Abstract
Chronic inflammation-associated bone diseases involve continuous destruction and impaired regeneration of bone. Mesenchymal stem cell (MSC)-based therapy has great potential to modulate inflammatory responses and enhance tissue regeneration. We previously showed that lipopolysaccharide (LPS) plus tumor necrosis factor alpha (TNFα)-preconditioned MSCs or genetically modified inflammation-sensing (driven by nuclear factor kappa-light-chain-enhancer of activated B cells [NFκB] activation) IL4-secreting MSCs enhanced immunomodulation of macrophages to the more desired tissue repaired M2 type. In the current study, the paracrine regulation of therapeutic MSCs on the proinflammatory response and osteogenesis of macrophage–MSC cocultures (representing endogenous cells) was examined using an in vitro transwell system. In the cocultures, IL4-secreting MSCs decreased TNFα and inducible nitric oxide synthase expression, and increased Arginase 1 and CD206 expression in the presence of LPS-contaminated polyethylene particles. The preconditioned MSCs decreased TNFα and CD206 expression in the bottom MSC–macrophage cocultures in the presence of contaminated particles. In osteogenesis assays, IL4-secreting MSCs decreased alkaline phosphatase (ALP) expression, but increased Alizarin Red staining in the presence of contaminated particles. The preconditioned MSCs increased ALP and osteocalcin expression, and had no significant effect on Alizarin Red staining. These results suggest that potential treatments using preconditioned MSCs at an earlier stage, or IL4-secreting MSCs at a later stage could enhance bone regeneration in inflammatory conditions, including periprosthetic osteolysis.
Impact Statement
Pathogen-associated molecular patterns, damage-associated molecular patterns, and other noxious stimuli activate macrophages to induce the proinflammatory responses. Modulation of inflammatory macrophages (M1) into an anti-inflammatory tissue repair macrophage (M2) phenotype at the appropriate time optimizes bone remodeling and regeneration. Simulating the proinflammatory stimuli by using preconditioned mesenchymal stem cells (MSCs) at an earlier stage, and alleviate the inflammation by using IL4-secreting MSCs at a later stage could further optimize bone regeneration in chronic inflammatory conditions, including periprosthetic osteolysis.
Keywords: mesenchymal stem cells, IL4, preconditioning, macrophage, osteogenesis, wear particles
Introduction
Wear particles generated from implanted devices, pathogen-associated molecular patterns, and damage-associated molecular patterns induce chronic inflammation and consequent periprosthetic osteolysis.1 Macrophages are the key cells mediating inflammation-associated tissue damage and osteoclast activation during disease progression.2 Modulation of inflammatory macrophages (M1) into an anti-inflammatory tissue repair macrophage (M2) phenotype at the appropriate time optimizes bone remodeling and regeneration.3
Mesenchymal stem cell (MSC)-based therapy has great potential for immunomodulation and tissue regeneration.4 Crosstalk between MSCs and macrophages are critical for successful bone remodeling.3,5,6 Acute transient inflammation promotes osteogenesis by MSCs and osteoprogenitors.5,7 Delayed treatment using IL4, which polarizes M1 macrophages into an M2 phenotype, further enhanced bone formation.5,8 To further enhance bone formation in the MSC-based therapy, we previously showed that preconditioned MSCs (using lipopolysaccharide [LPS] plus tumor necrosis factor alpha [TNFα])9 or genetically modified inflammation-sensing (driven by nuclear factor kappa-light-chain-enhancer of activated B cells [NFκB] activation) IL4-secreting MSCs10 enhanced immunomodulation of macrophages to the more desired M2 type. These immunomodulatory effects in response to wear particles were recently characterized using an in vitro acute phase model.11 However, the immunomodulation potential of these interventions during prolonged exposure to wear particles (chronic phase) and the consequent effects on the osteogenesis have not been characterized.
In the current study, we have established a transwell coculture model to mimic the crosstalk between endogenous MSCs and macrophages, and how the exogenous therapeutic MSCs affect osteogenic differentiation. Thus, we could distinguish the osteogenic changes in the transplanted MSCs versus endogenous MSCs. The effect of these novel MSC-based therapies on osteogenic differentiation was examined in an in vitro macrophage–MSC transwell coculture system with sterile or LPS-contaminated ultra-high-molecular-weight polyethylene (UHMWPE) wear particles.
