Abstract
Rotating wall vessel (RWV) bioreactors have been used to produce cell spheroids and organoids at a faster rate than in other bioreactor devices and with higher structural and functional fidelity. One of the limitations of traditional RWV systems is the well-documented tendency for air bubble formation during operation. The presence of these bubbles negates key features of the RWV environment, such as zero headspace, low-shear, and simulated microgravity. In this article, we describe the design, construction, and testing of a novel RWV capable of constantly removing air bubbles from the system without interfering with the fluid dynamics that produce optimized cell culture conditions. We modeled this capacity using computational fluid dynamics and then validated the model with alginate beads and spheroid cultures of A549 human lung adenocarcinoma cells. The areas of spheroids assembled from A549 cells in the novel bioreactor in the presence of air bubbles were an order of magnitude larger than in conventional bioreactors when bubbles were present. Our results demonstrate the ability of the novel design to remove and isolate bubbles while avoiding damage to spheroid assembly, as observed in conventional RWV bioreactors in the presence of bubbles. We anticipate that the novel design will increase experimental reproducibility and consistency when using RWV bioreactors.
Impact Statement
The rotating wall vessel (RWV) bioreactor is a powerful tool for the generation of sizeable, faster-growing organoids. However, the ideal, low-shear, modeled microgravity environment in the RWV is frequently disrupted by the formation of bubbles, a critical but understated failure mode. To address this, we have designed and fabricated a novel, modified RWV bioreactor capable of continuously removing bubbles while providing optimal fluid dynamics. We validated the capacity of this device with computational and empirical studies. We anticipate that our novel bioreactor will be more consistent and easier to use and may fill a unique and unmet niche in the burgeoning field of organoids.
Keywords: air bubble, computational fluid dynamics (CFD), high aspect ratio vessel (HARV), organoid, rotating wall vessel (RWV), modeled microgravity
Introduction
Organoids and spheroids in rotating wall vessel bioreactors
Two-dimensional (2D) cell culture, the gold standard of in vitro biology for nearly a century, has been used in virtually every major discovery in cell biology. However, increasing understanding of the complexity of biological systems has led to heightened awareness of the importance of model systems that more closely replicate the true three-dimensional (3D) environment of living tissues.1 While extensive progress has been made in engineered 3D scaffolds, organ recellularization, and bioprinting, recent studies demonstrate the unique potential of self-assembling spheroids and organoids for discoveries in disease modeling, drug discovery, and a host of other biomedical applications.2
Although several protocols exist for the generation and maintenance of spheroids and organoids, such as spontaneous self-assembly in nonadherent cell culture plates3 or the hanging drop method,4 culturing cells in the rotating wall vessel (RWV) bioreactor, with its improved nutrient exchange, yields consistent formation of organoids with superior morphology and unique, organotypic gene expression.5–11 The RWV bioreactor works by slowly rotating a liquid-filled cylinder on its axis, gently dragging the fluid, and any particles in it, in perfect circular paths. While alternate systems, like stirred-tank bioreactors, are also being used and optimized for organoid generation with high mass transfer,12 the RWV, with its lack of impellors or agitators, produces a lower shear, lower turbulence environment, reducing potential damage to forming tissues.13 This unique environment continues to provide high mass transfer associated with increased metabolic activity,14 altered differentiation with increased nutrient availability,15 modulation of pluripotency markers such as Oct4 linked to glycolytic enzymes such as pyruvate kinases,16 and differentiation associated with higher oxygen concentration17 both with pluripotent and multipotent cells mediated through hypoxia-inducible factor,18–21 In conjunction with high mass transfer, the low-shear environment of the RWV can improve culture condition of particular tissue types by eliminating detrimental shear effects, such as those driving cells toward a hematopoietic fate,22 inducing caspase-mediated apoptosis in some cell types,23 damaging delicate organoid substructures such as photoreceptor cilia,24 affecting cell metabolism,25 or simply failing to support the critical, low-shear biophysical environment required for certain types of differentiation.26 Recently, DiStefano et al. demonstrated that the tissue-like assembly of murine retinal organoids cultured into a tissue-like assembly with properly organized photoreceptor cells occurred significantly faster and the nascent organoids were significantly larger than in static cultures. Faster generation of larger, high-fidelity organoids in the RWV may facilitate the dissemination of these organoids for high-throughput tissue analysis in drug discovery and personalized medicine.27,28
Bubble formation in the RWV bioreactor and its impact
Initially developed by NASA in the 1980s, RWVs were originally designed to safely shuttle cells into space as scientists endeavored to discover the biological effects of microgravity and space radiation. However, it was quickly discovered that the RWV was also capable of supporting the formation of complex, 3D, tissue-like structures of high fidelity and relevance to biomedical research on the ground.29
Given that the low-shear, microgravity-simulating effect of the RWV relies on a circular, solid-body fluid path along its interior perimeter, the RWV requires a “zero headspace” condition, literally a continuous interface between the fluid and the wall with no air gap.29,30 When this interface is disrupted due to the presence of a bubble, buoyancy continually pushes the bubble to the highest point of the system despite the rotation of the device. The presence of a bubble at this locale interrupts the fluid path, adding turbulence and fluid shear stress, as the fluid is deflected around the bubble. Bubbles are easily formed because each RWV bioreactor has a gas exchange system for cellular respiration and for balancing the cell culture media pH with incubator CO2. When the incubator environment has insufficiently high humidity (below 100%), negative pressure can form as water vapor diffuses away from the bioreactor, inevitably causing dissolved gases to precipitate out, forming unwanted bubbles. The need to remove bubbles from the RWV system before and/or during use is widely discussed in literature,7,10,30–36 including a description of the issue in RWV bioreactors in low-Earth orbit.37 While the significance of failing to remove bubbles or an analysis of the change in shear due to the presence of a bubble is not widely discussed, it has been reported on occasion that bubble formation in the RWV may disrupt cell aggregation, decrease cell viability, and harm differentiation, subsequently harming organoid formation.7,35,38,39 Similarly, the literature is scant on providing a consistent way of preventing bubble formation.
