1) and FGF-10 (KGF-2) are postulated to play a role in re-epithelialization and later stages of angiogenesis by stimulation of endothelial cells and induction of VEGF expression.92 Despite this hypothesis, FGF-7-knockout mice showed normal wound healing.96 FGF-9 is necessary for the development of hair follicles as FGF-9 expressed by γδT cells upregulated Wnt expression in fibroblasts and subsequently induced hair follicle formation in a mouse model of wound healing.97 Furthermore, the overexpression of FGF-9 increased hair neogenesis by two- to threefolds.97 In human skin lesions, the formation of hair follicles does not occur after scarring, which can be attributed to the lack of FGF-9 expressing γδT cells in human dermis.97 Whitby and Ferguson reported that FGF-2 is absent in fetal wounds; however, their measurement method could not detect low levels of FGF-2.94 Later, it was reported that the expression of FGF-5, FGF-7, and FGF-10 increased during fetal skin development in rat while FGF-2 and FGF-9 remained unchanged.98 Furthermore, they showed that the overall expression of FGFs was downregulated in scarless fetal wound healing.
Vascular endothelial growth factor
VEGF family of growth factors are expressed by many cell types in the wound bed, including endothelial cells, fibroblasts, smooth muscle cells, platelets, keratinocytes, neutrophils, and macrophages.99 VEGF-A, which is generally called VEGF, is the key growth factor in the early stage of angiogenesis after tissue injury.8,92,99 Injury to the tissue leads to disruption of blood capillaries and hypoxia, which leads to the upregulation of HIF-1α expression and subsequent expression of VEGF and its receptors in the wound site to stimulate angiogenesis.99 VEGF is not only necessary in the early phase of angiogenesis, but it also contributes to scar formation. It is reported that the expression of VEGF was downregulated in scarless fetal wounds and the application of exogenous VEGF resulted in scar formation.100
Interleukins
IL-10 is known to mitigate fibrosis as a potent anti-inflammatory factor in different organs, including the heart, lung, kidney, liver, and intestine.101 IL-10 expression is upregulated in scarless fetal wounds, and wound healing in IL-10-deficient fetal mouse proceeds with scar formation.102 Recently, it has been shown that IL-10 stimulates HMW-HA production in adult fibroblasts through a signal transducer and activator of transcription-3-dependent signaling pathway, thus imparting a regenerative phenotype to the fibroblasts.103 Therefore, the antifibrotic effect of IL-10 is not solely due to its anti-inflammatory properties. It should be mentioned that the recombinant human IL-10 (Prevascar) clinical trial for wound healing in which the cytokine was injected intradermally to the wound edge was not successful.11 Although IL-10 therapy reduced scar formation after 1 month in clinical trials, scarring was similar to the control group over long-term.11 Lower levels of proinflammatory cytokines IL-6 and IL-8 are detected in fetal wounds compared with adults. Liechty et al. reported that IL-6 and IL-8 mRNA were detectable in adult wounds for up to 72 h compared with 12 h in fetal wounds, and the addition of IL-6 to fetal wounds led to scar formation.104,105 IL-6 is believed to promote inflammation through MCP-1 as well as direct activation of macrophages.104 IL-8 is a potent chemoattractant for neutrophils and stimulates re-epithelialization via increasing the migration and proliferation of keratinocytes.92 IL-8 also plays a role in the remodeling phase of wound healing by upregulating the expression of MMPs.92
Extracorporeal environment
Amniotic fluid (AF), which is enriched in HA and growth factors including epidermal growth factor (EGF), TGF-α, and insulin-like growth factor 1 (IGF-1), provides a sterile environment for fetal growth and development.106 Longaker et al. showed that AF not only contained high levels of HA, but it also stimulated HA production in the fetal wound.107 We previously discussed that HA has a major role in wound regeneration. It has been reported that stem cells derived from human AF, which were identified as CD-117-positive cells, accelerated re-epithelization after injection to the wound edge in a mouse model and attenuated scar formation.108 The AM is also shown to have antimicrobial and antifibrotic effects.109 Murphy et al. fabricated a hydrogel composed of solubilized AM and HA (SAM-HA) and investigated the regenerative properties of the hydrogel in a full-thickness mouse wound. They reported that the hydrogel accelerated wound re-epithelialization and closure. Higher amount of angiogenesis was observed in SAM-HA-treated wounds compared with nontreated or HA-only-treated wounds.110 Despite the regenerative properties of the AM and AF and their role in wound healing, it is well established that scarless wound healing is an intrinsic characteristic of the fetal skin.15 Longaker et al. made cutaneous wounds on adult sheep skin, which was transplanted into a fetus. They reported scar formation in the adult skin while the adjacent fetal skin healed without scarring.111
Advances in ECM-Based Pro-regenerative Materials
ECM not only provides structural support for the cells, but it also plays a critical role in cell fate determination through interactions with integrin receptors on the cell surface. These interactions regulate the strength of cell adhesion to ECM, cell polarization, migration, proliferation, differentiation, and cell phenotype.112,113 The ideal response to skin injury is regeneration and the formation of a normal tissue structure. However, our body's reaction to injury is repair rather than regeneration in which a fibrotic tissue (scar) replaces the normal skin tissue.114 In fetal wound healing during early gestation, a regenerative matrix is formed that leads to the formation of a scar-free normal skin tissue. Recapitulation of ECM properties of the fetal wound has been the subject of numerous studies.45,115 The list of clinically approved cellular and acellular regenerative constructs as skin substitutes is provided in Tables 1116–125 and 2,126–136 respectively. The focus of this section is on those combinations of biomaterials, cells, and growth factors that restore the normal architecture of the skin tissue after injury.
Table 1.
List of Clinically Approved Cellular Tissue Constructs for Skin Regeneration
| Product name | Comments | Refs. |
|---|---|---|
| Apligraf® | First FDA-approved bilayer skin substitute composed of neonatal fibroblasts derived from foreskin seeded in bovine type I collagen scaffold on which neonatal keratinocytes are cultured; used for venous ulcers and diabetic wounds | 129,130 |
| denovoSkin™ | Fibroblasts and keratinocytes harvested from the patient's skin, cultured in vitro, and seeded in a collagen scaffold; ongoing phase II clinical trial; used for burn injuries | 131 |
| Epicel® | Autologous keratinocytes cultured in vitro to produce sheets composed of 2–8 layers and bonded to petroleum gauze; used for deep full-thickness dermal burns in which at least 30% of the body surface area are affected by the injury | 132 |
| Orcel® | Allogeneic fibroblasts and keratinocytes cultured in a porous bovine collagen type I sponge as a bilayer skin substitute; approved by the FDA for donor site wounds in burn patients | 133 |
| StrataGraft™ | Adult progenitor keratinocytes cultured in vitro to form a fully stratified epidermal layer on a dermal matrix; used for deep burn injuries; ongoing phase III clinical trial | 134 |
| TransCyte® | Neonatal fibroblasts from foreskin seeded on a collagen-coated nylon mesh and bonded to a silicone substrate; temporary skin substitute for partial-thickness burns | 135 |
| Hyalograft 3D | Autologous fibroblasts seeded on a benzylic ester of hyaluronic acid scaffold; used as a skin substitute in combination with Laserskin® (autologous keratinocyte substitute) for chronic foot ulcers | 132,136 |
| Dermagraft® | Neonatal fibroblasts from foreskin cultured on a resorbable polyglactin mesh and cryopreserved; used for full-thickness burn wounds | 137 |
| Bioseed-S | Autologous cultured keratinocytes in a fibrin sealant; applied to chronic wounds | 138 |
Table 2.
