Abstract
Patient: Female, 61
Final Diagnosis: Pericardial effusion secondary to CLL
Symptoms: Shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Oncology
Objective:
Rare co-existance of disease or pathology
Background:
Small lymphocytic lymphoma (SLL) is a low-grade B-cell non-Hodgkin lymphoma and is the solid tumor equivalent of chronic lymphocytic leukemia (CLL) that is found in the peripheral blood. SLL typically presents with lymphadenopathy and is rarely associated with cardiac involvement. This report is of a case of lymphomatous pericardial effusion in a 61-year-old woman who presented with dyspnea.
Case Report:
A 61-year-old woman presented to the emergency department with a three-month history of worsening shortness of breath on exertion. Her symptoms progressed to shortness of breath at rest, with night sweats and chills. She had no weight loss. She was found to have a pericardial effusion, and an urgent pericardiocentesis was performed to prevent cardiac tamponade. Analysis of the pericardial fluid was consistent with a diagnosis of SLL. A bone marrow biopsy and a biopsy of a renal mass were consistent with a diagnosis of SLL. She was treated with rituximab and bendamustine with granulocyte-colony stimulating factor (G-CSF) support and was discharged home.
Conclusions:
A case is presented of a rare association between SLL and pericardial effusion with a favorable outcome following urgent pericardiocentesis to prevent cardiac tamponade followed by chemotherapy.
MeSH Keywords: Chemotherapy, Adjuvant; Leukemia, Lymphocytic, Chronic, B-Cell; Pericardial Effusion
Background
Small lymphocytic lymphoma (SLL) is a low-grade B-cell non-Hodgkin lymphoma (NHL) and is the solid tumor equivalent of chronic lymphocytic leukemia (CLL) that is found in the peripheral blood. SLL typically presents with lymphadenopathy and is rarely associated with cardiac involvement.
Primary malignancy involving the heart is rare with a prevalence of 0.05–4.8%, but the heart and pericardium can be involved with secondary malignancy, including deposits of lymphoma or leukemia [1–5]. Secondary cardiac malignancy occurs by hematogenous spread, with hematopoietic malignancy being a common secondary malignancy that involves the heart [1]. Secondary or metastatic cancers of the pericardium account for 75% of all cardiac malignancies [6]. Secondary tumors involving the heart include metastasis [5]. The pericardium, including the epicardium, is the most common location of cardiac involvement by secondary tumors, followed by the myocardium, and the endocardium [5]. This report is of a case of SLL involving the pericardium that was managed successfully with pericardiocentesis followed by chemotherapy.
Case Report
A 61-year-old woman presented to the emergency department with a three-month history of worsening shortness of breath on exertion, which progressed to shortness of breath at rest. She also complained of night sweats, chills, but no chest pain or weight loss. Her medical history included systemic hypertension, a 10 pack-year tobacco smoking history, and type 2 diabetes mellitus. Physical examination showed that her blood pressure was 104/64 mmHg, with a heart rate of 100 bpm, tachypnea with 30 breaths/min, and an oxygen saturation of 93% on 2L of oxygen administered by nasal cannula. She was afebrile with a temperature of 98.1°F (36.7°C). Her jugular venous pressure was >7 cm of H2O, she had sinus tachycardia with mildly muffled and distant heart sounds, but no pericardial friction rub, or peripheral edema. Chest percussion was dull over both lung fields. The hepatojugular reflex was absent.
Laboratory investigations showed a leukocytosis of 14×103 cell/µL, an absolute lymphocyte count of 3,440×103 cell/µL, a hemoglobin 12.2 g/dL, and a platelet count of 266×109/L. Her troponin levels were normal. The rest of her laboratory investigations were unremarkable.
