Abstract
Oscillatory activity within sensorimotor networks is characterized by time-varying changes in phase and power. The influence of interactions between sensorimotor oscillatory phase and power on human motor function, like corticospinal output, is unknown. We addressed this gap in knowledge by delivering transcranial magnetic stimulation (TMS) to the human motor cortex during electroencephalography recordings in 20 healthy participants. Motor evoked potentials, a measure of corticospinal excitability, were categorized offline based on the mu (8–12 Hz) and beta (13–30 Hz) oscillatory phase and power at the time of TMS. Phase-dependency of corticospinal excitability was evaluated across a continuous range of power levels using trial-by-trial linear mixed-effects models. For mu, there was no effect of PHASE or POWER (P > 0.51), but a significant PHASE × POWER interaction (P = 0.002). The direction of phase-dependency reversed with changing mu power levels: corticospinal output was higher during mu troughs versus peaks when mu power was high while the opposite was true when mu power was low. A similar PHASE × POWER interaction was not present for beta oscillations (P > 0.11). We conclude that the interaction between sensorimotor oscillatory phase and power gates human corticospinal output to an extent unexplained by sensorimotor oscillatory phase or power alone.
Keywords: electroencephalography, motor control, motor cortex, motor evoked potentials, transcranial magnetic stimulation
Introduction
Neuronal networks exhibit oscillatory activity that coordinates local processing and inter-regional communication through alternating windows of excitation and inhibition (Buzsáki 2006). Activity within these networks is characterized by time-varying changes in oscillatory phase and power. These oscillatory dynamics are known to impact brain function across cognitive domains, including perception (Romei et al. 2008; Busch et al. 2009; Dugué et al. 2011; Baumgarten et al. 2015; VanRullen 2016), attention (Busch and VanRullen 2010), and decision making (Drewes and VanRullen 2011; Wyart et al. 2012; Hamel-Thibault et al. 2016). For example, visual perception has repeatedly been shown to depend on the phase (Busch et al. 2009; Dugué et al. 2011; VanRullen 2016) and power (Mathewson et al. 2009, 2011; Romei et al. 2008; Romei et al. 2010) of occipital oscillatory activity.
Sensorimotor networks oscillate in the mu (8–12 Hz) and beta (13–30 Hz) ranges (Salmelin and Hari 1994; Jensen et al. 2005; Neuper et al. 2005). These oscillations originate from synchronous postsynaptic potentials in sensorimotor cortical pyramidal neurons (Pineda 2005; Buzsáki et al. 2012), and are functionally related to voluntary motor behavior: mu and beta oscillations are strongest at rest and desynchronize during voluntary motor activity (Pfurtscheller and Aranibar 1979; Pfurtscheller and Lopes da Silva 1999; Pfurtscheller et al. 2006). Despite their similarities, mu and beta oscillations reflect neural activity within separate sensorimotor networks, as indicated by their different generators (Costa et al. 2006; Mallet et al. 2008; Sherman et al. 2016), anatomical sources (Salmelin and Hari 1994), and somatotopic organization (Salmelin et al. 1995).
Invasive recordings have demonstrated that mu and beta oscillatory phases relate to variation in sensorimotor cortical activity (Haegens et al. 2011; Miller et al. 2012), with troughs (i.e., surface negativity) reflecting higher neuronal spiking rates (Haegens et al. 2011) and increased population-level activity (Miller et al. 2012) compared with peaks (i.e., surface positivity). Sensorimotor oscillatory power also reflects changing cortical activity levels: mu power is inversely related to neuronal spiking rates (Haegens et al. 2011), while beta power is inversely associated with population-level activity (Miller et al. 2012). Based on these findings, it is feasible that sensorimotor oscillatory power and phase could interact to shape human motor function.
The influence of sensorimotor oscillatory phase–power interactions on corticospinal excitability, an established noninvasive marker of human motor function in health and disease (Stinear et al. 2006; Hallett 2007; Reis et al. 2008; Dayan et al. 2013), is not known. Here, we addressed this gap in knowledge by evaluating the phase-dependency of corticospinal excitability across a continuous range of sensorimotor oscillatory power levels. Our results demonstrate that sensorimotor oscillatory phase–power interactions gate human corticospinal output to an extent unaccounted for by oscillatory phase or power alone.
Materials and Methods
Human subjects: 20 healthy subjects participated in this study (6 F, 14 M, age = 30 ± 1.59 [SE; Standard Error] years), during which single-pulse transcranial magnetic stimulation (TMS) was delivered during 32-channel electroencephalography (EEG) and electromyography (EMG) recordings. This study was approved by the National Institutes of Health Combined Neuroscience Section IRB, and all subjects provided their written informed consent before participating.
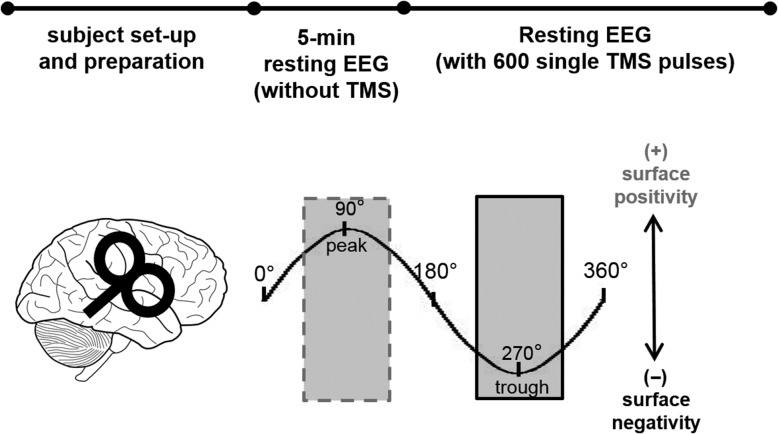
Experimental timeline: Subjects were instructed to sit quietly and fixate on a cross at eye level in front of them. Each experimental session started with a 5-minute resting-state EEG recording in the absence of TMS, followed by EEG recordings during delivery of 600 single TMS pulses to the right motor cortex (Fig. 1).
Figure 1.
Depiction of experimental approach. After subject set-up and preparation, 5 min of resting EEG was recorded without any TMS delivery. Then, 600 single TMS pulses were delivered to the right motor cortex during concurrent EEG recordings. Trials were categorized offline depending on the mu and beta oscillatory phase (peaks and troughs) at the time of the TMS pulse (see Materials and Methods). Shading depicts oscillatory phase ranges corresponding to peaks (dashed gray; surface positivity) and troughs (solid black; surface negativity).
EEG and EMG recordings: 32-channel EEG signals were recorded at 5 kHz (hardware filtering: DC—1 kHz) during TMS delivery using TMS-compatible amplifiers (BrainAmp MR+, Brain Vision) at 0.5 μV resolution. Impedances were maintained below 12 kΩ. Disposable adhesive electrodes arranged in a belly–tendon montage were used to record EMG signals at 5 kHz (hardware filtering: 5Hz–2 kHz; Signal, Cambridge Electronic Design, UK).