List of Clinically Approved Acellular Tissue Constructs for Skin Regeneration
| Product name | Comments | Refs. |
|---|---|---|
| Biobrane™ | A semi-permeable membrane composed of a nylon layer coated with peptides derived from porcine collagen type I and bonded to a thin porous silicone membrane; applied as a temporary dressing to superficial burn and donor site wounds | 139 |
| Integra® Omnigraft™ | A bilayer skin substitute composed of cross-linked bovine collagen and chondroitin-6-sulfate coated onto a silicone membrane to prevent fluid loss; first approved by the FDA for life-threatening burn injuries in 1996; approved by the FDA in 2012 for reconstruction of burn scars; approved by the FDA in 2016 for treating diabetic foot ulcers | 140,141 |
| Alloderm® | Human cadaveric skin processed to remove the epithelial layer and cells in the dermal layer without changing the matrix and basement membrane structure, and freeze-dried; approved by the FDA for burns; also used for abdominal wall and breast reconstruction | 142–144 |
| Hyalomatrix | Composed of a benzyl ester of hyaluronic acid (HYAFF) coated with a semi-permeable silicone membrane; HA provided by bacterial fermentation; approved by the FDA for partial- and full-thickness wounds. | 145 |
| Oasis® Wound Matrix | Derived from porcine acellular small intestinal mucosa; approved by the FDA for management of partial- and full-thickness wounds including pressure venous ulcers, diabetic ulcers, and traumatic and surgical wounds | 146 |
| PriMatrix | Derived from fetal bovine dermis; the only substitute with high level of collagen type III; approved by the FDA for the management of partial- and full-thickness wounds | 147–149 |
Hyaluronan
HA is synthesized in the plasma membrane by the action of three enzymes, namely hyaluronan synthase-1 (HAS-1), HAS-2, and HAS-3 with nucleotide sugars uridine diphosphate (UDP) glucuronic acid and UDP-N-acetyl-glucosamine as the substrate. HAS-1 and HAS-2 take part in the synthesis of HMW-HA, whereas HAS-3 is involved in the synthesis of LMW-HA.137 Secondary hydrogen bonding in the HA backbone creates hydrophobic patches, which form random coils or twists in the structure for interconnection with other HA chains to generate a network. HA interacts with cells through two surface receptors, namely CD44 and the receptor for HA-mediated motility (RHAMM).138 HA plays a crucial role in different stages of wound healing, but its biological function is dependent on it molecular weight. HMW-HA (>500 kDa) is present in normal skin to maintain moisture content. HMW-HA is associated with low inflammation, high Col III expression, and high TGF-β3 activity, whereas LMW-HA is associated with high inflammation, high Col I expression, and increased differentiation to myofibroblasts.13 Rayahin et al. reported that LMW-HA polarized macrophages to the proinflammatory phenotype, whereas HMW-HA induced the anti-inflammatory phenotype.139 Treatment of epidural fibrosis scars in a rat model of laminectomy and discectomy with HMW-HA reduced fibrosis and granulation tissue compared with the control group with no treatment.140 It has been shown that HMW-HA is fragmented to intermediate- and LMW-HA by the action of hyaluronidase and oxygen/nitrogen free radicals in the wound bed.141 RHAMM has a higher affinity for binding to LMW-HA compared with HWM-HA, and the disruption of this interaction by the application of topical RHAMM-mimetic peptides to a rat model of excisional wound decreased the number of macrophages, fibroblasts, and the expression levels of TGF-β1, α-SMA, and Col I through inhibition of the FAK pathway.142 As discussed in previous sections, the FAK pathway is associated with induction of fibrosis by the upregulation of MCP-1 and subsequent increase in inflammation.56 Scheibner et al. reported that LMW-HA stimulated the innate immune response via toll-like receptor-2 (TLR-2) activation, whereas HMW-HA inhibited TLR-2 signaling mediated inflammation.143
It is well established that HA plays a regenerative role in scarless fetal wound healing. The presence of HA in early blastoma of Xenopus tadpoles and zebrafish is indispensable for tail regeneration, and the inhibition of HA synthesis leads to impaired tail regeneration.62,144
HA used in biomedical applications is obtained from rooster combs or by bacterial fermentation.145 Hyalomatrix is a FDA-approved HA-based biomaterial for wound healing applications.146 Hyalomatrix is composed of two layers, a thin semi-permeable silicone membrane on the top that covers the skin and controls skin moisture and a matrix based on benzyl ester of HA, which is in direct contact with the wound bed. Hyalomatrix can be used in partial- or full-thickness wounds as a temporary dressing for wound healing before skin grafting.132 Voigt and Driver showed in a systematic review and meta-analysis of randomized clinical trials that HA and its derivatives have a positive effect on healing of burns, chronic and epithelial surgical wounds.147 Five dermal substitutes including Hyalomatrix were compared for scarring in a porcine model of full-thickness wound healing with no dermal substitute as the control group.148 No significant difference in scar formation was observed between the dermal substitutes and the control group. Hyalograft 3D is a cellular skin substitute in which autologous fibroblasts are seeded onto a benzylic ester of HA scaffold. Hyalograft 3D in combination with autologous keratinocytes were evaluated for wound healing in a randomized clinical trial for diabetic foot ulcers.123 According to the results, dorsal foot ulcers treated with Hyalograft 3D showed improved wound healing compared with the standard treatment while no improvement was observed in plantar ulcers. However, the extent of scarring was not reported in the clinical study. Hu et al. reported that strands of HA grafted to the wound bed improved healing in a rat model of full-thickness skin wound, suppressed TGF-β1 expression, and reduced scarring.149
Decorin
DCN, the most abundant PG in the adult human skin, is a member of the small leucine-rich proteoglycan (SLRP) family. DCN decorates collagen fibrils.13 DCN is composed of a protein core containing 12 leucine-rich repeats (LRRs) with a GAG chain (dermatan or chondroitin sulfate [CSCL]) attached to the N-terminus. DCN is believed to play a crucial role in collagen fibrillogenesis via LRR 4–6/Col I interaction.13,150,151 Furthermore, DCN protein core serves as a natural inhibitor of TGF-β1 interaction with cell surface receptors,13,150 leading to reduced fibrosis and scar formation.152 DCN is found in different layers of dermis, but its expression is highest in the papillary (superficial) layer, as papillary fibroblasts produce more DCN in vitro than fibroblasts isolated from the reticular dermis.63 Seidler et al. reported that intraperitoneal injection of DCN protein core in immune-deficient mouse with squamous carcinoma xenografts attenuated tumor growth with downregulation of epidermal growth factor receptor (EGFR) and increased tumor cell apoptosis.153 Furthermore, they demonstrated that DCN blocked the EGFR pathway and induced caspase-3 activity, a mediator of apoptosis, which eventually led to programmed cell death in tumor cells.
Persistence of fibroblasts in granulation tissue leads to scar formation, which is attributed to resistance of reticular fibroblasts to apoptotic effects of DCN. Honardoust et al. reported that DCN-treated superficial fibroblasts expressed higher levels of proapoptotic factors, including histone-1, caspase-1, caspase-8, and p53, and consequently experienced more apoptosis than deep dermal fibroblasts.154 The level of DCN produced by fibroblasts derived from HTSs in patients with grade II and III burns was lower than that of normal skin.155 Kwan et al. reported that deep dermal fibroblasts had higher expression of microRNA-181b than the superficial fibroblasts, and blocking the expression of this microRNA increased DCN expression.156 Taken together, these results explain why deep dermal injuries are more prone to fibrosis compared with superficial skin lesions.
DCN is known to suppress intracellular expression of β-catenin via interaction with MET receptor in cancer cells.157 As we previously discussed, the accumulation of β-catenin in fibroblasts is associated with fibrosis. Hence, the inhibition of fibrosis by DCN can be attributed to its interaction with the Wnt/β-catenin signaling pathway.
The antifibrotic effect of DCN in wound healing should be considered in designing biomaterials for skin tissue engineering. LRRs in the protein core of DCN interact with many growth factors and cell surface receptors.150 Therefore, biological function of DCN in the process of wound healing can be simulated with peptides that mimic specific amino acid sequences of the protein core in DCN. Jeon et al. demonstrated that a surgical glue based on fusion of the mussel adhesive protein and DCN-derived collagen binding peptides inhibited scar formation in a rat model of wound healing.158
Fibromodulin
FMOD, another member of the SLRP family, is isolated from bovine articular cartilage.159 FMOD structure is composed of a protein core containing 11–12 LRRs as binding sites for other proteins and GAG chains (keratin sulfate).160 FMOD binds to collagen through LRR11 site with high affinity or LRR7 site with low affinity and its role in collagen fibril assembly along with other SLRPs is well established. It has been shown that FMOD binding to collagen is essential in later stages of fibrillogenesis in the mouse tendon by acting as a cofactor for lysyl oxidase to regulate fiber thickness.161 FMOD as well as DCN have binding sites for TGF-β to sequester or modulate its function.162 The differential expression of FMOD in fetal and adult wounds is indicative of its role in scarless wound healing.64 Zheng et al. reported retarded wound closure and significant scarring in FMOD-deficient mouse.67 It was also reported that high expression of FMOD in mouse model of wound healing is associated with low levels of TGF-β1 and vice versa.65 Furthermore, Zheng et al. reported that FMOD enhanced migratory and contractile phenotype of fibroblasts by increasing the expression of CTGF through SMAD2/3 phosphorylation. FMOD also decreased scarring-associated factors by inhibition of noncanonical TGF-β1 signaling.