Some authors suggest a sufficiently high humidity incubator (several water pans used simultaneously) will prevent bubble formation.30 However, with the need to open the incubator for media changes or removal of samples at different time points, this becomes difficult to maintain in practice, resulting in the frequent formation of small bubbles within 24–48 h, which in turn can act as nucleation points for large bubble growth. Over longer time points, such as those required for organoid differentiation, bubble formation is inevitable. Nascent bubbles formed during extended culture can be removed manually by “alternating between driving plungers” of media-filled syringes.10 However, even short-term exposure to bubble-associated shear stress may be detrimental; genetic expression can be influenced by only minutes of exposure to elevated shear stress.40 With the increasing use of RWV bioreactor technology for organoid research, there is a need for an improved system capable of reproducibly neutralizing the impact of incidental bubbles by automatically removing them as they are formed.
In this study, we designed and implemented a modification to the high aspect ratio vessel (HARV)-type RWV, a low-volume version of the RWV, which resulted in the effective capture and removal of bubbles. Comparison between the modified and standard bioreactors showed similarities in both fluid dynamics and in the generation of organoids under ideal, nonbubble conditions. While the formation of spheroids/organoids was impaired in conventional RWVs in the presence of a bubble, it was fully maintained in the modified design.
Materials and Methods
Bioreactor design, modeling, and computational fluid dynamics
Potential bubble-segregating designs were drawn in 2D in AutoCAD 2018 software (Autodesk, Mill Valley, CA). The final design opted for a main body, similar to that of existing RWV bioreactors, with an additional thin, exterior, concentric channel running along ∼80% of the total perimeter and connected to the main body by a thin entrance. Conceptually, our design relies on buoyancy to continuously direct air bubbles out of the main volume into the channel. Additionally, a port added to the end of the channel aides in filling and emptying and can be used to remove bubbles during operation, ensuring long-term bubble-free culture. The volumes and dimensions of the main body were based on existing disposable 10 mL HARV models (Synthecon, Inc., Houston, TX), whereas the channel was given an additional volume of ∼3 mL.
Computational fluid dynamics (CFD) was employed to numerically evaluate the fluid dynamics of the new design. The “Fluent” software package (ANSYS 19.2; Ansys, Inc., Canonsburg, PA) was used to model fluid behavior in both the novel design and the existing 10 mL HARV-type RWV in a 3D environment with and without an added water vapor bubble. Unless noted otherwise, added bubbles were of a radius of 2 mm for a volume of ∼330 μL. Additional details are described in Supplementary Figure S1.
Bioreactor fabrication
The bubble-capturing bioreactors (BCBs) were designed to be leak proof, noncytotoxic, sterilizable, and gas permeable for cellular respiration/pH control. Additionally, separate “control” RWVs using identical materials were constructed emulating the existing HARV-type RWV bioreactors. The two designs are referred to as “novel” or “BCB” and “standard” or “HARV-type,” respectively (Fig. 1A, D show their finished construction respectively).
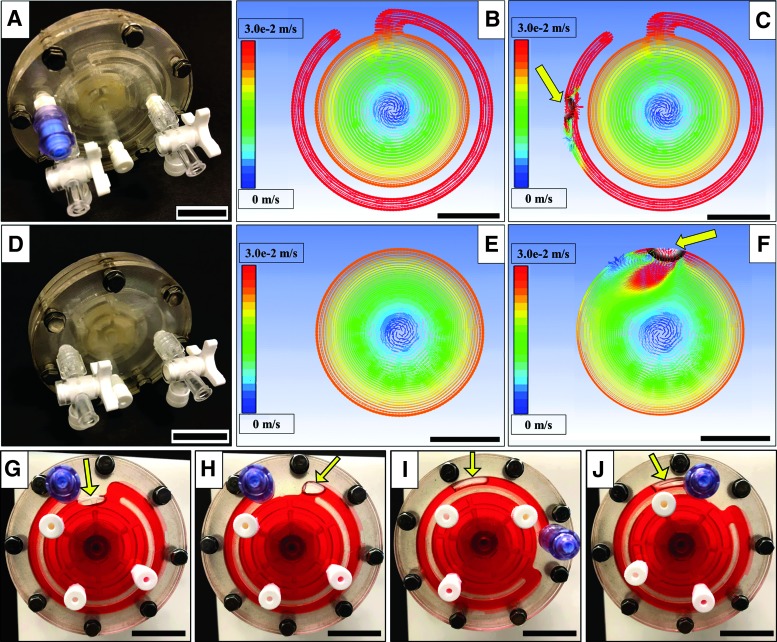
FIG. 1.
Fabrication and Modeling of the Novel and Standard Bioreactor. (A) A fully assembled novel bioreactor. (B, C) A front-viewed 3D model of stable fluid velocity vectors in a novel design bioreactor without bubbles and with, respectively. The bubble highlighted by the arrow is trapped in the channel without disrupting the velocity gradient in the main chamber. (D) A fully assembled standard bioreactor. (E, F) A front-viewed 3D model of stable fluid velocity vectors in a standard design bioreactor without bubbles and with, respectively. Note the significant increase in fluid velocity (exceeding the velocity scale by ∼10-fold, see text) after encountering the bubble (arrow). (G–J) Photographs of the movement of an actual bubble (arrow) in the novel design highlighted by colored liquid. (G, H) Bubble entering the channel. (I, J) A bubble moving through the channel to the back wall. All scale bars are 2.5 cm. 3D, three-dimensional. Color images are available online.