Transcranial magnetic stimulation (TMS): The scalp hotspot for the left first dorsal interosseous (FDI) muscle was identified over the hand representation of right motor cortex, and the resting motor threshold (RMT) was determined using an automatic threshold-tracking algorithm (adaptive PEST procedure, Awiszus and Borckhardt 2011). A figure-of-eight coil was held at ~45° relative to the midsagittal line (resulting in a posterior-to-anterior current direction in the brain), as this coil orientation most easily results in trans-synaptic activation of corticospinal neurons (Mills et al. 1992). In total, 600 single TMS pulses (MagStim 2002, MagStim Co. Ltd, UK) were delivered to the left FDI hotspot at a suprathreshold intensity of 120% RMT, using monophasic pulses (interpulse interval: 5 s with 15% jitter). This intensity was chosen to elicit a motor evoked potential (MEP) on each of the 600 trials. The average stimulation intensity was 54.25% ± 2.41% of maximum stimulator output, resulting in average MEP amplitudes of 1.41 ± 0.20 (SE) mV (see Table 1 for individual MEP amplitudes).
Table 1.
Average MEP amplitudes elicited by single-pulse TMS at 120% RMT for each subject. Average MEP amplitudes were calculated across all trials (excluding those contaminated by voluntary EMG activity), regardless of oscillatory phase during TMS delivery
| Subject | Average (mV) | SE (mV) |
|---|---|---|
| 1 | 1.44 | 0.05 |
| 2 | 3.99 | 0.04 |
| 3 | 0.49 | 0.03 |
| 4 | 0.63 | 0.02 |
| 5 | 0.53 | 0.02 |
| 6 | 0.43 | 0.02 |
| 7 | 1.70 | 0.03 |
| 8 | 1.07 | 0.03 |
| 9 | 1.48 | 0.06 |
| 10 | 1.90 | 0.06 |
| 11 | 0.72 | 0.03 |
| 12 | 0.69 | 0.03 |
| 13 | 1.48 | 0.06 |
| 14 | 1.84 | 0.04 |
| 15a | 1.95 | 0.06 |
| 16a | 3.00 | 0.07 |
| 17 | 1.81 | 0.07 |
| 18 | 1.34 | 0.03 |
| 19b | 0.52 | 0.02 |
| 20 | 1.26 | 0.05 |
| Average | 1.41 | |
| SE | 0.20 |
aSubjects not included in linear mixed-effects modeling due to due to excessively noisy EEG signals.
bSubject not included in linear mixed-effects modeling due to technical problems with neuronavigation-derived coil position data.
Coil position accuracy: Coil position was monitored online during TMS delivery using frameless neuronavigation (BrainSight, Rogue Research, Montreal). In addition to online monitoring, coil position in 4 software-defined dimensions (distance to target, target error, angular error, and twist error) relative to each subject’s FDI hotspot was recorded at the time of each TMS pulse. To control for coil position-related variability in MEP amplitudes, trial-by-trial measurements of the 4-coil position variables were included in the final statistical analysis as nuisance variables (see Experimental Design and Statistical Analysis). However, their inclusion did not affect the statistical results or conclusions of this study.
EMG processing: Offline processing of EMG data was performed in Matlab (TheMathWorks, Inc., Natick MA). Peak-to-peak MEP amplitudes were calculated as the difference between the maximum and minimum voltage deflections within 20–40 ms after each TMS pulse. EMG signal power was calculated in a −25 to −5 ms prestimulus window, and trials in which more than half of the samples exceeded an upper limit (defined as the 75th percentile+3*InterQuartile Range of EMG power) were excluded due to excessive EMG background activity. Trials where MEPs showed low correlations (r ≤ 0.40) with the mean MEP signal, reflecting further contamination from voluntary motor activity, were also excluded from analysis (~7% of all trials). For each subject, peak-to-peak MEP amplitudes were normalized to the mean MEP amplitude and ln-transformed to reduce skew (Lahr et al. 2016).
EEG processing: EEG was processed using FieldTrip (Oostenveld et al. 2011) and MNE Python (Gramfort et al. 2013; 2014). Data were segmented into 6 s trials and re-referenced to the average reference (Nunez and Srinivasan 2006). After eliminating trials containing artefacts (eye blinks, eye movements, large signal drifts, etc.), trials were Hjorth-transformed using sensors overlying right sensorimotor areas, with C4 as the central sensor and FC2, CP2, FC6, and CP6 as surround sensors (Hjorth 1975; Zrenner et al. 2018). Consistent with recent work (McFarland and Wolpaw 2017; Premoli et al. 2017; Zrenner et al. 2018), we defined mu and beta activity as occurring within 8–12 and 13–30 Hz, respectively. Although frequency definitions for mu and beta oscillations can vary across studies, we found that the frequency ranges chosen here captured the spectral activity in sensorimotor areas well (Figs 3C,D and 4C,D).
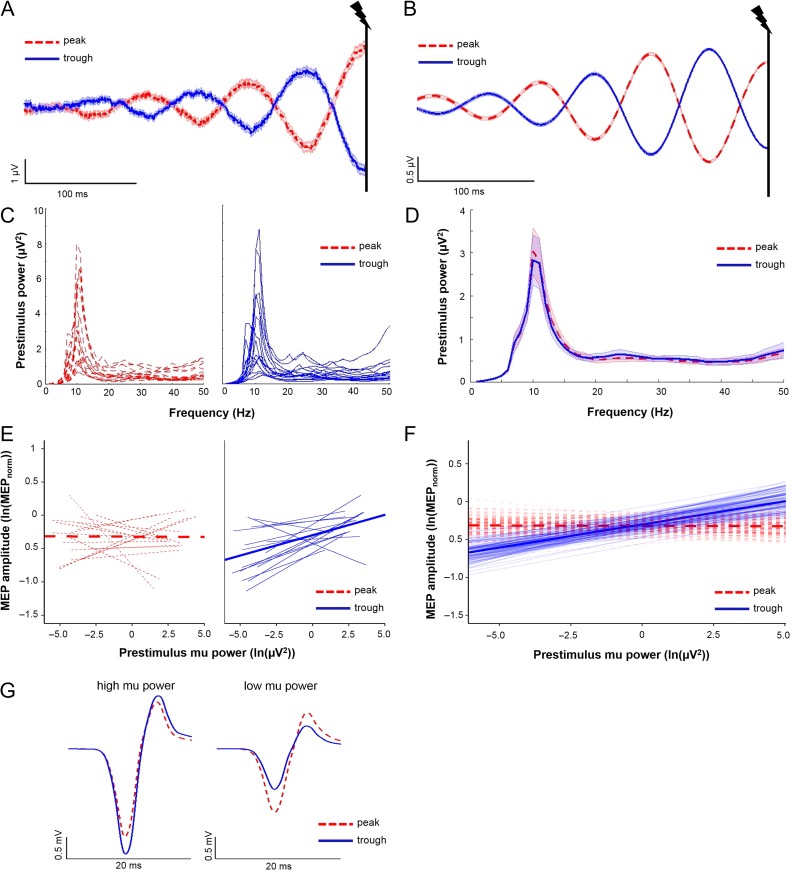
Figure 3.