These results indicate that FMOD enhances wound closure and attenuates scar formation by regulating the activity of TGF-β1.66 In that regard, FMOD or FMOD-mimetic peptides with binding sites for TGF-β1 could be beneficial to skin tissue engineering and wound healing. It has been demonstrated that the application of peptides with binding sites for TGF-β1 to skin wounds in animal models recapitulated ECM architecture and regeneration of the normal skin.163
Collagen type III
Among the 28 types of collagen in mammalian tissues, Col I and Col III are the most abundant in the skin tissue.164 Col III, which is a fibrillar collagen composed of three α1(III) chains, is present during embryonic development and wound healing, but it is replaced by Col I in the later stages of fetal development.165 Fleischmajer et al. demonstrated that Col I serves as the fibril building block and Col III serves as the regulator of fibril diameter and growth during fibrillogenesis in adult human skin.166 Liu et al. observed reduced number and increased size variability of collagen fibrils in Col III mutant mouse model of wound healing. They also observed that ∼60% of Col III-mutant mice developed spontaneous skin lesions.167 In humans, mutations in pro-collagen III gene leads to Ehlers–Danlos syndrome IV in which skin wounds heal but with higher scarring.168 Volk et al. reported higher number of myofibroblasts in granulation tissue of heterozygous Col III-deficient mouse after 7 days of wound healing compared with normal mouse, indicating a link between Col III and differentiation of myofibroblasts.169 It has been shown that the ratio of Col III to Col I is higher in the absence of myofibroblasts in scarless fetal wounds.170 Furthermore, the level of Col III increased in the mouse model of digit tip regeneration.57,171 Papillary dermis or the superficial layer of skin also contains elevated ratios of Col III to Col I compared with the deep reticular layer,172 which explains the scar-free or low-scar healing of superficial wounds. Taken together, it can be concluded that Col III plays a beneficial role in scar-free skin regeneration and as a skin substitute. Currently, the only commercially available skin substitute containing significant amounts of Col III (30%) is PriMatrix, which is derived from fetal bovine dermis. PriMatrix is shown to stimulate the formation of vascular granulation tissue in full-thickness wounds,134–136 and macrophages seeded on PriMatrix polarized to M2c phenotype, which is associated with less fibrosis.173 The application of PriMatrix to severely traumatic skin injuries resulted in complete re-epithelialization with minimal scarring.174
Steering Macrophages Toward Regeneration
Macrophages are the key mediators of inflammatory response after tissue injury. Macrophages can take a proinflammatory M1 phenotype or an anti-inflammatory M2. M1 phenotype in macrophages is induced by interferon-γ (IFNγ), lipopolysaccharides (LPS), TNF, and granulocyte–macrophage colony-stimulating factor. M1 macrophages secrete IL-1B, IL-6, and TNF-α, and they are identified by increased expression of IL-12, IL-23, CD80, and CD86. M2 phenotype in macrophages is induced by IL-4, IL-13 (M2a phenotype), immune complexes (M2b phenotype) and IL-10, and glucocorticoids (M2c phenotype). M2 macrophages are characterized by elevated expression of IL-10, CD163, CD204, and CD206. Each M2 subtype (M2a, M2b, and M2c) plays a unique role in resolution of inflammation, tissue remodeling, regeneration, fibrosis, angiogenesis, and promotion of wound healing.175–177 M1 macrophages are present in the early phase of inflammation to remove the microbial and tissue debris from the wound bed. M2 macrophages, which are present in the late phase of inflammation and referred to as “wound healing macrophages,” are involved in the formation of granulation tissue, remodeling, and termination of inflammation. Persistence of M1 macrophages in the wound bed leads to chronic inflammation and nonhealing wounds like chronic venous and diabetic ulcers. Macrophages are known to contribute to tissue regeneration after injury like limb regeneration in axolotl.178
Macrophages in wound healing can be classified, based on their function in scar formation or resolution, into profibrotic and antifibrotic phenotypes. Profibrotic phenotypes like M2a secrete anti-inflammatory and fibrotic factors such as IL-10 and TGF-β. Conversely, antifibrotic phenotypes secrete IL-10, MMPs, and tissue inhibitor of matrix metalloproteinases (TIMPs) that degrade the ECM.179–181 Fallowfield et al. showed in a mouse model of fibrotic liver that scar-associated macrophages (SAM) secrete MMP13 to resolve the fibrotic tissue.182 Furthermore, they observed that SAM-depleted mice had decreased MMP expression, which delayed resolution of the fibrosis.182 Ramachandran et al. identified a population of macrophages outside the M1/M2 classification with the ability to resolve hepatic fibrosis in a mouse model of the disease.183 This subpopulation, derived from LY-6Chigh circulatory monocytes, was characterized by CD11Bhigh/F4/80intermediate/LY-6Clow compared with the CD11Bintermediate/F4/80high resident hepatic macrophages in an uninjured control. It was shown that this macrophage subpopulation, which was involved in tissue remodeling and resolution of fibrosis, secreted high levels of MMP9, MMP12, IGF-1, and glycoprotein Nmb.
Lurier et al. analyzed different phenotypes of macrophages including M0, M1, M2a, and M2c by RNA sequencing and reported that M2c macrophages express higher levels of genes related to angiogenesis such as SERPINA1, SRPX2, MMP8, and VCAN.184 They also reported that M2c macrophages secrete higher levels of enzymes for matrix remodeling such as MMP7, MMP8, and TIMP1. They concluded that M2c macrophages could potentially attenuate fibrosis.184 M2c macrophages are considered as “deactivated” because they produce immunosuppressive factor IL-10 during wound healing.175 Grinberg et al. proposed a new subtype of angiogenic M2d macrophage phenotype such that M1 macrophages can be converted to M2d by co-stimulation with TLR and adenosine A2a receptor agonists, leading to high expression and production of IL-10 and VEGF.185,186
Material properties such as porosity and pore size, microstructure, hydrophobicity, surface chemistry, and mechanical properties affect polarization of macrophages.187 For example, Blakney et al. showed that decreasing compressive modulus of polyethylene glycol hydrogels conjugated with cell-adhesive RGD peptide (PEG-RGD) reduced the activation of the seeded macrophages.188 Cha et al. reported that encapsulation of human monocytes in gelatin methacryloyl (GelMA) hydrogels in the presence of IL-4 induced macrophage polarization to M2 phenotype, whereas encapsulation in polyethylene glycol diacrylate (PEGDA) hydrogel induced M1 polarization.189 They also showed that this effect was mediated by integrin α2β1 cell surface receptors interacting with ligands present in GelMA but not PEGDA. Inhibition of integrin-ligand interactions in GelMA reversed the macrophage phenotype to that observed in PEGDA.
It is known that scaffolds with 30–40 μm pore size induce M2 macrophage polarization, which leads to higher extent of angiogenesis and less fibrosis.190 This indicates the importance of scaffold porosity and pore size for induction of a regenerative response to wound healing. ECM-derived biomaterials are believed to polarize macrophages predominantly to M2 phenotype, although the method of processing and modification can mitigate this response.190 Badylak et al. compared the response of macrophages with scaffolds derived from porcine small intestinal submucosa (SIS) with or without cross-linking with carbodiimide (CDI-SIS) in a rat model.191 They reported that SIS scaffolds without treatment polarized macrophages to M2 phenotype, which in turn induced tissue remodeling, whereas the CDI-treated scaffolds polarized macrophages to M1 phenotype, which led to chronic inflammation. They attributed the change in macrophage phenotype to changes in collagen microstructure in SIS scaffolds with CDI cross-linking.