All systems were primarily constructed from 3/16′′ cast acrylic ACRYLITE® (Evonik Industries, Essen, Germany) and cut on a VLS 6.60 laser cutting table (Universal Laser Systems, Scottsdale, AZ). A gas-permeable membrane was produced by laminating a sheet of Tegaderm® (3M, Maplewood, MN), a known watertight, gas-permeable polymer, onto a flexible nylon mesh with a 300 μm pore size (McMaster-Carr, Elmhurst, IL) and tightly compressing under a vice overnight to ensure bonding.41 The nylon mesh-backed Tegaderm was then laser cut to match the size and bolt holes of the acrylic pieces. Finally, a 0.5 mm-thick heat-resistant silicone sheet (Laimeisi Silicone Co., Ltd., Shenzhen, China) was laser cut to match the shape of the central acrylic ring and to act as a compressible leak-resistant gasket. Stopcocks and Luer closures (McMaster-Carr) were added to enable filling. Additional information on the bioreactor fabrication can be found in Supplementary Figure S2.
Alginate bead fluid dynamics validation
To validate the predicted motion of spheroids in the bioreactors, we used low-density calcium alginate beads (∼2 mm in diameter). Approximately two dozen beads were loaded into both types of bioreactors with and without added air bubbles (∼500 μL) to replicate ideal and failure conditions and rotated at 25 rpm, a speed required for larger aggregates,42 until each system had achieved stable fluid dynamics, as inferred from reduced motion relative to any fixed position on the device. Motion and velocities of the beads at the exterior perimeter of the systems were captured, as previously described,7 using a tripod-mounted GoPro camera (GoPro, Inc., San Mateo, CA) at a rate of 60 frames per second. The captured images were subsequently analyzed using ImageJ43 Manual Tracking plugin. Particle velocity relative to bubbles and fixed locations on the bioreactor was calculated iteratively over the course of 1 s. Shear in the absence of bubbles was calculated according to Ramirez et al.,44 describing fluid shear on small particles in an RWV bioreactor, as shown in Equation 1,
 |
where 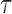 is fluid shear stress,
is fluid shear stress, 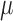 is the dynamic viscosity of water a 25°C, VF is the calculated velocity of the water at the particle's radial position if the fluid was acting as a solid body, VP is the observed velocity of the particle, and a is the radius of the particle.44 Because the VF where the fluid encounters the bubble cannot be calculated, the traditional fluid shear formula, Equation 2, was used when a particle interacted with a bubble,
is the dynamic viscosity of water a 25°C, VF is the calculated velocity of the water at the particle's radial position if the fluid was acting as a solid body, VP is the observed velocity of the particle, and a is the radius of the particle.44 Because the VF where the fluid encounters the bubble cannot be calculated, the traditional fluid shear formula, Equation 2, was used when a particle interacted with a bubble,
 |
where 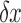 is the change in distance (velocity) and
is the change in distance (velocity) and 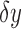 is the difference in location.44 Thus,
is the difference in location.44 Thus, 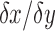 is the velocity gradient developed in the fluid between the wall and the particle. This was repeated for a dose-dependent evaluation of shear and circular fit45 for bubbles ranging from 0 to 500 μL in the standard bioreactor.
is the velocity gradient developed in the fluid between the wall and the particle. This was repeated for a dose-dependent evaluation of shear and circular fit45 for bubbles ranging from 0 to 500 μL in the standard bioreactor.
In line with the example of Ramirez et al., we used Equations 1 and 2, fully cognizant that these are substantial simplifications of the more complex 3D Navier–Stokes equation, the governing equation of motion in fluids.46–48 All shear values determined from the above equations are reported in dynes/cm2.
Cell culture for morphological assessment
For cell culture, a new sterilization and conditioning protocol based on an existing HARV protocol was developed and tested.7 This modified protocol is described further in the Supplementary Data. To assess the morphology of the cell-based organoids/spheroids generated in the RWV under ideal conditions, we paradigmatically used A549 human lung cancer cells (ATCC, Manassas, VA) that have been described extensively in tumor spheroid publications.49–51 Three milliliters of complete Dulbecco's Modified Eagle Medium (DMEM) were added to each bioreactor, followed by 1 mL of cell suspension at 1 × 106 cells/mL. The systems were then completely filled with media using 20 mL syringes (BD, Franklin Lakes, NJ) and carefully checked to ensure no bubbles were present. For the novel design, the filling was performed through the valve at the terminal end of the channel. Once cells were added, care was taken to ensure the entrance to the channel was located at the top to prevent cells incidentally entering the channel. Each bioreactor was then mounted on a 4RCCS system (Synthecon, Inc.), placed inside an incubator (37°C), and run at 10 rpm, a typical speed for cultures starting as single-cell suspensions in the RWV.7 After 72 h, the main volume of each bioreactor was collected into 15-mL conical centrifuge tubes (Corning, Corning, NY). Cells and aggregates were sedimented over 5 min and then collected with 1000 μL pipettes. Any standard design HARV bioreactor, which exhibited bubble formation, was excluded from these results so that the impact of bubble formation could be tested under more controlled conditions. Bubbles did also form in the novel BCB design, however, the design performed as intended and no bubbles were observed in the main volume of the BCB in any of our experiments.
In a separate set of studies, air bubbles were intentionally introduced to both the novel and standard bioreactors before culture to evaluate the effects of bubble formation on A549 aggregate formation and spheroid morphology. The above cell culture steps were followed precisely, including the complete filling of the bioreactors with media and removal of air bubbles. Subsequently, an air-filled 1-mL syringe (BD) was attached to one stopcock while an empty 1-mL syringe was attached to the other. Approximately 300 μL of air was added to each bioreactor to simulate a moderate-to-severe bubble failure. The bioreactors were then operated under precisely the same conditions as the ideal group. The two groups will be referred to as “without bubble” and “with bubble,” respectively. The cell experiment conditions are listed in Supplementary Table S1.