Interactions between mu oscillatory phase and power influence MEP amplitudes. (A and B) Averaged raw (A) and band-pass filtered (B) C4-Hjorth data for mu peak trials (dashed red lines) and mu trough trials (solid blue lines; 0.3 s of the 6 s shown). The vertical black lines indicate time of TMS delivery at 3 s. Shading, ± 1 SEM. Note the accurate categorization of mu peak and troughs in TMS stimulated trials. (C) Individual subject power spectral density estimates for mu peak trials (dashed red lines) and mu trough trials (solid blue lines). (D) Group averaged power spectral density estimates for mu peak trials (dashed red) and mu trough trials (solid blue). Shading, ± 1 SE. For (C and D), note that power spectral density estimates are close to zero between 1 and 6 Hz due to low frequency resolution below 6 Hz (window size for estimating PSD: 150 ms). (E) Individual subject regression lines (thin) and group-level model-fits (thick) depicting relationships between prestimulus mu power and MEP amplitude for mu peak trials (dashed red lines) and mu trough trials (solid blue lines). A greater proportion of subjects showed a positive relationship between mu power and MEP amplitudes when TMS occurred at mu troughs relative to mu peaks. (F) Group-level model fits (thick solid lines) depicting relationships between prestimulus mu power and MEP amplitudes for mu peak (dashed red lines) and mu trough (solid blue lines) trials, with 100 randomly chosen estimates of group-level model fits (generated using a bootstrapping procedure; thin transparent lines). Note that the group-level model fits intersect at mid-range power levels. (G) Averaged MEP traces from a representative subject obtained at mu troughs (solid blue line) and mu peaks (dashed red line) when mu power was highest (i.e., within the top 10% of all trials used for mu statistical modeling), and when mu power was lowest (i.e., within the bottom 10% of all trials used for mu statistical modeling). Note that the direction of mu phase-dependency of MEP amplitude reverses between high and low mu power levels. N = 17 for panels (A–F); 2 subjects excluded due to noisy EEG signals during TMS trials; 1 subject excluded due to technical problems with neuronavigation-derived coil position data.
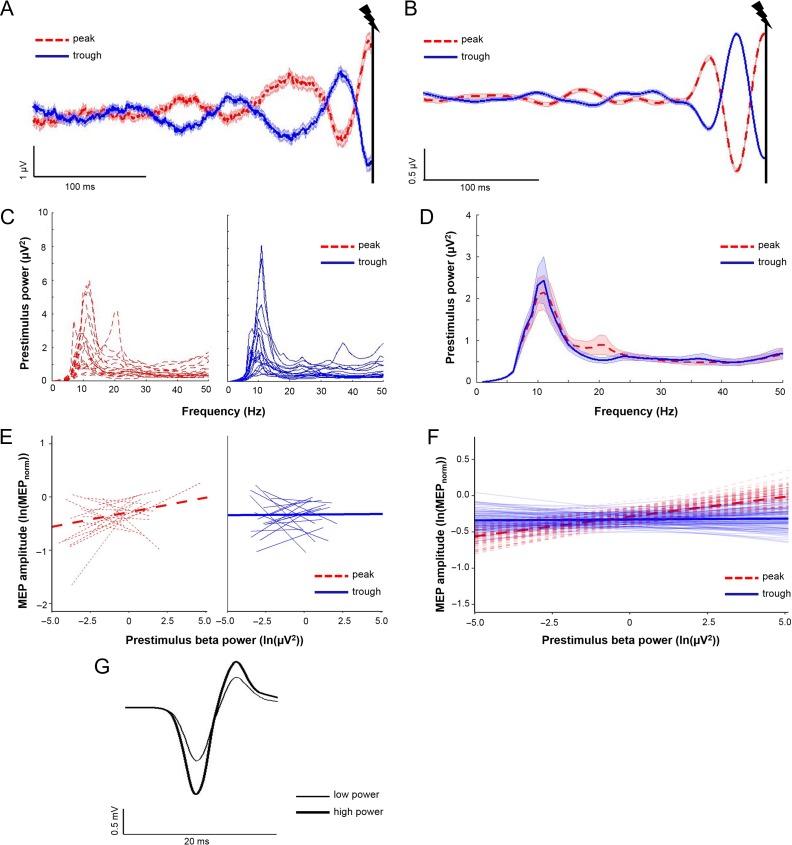
Figure 4.
Beta oscillatory power influences MEP amplitudes regardless of beta phase. (A and B) Averaged raw (A) and band-pass filtered (B) C4-Hjorth data for beta peak trials (dashed red lines) and beta trough trials (solid blue lines; 0.3 s of the 6 s shown). The vertical black lines indicate the time of TMS delivery at 3 s. Shading, ± 1 SE. (C) Individual subject power spectral density estimates for beta peak trials (dashed red lines) and beta trough trials (solid blue lines). (D) Group averaged power spectral density estimates for beta peak trials (dashed red lines) and beta trough trials (solid blue lines). Shading, ± 1 SE. For (C and D), power spectral density estimates are close to zero between 1 and 6 Hz due to low frequency resolution below 6 Hz (window size for PSD estimates: 150 ms). (E) Individual subject regression lines (thin) and group-level model-fits (thick) depicting relationships between prestimulus beta power and MEP amplitude for beta peak trials (dashed red lines) and beta trough trials (solid blue lines). A similar proportion of subjects showed positive relationships between prestimulus beta power and MEP amplitude for trials where TMS occurred during beta peaks and beta troughs. (F) Group-level model fits (thick solid lines) depicting relationships between prestimulus beta power and MEP amplitudes for beta peak trials (dashed red lines) and beta trough trials (solid blue lines), with 100 randomly chosen estimates of group-level model fits (thin transparent lines; generated using bootstrapping procedure). (G) Averaged MEP traces from the same subject from Figure 3G, obtained when beta power was highest (i.e., within the top 10% of all trials used for beta statistical modeling; thick black line) and lowest (i.e., within the bottom 10% of all trials used for beta; thin black line). Note that MEPs are larger during periods of high beta power. N = 17 for panels (A–F); 2 subjects excluded due to noisy EEG signals during TMS trials; 1 subject excluded due to technical problems with neuronavigation-derived coil position data.
EEG phase categorization in the absence of TMS: Delivering TMS during EEG recordings introduces large signal artefacts within EEG signals that can be removed using a variety of data acquisition and/or analytical procedures (i.e., sample and hold, interpolation of signals based on prestimulus and poststimulus EEG activity). Such procedures can bias oscillatory phase estimation at the exact moment of TMS delivery. Previous work evaluated the influence of sensorimotor oscillatory phase on corticospinal output, but these did not quantitatively test the accuracy of the phase estimation procedures (Mäki and Ilmoniemi 2010; Keil et al. 2013; Berger et al. 2014; Khademi et al. 2018). To avoid possible biases, we developed a novel artefact removal and phase estimation procedure, and then quantitatively evaluated its accuracy in EEG recordings obtained in the absence of any TMS.