Materials and Methods
Cells
The methods of isolating and characterizing murine bone marrow-derived MSCs and macrophages (male, Balb/c) have been described previously.12 Stanford's Administrative Panel on Laboratory Animal Care (APLAC) approved this isolation protocol (APLAC 17566) and Institutional Guidelines for the Care and Use of Laboratory Animals were observed in all aspects of this project. MSCs were cultured in Alpha-Minimum Essential Medium (α-MEM) supplied with 10% MSC-certified fetal bovine serum (FBS; Invitrogen) and antibiotic–antimycotic solution (100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B per milliliter; Hyclone, Thermo Scientific). The cells between passage 4 to 8 were used in the experiment. Macrophages were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, antibiotic/antimycotic solution, 30% of L929 cell-conditioned medium, and 10 ng/mL mouse macrophage colony stimulation factor (M-CSF; R&D Systems).
UHMWPE particles
Ceridust 3610 PE particles were filtered and sterilized as previously described.11 The sterility was confirmed by the endpoint chromogenic Limulus Amebocyte Lysate assay (Lonza, Portsmouth, NH). The particle size (4.62 ± 3.76 μm) was examined by an electron microscope in the Cell Science Image Facility at Stanford University. The particles were resuspended in phosphate-buffered saline (PBS) containing 5% bovine serum albumin with (contaminated) or without (sterile, data not shown) 10 ng/mL LPS. We focused on the results using contaminated particles since the sterile larger PE particles used in the current study induced marginal inflammatory response.11
MSC–macrophage coculture system
The preconditioned (20 ng/mL TNFα and 20 μg/mL LPS) or genetically modified MSCs were generated as previously described.9–11 The preconditioned MSCs were washed by PBS three times before seeded into the transwell plate. The MSC–macrophage coculture system is illustrated in Figure 1. Primary macrophages (5 × 104) were seeded in the bottom well of the transwell plate (0.4 μm polycarbonate membrane; Corning). Unmodified MSCs (1 × 104) were seeded 2 h later at the bottom well and incubated overnight. The ratio of cocultured macrophages and MSCs was determined based on our previous study.13 The upper chambers were seeded with unmodified MSCs, preconditioned MSCs, NFκB-IL4-secreting MSCs (2 × 104), or a control group without MSCs. The medium was replaced 2 h later by osteogenic medium (α-MEM [Thermo Scientific] supplemented with 10% FBS, 100 nM dexamethasone, 10 mM β-glycerol phosphate, and 50 μM ascorbate-2-phosphate; Sigma) containing 0.125% sterile, LPS-contaminated polyethylene particles, or vehicle control (no particles). Macrophage polarization status (M1: TNFα and inducible nitric oxide synthase [iNOS]; M2: Arginase 1 and CD206) was examined by quantitative polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), and immunofluorescence staining at day 1, 3, and 7 of coculture. Osteogenesis was examined by quantitative PCR (alkaline phosphatase [ALP]) at day 7 and Alizarin Red staining at week 3. Viable cell number of macrophage–MSC cocultures was analyzed by PicoGreen cell quantification assay.
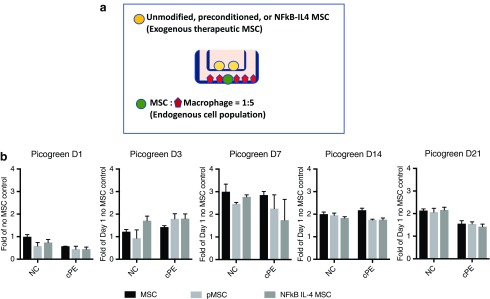
FIG. 1.
Illustration of MSC–macrophage coculture system. (a) MSCs (green circle) and macrophages (red pentagon) were cocultured with direct cell contact at the bottom chamber (represent endogenous cells) at a 1:5 ratio. The control, preconditioned, or IL4-secreting MSCs (yellow circle) were put on the upper chamber (represent therapeutic exogenous cells) and have the crosstalk with the bottom chamber through paracrine regulation. (b) Cell viability at bottom chamber at day 1, 3, 7, 14, and 21 were quantified by PicoGreen assay. NC: no particle control; cPE: contaminated polyethylene particles. MSCS, mesenchymal stem cells. Color images are available online.
RNA extraction and quantitative PCR
Cellular RNAs were extracted by using the RNeasy RNA Purification Kit (Qiagen, Valencia, CA). RNAs were reverse transcribed into complementary DNA (cDNA) using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Probes for 18s rRNA, TNF-α, iNOS, Arginase 1, CD206, Runt-related transcription factor 2 (Runx2), ALP, and osteocalcin were purchased from Applied Biosystems (Foster City, CA). Reverse transcriptase PCR was performed in an ABI 7900HT Sequencing Detection System (Applied Biosystems, Waltham, MA), using the 18s rRNA as the internal control. The −ΔΔCt relative quantitation method was used to evaluate the gene expression level.