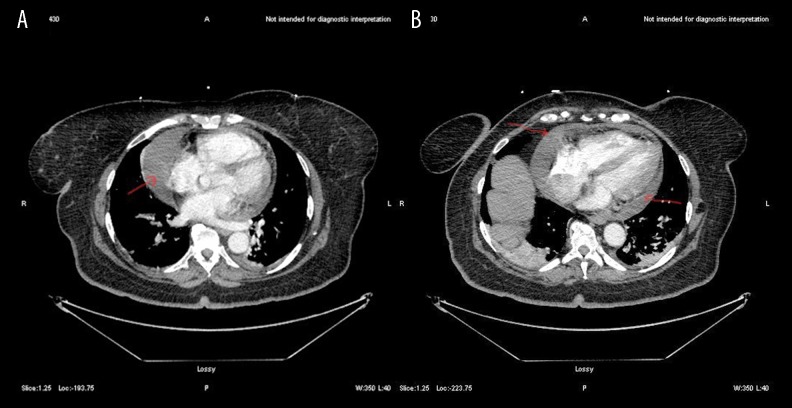
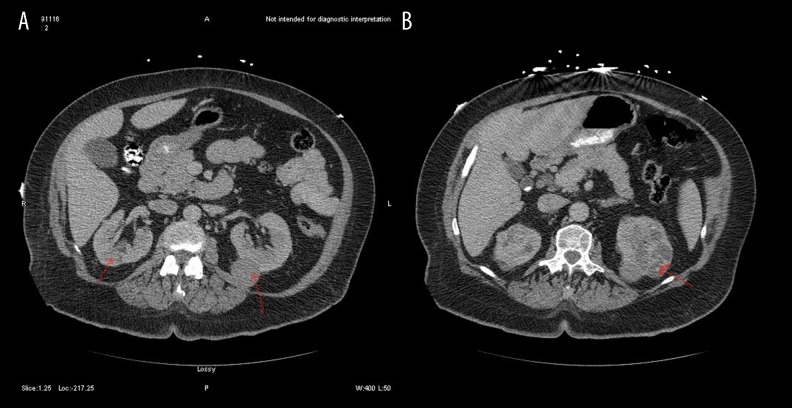
An electrocardiogram (ECG) showed sinus tachycardia with right axis deviation, no PR depression or ST changes. Chest X-ray showed an enlarged cardiac silhouette. Chest computed tomographic angiography (CTA) was negative for pulmonary embolism, but there was a significant moderate to large pericardial effusion (Figure 1). Echocardiography was significant for pericardial effusion with a compressed right atrium. Computed tomography (CT) of the abdomen and pelvis showed multiple solid and cystic heterogeneous lesions (Figure 2). The patient was resuscitated with intravenous fluids that included 4 liters of crystalloids, and she was taken to the operating room for emergency pericardiocentesis that drained 500 ml of serosanguinous fluid, and a pigtail drain was placed in situ.
Figure 1.
(A, B) Computed tomography (CT) angiography of the chest shows a moderate to large pericardial effusion (arrows).
Figure 2.
(A, B) Computed tomography (CT) of the abdomen and pelvis shows multiple solid and cystic lesions, some pf which appear heterogeneous (arrows).
Cytopathology of the pericardial fluid showed small lymphocytes consistent with a diagnosis of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) (Figure 3). Flow cytometry of the pericardial fluid and immunophenotyping showed a monoclonal B-cell population with positive immunostaining for CD5, CD23, CD8, CD19, CD20, and CD45, and negative immunostaining for CD33, CD34, and CD56. The biopsy of the renal was also consistent with SLL. Treatment commenced with rituximab and bendamustine with granulocyte-colony stimulating factor (G-CSF) support, and she was discharged home. Outpatient chemotherapy treatment was complicated by tumor lysis syndrome, and she was managed with allopurinol and hydration.
Figure 3.
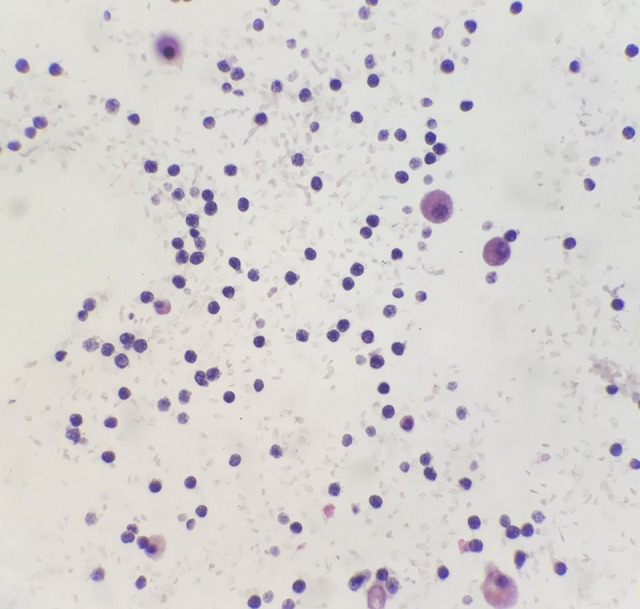
Immunohistochemical staining shows that the lymphoma cells are positive for CD79a, PAX-5, and CD5, and negative for CD3, Bcl-6, Bcl-2, CD20, and Bcl-1, with a low cell proliferation rate on immunostaining for Ki67. Immunohistochemistry for the proliferation index shows that 1–2% of the lymphoma cells are Ki-67 positive. The cell morphology, immunohistochemistry, and flow cytometry findings support the diagnosis of a CD5-positive small lymphocytic lymphoma (SLL), which is a low-grade, B-cell, non-Hodgkin lymphoma (NHL).