Imaging and evaluation
For both the with bubble and without bubble groups, the morphology of the ensuing organoids/spheroids was evaluated by standard image analysis of phase-contrast micrographs. At the end of each culture, the contents of each bioreactor were collected, as described above. Using this method, virtually all aggregates and single cells were collected, which was confirmed by visualizing the supernatant. After sedimentation, 300 μL of spheroid-containing media was collected from the bottom of the tubes and gently transferred to the 14-mm centers of 35-mm glass-bottom Petri dishes (P35G-1.5-14-C; MatTek, Ashland, MA). At least 10 random images per aliquot were taken at 10 × magnification of all available spheroids, aggregates, or individual cells. Images were exported to ImageJ, all identifiable spheroids were manually outlined, and data were recorded on each image for area and circularity. Further information on determining the circularity of the spheroids is provided in the Supplementary Data. Aggregates stained for fluorescent live/dead imaging were placed on poly-L-lysine-coated MatTek dishes and treated sequentially with 3 μM Calcein AM (Molecular Probes, Eugene, OR) and 3 μM Propidium Iodide (Molecular Probes) for 30 min each and rinsed three times with phosphate-buffered saline before imaging with an FSX100 Fluorescent Microscope (Olympus Life Science, Waltham, MA).
Novel design failure mode analysis
One possible limitation of this device is the probability of cells entering the channel before the establishment of solid-body rotation. To evaluate the probability of this occurrence, human dermal fibroblasts (HDFBs from ATCC) were cultured in complete DMEM (Gibco), as described previously.52 One hundred thousand HDFBs were added to each of the bioreactors and incubated on the 4RCCS at 10 rpm for 24 h. HDFBs were selected due to their tendency not to form aggregates, thus reducing error due to changing sedimentation rates. After 24 h, the media in the main volume and the channel volume were collected into separate conical tubes through the main and the channel ports, respectively, spun down, and resuspended in 1 mL of media. Cell counting was then performed three times on each sample and averages were taken of the totals.
Statistics
Unless stated otherwise, a minimum of three independent experiments (n = 3) were performed for each parameter tested. The calculated areas of the organoids/spheroids are presented as medians ± the standard deviation of the medians. Circularity and cell count values are shown as averages ± the standard deviation of the averages. Significance was evaluated with a two-tailed student t-test and results were considered significant with a p-value less than 0.05 after post hoc correction using the Bonferroni method.
Results
Design, modeling, and CFD
Figure 1 shows the design of the novel bioreactor and the results of a CFD analysis, comparing the standard and novel designs under ideal and nonideal (i.e., in the presence of a bubble) conditions. Panels B, C, E, and F show CFD-modeled fluid velocity vectors of the 3D systems viewed from the front. Figure 1B and C model the novel bioreactor design without and with a bubble (as indicated by each arrow), respectively. Figure 1E and F model the standard design without and with a bubble, respectively. Comparison of Figure 1E and F shows that the introduction of a bubble to a standard RWV bioreactor system yields a large high-velocity zone around the bubble that deforms the classic RWV circular fluid paths and creates a noticeable low-velocity eddy directly behind the bubble. For the sake of comparison between the various images, the fluid velocity vector scale bars for all CFD images are shown from 0 to 0.03 m/s. Notably, the red oblong area shown below the bubble in Figure 1F significantly exceeds this range with maximum values in excess of 0.25 m/s. Comparing the results of Figure 1B and C to those of Figure 1F and E demonstrates the expected physical behavior of the novel design. The results of either condition of the novel design show circular fluid velocity paths that are remarkably similar to that of the standard design under ideal, bubble-free conditions. Figure 1G and H demonstrate how a bubble is captured through the channel entrance and Figure 1I and J show that the bubbles move toward the back wall of the channel as the novel bioreactor rotates.
Alginate bead fluid dynamics validation