Enzyme-linked immunosorbent assay
ELISA Kits for IL4, TNF, and PGE2 DuoSet ELISA Kits were purchased from R&D Systems. The manufacturer's protocols were followed carefully. The optical densities were determined using a Bio-Rad 3550-UV microplate reader (Bio-Rad, Hercules, CA) set at 450 nm.
Immunofluorescent staining
Macrophages marker F4/80 (1 μg/mL, Alexa Fluor 647-conjugated, monoclonal rat anti-mouse IgG2b; Bio-Rad) and their polarization markers, including CD206 (1 μg/mL, allophycocyanin-conjugated, monoclonal rat anti-mouse IgG2a κ; BioLegend, San Diego, CA), Arginase 1 (5 μg/mL, unconjugated, polyclonal rabbit anti-mouse IgG; Abcam, Cambridge, United Kingdom) were stained as previously described.11 The images were captured using a fluorescence microscope (Axio Observer 3.1; Zeiss, Oberkochen, Germany) in three randomly selected fields of view, and the signals of each marker were quantified by using NIH ImageJ.
Statistical analysis
Unpaired t-tests were performed for data with two groups, and a one-way analysis of variance (ANOVA) with Tukey's post hoc test was performed for data with three or more groups. The statistical analysis was conducted using Prism 7 (GraphPad Software, San Diego, CA). Data are reported as mean ± standard deviations. p < 0.05 was chosen as the threshold of statistical significance.
Results
In vitro therapeutic model in a MSC–macrophage coculture system
The MSC–macrophage transwell coculture system (Fig. 1a) was used (1) to examine how contaminated UHMWPE particles affect osteogenesis in the cocultured MSCs–macrophages (bottom chamber, represent endogenous cells); and (2) to evaluate the therapeutic intervention of preconditioned or IL4-secreting MSCs (upper chamber, represent exogenous implanted cells). The cell numbers of cocultured MSCs–macrophages at the bottom chamber showed a peak at day 7 and gradually decreased afterward (Fig. 1b). No significant effects of therapeutic MSCs (upper chamber) on the MSC–macrophage viability was observed.
Immunomodulation of the inflammatory response in MSC–macrophage cocultures by the preconditioned or IL4-secreting MSCs
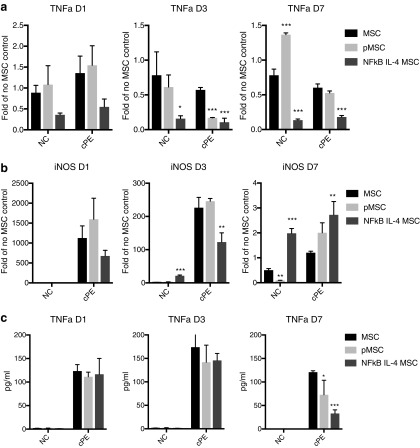
In the presence of contaminated particles, IL4-secreting MSCs decreased TNFα transcription (after day 3, Fig. 2a and Supplementary Fig. S1a) and showed distinct effects on iNOS transcription at different time points of MSC–macrophage cocultures (Fig. 2b). IL4-secreting MSCs suppressed TNFα secretion induced by contaminated particles at day 7 (Fig. 2c). The preconditioned MSCs transiently decreased TNFα transcription at day 3 (Fig. 2a) and protein secretions at day 7 when exposed to the contaminated particles (Fig. 2c).
FIG. 2.
Expression of proinflammatory M1 markers in the MSC–macrophage cocultured cells. The RNA expression of TNFα (a) and iNOS (b) at day 1, 3, and 7 in the MSC–macrophage at the bottom chamber were examined by quantitative PCR. (c) TNFα secretions at day 1, 3, and 7 were examined by ELISA. NC: no particle control; cPE: contaminated polyethylene particles. *p < 0.05; **p < 0.01; ***p < 0.005. TNFα, tumor necrosis factor alpha; iNOS, inducible nitrix oxide synthase; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.