In another study, murine bone marrow-derived macrophages were seeded on matrices derived from digested, decellularized porcine small intestinal submucosa (SIS), brain, urinary bladder, liver, skeletal muscle, esophagus, colon, and skin.192 The matrices derived from the brain, SIS, esophagus, urinary bladder, and colon polarized macrophages to the anti-inflammatory M2 phenotype, whereas the dermal matrix polarized the macrophages to the proinflammatory M1 phenotype. He et al. reported that acellular mouse dermal matrices increased M2 macrophage polarization with the upregulation of MMP3, MMP9, EGF, IGF, PDGF, TGF-β, and VEGF expression.193 They further showed that the degradation of collagen in dermal matrices produced peptides that led to the activation of acid-sensing pathway-associated lysosomal adaptor protein (LAMTOR1), which in turn mediated a regenerative response during wound healing by M2 macrophage polarization in the presence of IL-4. Vasconcelos et al. observed different macrophage responses to chitosan in a mouse model of wound healing depending on the degree of acetylation (DA; 5% and 15%).194 Based on their results, porous chitosan scaffolds with 5% DA polarized macrophages to M2 phenotype with low expression of proinflammatory cytokines such as IL-6 and TNF-α, whereas the opposite response was observed in 15% DA scaffolds (polarization to M1 phenotype). Furthermore, the macrophage phenotype in 15% DA chitosan scaffolds was switched to M2 by conjugation of the scaffolds with the anti-inflammatory or pro-resolution Resolvin D1 (RvD1) chemical mediator.195 They concluded that immune response of biomaterials can be modulated toward regeneration by conjugation with pro-resolution agents.
Keratin is also shown to induce regeneration after injury with less scarring.196 Fearing and Van Dyke compared macrophage polarization on chamber slides coated with keratin with those coated with collagen in vitro and reported that keratin induced M2 polarization with higher IL-10 and lower IL-6 and IL-1B expressions.196 Taraballi et al. reported that macrophages seeded on collagen scaffolds functionalized with CSCL had higher expression of anti-inflammatory cytokines IL-10, mannose receptor-1, arginase-1, and TGF-β compared with the unmodified collagen scaffold.197,198 Furthermore, they showed that the CSCL effect can be attributed to inhibition of the LPS/CD44/NF-κB signaling pathway. In the absence of CSCL, LPS bind to CD44 receptors on the surface of macrophages leading to phosphorylation of NF-κB and activation of proinflammatory genes. In CSCL scaffolds, CSCL competes with LPS and disrupts the signaling pathway for activation of proinflammatory genes.197,198 Castellano et al. showed that electrospun polyhydroxybutyrate (PBH) scaffolds polarize macrophages to M2 phenotype in a rat model.199 They also observed that M1 macrophages, initially present in the wound bed of PBH-implanted animals, were depleted after 3 days. However, M1 macrophage depletion did not happen in the control animals implanted with Matriderm scaffolds, which led to further scarring. They reported that combination of PBH scaffold and human dermoepidermal skin equivalent implanted in the wound resulted in higher neoangiogenesis and graft survival.
Prospect
The worldwide market for scar treatment has been predicted to reach $35 billion by 2023.200 The largest share of this market belongs to postsurgical scars and topical treatments.201 Among topical products, silicone-based matrices are shown to have the fastest growth rate in the market.201 Currently, the most common approaches to reduce scarring, based on scar management guidelines, are pressure therapy, silicone gels and sheets, intra-lesion corticosteroid injection, and surgical removal of the scar.202 Approximately 32–72% of burn injuries in the United States lead to scarring.2,4 The gold standard for treatment of full- and deep partial-thickness burn wounds is skin grafting.203 Lack of adequate skin grafts and donor site complications have increased the demand for tissue engineered skin substitutes.204
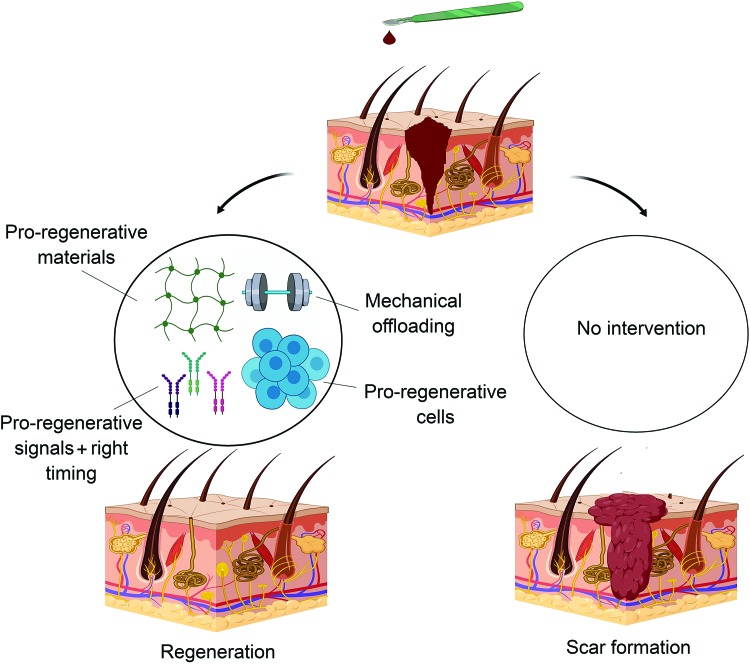
The role of skin substitutes in wound injuries is to accelerate healing while minimizing scar formation. Although commercially available skin substitutes protect the wound from infection and regulate moisture content, they lack optimization with respect to regeneration and scarring. The ideal construct for skin regeneration should be cellular to regenerate the lost cells and should contain growth factors encapsulated in a skin-mimetic matrix to minimize scaring and optimize mechanical and barrier properties (Fig. 4). Currently, autologous and neonatal foreskin-derived fibroblasts are being used as the cell source in skin tissue constructs.205–207
FIG. 4.
Strategies for inducing regeneration rather than repair after skin injury. The combination of pro-regenerative materials with optimum mechanical properties along with the right cell types and soluble molecules paves the way toward regeneration. Color images are available online.
As discussed in previous sections, fibroblasts selected based on the expression of embryonic marker Engrailed-1 (En1) and characterized by CD26 surface marker are involved in deposition major connective tissue, scar formation, and transition from scar-free to scar-forming wound healing in murine fetal skin.15,208 It is reported that the En1 fibroblasts increase from <1% in early gestation to ∼75% postnatally.15 In late gestation (transition time), ∼22% of the fibroblasts are En1 positive (Fig. 3),15 and majority of fibroblasts in the adult skin express CD26 surface marker.209
Considering these facts, a question that comes to mind is what is the role of CD26-negative fibroblasts in wound healing? Lichtenberger et al. showed by flow cytometry that CD26+/Sca1+ fibroblasts were the most abundant cells in the regression (telogen) phase of hair follicles and their population decreased in the growth (anagen) phase. Conversely, CD26−/Sca1− fibroblasts were the most abundant cells in the anagen phase.210 These findings indicate a possible role for CD26−/Sca1− fibroblasts in wound healing and skin regeneration. Lineage tracing experiments have identified multiple subpopulation of MSCs that can function as progenitors for myofibroblasts.211 These include (a) CD26+ fibroblasts, (b) PDGFRα+/Sca1+/Dlk1+ fibroblasts, (c) PDGFRα+/Sca1+/ADAM12+ perivascular mesenchymal cells, and (d) adiponectin+ adipocytes. These findings suggest that the regenerative properties of skin substitutes can be improved by optimizing the ratio of different fibroblast subpopulations in the construct. For example, lower ratios of CD26+ fibroblasts in the skin substitute could lead to scar-free skin wound healing. Future studies should be focused on understanding of the effect of different subpopulations of fibroblasts on scarring and the fate of wound healing.