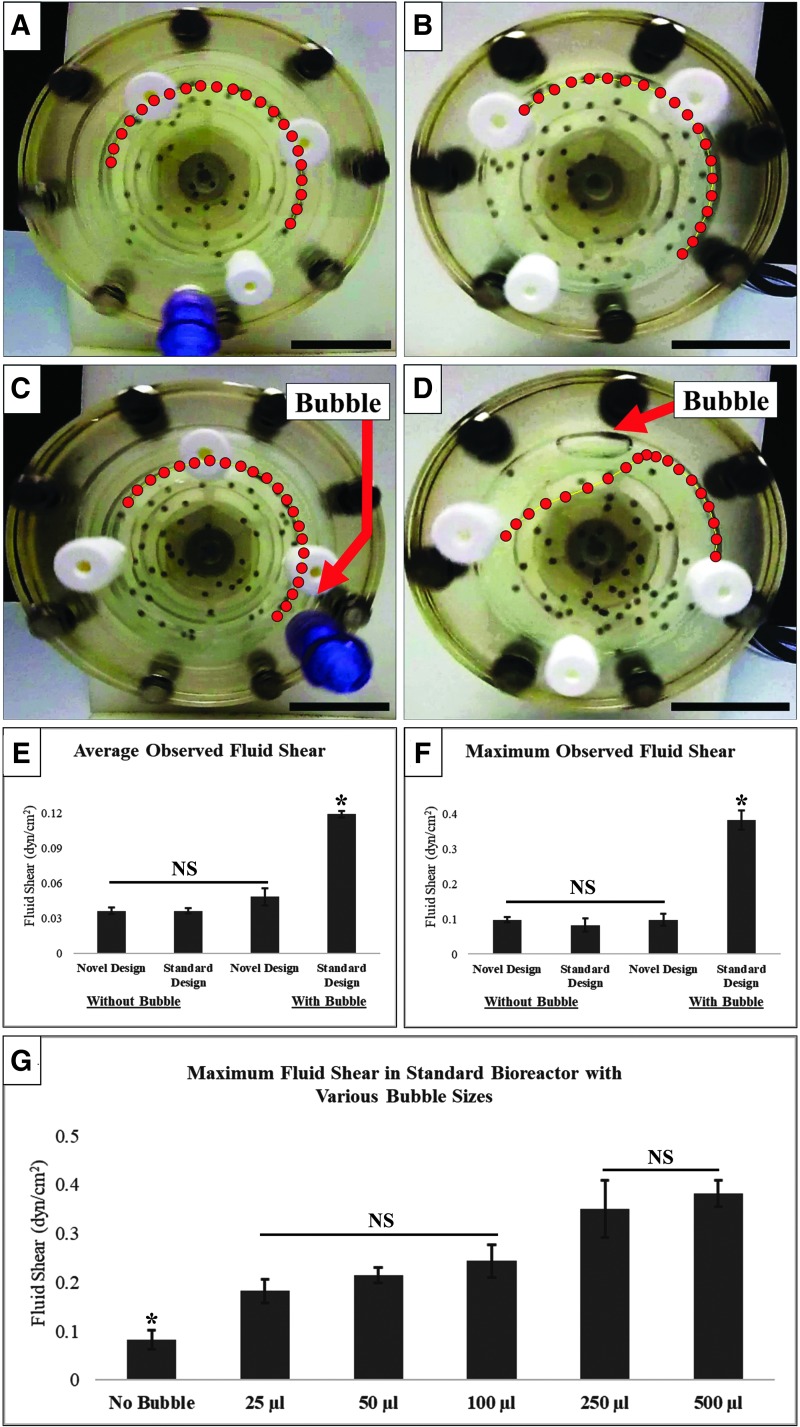
Figure 2 shows the change in the position of a single alginate bead over 1 s of rotation of the novel (Fig. 2A, C) and the standard bioreactor (Fig. 2B, D), with and without bubbles, after each system had reached equilibrium. The red dots represent the position of that one particle every 1/20th of a second (Fig. 2A–D). Shear stress values calculated from Equation 1 are shown in Figure 2E and F. Notably, the deflection of the path of the bead in Figure 2D (standard bioreactor with bubble) indicates the fluid dynamic disruption in the RWV by a single bubble and very closely resembles the shape of the fluid velocity vectors in Figure 1F. Although initially the path appears similar to the other three conditions, as soon as the particle interacts with the bubble, it slows, is deflected off of its course, and then accelerated into a new position. This altered movement destroys the condition of whole-body rotation/microgravity simulation due to the disruption of the circular fluid path, while also increasing the maximum shear force as much as fivefold (Fig. 2F). The particle paths in Figure 2A–C closely resemble one another, indicating that the BCB maintains the ideal conditions of zero headspace/whole-body rotation by effectively removing the intentionally introduced bubble. Figure 2E and F demonstrate the insignificant differences in both average and maximum shear experienced in the above three “ideal” conditions. Although a bubble is not readily visible in Figure 2C, it is present at the back of the channel (red arrow) and is of the same size as the one in Figure 2D. Figure 2G shows the calculated maximum shear stress of the standard HARV design seeded with bubbles ranging from 25 to 500 μL. All shear results are listed in Supplementary Table S2. The circular residuals resulting from path disruption for various bubble sizes are shown in Supplementary Figure S3. The path disruption images indicate that particles may spend upward of 25% of their cycle in an abnormal shear state.
FIG. 2.
Alginate bead shear modeling. (A–D) The position of alginate beads over 1 s marked every 1/20th of a second in RWV bioreactors rotating counterclockwise under the four conditions defined in the text. The bubble is shown by the red arrows in (C, D). (E) The average fluid shear stress experienced by the beads over the course of the path. The shear stress in the novel design with bubbles is not significantly different from that in the first two instances. (F) The maximum shear stress experienced by the beads during the observed path. (G) Maximum shear values observed in the standard bioreactor with increasing bubble size. Shear stress measurements are detailed in the text. All scale bars are 2.5 cm. *Significant at p < 0.05. NS, not significant; RWV, rotating wall vessel. Color images are available online.
Cell culture
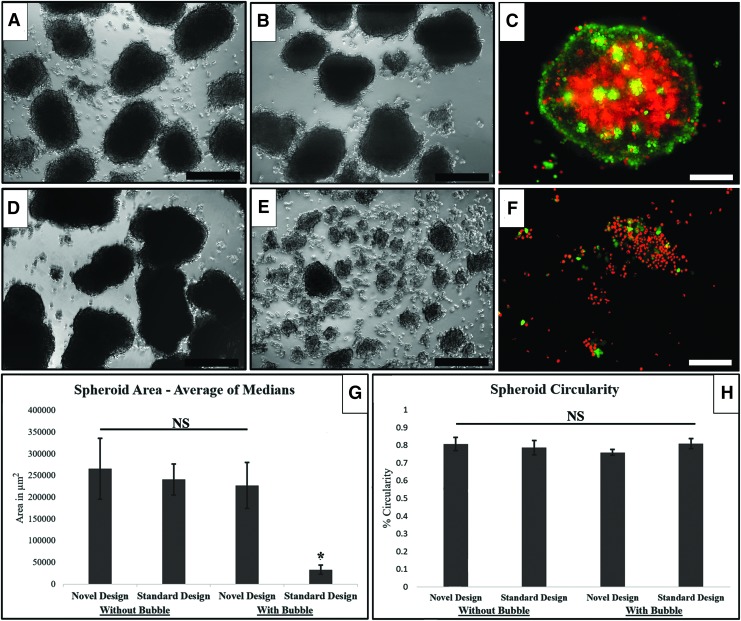
Shown in Figure 3 are representative micrographs of A549 spheroid cultures from each of the four conditions described above for 72 h. Figure 3A and B are representative of typical spheroids from the novel and standard bioreactor without air bubbles, whereas Figure 3D and E show the results from BCB and HARV bioreactors initialized with an air bubble. The images in Figure 3A, B, D shows similar results in terms of spheroid size and circularity. By contrast, only either small aggregates or single cells were found in the standard bioreactor in the presence of bubbles (Fig. 3E). Figure 3C and F show fluorescent live/dead staining of aggregates/cells taken from the novel and standard bioreactors, respectively, when seeded with bubbles. The spheroids taken from the novel bioreactor demonstrate a healthy, live exterior with a “dying core,” consistently mirroring the anoxic and subsequently necrotic core of spheroids, as previously demonstrated by others in the RWV.9 As summarized in Figure 3G, introduction of a bubble reduced spheroid area by ∼10 × in a standard bioreactor, but not in the novel design. Interestingly, the circularity of the spheroids was unaffected between the four groups, regardless of condition or spheroid size (Fig. 3H). Of note, ∼40% of all experiments performed in the standard design without a bubble were rejected due to bubbles forming during operation. In our experiments, the BCB design captured and permanently segregated all bubbles within a single rotation after startup.