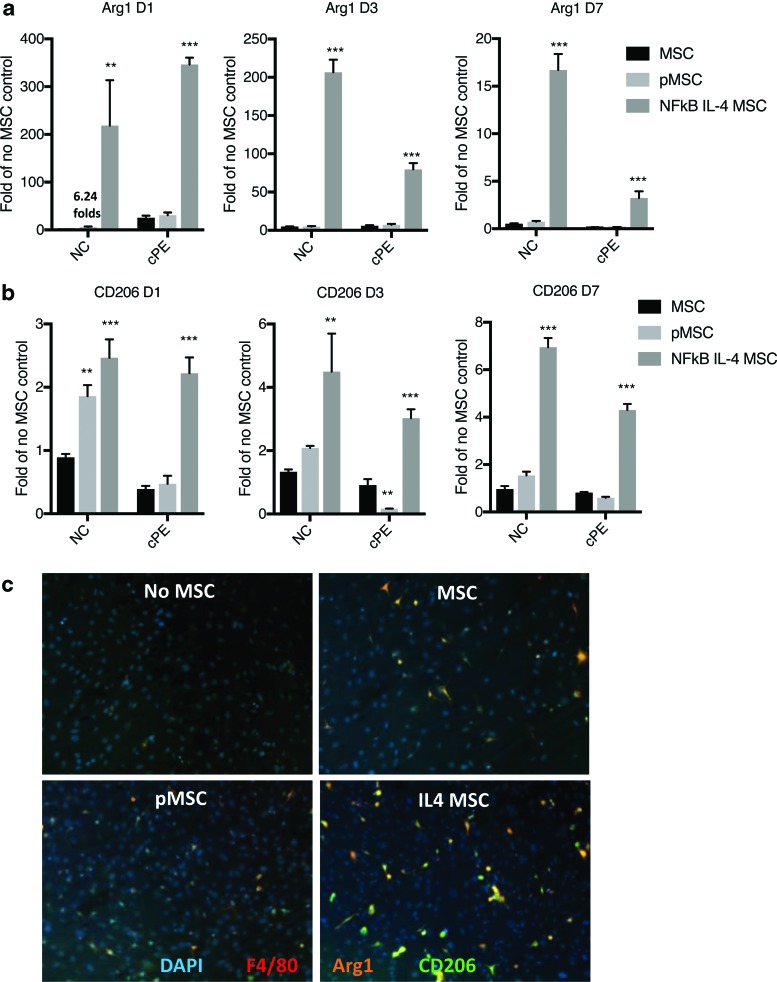
In the anti-inflammatory (M2) marker expression, IL4-secreting MSCs increased Arginase 1 and CD206 transcription regardless of particle exposure (Fig. 3a, b and Supplementary Fig. S1b). The preconditioned MSCs increased CD206 transcription in the absence of particles on day 1 (Fig. 3b). The increased Arginase 1 transcription at the same time point was negligible compared with the group of IL4-secreting MSCs (Fig. 3a). Protein expression of Arginase1 and CD206 was further examined by immunofluorescent staining (Fig. 3c).
FIG. 3.
Expression of anti-inflammatory M2 markers in the MSC–macrophage cocultured cells. The RNA expression of Arginase 1 (Arg1) (a) and CD206 (b) at day 1, 3, and 7 in the MSC–macrophage at bottom chamber were examined by quantitative PCR. (c) The bottom cells were stained by DAPI (nucleus staining), F4/80 (macrophage), Arg1, and CD206. NC: no particle control; cPE: contaminated polyethylene particles. **p < 0.01; ***p < 0.005. Color images are available online.
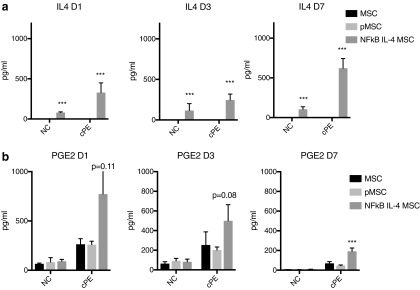
We also examined IL4 and PGE2 secretion in the coculture system to clarify how MSCs on the upper chamber affected the MSC–macrophage cocultures (Fig. 4). IL4 secretion was only found in the IL4-secreting MSC groups and was further increased in the presence of contaminated particles (Fig. 4a and Supplementary Fig. S1c). Exposure of MSC–macrophage cocultures to the contaminated particles increased PGE2 production. IL4-secreting MSCs further increased PGE2 production in the presence of contaminated particles at day 7 and 14 of cocultures (Fig. 4b and Supplementary Fig. S1c). Preconditioned MSCs showed no significant effect on PGE2 production.
FIG. 4.