It has been reported that the number of stem cells in scar-free fetal wounds is higher than that in adult wounds. However, embryonic stem (ES) and induced pluripotent stem (iPS) cells are limited by teratoma formation in vivo and tumorigenesis.212 MSCs are known to increase angiogenesis and attenuate inflammation and scar formation in chronic wounds, but they are limited by their heterogeneity with variable proliferative rates and differentiation ability.213 Kuroda et al. identified multipotent cells, called multilineage-differentiating stress enduring (MUSE) cells, from human MSCs including dermal fibroblasts, adipose derived MSCs, and bone marrow-derived MSCs, which are characterized by the expression of stage-specific embryonic antigen-3 marker. They reported that MUSE cells have the ability to differentiate into the three different germ layers, namely ectoderm, mesoderm, and endoderm, but they do not produce teratoma in mouse unlike ES and iPS cells.214 It was shown that keratinocytes, fibroblasts, and melanocytes generated from differentiation of MUSE cells can be used to reconstitute a skin substitute.215,216
In addition to optimizing cell types and their ratio, the matrix in a skin substitute for wound healing should be engineered for regeneration. A variety of natural materials like collagen, HA, fibrin, chitosan, and alginate as well as synthetic materials like polypyrrole, poly(ɛ-caprolactone), and polylactic-glycolic acid have been developed for generating a skin substitute for wound healing.118,217 Among synthetic materials, silicone-based polymers have been used extensively in skin substitutes as a stand-alone matrix or as a part of the tissue construct. Silicone gel sheets are reported to reduce scarring in clinical applications.218 As mentioned in previous sections, biomaterials affect the process of wound healing by altering the gene expression profile of the cells through stimulation or inhibition of cell surface receptors and control of specific intracellular pathways. Therefore, selection of optimum biomaterial composition like combination of HMW-HA, high ratio of Col III to Col I, and conjugation of fibromodulin- and DCN -mimetic peptides can significantly affect the resolution of wound healing. Mechanical off-loading devices are shown to be effective in minimizing scar formation. Mechanical properties of the matrix are important factors that should be considered in the selection of biomaterial for skin wound healing. Longaker et al. showed that the application of a stress-shielding silicone device on postsurgical wounds decreased scar formation in a controlled randomized clinical trial.219
To summarize, pro-regenerative matrices combined with engineered cells with less intrinsic potential for fibrogenesis bring us one step closer to scar-free skin tissue regeneration.
Acknowledgments
This work was supported by research grants to E.J. from the National Science Foundation under Award Number CBET1403545 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR063745. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1. World Health Organization. Burns. 2018. Available at: http://www.who.int/en/news-room/fact-sheets/detail/burns Accessed March20, 2019
- 2. American Burn Association. Burn Incidence and Treatment in the United States. 2016. Available at: http://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/ Accessed March20, 2019
- 3. Aarabi S., Longaker M.T., and Gurtner G.C. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 4, e234, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence J.W., Mason S.T., Schomer K., and Klein M.B. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res 33, 136, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Sen C.K., Gordillo G.M., Roy S., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17, 763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mirastschijski U., Sander J., Zier U., Rennekampff H., Weyand B., and Vogt P.M. The cost of post-burn scarring. Ann Burns Fire Disasters 28, 215, 2015 [PMC free article] [PubMed] [Google Scholar]
- 7. Bock O., Schmid-Ott G., Malewski P., and Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297, 433, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Kumar V., Abbas A.K., Aster J.C., and Perkins A.J. Inflammation and Repair. Robbins Basic Pathology. New York, NY: Elsevier, 2018, p. 57 [Google Scholar]
- 9. Burrington J.D. Wound healing in the fetal lamb. J Pediatr Surg 6, 523, 1971 [DOI] [PubMed] [Google Scholar]
- 10. Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 381, 353, 1979 [DOI] [PubMed] [Google Scholar]
- 11. Rose L.F., and Chan R.K. The burn wound microenvironment. Adv Wound Care 5, 106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 22, 407, 2013 [DOI] [PubMed] [Google Scholar]
- 13. Tracy L.E., Minasian R.A., and Caterson E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care 5, 119, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Driskell R.R., Lichtenberger B.M., Hoste E., et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rinkevich Y., Walmsley G.G., Hu M.S., et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang D., Correa-Gallegos D., Christ S., et al. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol 20, 422, 2018 [DOI] [PubMed] [Google Scholar]
- 17. Klemann C., Wagner L., Stephan M., and von Horsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol 185, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadir R., Imberty A., Baleux F., and Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem 279, 43854, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Ceradini D.J., Kulkarni A.R., Callaghan M.J., et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10, 858, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kitaori T., Ito H., Schwarz E.M., et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60, 813, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Ghadge S.K., Messner M., Van Pham T., et al. Prolyl-hydroxylase inhibition induces SDF-1 associated with increased CXCR4+/CD11b+ subpopulations and cardiac repair. J Mol Med 95, 825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petit I., Jin D., and Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28, 299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rabbany S.Y., Pastore J., Yamamoto M., et al. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant 19, 399, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Hu M.S., Borrelli M.R., Januszyk M., et al. Pathway analysis of gene expression of E14 versus E18 fetal fibroblasts. Adv Wound Care 7, 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ding J., Hori K., Zhang R., et al. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair Regen 19, 568, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Larson B.J., Longaker M.T., and Lorenz H.P. Scarless fetal wound healing: a basic science review. Plastic Reconstr Surg 126, 1172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yagi L.H., Watanuki L.M., Isaac C., Gemperli R., Nakamura Y.M., and Ladeira P.R.S. Human fetal wound healing: a review of molecular and cellular aspects. Eur J Plastic Surg 39, 239, 2016 [Google Scholar]
- 28. Plikus M.V., Guerrero-Juarez C.F., Ito M., et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dunkin C.S.J., Pleat J.M., Gillespie P.H., Tyler M.P.H., Roberts A.H.N., and McGrouther D.A. Scarring occurs at a critical depth of skin injury: precise measurement in a graduated dermal scratch in human volunteers. Plastic Reconstr Surg 119, 1722, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Wang J.F., Dodd C., Shankowsky H.A., Scott P.G., Tredget E.E., and Grp W.H.R. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest 88, 1278, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Wilgus T.A., Roy S., and McDaniel J.C. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care 2, 379, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dovi J.V., Szpaderska A.M., and DiPietro L.A. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost 92, 275, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Naik-Mathuria B., Gay A.N., Zhu X., Yu L., Cass D.L., and Olutoye O.O. Age-dependent recruitment of neutrophils by fetal endothelial cells: implications in scarless wound healing. J Pediatric Surg 42, 166, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Minutti C.M., Knipper J.A., Allen J.E., and Zaiss D.M. Tissue-specific contribution of macrophages to wound healing. Sem Cell Dev Biol 61, 3, 2017 [DOI] [PubMed] [Google Scholar]
- 35. Cowin A., Brosnan M., Holmes T., and Ferguson M. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn 212, 385, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Wulff B.C., Parent A.E., Meleski M.A., DiPietro L.A., Schrementi M.E., and Wilgus T.A. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 132, 458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeong W., Yang C.E., Roh T.S., Kim J.H., Lee J.H., and Lee W.J. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound-healing model. Intl J Mol Sci 18, e1698, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walraven M.l., Talhout W., Beelen R.H., van Egmond M., and Ulrich M.M. Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 24, 533–541, 2016 [DOI] [PubMed] [Google Scholar]
- 39. Glim J.E., Beelen R.H., Niessen F.B., Everts V., and Ulrich M.M. The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Arch Oral Biol 60, 272, 2015 [DOI] [PubMed] [Google Scholar]
- 40. Wilgus T.A., Bergdall V.K., Tober K.L., et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol 165, 753, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olutoye O.O., Barone E.J., Yager D.R., Uchida T., Cohen I.K., and Diegelmann R.F. Hyaluronic acid inhibits fetal platelet function: implications in scarless healing. J Pediatric Surg 32, 1037, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Kushida S., Kakudo N., Suzuki K., and Kusumoto K. Effects of platelet-rich plasma on proliferation and myofibroblastic differentiation in human dermal fibroblasts. Ann Plastic Surg 71, 219, 2013 [DOI] [PubMed] [Google Scholar]