In doing so, we estimated the instantaneous phase angle at the 3 s time point (i.e., the mid-point of each trial) of each 6 s EEG trial obtained during the first 5 min of each experimental session (see Experimental Timeline and Fig. 1). EEG was preprocessed (see EEG Processing), and then concatenated across all subjects to create a single “super subject” containing 668 6 s trials. In a first step, the value of the C4-Hjorth transformed data at 2.998 s into each 6 s trial was identified, and the remainder of each trial (2.998 s to 6 s) was clamped to this value. After clamping, all trials were band-pass filtered into the mu (8–12 Hz) and beta (13–30 Hz) frequency bands using a one-pass, causal FIR filter (Blackmann–Harris window, window size = 250 ms, order = 2500) with phase-delay correction based on the window size. Estimates of instantaneous oscillatory phase at 3 s into each 6 s trial were subsequently calculated using the Hilbert transform. Depending on the phase angle, trials were assigned to “peak” (45–135°) or “trough” (225–315°) categories, resulting in a phase window more conservative and closer to actual peaks and troughs (90° and 270°, see Fig. 1) than previous work (Zrenner et al. 2018). For the mu band, this procedure led to the categorization of 132 of 668 trials as mu peaks and 116 of 668 trials as mu troughs. Overall, 420 of 668 trials did not fit either mu phase category (outside the shaded areas depicted in Fig. 1). For the beta band, this procedure led to the categorization of 124 of 668 beta peak trials, 126 of 668 beta trough trials, and 418 trials that did not fit either beta phase category. In a second step, the actual oscillatory phase was then determined by band-pass filtering each 6 s trial using identical parameters without clamping (Fig. 2A,B; Bergmann et al. 2012; Zrenner et al. 2018). The extent to which the actual oscillatory phases clustered near 90° for peak trials and 270° for trough trials was used to quantify the accuracy of the phase estimation approach used here (see Experimental Design and Statistical Analysis and Fig. 2).
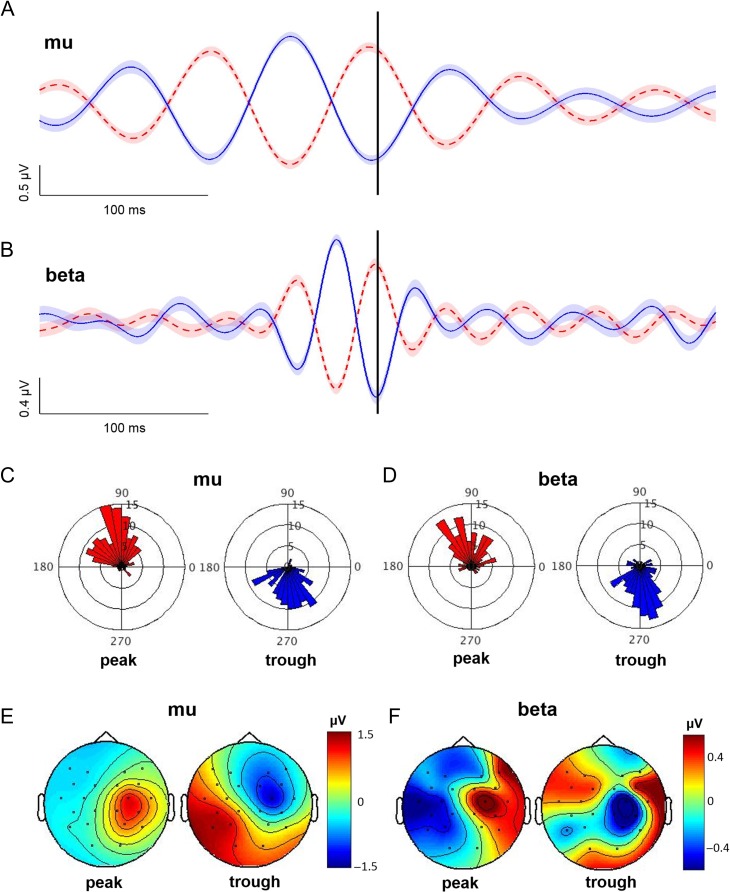
Figure 2.
EEG phase categorization in the absence of TMS. (A and B) Averaged peak and trough trials (20 subjects combined to create a single “super subject”; 0.4 of the 6 s are shown). The vertical black line shows the 3 s time point at which oscillatory phase was estimated (see Methods). Note the accurate identification of peak (dashed red) and trough (solid blue) phases for both mu (A) and beta (B) frequency bands. Shading, ± 1 SE. (C and D) Phase angle histograms reflecting the actual oscillatory phase at the 3 s time point for mu (C) and beta (D) oscillatory peak and trough trials. Each division reflects 5 trials. Note that peak trials (red) cluster near 90° while trough trials (blue) cluster near 270° for both frequency bands. (E and F) Scalp distribution of EEG activity during mu (E) and beta (F) oscillatory peaks and troughs obtained from C4-Hjorth transformed and band-pass filtered EEG data recorded in the absence of TMS. These topographies reflect average scalp-recorded EEG signals within a 3 ms window centered on mu and beta oscillatory peaks and troughs. Note that mu and beta peaks are associated with positive EEG amplitudes spatially localized to right central regions, while mu and beta troughs are associated with negative EEG amplitudes that also localized to right central regions. These topographies confirm the spatial specificity of the EEG signals used to identify mu and beta peaks and troughs.
EEG phase categorization in the presence of TMS: After evaluating the accuracy of the procedure presented above, the same approach was used to characterize the instantaneous phase at the time of TMS delivery (see Experimental Timeline and Fig. 1). That is, the value of the C4-Hjorth transformed data at 2.998 s (i.e., 0.2 ms before TMS delivery) was identified, and the remainder of each trial was clamped to this value. All trials were subsequently band-pass filtered into the mu and beta ranges (filter parameters detailed above). Estimates of instantaneous oscillatory phase at 3 s into each 6 s trial (i.e., at the exact time of TMS delivery) were calculated using the Hilbert transform. Trials were then categorized as “peak” (45–135°) and “trough” (225–315°) trials. For the mu band, this procedure led to identification of, on average, 90.35 ± 4.38 (SE) mu peak trials, 90.65 ± 3.99 mu trough trials, 86.8 ± 4.64 beta peak trials, and 96.05 ± 5.76 beta trough trials (Table 2).
Table 2.
Number of trials identified for each phase condition per subject
| Subject | Mu peak | Mu trough | Beta peak | Beta trough |
|---|---|---|---|---|
| 1 | 118 | 91 | 100 | 102 |
| 2 | 103 | 92 | 81 | 135 |
| 3 | 71 | 80 | 65 | 77 |
| 4 | 72 | 63 | 59 | 57 |
| 5 | 90 | 87 | 93 | 75 |
| 6 | 79 | 98 | 80 | 81 |
| 7 | 89 | 115 | 82 | 135 |
| 8 | 105 | 89 | 93 | 108 |
| 9 | 107 | 116 | 100 | 152 |
| 10 | 97 | 104 | 109 | 98 |
| 11 | 99 | 95 | 101 | 107 |
| 12 | 99 | 86 | 90 | 105 |
| 13 | 106 | 98 | 121 | 66 |
| 14 | 62 | 58 | 53 | 66 |
| 15a | 53 | 62 | 57 | 72 |
| 16a | 62 | 67 | 59 | 73 |
| 17 | 97 | 107 | 88 | 108 |
| 18 | 126 | 115 | 113 | 106 |
| 19b | 97 | 106 | 117 | 112 |
| 20 | 75 | 84 | 75 | 86 |
| Average | 90.35 | 90.65 | 86.8 | 96.05 |
| SE | 4.38 | 3.99 | 4.64 | 5.76 |
aSubjects not included in linear mixed-effects modeling due to excessively noisy EEG signals.
bSubject not included in linear mixed-effects modeling due to technical problems with neuronavigation-derived coil position data.