Discussion
In the current classification of lymphoma, small lymphocytic lymphoma (SLL) is a low-grade B-cell non-Hodgkin lymphoma (NHL) that usually presents with lymphadenopathy, and is the equivalent of chronic lymphocytic leukemia (CLL) that occurs in the peripheral blood. SLL is rarely associated with cardiac involvement and is a low-grade NHL characterized by a monoclonal population of mature B-cell lymphocytes [1]. Because of its indolent course, SLL is often diagnosed incidentally.
Bone marrow-derived B-cell CLL is the most common type of leukemia in western countries and represents between 25–30% of all cases of leukemia the US [6]. The average incidence ranges from <0.01% in patients from Asia to approximately 0.06% in patients from the US [7]. One of the key diagnostic criteria for CLL involves an absolute lymphocyte count >5×109/L, which this patient did not have. However, flow cytometry and immunostaining of the cells in the pericardial fluid can be diagnostic for SLL.
The symptoms of SLL typically range from constitutional symptoms, such as night sweats, weight loss, and fatigue, to symptomatic enlargement of lymphoid tissue [1].
Pericardial involvement in SLL is extremely rare. In 2014, two previous cases of B-cell CLL/SLL were reported with pericardial involvement [1,8,9]. These cases presented with constrictive pericarditis [8,9], and although our patient had pericardial effusion with impending cardiac tamponade, she did not have constrictive pericarditis. Pericardial effusion can be due to several conditions, such as cell infiltrates from extramedullary hematopoiesis, hemorrhagic diathesis due to thrombocytopenia, and in patients receiving chemotherapy pericardial effusion may be a side effect of treatment, or could be a result of an infection [1,2].
Echocardiography is the initial diagnostic test of choice for pericardial effusion, while magnetic resonance imaging (MRI) can provide more information on the characterization of the tissue structure and also provides characterization of the pericardium [1]. The treatment of malignant pericardial effusion should be individualized. However, pericardiocentesis with an indwelling catheter reduces the effusion and prevents cardiac tamponade, as shown in this patient. Systemic tumor chemotherapy reduces the re-occurrence of the pericardial effusion and reduces patient morbidity and mortality. Other advanced techniques, including pericardiotomy or the use of subxiphoid percutaneous balloon pericardiotomy, can be effective. This patient had a successful outcome following percutaneous pericardiocentesis and initiation of chemotherapy.
Conclusions
A rare case of lymphomatous pericardial effusion is presented that was associated with lymphocytic lymphoma (SLL), which is a low-grade B-cell non-Hodgkin lymphoma (NHL). It is rare for SLL to present with pericardial effusion, but as this case had shown, urgent diagnosis and treatment with pericardiocentesis is required to prevent cardiac tamponade.
Footnotes
Conflict of interest
None.
References:
- 1.Ho N, Myles JL, Johnston DR, et al. Pericardial involvement with chronic lymphocytic leukemia/small lymphocytic lymphoma: A rare case of constrictive pericarditis. CASE (Phila) 2018;2(4):147–50. doi: 10.1016/j.case.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurber DL, Edwards JE, Achor RWP. Secondary malignant tumors of the pericardium. Circulation. 1962;26:228–41. doi: 10.1161/01.cir.26.2.228. [DOI] [PubMed] [Google Scholar]
- 3.Butany J, Leong S, Carmichael K, Komeda MA. 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675–80. [PubMed] [Google Scholar]
- 4.MacGee W. Metastatic and invasive tumours involving the heart in a geriatric population: a necropsy study. Virch Arch Pathol Anat. 1991;419:183–89. doi: 10.1007/BF01626346. [DOI] [PubMed] [Google Scholar]
- 5.Lam KY, Dickens P, Chan ACL. Tumors of the heart: A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–31. [PubMed] [Google Scholar]
- 6.Atwal D, Raval M, Firwana B, et al. An unusual presentation of chronic lymphocytic leukemia. Avicenna J Med. 2017;7(3):133–36. doi: 10.4103/ajm.AJM_171_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukemia. Nat Rev Dis Prim. 2017;3:17008. doi: 10.1038/nrdp.2017.8. [DOI] [PubMed] [Google Scholar]
- 8.Habboush HW, Dhundee J, Okati DAL, Davies AG. Constrictive pericarditis in B cell chronic lymphatic leukemia. Clin Lab Haem. 1996;18:117–19. doi: 10.1046/j.1365-2257.1996.00164.x. [DOI] [PubMed] [Google Scholar]
- 9.Danilova OV, Danilov AV. Pericardial involvement by chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2015;126:424. doi: 10.1182/blood-2015-04-641571. [DOI] [PMC free article] [PubMed] [Google Scholar]