- 43. Caceres M., Hidalgo R., Sanz A., MartÃ-nez J., Riera P., and Smith P.C. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol 79, 714, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Chellini F., Tani A., Vallone L., et al. Platelet-rich plasma prevents in vitro transforming growth factor-Î21-induced fibroblast to myofibroblast transition: involvement of vascular endothelial growth factor (VEGF)-A/VEGF receptor-1-mediated signaling. Cells 7, e142, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walmsley G.G., Maan Z.N., Wong V.W., et al. Scarless wound healing: chasing the holy grail. Plastic Reconstr Surg 135, 907, 2015 [DOI] [PubMed] [Google Scholar]
- 46. Wang L., Hu L., Zhou X., et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep 7, 13321, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim J., and Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37, 1445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabapathy V., Sundaram B., Sreelakshmi V.M., Mankuzhy P., and Kumar S. Human Wharton's jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One 9, e93726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu S.Y., Jiang L., Li H.J., et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol 134, 2648, 2014 [DOI] [PubMed] [Google Scholar]
- 50. Li Y., Zhang W., Gao J.X., et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther 7, e102, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doi H., Kitajima Y., Luo L., et al. Potency of umbilical cord blood-and Wharton's jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep 6, 18844, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colwell A.S., Krummel T.M., Longaker M.T., and Lorenz H.P. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plastic Reconstr Surg 118, 1125, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Smithmyer M.E., Sawicki L.A., and Kloxin A.M. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater Sci 2, 634, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hinz B. Matrix mechanics and regulation of the fibroblast phenotype. Periodontology 2000 63, 14, 2013 [DOI] [PubMed] [Google Scholar]
- 55. Goffin J.M., Pittet P., Csucs G., Lussi J.W., Meister J.J., and Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172, 259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wong V.W., Rustad K.C., Akaishi S., et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18, 148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marrero L., Simkin J., Sammarco M., and Muneoka K. Fibroblast reticular cells engineer a blastema extracellular network during digit tip regeneration in mice. Regeneration 4, 69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kavasi R.M., Berdiaki A., Spyridaki I., et al. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol 101, 128, 2017 [DOI] [PubMed] [Google Scholar]
- 59. Longaker M.T., Chiu E.S., Adzick N.S., Stern M., Harrison M.R., and Stern R. Studies in fetal wound-healing. 5. a prolonged presence of hyaluronic-acid characterizes fetal wound fluid. Ann Surg 213, 292, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leung A., Crombleholme T.M., and Keswani S.G. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatrics 24, 371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vitulo N., Dalla Valle L., Skobo T., Valle G., and Alibardi L. Transcriptome analysis of the regenerating tail vs. the scarring limb in lizard reveals pathways leading to successful vs. unsuccessful organ regeneration in amniotes. Dev Dyn 246, 116, 2017 [DOI] [PubMed] [Google Scholar]
- 62. Ouyang X.H., Panetta N.J., Talbott M.D., et al. Hyaluronic acid synthesis is required for zebrafish tail fin regeneration. PLoS One 12, e0171898, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schonherr E., Beavan L.A., Hausser H., Kresse H., and Culp L.A. Differences in decorin expression by papillary and reticular fibroblasts in vivo and in vitro. Biochem J 290, 893, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soo C., Hu F.Y., Zhang X.L., et al. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol 157, 423, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zheng Z., Zhang X.L., Dang C., et al. Fibromodulin is essential for fetal-type scarless cutaneous wound healing. Am J Pathol 186, 2824, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng Z., James A.W., Li C., et al. Fibromodulin reduces scar formation in adult cutaneous wounds by eliciting a fetal-like phenotype. Signal Transduct Target Ther 2, 17050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng Z., Nguyen C., Zhang X., et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-beta3 signaling. J Invest Dermatol 131, 769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coolen N.A., Schouten K.C., Middelkoop E., and Ulrich M.M. Comparison between human fetal and adult skin. Arch Dermatol Res 302, 47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Almine J.F., Wise S.G., and Weiss A.S. Elastin signaling in wound repair. Birth Defects Res C Embryo Today 96, 248, 2012 [DOI] [PubMed] [Google Scholar]
- 70. Stenman S., and Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med 147, 1054, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lenselink E.A. Role of fibronectin in normal wound healing. Int Wound J 12, 313, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lo D.D., Zimmermann A.S., Nauta A., Longaker M.T., and Lorenz H.P. Scarless fetal skin wound healing update. Birth Defects Res Part C Embryo Today Rev 96, 237, 2012 [DOI] [PubMed] [Google Scholar]
- 73. Longaker M.T., Whitby D.J., Ferguson M.W.J., et al. Studies in fetal wound-healing. 3. early deposition of fibronectin distinguishes fetal from adult wound-healing. J Pediatric Surg 24, 799, 1989 [DOI] [PubMed] [Google Scholar]
- 74. Serezani A.P.M., Bozdogan G., Sehra S., et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J Allergy Clin Immunol 139, 142, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levesque M., Gatien S., Finnson K., et al. Transforming growth factor-beta signaling is essential for limb regeneration in axolotls. PLoS One 2, e1227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pakyari M., Farrokhi A., Maharlooei M.K., and Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2, 215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Penn J.W., Grobbelaar A.O., and Rolfe K.J. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma 2, 18, 2012 [PMC free article] [PubMed] [Google Scholar]
- 78. Martin P. Wound healing: aiming for perfect skin regeneration. Science 276, 75, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Mori T., Kawara S., Shinozaki M., et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol 181, 153, 1999 [DOI] [PubMed] [Google Scholar]
- 80. Shah M., Foreman D.M., and Ferguson M.W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 108, 985, 1995 [DOI] [PubMed] [Google Scholar]
- 81. Lichtman M.K., Otero-Vinas M., and Falanga V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen 24, 215, 2015 [DOI] [PubMed] [Google Scholar]
- 82. MacDonald B.T., Tamai K., and He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17, 9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beyer C., Schramm A., Akhmetshina A., et al. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71, 761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carthy J.M., Garmaroudi F.S., Luo Z., and McManus B.M. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS One 6, e19809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carre A.L., James A.W., MacLeod L., et al. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg 125, 74, 2010 [DOI] [PubMed] [Google Scholar]
- 86. Collins C.A., Kretzschmar K., and Watt F.M. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development 138, 5189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen D., Jarrell A., Guo C., Lang R., and Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 139, 1522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Whyte J.L., Smith A.A., Liu B., et al. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One 8, e76883, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rognoni E., Gomez C., Pisco A.O., et al. Inhibition of beta-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143, 2522, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shirakata Y., Kimura R., Nanba D., et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 118, 2363, 2005 [DOI] [PubMed] [Google Scholar]
- 91. Peled Z.M., Rhee S.J., Hsu M., Chang J., Krummel T.M., and Longaker M.T. The ontogeny of scarless healing II: EGF and PDGF-B gene expression in fetal rat skin and fibroblasts as a function of gestational age. Ann Plast Surg 47, 417, 2001 [DOI] [PubMed] [Google Scholar]
- 92. Barrientos S., Stojadinovic O., Golinko M.S., Brem H., and Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 16, 585, 2008 [DOI] [PubMed] [Google Scholar]
- 93. Bonner J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15, 255, 2004 [DOI] [PubMed] [Google Scholar]
- 94. Whitby D.J., and Ferguson M.W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 147, 207, 1991 [DOI] [PubMed] [Google Scholar]
- 95. Ortega S., Ittmann M., Tsang S.H., Ehrlich M., and Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A 95, 5672, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guo L., Degenstein L., and Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10, 165, 1996 [DOI] [PubMed] [Google Scholar]
- 97. Gay D., Kwon O., Zhang Z., et al. FGF9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med 19, 916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dang C.M., Beanes S.R., Soo C., et al. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plast Reconstr Surg 111, 1969, 2003 [DOI] [PubMed] [Google Scholar]