EEG power calculation: EEG power was calculated within 150 ms preceding TMS delivery (time window = 2.848 s to 2.998 s within each 6 s trial). This time window was chosen to cover at least a full cycle of oscillatory activity above 6 Hz, while also providing an estimate of mu and beta oscillatory states that are closely temporally linked to the measured MEPs. Unfiltered C4-Hjorth data were downsampled to 500 Hz, and the power spectral density between 1 and 100 Hz (excluding line noise: 58–62 Hz) was estimated using an autoregressive model approach (Burg’s method, model order: 26, frequency resolution: 1 Hz; Krusienski et al. 2006; McFarland and Wolpaw 2008). For each trial, the resulting power spectrum was whitened (i.e., normalized to 1/f) and averaged within 8–12 Hz (mu) and 13–30 Hz (beta). Data were subsequently ln-transformed to reduce skew.
Experimental design and statistical analysis: EEG phase categorization in the absence of TMS was assessed using V-tests for circular uniformity, with expected mean angles equal to 90° and 270° for peak and trough trials, respectively. Differences in mean phase angles between peak and trough trials were assessed using the Watson–Williams test (CircStat Toolbox, Berens 2009).
Group-level, within-subject variation in ln-transformed MEP amplitudes was evaluated by fitting separate trial-by-trial linear mixed effects models for each frequency band (Pinheiro and Bates 2000). Models were specified with fixed effects of PHASE (repeated measures, categorical variable), ln-transformed POWER (repeated measures, continuous variable), and the PHASE × POWER interaction, with the random intercept of SUBJECT included to account for interindividual variability in MEP amplitudes. Coil position variables were included in the statistical models as nuisance variables to control for coil position-related variability in MEP amplitudes. Group-level estimates were calculated with all coil position variables set to zero. Additional models for each frequency band were run specifying a random slope for ln-transformed POWER to ensure the best model fit. Models were compared using likelihood ratio tests. If significant improvement in model fit using a random slope was shown through the likelihood ratio test, then this specification was used as the final model. 95% Confidence intervals (CI) for group-level model fits were obtained from the final model using a bootstrapping procedure with 10 000 iterations. Alpha was equal to 0.05 for all analyses, and all statistical analyses were performed in R (R Studio Team 2015) and Matlab.
Results
Accuracy of Sensorimotor Oscillatory Phase Estimation
To evaluate the accuracy of the EEG phase categorization approach used here, we statistically tested the extent to which the actual oscillatory phases clustered near 90° for peak and 270° for peak and trough trials (see Materials and Methods; EEG phase categorization). Trials categorized as peaks and troughs were first identified (see Fig. 2A,B for averages, and Fig. 2C,D for phase angle histograms) and then evaluated for nonuniformity using V-tests. The distribution of phase angles for peak and trough trials were both nonuniform, indicating significant clustering near 90° and 270°, respectively (P < 0.001 for all, mu peak mean angle = 103.93° [95% CI: 95.26–112.61°], V = 89.42; beta peak mean angle = 91.42° [95% CI = 78.94–103.90°], V = 66.34; mu trough mean angle = 276.13° [95% CI = 266.61–285.65°], V = 78.87; beta trough mean angle = 272.27° [95% CI = 261.93–282.61°], V = 78.11). Moreover, mean phase angles differed significantly between peak and trough trials for both frequency bands (P < 0.001 for both; mu F1,246 = 590.60, beta F1,248 = 394.20). EEG activity during mu and beta peak and trough trials was localized to right central regions (Fig. 2E,F). These data confirm that the EEG phase categorization procedure used here accurately identified the phase of sensorimotor mu and beta oscillations at a single, predetermined point in time.
Influence of Sensorimotor Oscillatory Phase–Power Interactions on Corticospinal Excitability
We then evaluated the influence of mu and beta oscillatory phase on MEP amplitudes across a continuous range of power levels. Average prestimulus mu oscillatory activity showed accurate identification of mu oscillatory phase at the time of TMS delivery (Fig. 3A,B depict raw and filtered data, respectively). Likelihood ratio testing showed that an intercept-only model was preferable (P = 0.94), so this formulation was used. Trial-by-trial linear mixed-effect modeling (3173 trials total) revealed no main effects of PHASE or POWER (P = 0.513 and P = 0.950, respectively) on MEP amplitudes. However, there was a significant PHASE × POWER interaction (P = 0.002), indicating that mu phase and oscillatory power interacted to modulate MEP amplitude. This was evident as a significantly more positive relationship between prestimulus mu power and MEP amplitude for trough relative to peak trials (slope for peak trials = −0.0009 [95% CI = -0.021–0.028]; slope for trough trials = 0.061 [95% CI = 0.032–0.091], Fig. 3C,D). When mu power was highest (i.e., within the top 10% of all 3173 trials used for mu statistical modeling), MEP amplitudes were 20.12 ± 12.35% larger at mu troughs than peaks. When mu power was lowest (i.e., within the bottom 10% of all 3173 trials used for mu statistical modeling), MEP amplitudes were 10.83 ± 11.54% smaller at mu troughs than peaks (Fig. 3G shows averaged MEP traces from a representative subject). Overall, 15 of 17 subjects showed a positive relationship between prestimulus mu power and MEP amplitude when TMS occurred during mu troughs, while 9 of 17 subjects showed a positive relationship between prestimulus mu power and MEP amplitude when TMS occurred during mu peaks. For a full description of the statistical model and results, see Table 3.
Table 3.
Statistical results for linear mixed-effects models. Fixed effects of coil1–coil4 (italicized) were included in the models as nuisance variables to control for coil position-related MEP amplitude variability. Note that including fixed effects of coil1–coil4 did not modify either the statistical results or the conclusions of this study. Two subjects were excluded from linear mixed-effects modeling due to excessively noisy EEG signals, and one subject was excluded due to technical problems with neuronavigation-related coil position data (total N = 17)
| Variance | Β | Standard error | t-Value | P | |
|---|---|---|---|---|---|
| Mu (N = 17) | |||||
| Random effects | |||||
| Intercept | 0.06 | ||||
| Fixed effects | |||||
| Intercept | −0.319 | 0.085 | −3.743 | <0.001 | |
| Phase | 0.021 | 0.032 | 0.654 | 0.513 | |
| Power | −0.001 | 0.015 | −0.062 | 0.950 | |
| Phase × Power | 0.062 | 0.020 | 3.098 | 0.002a | |
| Coil1 (dist. to target) | 0.136 | 0.031 | 4.349 | <0.001 | |
| Coil2 (target error) | −0.102 | 0.042 | −2.430 | 0.015 | |
| Coil3 (angular error) | −0.037 | 0.009 | −3.928 | <0.001 | |
| Coil4 (twist error) | 0.013 | 0.004 | 3.530 | <0.001 | |
| Beta (N = 17) | |||||
| Random effects | |||||
| Intercept | 0.05 | ||||
| Fixed effects | |||||
| Intercept | −0.285 | 0.077 | −3.735 | <0.001 | |
| Phase | −0.043 | 0.040 | −1.061 | 0.289 | |
| Power | 0.055 | 0.024 | 2.254 | 0.024a | |
| Phase × Power | −0.052 | 0.033 | −1.584 | 0.113 | |
| Coil1 (dist. to target) | 0.124 | 0.030 | 4.193 | <0.001 | |
| Coil2 (target error) | −0.173 | 0.039 | −4.440 | <0.001 | |
| Coil3 (angular error) | −0.026 | 0.009 | −2.765 | 0.006 | |
| Coil4 (twist error) | 0.005 | 0.003 | 1.270 | 0.204 |
aMain effects or interactions reaching significance.