FIG. 3.
A549 cell culture results for spheroid morphology after 72 h. (A) Spheroid formation in the novel design with no bubble present. (B) Spheroid formation in the standard design with no bubble present. (C) Calcein AM/Propidium Iodide staining of a spheroid from the novel design with a bubble present. (D) Spheroid formation in the novel design with a bubble present. (E) Spheroid formation in the standard design with a bubble present. (F) Calcein AM/Propidium Iodide staining of a spheroid from the standard design with a bubble present. (G) Combined quantified results for spheroid areas for each of the above conditions taken as the average of medians, for details, see text. (H) Combined quantified results for spheroid circularity taken as an average of averages of independent triplicate data, for details, see text. Scale bars for (A, B, D, E) are 200 μm, scale bars for C and F are 100 μm. *Significant at p < 0.05. NS, not significant. Color images are available online.
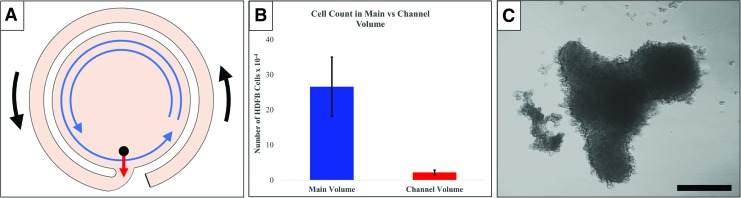
We also collected the contents of the channels from the novel design bioreactors. While there were generally little to no aggregates nor single cells present, on occasion, a few very large spheroids (typically >500 μm) were found. Phase-contrast microscopy images of typical aggregates found in the BCB channel (Fig. 4C), demonstrate the large, anisotropic characteristics of aggregates captured in the channel. This may indicate a tendency for larger spheroids to irreversibly enter the channel only once their sedimentation rates exceed the maximum velocity of the rotating media.
FIG. 4.
Experimental Analysis of a Failure Mode. (A) Conceptual representation of the path by which the “channel capture” failure mode may occur during initialization of reactor rotation. (B) Experimental validation: The panel shows the incidence of channel capture of human dermal fibroblasts cultured for 24 h. (C) Representative images of A549 aggregates of aggregates taken from the channel volume of the novel bioreactor after 72 h in culture. Scale bar for (C) is 200 μm. Color images are available online.
Novel design failure mode analysis
Figure 4 depicts the analysis of the potential failure mode of the novel bioreactor, that is, of cells irreversibly entering the channel irreversibly (Fig. 4A). To assess this possibility, HDFBs were seeded into the main volume and cultured for 24 h, before harvesting and counting the cells to determine the totals in, respectively, the main and channel volumes. As shown in Figure 4B, less than 2% of all cells entered the channel over the course of 24 h.
Discussion
The aim of this study was to design a modified RWV, capable of producing the same low-shear, microgravity-simulating environment as a typical RWV, while capturing and removing nascent bubbles, thus maintaining the zero headspace condition, critical to organoid formation. Based on the results shown in Figure 1, we hypothesized that both the standard and novel bioreactor designs would produce similar organoids under bubble-free conditions. However, when a bubble was introduced, organoid formation would be significantly disrupted in the standard bioreactor, while no difference would be observed in the novel BCB. The results in Figures 2 and 3 indeed demonstrate that the modified RWV can produce and maintain the required zero headspace conditions, while actively removing bubbles and producing large spheroids/organoids, like traditional RWV devices under optimal, bubble-free conditions.
Although the equations used to calculate shear in the alginate bead systems are substantial simplifications, the average experimental values of ∼0.04 dynes/cm2, under ideal conditions, very closely approximated the previously published ideal range of ∼0.044 dynes/cm2 under zero headspace conditions.46 By contrast, in the presence of a bubble (Fig. 2D), the maximum fluid shear stress increases ∼10-fold relative to the average to 0.4 dynes/cm2. This order-of-magnitude increase appears to be sufficient to disrupt aggregate formation, but may not yet ensure cell damage, which reportedly requires 0.92 dynes/cm2.53 When taken contextually with Figure 3E, the appearance of single cells and very small aggregates indicates that spheroid formation was disrupted as a result of increased shear forces. By contrast, the similarity (Fig. 3G, H) in aggregate sizes in the novel design with and without bubbles indicates that this novel technology achieved its intended goal of reducing the impact of bubbles on aggregate formation. In our use, bubbles did not grow sufficiently large as to overfill the channel or reenter the system over the course of 72 h. However, should this issue arise with longer-term culture, bubbles can be easily removed from the channel through its Luer port with a simple syringe during operation. Our results demonstrate that the BCB design may combine enhanced reproducibility of organoid generation with improved ease of use and a decreased likelihood of experiment failure. Although the probability of channel capture may increase as spheroids grow and sedimentation rates change, this issue can be resolved by increasing the rotational speed of the device.
Our cell culture results, taken in conjunction with the shear values obtained with even small bubbles (Fig. 2G), demonstrate that the prevention of bubble formation is important for the outcome and reproducibility of experiments using the RWV, yet, it is seemingly underreported in the literature.30 Because bubble formation is frequent, and its prevention is anecdotal at best, researchers are likely to collect results from RWVs that may intermittently form bubbles (e.g., overnight), not realizing that such an experiment may be compromised. It is one of our intentions that the novel BCB design may be able to prevent false-positive/negative results and increase the reproducibility of the system.