- 99. Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., and Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 153, 347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wilgus T.A., Ferreira A.M., Oberyszyn T.M., Bergdall V.K., and Dipietro L.A. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 88, 579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sziksz E., Pap D., Lippai R., et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm 2015, e764641, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liechty K.W., Kim H.B., Adzick N.S., and Crombleholme T.M. Fetal wound repair results in scar formation in interleukin-10 deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatric Surg 35, 866, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Balaji S., Wang X., King A., et al. Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J 31, 868, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liechty K.W., Adzick N.S., and Crombleholme T.M. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 12, 671, 2000 [DOI] [PubMed] [Google Scholar]
- 105. Liechty K.W., Crombleholme T.M., Cass D.L., Martin B., and Adzick N.S. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res 77, 80, 1998 [DOI] [PubMed] [Google Scholar]
- 106. Underwood M.A., Gilbert W.M., and Sherman M.P. Amniotic fluid: not just fetal urine anymore. J Perinatol 25, 341, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Longaker M.T., Adzick N.S., Hall J.L., et al. Studies in fetal wound healing, VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatric Surg 25, 430, 1990 [DOI] [PubMed] [Google Scholar]
- 108. Fukutake M., Ochiai D., Masuda H., et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Human Cell 32, 51, 2019 [DOI] [PubMed] [Google Scholar]
- 109. Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., and Seifalian A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater 15, 88, 2008 [DOI] [PubMed] [Google Scholar]
- 110. Murphy S.V., Skardal A., Song L., et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med 6, 2020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Longaker M.T., Whitby D.J., Ferguson M., Lorenz H.P., Harrison M.R., and Adzick N.S. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 219, 65, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hynes R.O. The extracellular matrix: not just pretty fibrils. Science 326, 1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ringer P., Colo G., Fassler R., and Grashoff C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol 64, 6, 2017 [DOI] [PubMed] [Google Scholar]
- 114. Reinke J.M., and Sorg H. Wound repair and regeneration. Eur Surg Res 49, 35, 2012 [DOI] [PubMed] [Google Scholar]
- 115. Adzick N.S., and Longaker M.T. Scarless fetal healing. Therapeutic implications. Ann Surg 215, 3, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zaulyanov L., and Kirsner R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2, 93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dodson B.P., and Levine A.D. Challenges in the translation and commercialization of cell therapies. BMC Biotechnol 15, 70, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vig K., Chaudhari A., Tripathi S., et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci 18, e789, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shevchenko R.V., James S.L., and James S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 7, 229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Halim A.S., Khoo T.L., and Mohd Yussof S.J. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg 43, S23–S28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Centanni J.M., Straseski J.A., Wicks A., et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg 253, 672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Noordenbos J., Dore C., and Hansbrough J.F. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil 20, 275, 1999 [DOI] [PubMed] [Google Scholar]
- 123. Caravaggi C., De Giglio R., Pritelli C., et al. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 26, 2853, 2003 [DOI] [PubMed] [Google Scholar]
- 124. Marston W.A., Hanft J., Norwood P., and Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701, 2003 [DOI] [PubMed] [Google Scholar]
- 125. Vanscheidt W., Ukat A., Horak V., et al. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: a multinational randomized controlled clinical trial. Wound Repair Regen 15, 308, 2007 [DOI] [PubMed] [Google Scholar]
- 126. Greenwood J.E., Clausen J., and Kavanagh S. Experience with biobrane: uses and caveats for success. Eplasty 9, e25, 2009 [PMC free article] [PubMed] [Google Scholar]
- 127. Heimbach D.M., Warden G.D., Luterman A., et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 24, 42, 2003 [DOI] [PubMed] [Google Scholar]
- 128. Dantzer E., and Braye F.M. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg 54, 659, 2001 [DOI] [PubMed] [Google Scholar]
- 129. Wainwright D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21, 243, 1995 [DOI] [PubMed] [Google Scholar]
- 130. Breuing K.H., and Colwell A.S. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 59, 250, 2007 [DOI] [PubMed] [Google Scholar]
- 131. Buinewicz B., and Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 52, 188, 2004 [DOI] [PubMed] [Google Scholar]
- 132. Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma 2, 162, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 28, 3587, 2007 [DOI] [PubMed] [Google Scholar]
- 134. Cornwell K.G., Landsman A., and James K.S. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 26, 507, 2009 [DOI] [PubMed] [Google Scholar]
- 135. Parcells A.L., Karcich J., Granick M.S., and Marano M.A. The use of fetal bovine dermal scaffold (primatrix) in the management of full-thickness hand burns. Eplasty 14, e36, 2014 [PMC free article] [PubMed] [Google Scholar]
- 136. Neill J.S., and Lineaweaver W.C. Tissue response to bovine fetal collagen extracellular matrix in full-thickness skin wounds. Am J Clin Pathol 140, 248, 2013 [DOI] [PubMed] [Google Scholar]
- 137. Dicker K.T., Gurski L.A., Pradhan-Bhatt S., Witt R.L., Farach-Carson M.C., and Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10, 1558, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Misra S., Hascall V.C., Markwald R.R., and Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 6, e201, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rayahin J.E., Buhrman J.S., Zhang Y., Koh T.J., and Gemeinhart R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 1, 481, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Isik S., Taskapilioglu M.O., Atalay F.O., and Dogan S. Effects of cross-linked high-molecular-weight hyaluronic acid on epidural fibrosis: experimental study. J Neurosurg Spine 22, 94, 2015 [DOI] [PubMed] [Google Scholar]
- 141. Monslow J., Govindaraju P., and Pure E. Hyaluronan-a functional and structural sweet spot in the tissue microenvironment. Front Immunol 6, e231, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tolg C., Hamilton S.R., Zalinska E., et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol 181, 1250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Scheibner K.A., Lutz M.A., Boodoo S., Fenton M.J., Powell J.D., and Horton M.R. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177, 1272, 2006 [DOI] [PubMed] [Google Scholar]
- 144. Contreras E.G., Gaete M., Sanchez N., Carrasco H.C., and Larrain J. Early requirement of Hyaluronan for tail regeneration in Xenopus tadpoles. Development 136, 2987, 2009 [DOI] [PubMed] [Google Scholar]
- 145. Sze J.H., Brownlie J.C., and Love C.A. Biotechnological production of hyaluronic acid: a mini review. 3 Biotech 6, e67, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Altinteb. Hyaluronic-acid based dermal substitute. 2018. Available at: www.altinteb.com/hyqlomatrix.pdf Accessed March20, 2019
- 147. Voigt J., and Driver V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen 20, 317, 2012 [DOI] [PubMed] [Google Scholar]
- 148. Philandrianos C., Andrac-Meyer L., Mordon S., et al. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 38, 820, 2012 [DOI] [PubMed] [Google Scholar]
- 149. Hu M., Sabelman E.E., Cao Y., Chang J., and Hentz V.R. Three-dimensional hyaluronic acid grafts promote healing and reduce scar formation in skin incision wounds. J Biomed Mater Res Part B Appl Biomater 67, 586, 2003 [DOI] [PubMed] [Google Scholar]
- 150. Gubbiotti M.A., Vallet S.D., Ricard-Blum S., and Iozzo R.V. Decorin interacting network: a comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol 55, 7, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Iozzo R.V., and Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42, 11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Baghy K., Iozzo R.V., and Kovalszky I. Decorin–TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem 60, 262-268, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Seidler D.G., Goldoni S., Agnew C., et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem 281, 26408, 2006 [DOI] [PubMed] [Google Scholar]
- 154. Honardoust D., Ding J., Varkey M., Shankowsky H.A., and Tredget E.E. Deep dermal fibroblasts refractory to migration and decorin-induced apoptosis contribute to hypertrophic scarring. J Burn Care Res 33, 668, 2012 [DOI] [PubMed] [Google Scholar]
- 155. Scott P., Dodd C., Ghahary A., Shen Y., and Tredget E. Fibroblasts from post-burn hypertrophic scar tissue synthesize less decorin than normal dermal fibroblasts. Clin Sci 94, 541, 1998 [DOI] [PubMed] [Google Scholar]
- 156. Kwan P., Ding J., and Tredget E.E. MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLos One 10, e0123054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Goldoni S., Humphries A., Nystrom A., et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 185, 743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jeon E.Y., Choi B.-H., Jung D., Hwang B.H., and Cha H.J. Natural healing-inspired collagen-targeting surgical protein glue for accelerated scarless skin regeneration. Biomaterials 134, 154, 2017 [DOI] [PubMed] [Google Scholar]
- 159. Heinegard D., Larsson T., Sommarin Y., Franzen A., Paulsson M., and Hedbom E. Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J Biol Chem 261, 13866, 1986 [PubMed] [Google Scholar]
- 160. Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J 19, 287, 2002 [DOI] [PubMed] [Google Scholar]
- 161. Kalamajski S., and Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 29, 248, 2010 [DOI] [PubMed] [Google Scholar]