Average prestimulus beta oscillatory activity showed accurate identification of beta oscillatory phase at the time of TMS delivery (Fig. 4A,B depict raw and filtered data, respectively). Likelihood ratio testing showed that an intercept-only model was preferable (P = 0.08), so this formulation was used. Trial-by-trial linear mixed-effect modeling (3167 trials total) revealed a significant effect of POWER (P = 0.024) on MEP amplitudes, but no main effect of PHASE nor a PHASE x POWER interaction (P = 0.289 and P = 0.113, respectively). There was a significant positive relationship between prestimulus beta power and MEP amplitude that was similar for peak and trough trials (slope values for peak trials = 0.055 [95% CI = 0.006–0.102], slope value for trough trials = 0.002 [95% CI = −0.048–0.053], Fig. 4C,D). MEP amplitudes were on average 18.03 ± 19.50% larger when beta power was highest (i.e., within the top 10% of all 3167 trials used for beta statistical modeling) compared when it was lowest (i.e., within the bottom 10% of all trials used for beta statistical modeling). Figure 4G shows averaged MEP traces from a representative subject. At the individual subject level, 9 of 17 subjects showed a positive relationship between prestimulus beta power and MEP amplitude when TMS occurred at beta peaks, whereas 10 of 17 subjects showed a positive relationship between prestimulus beta power and MEP amplitude when TMS occurred at beta troughs. See Table 3 for a full description of the statistical model and results.
Discussion
This study is the first to evaluate the influence of interactions between sensorimotor oscillatory phase and power on human corticospinal output, demonstrating that these interactions gate corticospinal excitability to an extent unexplained by oscillatory phase or power alone. Specifically, the influence of mu oscillatory phase on corticospinal output varied with mu power, such that the direction of mu phase-dependency reversed with changing mu power levels. This reversal was characterized by higher corticospinal output during mu troughs when mu power was high, while the opposite was true when mu power was low. Moreover, corticospinal output was similar between troughs and peaks at mid-range mu power levels. The interactive effect between oscillatory phase and power was not present for the beta oscillation, as beta power was positively associated with corticospinal output independent of beta phase.
Phase–Power Interactions Differentially Gate Corticospinal Output
Sensorimotor oscillatory activity between 8 and 30 Hz can be functionally subdivided into mu (8–12 Hz) and beta (13–30 Hz) rhythms (Salmelin and Hari 1994; Jensen et al. 2005; Neuper et al. 2005). Mu and beta oscillatory activity reflects synchronization of postsynaptic potentials within sensorimotor pyramidal neurons (Pineda 2005; Buzsáki et al. 2012), is strongly modulated by motor activity, and desynchronizes during motor preparation, imagery, and movement (Pfurtscheller and Aranibar 1979; Pfurtscheller and Lopes da Silva 1999; Pfurtscheller et al. 2006). Consistently, mu and beta power are inversely related to brain oxygen-level dependent (BOLD) activation of sensorimotor areas (Ritter et al. 2009; Yuan et al. 2010). Mu oscillations are generated by cortical cells under the influence of thalamic nuclei (Hughes and Crunelli 2005; Haegens et al. 2015), localize to the postcentral gyrus (Salmelin and Hari 1994), and do not show clear somatotopy during movement (Salmelin et al. 1995). In contrast, beta oscillations are generated by cortical cells under the influence of corticostriatal circuits (Costa et al. 2006; Mallet et al. 2008; Sherman et al. 2016), localize cortically to the precentral gyrus (Salmelin and Hari 1994), and are more somatotopically organized than mu oscillations (Salmelin et al. 1995).
Previous work evaluated the influence of mu phase on corticospinal output without considering trial-by-trial variation in oscillatory power (Mäki and Ilmoniemi 2010; Berger et al. 2014; Khademi et al. 2018; Zrenner et al. 2018). Perhaps unsurprising given our current findings, these studies rendered inconsistent results. Here, we implemented an experimental design and analytical approach that enabled the measurement of corticospinal output across a continuous range of mu power levels. Using this approach, we found that corticospinal output was highest at mu troughs during periods of high mu power. Further, this pattern of phase-dependency completely reversed during periods of low mu power. What could explain this striking reversal? Crucially, the interactive relationship between mu phase and power was driven by a positive relationship between mu power and corticospinal excitability when TMS occurred at mu troughs, but no clear relationship (i.e., slope near zero) between mu power and corticospinal excitability when TMS occurred at mu peaks (Fig. 3E,F). Previous work suggests that high power mu troughs reflect periods during which excitatory thalamocortical inputs onto sensorimotor cortical pyramidal cells are highly synchronized (Hughes and Crunelli 2005; Pineda 2005; Buzsáki et al. 2012; Haegens et al. 2015), generating strong depolarizing currents. Such currents then bring cells closer to their firing threshold, raising their overall excitability levels and conceivably leading to a greater proportion of these cells being activated by TMS. Given that neuronal spiking rates in sensorimotor regions are highest at mu troughs (Haegens et al. 2011), it is likely that time-related variation in mu power is predominantly influenced by variation in the synchronization of excitatory thalamocortical inputs onto pyramidal neurons during mu troughs. Our results therefore suggest that when mu power is low, synchronized excitatory thalamocortical input to pyramidal cells is also low, leading to fewer cells being close to firing threshold, fewer cells being activated by TMS, and lower overall corticospinal output. In contrast, when mu power is high, synchronized excitatory thalamocortical input onto sensorimotor pyramidal cells is also high, leading to more cells being close to firing threshold, resulting in stronger corticospinal output. We therefore suggest that the mu rhythm only impacts corticospinal excitability during its excitatory phase (i.e., troughs), with mu peaks reflecting a neutral excitability state that is not strongly affected by synchronization of corticothalamic inputs to sensorimotor cortical pyramidal cells. Overall, our results indicate that the magnitude and direction of mu phase-dependency of corticospinal output is dynamically shaped by changing mu power levels, explaining previous seemingly contradictory reports (Mäki and Ilmoniemi 2010; Berger et al. 2014; Khademi et al. 2018; Zrenner et al. 2018).