While under ideal conditions the calculated shear stresses and the observed cell spheroid sizes were statistically indistinguishable between the “standard without bubble” group and either of the “novel” groups, there are distinct differences between the standard and novel designs. As described in Figure 4, there is a probability of a small subpopulation of the cells irreversibly entering the novel channel during initialization. However, after initialization, channel capture is only likely to occur if the cells or aggregates tend to drift toward the exterior perimeter of the main volume, with the probability of that tendency increasing if the rotational velocity is incorrect, as seen with the abnormally large aggregates seen in Figure 4C. In the standard system, a device set to the incorrect speed would still contain all its cell contents. In the novel system, if an incorrect rotational speed is set, aggregates would tend to be captured in the channel, thus providing tangible early feedback that the system settings are inadequate, allowing the speed to be increased as aggregates grow. In a standard system, particles interacting with the wall may be thrown off their circular path, thus negating the modeled microgravity (whole-body rotation, zero headspace) environment. However, these cells would still be collected together with the contents of the entire bioreactor, potentially skewing mechanistic analyses, such as global gene expression profiling in the wake of organoid assembly and differentiation. In the novel system, cells in the channel volume can easily be collected separately and discarded from those in the main volume, ensuring the cells tested are only the ones that did not tend toward the wall, decreasing skew and increasing the consistency of results.
In summary, we engineered and tested a modification to the traditional HARV-type RWV bioreactor, which results in the effective capture and removal of bubbles. In this article, we compared the modified BCB and standard HARV bioreactors side by side and found similarities in both fluid dynamics and in the ability to generate sizeable cancer spheroids in the absence of bubbles. However, in the presence of a bubble, the spheroid/organoid formation is impaired in conventional RWVs, while it is fully maintained in the modified design. We anticipate that the novel design will increase experimental reproducibility and consistency when using these kinds of rotatory bioreactors.
Supplementary Material
Acknowledgments
The authors wish to thank Mr. Robert Redden and Mrs. Helen Freitas (Dept. Bioengineering) for their guidance and support. The authors thank Kelsey Manahan-Phelan (Library and Archives, Academy of Natural Sciences, Philadelphia) for her assistance in sourcing references. Their research is supported by the National Aeronautics and Space Agency (NASA), Grant # 80NSSC18K1480 and the Intramural Research Program of the National Eye Institute (EY000450, EY000 474, EY000546).
Disclosure Statement
Temple University has filed a patent application for the novel bioreactor design, with M.A.P. and P.I.L. as named coinventors.
Supplementary Material
References
- 1. Antoni D., Burckel H., Josset E., and Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16, 5517, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xinaris C., Brizi V., and Remuzzi G. Organoid models and applications in biomedical research. Nephron 130, 191, 2015 [DOI] [PubMed] [Google Scholar]
- 3. Fang Y., and Eglen R.M. Three-dimensional cell cultures in drug discovery and development. SLAS Discov 22, 456, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eder T., and Eder I.E. 3D Hanging Drop Culture to Establish Prostate Cancer Organoids. New York, NY: Humana Press, 2017, pp. 167–175 [DOI] [PubMed] [Google Scholar]
- 5. Lelkes P.I., Galvan D.L., Thomas Hayman G., et al. Simulated microgravity conditions enhance differentiation of cultured PC12 cells towards the neuroendocrine phenotype. In Vitro Cell Dev Biol Anim 34, 316, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Gerecht-Nir S., Cohen S., and Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. 86, 493, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Botta G.P., Manley P., Miller S., and Lelkes P.I. Real-time assessment of three-dimensional cell aggregation in rotating wall vessel bioreactors in vitro. Nat Protoc 1, 2116, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Redden R.A., and Doolin E.J. Microgravity assay of neuroblastoma: in vitro aggregation kinetics and organoid morphology correlate with MYCN expression. 47, 312, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Redden R.A., Iyer R., Brodeur G.M., and Doolin E.J. Rotary bioreactor culture can discern specific behavior phenotypes in Trk-null and Trk-expressing neuroblastoma cell lines. In Vitro Cell Dev Biol Anim 50, 188, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radtke A.L., and Herbst-Kralovetz M.M. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J Vis Exp 62, e3868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattei C., Alshawaf A., D'abaco G., Nayagam B., and Dottori M. Generation of neural organoids from human embryonic stem cells using the rotary cell culture system: effects of microgravity on neural progenitor cell fate. Stem Cells Dev 27, 848, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qian X., Jacob F., Song M.M., Nguyen H.N., Song H., and Ming G. Generation of human brain region—specific organoids using a miniaturized spinning bioreactor. Nat Protoc 13, 565, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villa A., Versari S., Maier J.A.M., and Bradamante S. Cell behavior in simulated microgravity: a comparison of results obtained with RWV and RPM. Gravit Space Biol Bull 18, 89, 2005 [PubMed] [Google Scholar]
- 14. Catapano G., Speranza G., Maniglio D., DeBartolo L., and Della Volpe C. Bioreactor type and operating conditions influence cell response to polymeric material properties. In: Proceedings of the IEEE-EMBS Special Topic Conference on Molecular, Cellular and Tissue Engineering. IEEE; 2002:60–61; DOI: 10.1109/MCTE.2002.1175004 [DOI] [Google Scholar]
- 15. Wells A., Rodrigues M., Wells A.W., and Nuschke A. Starvation as an initiator of mesenchymal stem cell/multipotent stromal cell differentiation. J Stem Cell Res Ther 1, 2016; DOI: 10.15406/jsrt.2016.01.00020 [DOI] [Google Scholar]