Secretion of immunomodulatory factors by the preconditioned or IL4-secreting MSCs. (a) Secretion of IL4 by the genetically modified MSCs in the coculture system. (b) Generation of PGE2 in the cocultured system. NC: no particle control; cPE: contaminated polyethylene particles.***p < 0.005.
Modulation of osteogenesis of MSC–macrophage cocultured cells by the preconditioned or IL4-secreting MSCs
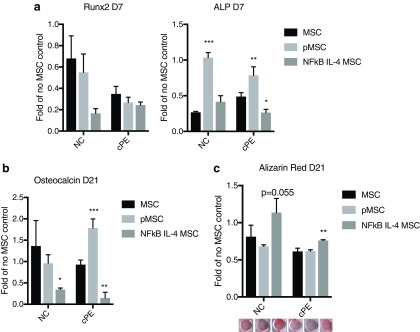
In osteogenesis assays, IL4-secreting MSCs decreased ALP expression (day 7) in the presence of contaminated particles (Fig. 5a). The preconditioned MSCs increased ALP expression (Fig. 5a) in both the absence or presence of contaminated particles. No significant change in Runx2 expression was observed. At the later time point of osteogenesis (day 21), the preconditioned MSCs increased osteocalcin expression (Fig. 5b), and IL4-secreting MSC increased Alizarin Red staining when exposed to the contaminated particles (Fig. 5c).
FIG. 5.
Osteogenic differentiation at earlier and later stage in the MSC–macrophage cocultured cells. (a) RNA expression of early osteogenic marker (Runx2 & ALP at day 7) in the MSC–macrophage at the bottom chamber were examined by quantitative PCR. The osteogenesis at a later stage (day 21) was examined by osteocalcin expression (b) and Alizarin Red staining (c). NC: no particle control; cPE: contaminated polyethylene particles. *p < 0.05; **p < 0.01; ***p < 0.005. ALP, alkaline phosphatase; Runx2, Runt-related transcription factor 2. Color images are available online.
Discussion
Genetically altered and preconditioned MSCs have the potential for enhanced immunomodulation in different inflammatory scenarios. IL4-secreting MSCs showed stronger immunomodulation compared with the preconditioned MSCs and control MSCs. IL4-secreting MSCs and preconditioned MSCs enhanced osteogenesis on MSC–macrophage cocultures at different stages. Compared with our previous study using the acute inflammatory macrophages,11 the current model examined the paracrine regulation of therapeutic MSCs on MSC–macrophage cocultures under chronic inflammatory conditions.
We previously reported that primary macrophages only live for ∼1 week in the coculture model, and the life-span was even shorter for M1 macrophages.5 This phenomenon was confirmed by reduced cell viability and lower macrophage marker expressions with prolonged exposure to the contaminated particles. Nevertheless, macrophages could still affect osteogenesis despite their short-living nature in the cocultures.5,6
IL4-secreting MSCs demonstrated a stronger immunomodulating ability compared with the preconditioned or control MSCs. Compared with our previous study,11 the current findings demonstrate that IL4-secreting MSCs continuously produced IL4 in response to contaminated particles, and suppressed proinflammatory reaction at chronic phase over a 3-week period (Supplementary Fig. S1). IL4-secreting MSCs suppressed TNFα transcription but had no significant effect on protein expression in the previous acute phase model (3 days).11 In this study, we confirmed that IL4-secreting MSCs suppressed TNFα secretion 7 days after coculture (Fig. 2), which could sustain the acute inflammatory signals required for optimal bone regeneration at an earlier phase.
PGE2 is both a mediator of the inflammatory response and has an essential role in resolving inflammation.14 PGE2 is secreted from many cell types, including macrophages and MSCs.15,16 A previous study showed that LPS induced PGE2 secretion in macrophage–MSC cocultures, and is essential for MSC-mediated immunomodulation and M2 macrophage transition.15 Alternatively, IL4-treated macrophages have reduced PGE2 production due to inhibition of COX2 expression.17,18 Our data demonstrated that PGE2 secretion was induced in macrophage–MSC cocultures exposed to contaminated particles. Interestingly, IL4-secreting MSCs further increased PGE2 production in the cocultures (Fig. 4). Our data did not clarify whether macrophages, MSCs, or both, contributed to the increased PGE2 production.