In the current study, we identified a significant influence of beta power, but not beta phase nor phase–power interactions, on corticospinal output. There are multiple possible explanations for this finding. First, interactions between beta oscillatory phase and power may not influence corticospinal output, suggesting that phase–power interactions only affect corticospinal output for the mu band. Such a scenario might indicate that local cortical excitability is primarily influenced by the most spectrally dominant rhythm within a given cortical region; for sensorimotor areas, this is the mu rhythm (Assenza et al. 2017; see Fig. 3C,D). Indeed, previous work has shown that the dominant frequency of TMS-induced oscillatory activity varies across cortical regions (Rosanova et al. 2009), and that different oscillatory frequencies underlie natural variation in cortical excitability within separate cortical sites in the human visual system (Samaha et al. 2017). Our results therefore suggest that although beta oscillatory activity does index corticospinal output to some degree, this effect is overshadowed by the more dominant mu rhythm. Second, the recording and stimulation procedures used here, while suitable for uncovering phase–power relationships in the mu band, may not have been optimal for identifying phase or phase–power interactions within the beta band. Previous work examining beta phase-dependency of corticospinal output has generated mixed findings (Mäki and Ilmoniemi 2010; Keil et al. 2013; Berger et al. 2014; Khademi et al. 2018), although most reported some phase-dependency. However, those that did report such effects tested corticospinal excitability using threshold-level stimulation (i.e., 100% RMT), which is known to activate different neuronal populations compared with the suprathreshold intensity used here (i.e., 120% RMT; Di Lazzaro et al. 2004; D’Ostilio et al. 2016). Further, it was recently demonstrated that beta oscillatory influences on corticospinal excitability are only detectable using threshold-level stimulation (Raco et al. 2017; Khademi et al. 2018). Physiologically speaking, it is feasible that only certain sensorimotor cortical neuronal subpopulations interact strongly with beta oscillatory activity, a scenario which could explain conflicting results observed obtained across different stimulation intensities (Keil et al. 2013; Khademi et al. 2018; and the current findings). However, systematic evaluation of beta phase–power interactions at multiple stimulation intensities is needed to appropriately address this issue. Third, we cannot rule out that interactions between beta phase and power influence human corticospinal output. This possibility is supported by a weak trend towards an interaction between beta phase and power (Fig. 4E). Future work with larger sample sizes may be needed to definitively determine whether beta phase and power interact to shape human corticospinal output.
Interdependence of Oscillatory Phase and Power
Although oscillatory phase and power are often measured and considered separately, they are not completely independent. This is because the presence of phase information relies on the ongoing oscillatory activity having some measurable, nonzero power. Considering this relationship, it follows that phase-dependency in human motor function might only be detectable when oscillatory power is nonzero, and one would therefore expect phase-dependency of corticospinal output to scale linearly with power as seen previously in studies of visual perception (Mathewson et al. 2009). Consistent with this, Zrenner et al. (2018) recently reported higher corticospinal excitability levels during mu troughs relative to peaks when mu power exceeded a predefined threshold in preselected individuals. Here, we studied phase-related differences in corticospinal excitability over a continuous range of power levels, allowing us to capture the full range of the influence of interactions between oscillatory phase and power on descending motor output. Our findings provide new information on the nature of interactions between oscillatory strength and phase-dependency of corticospinal output, indicating that mu phase is a biomarker of the sensorimotor cortical state even during weak mu rhythms.
Interactions between oscillatory phase and power also influence cognitive function. Mathewson et al. reported improved visual perception during weak compared with strong occipital alpha oscillations; that is, visual perception was better when occipital alpha power was low. Further, when alpha power was high, visual perception was better during occipital alpha troughs relative to peaks, suggesting that occipital alpha reflects “pulsed inhibition” of visual perception (Mathewson et al. 2009, 2011). Thus, phase-dependency of visual perception was only present when occipital alpha rhythms were sufficiently strong (i.e., power was high). Our current findings show that the mu oscillation similarly impacts corticospinal output, suggesting that oscillatory phase–power interactions fundamentally organize cortical activity across multiple neural domains. However, Mathewson et al. observed that oscillatory phase did not influence visual perception when occipital alpha oscillations were weak, whereas we found that phase influenced motor output during both strong and weak mu oscillations. These disparate findings may relate to modality-specific differences in brain function or to the use of different methodological approaches (i.e., Mathewson et al. used a median split to test low vs. high power trials whereas we examined phase-dependency across a continuous range of power levels). Overall, our results suggest that considering phase–power interactions, rather than phase or power alone, is critical for accurate evaluation of oscillatory control of motor output.
Corticospinal Output as a Marker of Human Motor Function
Here, we evaluated oscillatory gating of corticospinal excitability because it is a well-established, noninvasive marker of human motor function in both health and disease (Stinear et al. 2006; Hallett 2007; Reis et al. 2008), and because it reflects the integrity of the corticospinal tract with high temporal resolution (Dayan et al. 2013). The corticospinal tract contributes heavily to hand function in humans (Lemon 2008), and its integrity is closely associated with the potential for motor recovery after stroke (Stinear et al. 2006), indicating its fundamental role in coordinating voluntary motor behavior. Second, corticospinal excitability relates to motor performance (Greenhouse et al. 2017) and motor learning (Muellbacher et al. 2001; Ziemann et al. 2004; Rosenkranz et al. 2007). For example, motor execution is faster in individuals with higher resting corticospinal excitability (Greenhouse et al. 2017), motor learning increases corticospinal excitability (Muellbacher et al. 2001; Ziemann et al. 2004; Rosenkranz et al. 2007), and brain stimulation protocols that enhance corticospinal excitability can promote motor learning (Jung and Ziemann 2009; Reis et al. 2009; Teo et al. 2010; Galea et al. 2011). Given these relationships, our results raise the intriguing possibility that brain states characterized by specific sensorimotor oscillatory phase–power interactions provide windows of opportunity where the motor system is more likely to undergo adaptive plasticity. Thus, application of neuromodulatory interventions during which stimulation is precisely timed to occur during these windows of opportunity (i.e., brain state-dependent neuromodulation) could more strongly engage sensorimotor networks compared with delivering such interventions outside of these windows, thus optimally promoting motor performance, learning and neurorehabilitation. Additionally, it is conceivable that high power mu troughs could promote motor execution or learning (Chen et al. 1998; Leocani et al. 2000; Rosenkranz et al. 2007) to a greater extent than mu peaks.
Conclusions
The current study is the first to systematically evaluate the impact of interactions between oscillatory phase and power on human corticospinal excitability. We report that these interactions influence the functional state of the human motor system, such that mu phase-dependency of corticospinal excitability varies significantly according to mu power. This variation led to a complete reversal of phase-dependency with changing mu power levels, which was driven by a positive relationship between mu power and corticospinal output at mu troughs, but no clear relationship (i.e., near zero slope) at mu peaks. Further, beta power was positively associated with corticospinal excitability independent of beta phase. We therefore conclude that sensorimotor oscillatory phase and power interact to gate human corticospinal output to an extent unaccounted for by phase or power alone.
Funding
Intramural Research Program of the National Institute of Neurological Disorders and Stroke. S.J.H. is supported by an National Institute of Neurological Disorders and Stroke Intramural Competitive Fellowship.
Note
Conflict of Interest: The authors declare no competing financial interests.
References
- Assenza G, Capone F, di Biase L, Ferri F, Florio L, Guerra A, Marano M, Paolucci M, Ranieri F, Salomone G, et al. 2017. Oscillatory activities in neurological disorders of elderly: biomarkers to target for neuromodulation. Front Aging Neurosci. 9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awiszus F, Borckhardt JJ. 2011. TMS motor threshold assessment tool (MTAT 2.0). Brain Stimulation Laboratory, Medical University of South Carolina, USA.