- 16. Dahan P., Lu V., Nguyen R.M.T., Kennedy S.A.L, and Teitell M.A. Metabolism in pluripotency: both driver and passenger? J Biol Chem 294, 5420, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hubert C.G., Rivera M., Spangler L.C., et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res 76, 2465, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forristal C.E., Wright K.L., Hanley N.A., Oreffo R.O.C., and Houghton F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139, 85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ayabe H., Anada T., Kamoya T., et al. Optimal hypoxia regulates human iPSC-derived liver bud differentiation through intercellular TGFB signaling. Stem Cell Reports 11, 306, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grebenyuk S., and Ranga A. Engineering organoid vascularization. Front Bioeng Biotechnol 7, 39, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y., Wang L., Guo Y., Zhu Y., and Qin J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv 8, 1677, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfe R.P., and Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng 110, 1231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regmi S., Fu A., and Luo K.Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep 7, 39975, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ovando-Roche P., West E.L., Branch M.J., et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther 9, 156, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frangos J.A., McIntire L.V., and Eskin S.G. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 32, 1053, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Poli D., Magliaro C., and Ahluwalia A. Experimental and computational methods for the study of cerebral organoids: a review. Front Neurosci 13, 162, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiStefano T., Chen H.Y., Panebianco C., et al. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports 10, 300, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phelan M.A., Lelkes P.I., and Swaroop A. Mini and customized low-cost bioreactors for optimized high-throughput generation of tissue organoids. Stem Cell Investig 5, 33, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwarz R.P., Goodwin T.J., and Wolf D.A. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods 14, 51, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Hammond T.G., and Hammond J.M. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol 281, F12, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Hammond T., Allen P., and Birdsall H. Is there a space-based technology solution to problems with preclinical drug toxicity testing? Pharm Res 33, 1545, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. King J.A., and Miller W.M. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol 11, 394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pollack S.R., Meaney D.F., Levine E.M., Litt M., and Johnston E.D. Numerical model and experimental validation of microcarrier motion in a rotating bioreactor. Tissue Eng 6, 519, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Salerno-Goncalves R., Fasano A., and Sztein M.B. Development of a multicellular three-dimensional organotypic model of the human intestinal mucosa grown under microgravity. J Vis Exp 2016; DOI: 10.3791/54148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varley M.C., Markaki A.E., and Brooks R.A. Effect of rotation on scaffold motion and cell growth in rotating bioreactors. Tissue Eng Part A 23, 522, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wuest S.L., Richard S., Kopp S., Grimm D., and Egli M. Simulated microgravity: critical review on the use of random positioning machines for mammalian cell culture. Biomed Res Int 2015, 971474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Niederhaus C., Nahra H., Gonda S., et al. Bubble experiments on the hydrodynamic focusing bioreactor-space (HFB-S). In: New London, CT: Gordon Conference on Gravitational Effects on Physico-Chemical Systems, 2002. https://ntrs.nasa.gov/search.jsp?R=20040000164 Accessed June27, 2019
- 38. Lelkes P.I., and Unsworth B.R. Neuroectodermal cell culture. In: Methods of Tissue Engineering. Elsevier, 2002, pp. 371–382 [Google Scholar]
- 39. Sanford G.L., Ellerson D., Melhado-Gardner C., Sroufe A.E., and Harris-Hooker S. Three-dimensional growth of endothelial cells in the microgravity-based rotating wall. In Vitro Cell Dev Biol Anim 38, 493, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Choi C.K., and Helmke B.P. Short-term shear stress induces rapid actin dynamics in living endothelial cells. Mol Cell Biomech 5, 247, 2008 [PMC free article] [PubMed] [Google Scholar]
- 41. Yu-shuang L., and Jiong C. Moisture vapor transmission rates of various transparent dressings at different temperatures and humidities. Chin Med J (Engl) 122, 927, 2009 [PubMed] [Google Scholar]
- 42. Botchwey E.A., Pollack S.R., Levine E.M., and Laurencin C.T. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res 55, 242, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramirez L.E.S., Lim E.A., Coimbra C.F.M., and Kobayashi M.H. On the dynamics of a spherical scaffold in rotating bioreactors. Biotechnol Bioeng 84, 382, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Brown R. fitcircle.m. MATLAB Central File Exchange. https://www.mathworks.com/matlabcentral/fileexchange/15060-fitcircle-m Accessed June28, 2019
- 46. Begley C.M., and Kleis S.J. The fluid dynamic and shear environment in the NASA/JSC rotating-wall perfused-vessel bioreactor. Biotechnol Bioeng 70, 32, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Munson B.R., Rothmayer A.P., Okiishi T.H., and Huebsch W.W. Fluid Mechanics. 7th ed. John Wiley & Sons, Inc., 2012. www.wileyplus.com Accessed August2, 2018 [Google Scholar]
- 48. Wolf D.A., and Schwarz R.P. Analysis of gravity-induced particle motion and fluid perfusion flow in the NASA-designed rotating zero-head-space tissue culture vessel. October 1991. https://ntrs.nasa.gov/search.jsp?R=19920004122 Accessed May28, 2018
- 49. Sambale F., Lavrentieva A., Stahl F., et al. Three dimensional spheroid cell culture for nanoparticle safety testing. J Biotechnol 205, 120, 2015 [DOI] [PubMed] [Google Scholar]
- 50. Zuchowska A., Jastrzebska E., Chudy M., Dybko A., and Brzozka Z. 3D lung spheroid cultures for evaluation of photodynamic therapy (PDT) procedures in microfluidic Lab-on-a-Chip system. Anal Chim Acta 990, 110, 2017 [DOI] [PubMed] [Google Scholar]
- 51. Maruhashi R., Akizuki R., Sato T., et al. Elevation of sensitivity to anticancer agents of human lung adenocarcinoma A549 cells by knockdown of claudin-2 expression in monolayer and spheroid culture models. Biochim Biophys Acta Mol Cell Res 1865, 470, 2018 [DOI] [PubMed] [Google Scholar]
- 52. Lin L., Perets A., Har-El Y.-E., et al. Alimentary “green” Proteins as Electrospun Scaffolds for Skin Regenerative Engineering. J Tissue Eng Regen Med 7, 994, 2013 [DOI] [PubMed] [Google Scholar]
- 53. Goodwin T.J., Prewett T.L., Wolf D.A., and Spaulding G.F. Reduced shear stress: a major component in the ability of mammalian tissues to form three-dimensional assemblies in simulated microgravity. J Cell Biochem 51, 301, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.