MSCs have multilineage differentiation abilities in vitro. Transplantation of MSC enhances tissue regeneration, including bone and cartilage in translational models.19,20 However, the mechanism of MSC-based cell therapy, either through direct differentiation of transplanted MSC or indirect paracrine regulation on endogenous cells, remains debatable. Osteogenic differentiation in single cultured IL4-secreting MSC was reduced in the absence of macrophages.10 In the current study, IL4-secreting MSCs increased the osteogenic differentiation at a later stage in MSC–macrophage cocultures exposed to the contaminated particles, suggesting that crosstalk between macrophages and MSCs results in paracrine cues that alter bone formation. The early osteogenic marker ALP was reduced in the IL4-secreting MSC cocultured cells, indicating that the IL4-mediated suppressive effect existed at an earlier stage. Sequential polarization of proinflammatory M1 macrophages into anti-inflammatory M2 types optimized osteogenesis in the coculture model.5,8 Our data showed that IL4-secreting MSCs suppressed TNFα secretion at day 7. The results suggest that enhanced osteogenesis at a later stage could be associated with the M1 to M2 macrophage transition during the coculture.
Induction of PGE2 is associated with increased osteogenesis in preconditioned MSCs.9 The absence of PGE2 induction in the current coculture model (Fig. 4) may be due to continuous exposure to LPS, or the crosstalk between macrophages and MSCs with direct contact generated a comparable amount of PGE2 to the preconditioned MSCs. The preconditioned MSCs provided paracrine signals to increase early osteogenic marker expression in the macrophage–MSC cocultures, which was impaired in the IL4-secreting MSC groups (Fig. 5). Thus, it may be intriguing to examine a combined strategy of preconditioning and genetically modified MSCs. The therapeutic effects of the preconditioned MSCs, IL4-secreting MSCs, or combined strategies at different stages will be further evaluated in translational in vivo models.
In conclusion, potential treatments using preconditioned MSCs at an earlier stage, and IL4-secreting MSCs at a later stage could further optimize bone regeneration in inflammatory conditions, including periprosthetic osteolysis.
Supplementary Material
Acknowledgments
This work was supported by NIH grant 2R01 AR055650 and 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko foundation.
Disclosure Statement
The authors have no conflict of interest to declare.
Supplementary Material
References
- 1. Goodman S.B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 28, 5044, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ingham E., and Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 26, 1271, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Loi F., Cordova L.A., Pajarinen J., Lin T.H., Yao Z., and Goodman S.B. Inflammation, fracture and bone repair. Bone 86, 119, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Squillaro T., Peluso G., and Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25, 829, 2016 [DOI] [PubMed] [Google Scholar]
- 5. Loi F., Cordova L.A., Zhang R., et al. . The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther 7, 15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu L.Y., Loi F., Nathan K., et al. . Pro-inflammatory M1 macrophages promote osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res 35, 2378, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vi L., Baht G.S., Whetstone H., et al. . Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 30, 1090, 2015 [DOI] [PubMed] [Google Scholar]
- 8. Nathan K., Lu L., Pajarinen J., et al. . Temporal modulation of macrophage polarization enhances osteogenesis in primary mesenchymal stem cells. ORS Conference Abstract 0765, 2017 [Google Scholar]
- 9. Lin T., Pajarinen J., Nabeshima A., et al. . Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res Ther 8, 277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin T., Pajarinen J., Nabeshima A., et al. . Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 19, 1025, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin T., Kohno Y., Huang J.F., et al. . NFkappaB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles. J Biomed Mater Res A 106, 2744, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin T.H., Sato T., Barcay K.R., et al. . NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng Part A 21, 875, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu L.Y., Loi F., Nathan K., et al. . Pro-inflammatory M1 macrophages promote osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res 35, 2378, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ricciotti E., and FitzGerald G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31, 986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nemeth K., Leelahavanichkul A., Yuen P.S., et al. . Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15, 42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ylostalo J.H., Bartosh T.J., Coble K., and Prockop D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30, 2283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart P.H., Cooper R.L., and Finlay-Jones J.J. IL-4 suppresses IL-1 beta, TNF-alpha and PGE2 production by human peritoneal macrophages. Immunology 72, 344, 1991 [PMC free article] [PubMed] [Google Scholar]
- 18. Shay A.E., Diwakar B.T., Guan B.J., Narayan V., Urban J.F., Jr, and Prabhu K.S. IL-4 up-regulates cyclooxygenase-1 expression in macrophages. J Biol Chem 292, 14544, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knight M.N., and Hankenson K.D. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle) 2, 306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahlin R.L., Kinard L.A., Lam J., et al. . Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials 35, 7460, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.