- Baumgarten TJ, Schnitzler A, Lange K. 2015. Beta oscillations define discrete perceptual cycles in the somatosensory domain. Proc Nat Acad Sci USA. 112(39):12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw. 31(10):1–21. [Google Scholar]
- Berger B, Minarik T, Liuzzi G, Hummel FC, Sauseng P. 2014. EEG oscillatory phase-dependent markers of corticospinal excitability in the resting brain. Biomed Res Int. 2014:936096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, Siebner HR. 2012. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci. 32(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. 2009. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 29(24):7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. 2010. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci USA. 107(37):16048–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. 2006. Rhythms of the brain. New York, NY: Oxford University Press. [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13(6):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. 1998. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 44(3):317–325. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. 2006. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 52(2):359–369. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. 2013. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 16(7):838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. 2004. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 115(2):255–266. [DOI] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. 2011. is the rhythm of your eyes: the phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. J Neurosci. 31(12):4698–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R. 2011. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J Neurosci. 31(33):11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ostilio K, Goetz SM, Hannah R, Ciocca M, Chieffo R, Chen JCA, Peterchev AV, Rothwell JC. 2016. Effect of coil orientation on strength–duration time constant and I-wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 127(1):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, Orban de Xivry JJ, Celnik P. 2011. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 21(8):1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Goj R, Jas M, Brooks T, Parkkonen L, et al. 2013. MEG and EEG data analysis with MNE-Python. Front Neurosci. 7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Parkkonen L, Hämäläinen MS. 2014. Software for processing MEG and EEG data. Neuroimage. 86:446–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Kind M, Noah S, Maddock RJ, Ivry RB. 2017. Individual differences in resting corticospinal excitability are correlated with reaction time and GABA content in motor cortex. J Neurosci. 27(10):2686–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Barczak A, Musacchia G, Lipton ML, Mehta AD, Lakatos P, Schroeder CE. 2015. Laminar profile and physiology of the α rhythm in primary visual, auditory, and somatosensory regions of neocortex. J Neurosci. 25(42):14341–14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. 2011. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci. 108(48):19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. 2007. Transcranial magnetic stimulation: a primer. Neuron. 55(2):187–199. [DOI] [PubMed] [Google Scholar]
- Hamel-Thibault A, Thénault F, Whittingstall K, Bernier PM. 2016. Delta-band oscillations in motor regions predict hand selection for reaching. Cereb Cortex. 28(2):574–584. [DOI] [PubMed] [Google Scholar]
- Hjorth B. 1975. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol. 29(5):526–530. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. 2005. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 11(4):357–372. [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. 2005. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 26(2):347–355. [DOI] [PubMed] [Google Scholar]
- Jung P, Ziemann U. 2009. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 29(17):5597–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil J, Timm J, SanMiguel I, Schulz H, Obleser J, Schönwiesner M. 2013. Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J Neurophysiol. 111(3):513–519. [DOI] [PubMed] [Google Scholar]
- Khademi F, Royter V, Gharabaghi A. 2018. Distinct beta-band oscillatory circuits underlie corticospinal gain modulation. Cereb Cortex. 28(4):1502–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusienski DJ, McFarland DJ, Wolpaw JR. 2006. An evaluation of autoregressive spectral estimation model order for brain-computer interface applications. Eng in Med and Biol Society. 28th Annual Conference of the IEEE. pp. 1323–1326. [DOI] [PubMed]
- Lahr J, Paßmann S, List J, Vach W, Flöel A, Klöppel S. 2016. Effects of different analysis strategies on paired associative stimulation. A pooled data analysis from three research labs. PLoS One. 11(5):e0154880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. 2008. Descending pathways in motor control. Annu Rev Neurosci. 31:195–218. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. 2000. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 123(6):1161–1173. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. 2008. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 28(18):4795–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. 2009. To see or not to see: prestimulus α phase predicts visual awareness. J Neurosci. 29(9):2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. 2011. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR. 2008. Sensorimotor rhythm-based brain-computer interface (BCI): model order selection for autoregressive spectral analysis. J Neural Eng. 5(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR. 2017. EEG-based brain-computer interfaces. Curr Opin Biomed Eng. 4:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, Ojemann JG, Fetz EE. 2012. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 8(9):e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. 1992. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalog Clin Neurophysiol. 85(1):17–21. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. 2001. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 136(4):431–438. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. 2010. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol. 121(4):492–501. [DOI] [PubMed] [Google Scholar]
- Neuper C, Grabner RH, Fink A, Neubauer AC. 2005. Long-term stability and consistency of EEG event-related (de-)synchronization across different cognitive tasks. Clin Neurophysiol. 116(7):1681–1694. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. 2006. Electric fields of the brain: the neurophysics of EEG. USA: Oxford University Press. [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. 1979. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr Clin Neurophysiol. 46(2):138–146. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Da Silva FL. 2006. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage. 31(1):153–159. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- Pineda JA. 2005. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Rev. 50(1):57–68. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. 2000. Linear mixed-effects models: basic concepts and examples Mixed-effects models in S and S-Plus. 3–56. [Google Scholar]
- Premoli I, Bergmann TO, Fecchio M, Rosanova M, Biondi A, Belardinelli P, Ziemann U. 2017. The impact of GABAergic drugs on TMS-induced brain oscillations in human motor cortex. Neuroimage. 163:1–12. [DOI] [PubMed] [Google Scholar]
- R Studio Team 2015. R Studio: Integrated Development for R. R Studio, Inc., Boston, MA. URL http://www.rstudio.com/.
- Raco V, Bauer R, Norim S, Gharabaghi A. 2017. Cumulative effects of single TMS pulses during beta-tACS are stimulation intensity-dependent. Brain Stimul. 10(6):1055–1060. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik P, Krakauer JW. 2009. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 106(5):1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. 2008. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 586(2):325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. 2009. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI‐BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 30(4):1168–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. 2010. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 30(25):8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G. 2008. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 19(2):203–208. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. 2009. Natural frequencies of human corticothalamic circuits. J Neurosci. 29(24):7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. 2007. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 27(44):12058–12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hari R. 1994. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 60(2):537–550. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hámáaláinen M, Kajola M, Hari R. 1995. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage. 2(4):237–243. [DOI] [PubMed] [Google Scholar]
- Samaha J, Gosseries O, Postle BR. 2017. Distinct oscillatory frequencies underlie excitability of human occipital and parietal cortex. J Neurosci. 37(11):2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MA, Lee S, Law R, Haegens S, Thorn CA, Moore CI, Jones SR. 2016. Neural mechanisms of transient neocortical beta rhythms: converging evidence from humans, computational modeling, monkeys, and mice. Proc Natl Acad Sci USA. 113(33):e4885–e4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale P, Coxon JP, Fleming MK, Byblow WD. 2006. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 130(1):170–180. [DOI] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Cheeran B, Greenwood RK, Rothwell JC. 2010. Human theta, stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex. 21(7):1627–1638. [DOI] [PubMed] [Google Scholar]
- VanRullen R. 2016. Perceptual cycles. Trends Cogn Sci. 20(10):723–735. [DOI] [PubMed] [Google Scholar]
- Wyart V, De Gardelle V, Scholl J, Summerfield C. 2012. Rhythmic fluctuations in evidence accumulation during decision making in the human brain. Neuron. 76(4):847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B. 2010. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements. Neuroimage. 49(3):2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Iliać TV, Pauli C, Meintzschel F, Ruge D. 2004. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 24(7):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner C, Desideri D, Belardinelli P, Ziemann U. 2018. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul. 11:374–389. [DOI] [PubMed] [Google Scholar]