- 162. Hildebrand A., Romaris M., Rasmussen L.M., et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302, 527, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Soo B.C., Ting K., and Zheng Z. Fibromodulin peptide. U.S. Patent 9,409, 963, 2016 [Google Scholar]
- 164. Xue M., and Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care 4, 119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Des Parkin J., San Antonio J.D., Persikov A.V., et al. The collagen III fibril has a “flexi-rod” structure of flexible sequences interspersed with rigid bioactive domains including two with hemostatic roles. PLoS One 12, e0175582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fleischmajer R., Macdonald E.D., Perlish J.S., Burgeson R.E., and Fisher L.W. Dermal collagen fibrils are hybrids of type-I and type-III collagen molecules. J Struct Biol 105, 162, 1990 [DOI] [PubMed] [Google Scholar]
- 167. Liu X., Wu H., Byrne M., Krane S., and Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 94, 1852, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Germain D.P. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis 2, 32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Volk S.W., Wang Y., Mauldin E.A., Liechty K.W., and Adams S.L. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 194, 25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cuttle L., Nataatmadja M., Fraser J.F., Kempf M., Kimble R.M., and Hayes M.T. Collagen in the scarless fetal skin wound: detection with Picrosirius-polarization. Wound Repair Regen 13, 198, 2005 [DOI] [PubMed] [Google Scholar]
- 171. Simkin J., Sammarco M.C., Dawson L.A., et al. Epidermal closure regulates histolysis during mammalian (Mus) digit regeneration. Regeneration 2, 106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sriram G., Bigliardi P.L., and Bigliardi-Qi M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94, 483, 2015 [DOI] [PubMed] [Google Scholar]
- 173. Witherel C.E., Graney P.L., Freytes D.O., Weingarten M.S., and Spiller K.L. Response of human macrophages to wound matrices in vitro. Wound Repair Regen 24, 514, 2016 [DOI] [PubMed] [Google Scholar]
- 174. Hayn E. Successful treatment of complex traumatic and surgical wounds with a foetal bovine dermal matrix. Int Wound J 11, 675, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Martinez F.O., and Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Martinez F.O., Sica A., Mantovani A., and Locati M. Macrophage activation and polarization. Front Biosci 13, 453, 2008 [DOI] [PubMed] [Google Scholar]
- 177. Murray P.J. Macrophage polarization. Annu Rev Physiol 79, 541, 2016 [DOI] [PubMed] [Google Scholar]
- 178. Brown B.N., Sicari B.M., and Badylak S.F. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol 5, e510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Snyder R.J., Lantis J., Kirsner R.S., Shah V., Molyneaux M., and Carter M.J. Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen 24, 613, 2016 [DOI] [PubMed] [Google Scholar]
- 180. Wynn T.A., and Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lech M., and Anders H.J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta 1832, 989, 2013 [DOI] [PubMed] [Google Scholar]
- 182. Fallowfield J.A., Mizuno M., Kendall T.J., et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178, 5288, 2007 [DOI] [PubMed] [Google Scholar]
- 183. Ramachandran P., Pellicoro A., Vernon M.A., et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A 109, E3186–E3195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lurier E.B., Dalton D., Dampier W., et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 222, 847, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ferrante C.J., and Leibovich S.J. Regulation of macrophage polarization and wound healing. Adv Wound Care 1, 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Grinberg S., Hasko G., Wu D., and Leibovich S.J. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am J Pathol 175, 2439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sridharan R., Cameron A.R., Kelly D.J., Kearney C.J., and O'Brien F.J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today 18, 313, 2015 [Google Scholar]
- 188. Blakney A.K., Swartzlander M.D., and Bryant S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res Part A 100A, 1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cha B.H., Shin S.R., Leijten J., et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv Healthc Mater 6, 289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Brown B.N., Ratner B.D., Goodman S.B., Amar S., and Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in and regenerative medicine. Biomaterials 33, 3792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., and Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008 [DOI] [PubMed] [Google Scholar]
- 192. Dziki J.L., Wang D.S., Pineda C., Sicari B.M., Rausch T., and Badylak S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J Biomed Mater Res Part A 105, 138, 2017 [DOI] [PubMed] [Google Scholar]
- 193. He C.M., Yang Z., Jin Y., Qi X.Y., Chu J., and Deng X.Y. ADM scaffolds generate a pro-regenerative microenvironment during full-thickness cutaneous wound healing through M2 macrophage polarization via Lamtor1. Front Physiol 9, 657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Vasconcelos D.P., Fonseca A.C., Costa M., et al. Macrophage polarization following chitosan implantation. Biomaterials 34, 9952, 2013 [DOI] [PubMed] [Google Scholar]
- 195. Vasconcelos D.P., Costa M., Amaral I.F., Barbosa M.A., Aguas A.P., and Barbosa J.N. Development of an immunomodulatory biomaterial: using resolvin D1 to modulate inflammation. Biomaterials 53, 566, 2015 [DOI] [PubMed] [Google Scholar]
- 196. Fearing B.V., and Van Dyke M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater 10, 3136, 2014 [DOI] [PubMed] [Google Scholar]
- 197. Taraballi F., Corradetti B., Minardi S., et al. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J Tissue Eng 7, 2041731415624667, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Corradetti B., Taraballi F., Corbo C., et al. Immune tuning scaffold for the local induction of a pro-regenerative environment. Sci Rep 7, 17030, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Castellano D., Sanchis A., Blanes M., et al. Electrospun poly(hydroxybutyrate) scaffolds promote engraftment of human skin equivalents via macrophage M2 polarization and angiogenesis. J Tissue Eng Regen Med 12, E983–E994, 2018 [DOI] [PubMed] [Google Scholar]
- 200. Prescienta & Strategic intelligence. Scar Treatment Market to Reach $34.9 Billion by 2023. 2018. Available at: https://www.psmarketresearch.com/press-release/global-scar-treatment-market Accessed March20, 2019
- 201. Prescienta & Strategic intelligence. Scar Treatment Market Overview. 2018. Available at: https://www.psmarketresearch.com/market-analysis/scar-treatment-market Accessed March20, 2019
- 202. Monstrey S., Middelkoop E., Vranckx J.J., et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plastic Reconstr Aesthet Surg 67, 1017, 2014 [DOI] [PubMed] [Google Scholar]
- 203. Rowan M.P., Cancio L.C., Elster E.A., et al. Burn wound healing and treatment: review and advancements. Critical Care 19, 243, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bello Y.M., Falabella A.F., and Eaglstein W.H. Tissue-engineered skin. Am J Clin Dermatol 2, 305, 2001 [DOI] [PubMed] [Google Scholar]
- 205. Debels H., Hamdi M., Abberton K., and Morrison W. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plastic Reconstr Surg Glob Open 3, e284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Varkey M., Ding J., and Tredget E.E. Advances in skin substitutes-potential of tissue engineered skin for facilitating anti-fibrotic healing. J Funct Biomater 6, 547, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Chua A.W., Khoo Y.C., Tan B.K., Tan K.C., Foo C.L., and Chong S.J. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 4, 3, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hu M.S., Walmsley G.G., Litzenburger U., et al. ATAC-seq reveals heterogeneity of fibroblasts during transition from scarless fetal to scar-forming adult wound repair. Plastic Reconstr Surg Glob Open 4, 128, 2016 [Google Scholar]
- 209. Mah W., Jiang G., Olver D., et al. Elevated CD26 expression by skin fibroblasts distinguishes a profibrotic phenotype involved in scar formation compared to gingival fibroblasts. Am J Pathol 187, 1717, 2017 [DOI] [PubMed] [Google Scholar]
- 210. Lichtenberger B.M., Mastrogiannaki M., and Watt F.M. Epidermal beta-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun 7, 10537, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Di Carlo S.E., and Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest 128, 54, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wei X., Yang X., Han Z.P., Qu F.F., Shao L., and Shi Y.F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sinica 34, 747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 10, 29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kuroda Y., Kitada M., Wakao S., et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A 107, 8639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Yamauchi T., Yamasaki K., Tsuchiyama K., Koike S., and Aiba S. A quantitative analysis of multilineage-differentiating stress-enduring (MUSE) cells in human adipose tissue and efficacy of melanocytes induction. J Dermatol Sci 86, 198, 2017 [DOI] [PubMed] [Google Scholar]
- 216. Yamauchi T., Yamasaki K., Tsuchiyama K., Koike S., and Aiba S. The potential of muse cells for regenerative medicine of skin: procedures to reconstitute skin with MUSE cell-derived keratinocytes, fibroblasts, and melanocytes. J Invest Dermatol 137, 2639, 2017 [DOI] [PubMed] [Google Scholar]
- 217. Moore A.L., Marshall C.D., and Longaker M.T. Minimizing skin scarring through biomaterial design. J Funct Biomater 8, 3, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Mustoe T.A. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg 32, 82, 2008 [DOI] [PubMed] [Google Scholar]
- 219. Longaker M.T., Rohrich R.J., Greenberg L., et al. A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg 134, 536, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]